Abstract.
Cystic echinococcosis (CE) is a chronic, complex, and overlooked zoonotic disease caused by Echinococcus granulosus. In humans, it may result in a wide spectrum of clinical manifestations depending on the type of complications, ranging from asymptomatic infection to fatal disease. The primary complications and risk factors associated with CE are not well defined. We performed a retrospective, observational study of inpatients diagnosed with CE from January 1998 to December 2017 in the public health-care system of western Spain. Five hundred and six cases were analyzed. More than half of the patients (302 [59.7%]) were asymptomatic, and the diagnoses were made incidentally. A total of 204 (40.3%) patients had complications associated with CE; 97 (47.5%) were mechanical, 62 (30.4%) were infectious, 15 (7.3%) were immunoallergic, and 30 (14.7%) involved a combination of complications. Mortality was higher in patients with mechanical complications (9.4%) than in patients with infectious complications (5.6%) and in patients with allergic complications (0%) (odds ratio = 19.7, 95% CI, 4.3–89.1, P < 0.001). In summary, CE frequently results in complications, especially in the liver in younger patients and, regardless of other variables, such as size or stage of cyst. Mechanical problems and superinfection are the most frequent complications. CE is an obligatory diagnosis in patients with urticarial or anaphylactoid reactions of unknown cause in endemic areas.
INTRODUCTION
Cystic echinococcosis (CE) is a parasitic disease with a worldwide distribution caused by the larval forms of the tapeworm Echinococcus.1 Echinococcus granulosus sensu lato and E. multilocularis are the most important species because of their geographical distribution and economic impact worldwide, causing CE and alveolar echinococcosis, respectively. Eurasia, Africa, Australia, and South America are the areas with the highest prevalence of CE, mainly because of sheep farming. CE in Europe is prevalent in countries around the Mediterranean, such as Greece, Italy, Portugal, and Spain.2 In Spain, the most affected regions were those of the northeastern, central, and western parts of the country (the autonomous regions of Aragon, Castile-La Mancha, Castile-Leon, Extremadura, Navarre, and La Rioja), where human CE incidence rates range from 1.1 to 3.4 cases per 105 inhabitants, as well as ovine/bovine CE prevalence rates of up to 23% are observed.3,4 Human infection causes cyst growth in any organ; the most common locations are the liver (> 65%) and lungs (25%). Spanish authors such as Hidalgo Pascual et al.5 and Pozo et al.6 describe the clinical course of CE in previous works. Cysts can grow approximately 1–50 mm/year and persist unchanged over the years, or they may exhibit spontaneous rupture, collapse, and disappear.7 The initial phase of primary infection is most often asymptomatic. Many infections are acquired in childhood but do not cause clinical manifestations until adulthood. Latency of more than 50 years before symptoms arise has been reported, whereas approximately 50% of detected cases occur in asymptomatic patients.8,9 Moreover, other cases remain undiagnosed or are incidentally found during autopsy.10 On the other hand, CE can frequently become complicated, causing several manifestations, including death.11 Complications frequently occur secondary to CE treatment, which is associated with surgical interventions or other therapeutic procedures, such as puncture, aspiration, injection, reaspiration.12,13 Moreover, primary complications have also been frequently described in the literature because of rupture of the cyst, superinfection, or immunological reactions. Nevertheless, the data on the incidence of these complications and the associated risk factors have not been well defined. Thus, the aim of this study was to analyze the primary complications as related to initial presentation in patients hospitalized with CE during the years 1998–2017 in a tertiary hospital to define the frequency and the risk factors involved with CE.
Patients and methods.
The design was a descriptive retrospective study. We reviewed all patients diagnosed with CE according to the ICD-9 (code 122.0 to 122.9) criteria who were admitted to the Complejo Asistencial Universitario de Salamanca (CAUSA) between January 1998 and December 2017. Complejo Asistencial Universitario de Salamanca is a tertiary care hospital; it covers an area of 12,350 km2 with 333,603 inhabitants as of 2017 (National Institute of Statistics (INE; http://www.ine.es/),14 and it is located in western Spain. The initial screening of patients was carried out using the Hospital Discharge Reports. It is an official and mandatory document that summarizes hospital care after admission and includes final diagnoses, the procedures used, and the main recommendations that the patient should follow. The clinical and epidemiological data were collected after review of the medical records. A data collection protocol was designed to collect data from medical records. We defined a primary complication of CE if a patient with CE presented with a symptom or sign attributable to CE. Patients with complications secondary to the surgical or other treatments for CE and those with missing data were excluded from the study. The Ethics Committee of CAUSA approved this study. All the data analyzed were anonymous.
Statistical analysis.
The results are expressed as frequencies for categorical variables and as the mean and ±SD for continuous variables. A chi-square test was used to compare the association between the categorical variables, such as the clinical and demographic variables, and the measured outcomes are expressed as the odds ratios (ORs) together with the 95% CIs. The continuous variables were compared with Student’s t-test or the Mann–Whitney test for two groups, depending on their normal or non-normal distribution, respectively. In addition, we applied corresponding regression models for the multivariate analysis. We considered a statistically significant difference at a P-value < 0.05.
The annual/period incidence rate of CE was calculated by dividing the number of new cases observed in the defined period (1 year or 20 years) by the total disease-free periods of disease-person time during the observation period defined in the study, multiplied by 100,000 and expressed as “cases per 105 person-years.” As it is not possible to accurately measure disease-free periods, the total figure of person-time at risk can be approximately estimated and is satisfactory when the size of the population is stable; therefore, the estimate is achieved by multiplying the average population size studied by the duration of the observation period. Thus, the denominators were obtained from population counts for each year at the municipality level of the National Institute of Statistics. All of the data were analyzed with SPSS 23.0 (Statistical Package for the Social Sciences, IBM Corp., Armonk, NY).
RESULTS
Main data.
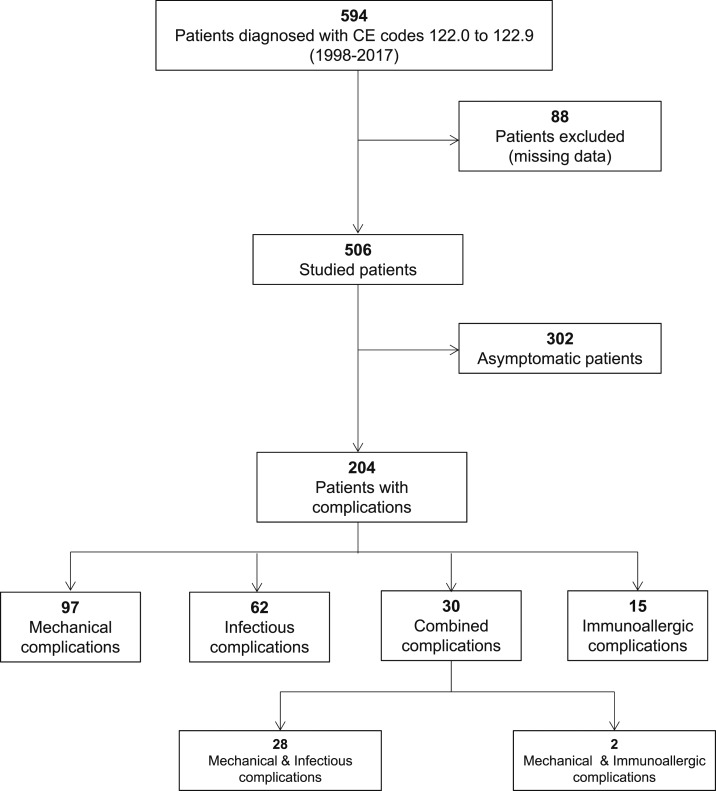
Between January 1998 and December 2017, 594 patients with new CE-related diagnosis codes 122.0 to 122.9 were registered at CAUSA, and 88 (14.8%) patients with missing data were excluded from the study. Figure 1 shows the flowchart of participants in the study. A total of 506 patients met the inclusion criteria. Table 1 shows the primary data of patients with CE.
Figure 1.
Participant profile.
Table 1.
Main clinical characteristics of the CE patients included in the present study attending at the associated complications
| Characteristics | 506 patients, n (%) |
|---|---|
| Age, mean ± SD | 59.3 ± 20.3 |
| Male | 298 (58.9) |
| Rural areas | 351 (69.4) |
| Contact with animals | 117 (23.1) |
| Other comorbidity | 239 (47.2) |
| Cyst multiple | 159 (31.4) |
| Cyst size mean ± SD | |
| Cyst size ≥ 7 cm | 228 (45.1) |
| Location CE | |
| Liver | 422 (83.4) |
| Lung | 71 (14.0) |
| Other/disseminated | 64 (12.6) |
| WHO stages | 422 (83.4) |
| I | 18/422 (4.3) |
| II | 109/422 (25.8) |
| III | 61/422 (14.5) |
| IV | 77/422 (18.2) |
| V | 157/422 (37.2) |
| Complications | 204 (40.3) |
| Eosinophilia (≥ 450/mm3) | 118 (23.3) |
| Positive serology (haemaglutination > 1/80) | 172 (34.0) |
| Hyper-IgE (IgE > 100 UI/mL) | 34 (6.7) |
| Curative treatment (surgery, surgery & drugs, and puncture, aspiration, injection, reaspiration) | 356 (70.4) |
| Only medical treatment | 17 (3.4) |
| Watch & wait | 133 (26.3) |
| Recurrences | 53 (10.5) |
| Overall mortality | 81 (16.0) |
| Attributable mortality CE | 14 (2.8) |
| Hospital stay, mean ± SD | 16.3 ± 82.0 |
| Hospitalization expense per individual of the cohort* (€ 404.69/day × 16.3 days) | 6,596.45/patient |
* Price per hospitalization per day of stay and occupied bed, according to the classification of hospitals by sections, in the CAUSA (Section 1) = 404.69 €/day.
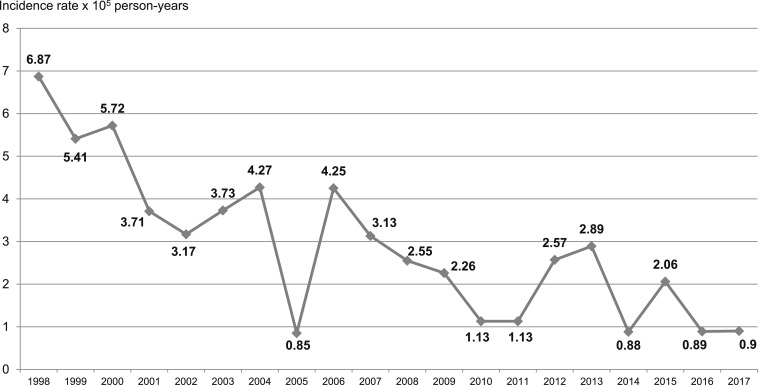
A total of 302 (59.7%) patients were asymptomatic at the time of diagnosis or during the follow-up, and the diagnosis in these cases was made incidentally. In total, 204 (40.3%) patients presented clinical data that suggested different complications of CE (Figure 2). The incidence rate during the study period (1998–2017) was 2.93 cases ×105 person-years. Figure 3 shows the progression of the annual incidence rates of CE complications over the 20 years.
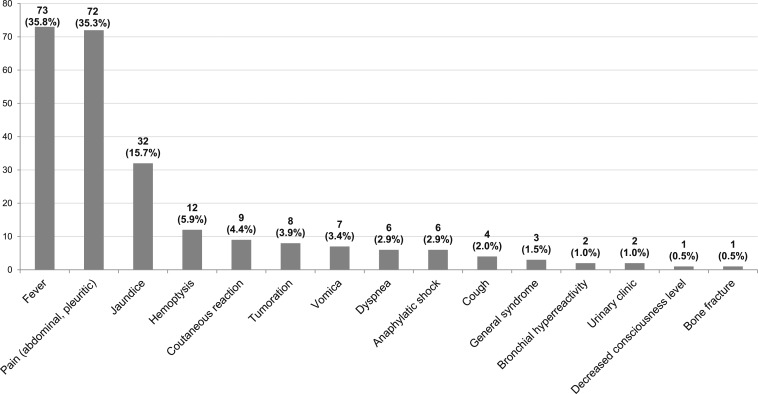
Figure 2.
Main clinical manifestations associated with complications of CE (percentage was calculated on the 204 patients who had complications).
Figure 3.
Evolution of annual incidence rates (×105 person-years) of complications of CE during the period (1998–2017).
Risk factors for complications.
Younger patients (< 60 years old) show a higher risk complication than older patients did (45.1% vs. 36.8%, P = 0.058). Table 2 shows the association analysis between the risk factors and types of complications. Allergic complications were more frequent in younger patients (< 60 years old) than in older patients (88.2% vs. 11.8%, respectively, [OR = 10.8, 95% CI, 2.4–47.7, P < 0.001] and in patients from urban areas than in patients from rural areas (58.8% vs. 41.2%, respectively) [OR = 1.6, 95% CI, 1.1–2.4, P = 0.014]. The liver was the most common location of cysts among the three groups of patients based on complications, and the incidence percentages showed significant differences. A total of 94.1% of patients had liver cysts in the allergic complications group, 83.3% in the infectious complications group, and 68.5% in the mechanical complications group (P < 0.001). In patients with allergic complications, the percentage of patients with multiple cysts was higher (52.9%, P = 0.050) than that of patients with infectious complications (28.9%) and of patients with mechanical complications (32.3%). In these patients, single cysts were more commonly detected. We did not find a statistically significant difference in the complication of CE in variables such as cyst size or WHO classification (P < 0.05).
Table 2.
Association analysis of the main complications of CE (dependent variable) and the clinical variables (independent variables) included in this study
| Independent variables | Mechanical complications (N = 127) | Infectious complications (N = 90) | Allergic complications (N = 17) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | P-value* | Or (95% CI) | n (%) | P-value* | Or (95% CI) | n (%) | P-value* | Or (95% CI) | |
| Gender, male | 80 (63.0) | 0.278 | 1.2 (0.8–1.9) | 52 (57.8) | 0.793 | 0.9 (0.6–1.5) | 11 (64.7) | 0.627 | 1.3 (0.5–3.5) |
| Age, ≥ 60 years | 66 (52.0) | 0.144 | 0.7 (0.5–1.1) | 56 (62.2) | 0.310 | 1.3 (0.8–2.0) | 2 (11.8) | < 0.001* | 0.1 (0.0–0.4) |
| Rural areas | 96 (75.6) | 0.079 | 1.5 (0.9–2.4) | 70 (77.8) | 0.055 | 1.7 (0.9–2.9) | 7 (41.2) | 0.014* | 0.6 (0.2–0.8) |
| Comorbidity | 41 (32.3) | < 0.001* | 0.4 (0.3–0.6) | 43 (47.8) | 0.892 | 1.1 (0.6–1.6) | 1 (5.9) | 0.001* | 0.1 (0.0–0.5) |
| Immunodepression | 14 (11.0) | 0.005* | 0.4 (0.2–0.8) | 15 (16.7) | 0.439 | 0.8 (0.4–1.4) | 0 | 0.038* | – |
| Single cyst | 86 (67.7) | 0.809 | 1.1 (0.7–1.6) | 64 (71.1) | 0.588 | 1.1 (0.7–1.9) | 8 (47.1) | 0.050 | 2.5 (0.9–6.7) |
| Multiple cysts | 41 (32.3) | 26 (28.9) | 9 (52.9) | ||||||
| Liver localization | 87 (68.5) | < 0.001* | 0.3 (0.2–0.5) | 75 (83.3) | 0.993 | 1.0 (0.5–1.8) | 16 (94.1) | 0.226 | 3.2 (0.4–25.0) |
| Lung localization | 33 (26.0) | < 0.001* | 3.1 (1.9–5.3) | 14 (15.6) | 0.652 | 1.1 (0.6–2.2) | 1 (5.9) | 0.324 | 0.4 (0.1–2.8) |
| Others localization | 24 (18.9) | 0.014* | 2.0 (1.1–3.4) | 11 (12.2) | 0.887 | 0.9 (0.5–1.9) | 1 (5.9) | 0.392 | 0.4 (0.1–3.2) |
| Size cyst < 7 cm | 67 (53.6) | 0.973 | 1.0 (0.7–1.5) | 36 (42.4) | 0.024* | 1.7 (1.1–2.7) | 10 (58.8) | 0.652 | 1.2 (0.5–3.3) |
| Size cyst > 7 cm | 58 (46.4) | 49 (57.6) | 7 (41.2) | ||||||
| WHO-1 | 6 (6.9) | 0.084 | – | 4 (5.3) | 0.812 | – | 1 (6.3) | 0.160 | – |
| WHO-2 | 29 (33.3) | 22 (29.3) | 4 (25.0) | ||||||
| WHO-3 | 15 (17.2) | 12 (16.0) | 5 (31.3) | ||||||
| WHO-4 | 11 (12.6) | 13 (17.3) | 0 | ||||||
| WHO-5 | 26 (29.9) | 24 (32.0) | 6 (37.5) | ||||||
| Positive serology (> 1/80) | 55 (43.3) | 0.010* | 1.7 (1.1–2.6) | 36 (40.0) | 0.190 | 1.4 (0.8–2.2) | 13 (76.5) | < 0.001* | 6.7 (2.1–20.9) |
| Hyper-IgE | 9 (7.1) | 0.159 | 4.3 (0.5–38.1) | 6 (6.7) | 0.854 | 1.2 (0.2–6.7) | 10 (58.8) | 0.028* | 0.7 (0.5–0.9) |
| Pathological eosinophilia (≥ 450) | 44 (36.4) | 0.001* | 2.2 (1.4–3.4) | 30 (34.5) | 0.022* | 1.8 (1.1–2.9) | 7 (46.7) | 0.048* | 2.7 (1.1–7.7) |
| Tumor associated | 2 (1.6) | 0.009* | 0.2 (0.4–0.7) | 3 (3.3) | 0.175 | 0.4 (0.1–1.5) | 0 | 0.267 | – |
| Thrombosis associated | 0 | 0.028* | – | 1 (1.1) | 0.290 | 0.3 (0.1–2.7) | 1 (5.9) | 0.427 | 2.3 (0.3–18.5) |
| Preoperative fistula | 41 (32.3) | < 0.001* | 3.3 (2.1–5.4) | 28 (31.1) | < 0.001* | 2.6 (1.5–4.5) | 4 (23.5) | 0.500 | 1.5 (0.5–4.6) |
| Curative treatment (surgery + puncture, aspiration, injection, reaspiration) | 109 (85.8) | < 0.001* | 3.2 (1.9–5.5) | 61 (67.8) | 0.533 | 0.8 (0.5–1.4) | 17 (100.0) | 0.007* | – |
| Drugs or Watch & Wait | 18 (14.2) | 29 (32.2) | 0 | ||||||
| Recurrences | 14 (11.0) | 0.815 | 1.1 (0.6–2.1) | 11 (12.2) | 0.555 | 1.2 (0.6–2.5) | 3 (17.6) | 0.328 | 1.8 (0.5–6.7) |
| Cohort mortality | 18 (14.2) | 0.515 | 0.8 (0.5–1.5) | 17 (18.9) | 0.416 | 1.3 (0.7–2.3) | 0 | 0.067 | – |
| CE disease mortality | 12 (9.4) | < 0.001* | 19.7 (4.3–89.1) | 5 (5.6) | 0.076 | 2.6 (0.8–8.1) | 0 | 0.479 | – |
* Statistical significance level of 5% (P < 0.05).
Mechanical complications.
In our cohort, the macroscopic rupture of CE and secondary fistula were the most frequent complications detected in 17.4% (88) of the patients (Table 3). The most frequent fistulas were biliocystic in 68/88 (77.2%) patients, followed by bronchial fistulas in 12/88 (13.6%) cases, pleural fistulas in 6/88 (6.8%) cases, and pericardial and cutaneous in 1/88 (1.14%) patient with liver and thoracic CE.
Table 3.
Description of the main types of complications associated with CE
| Type of complications | N = 506 patients n (%) |
|---|---|
| Mechanical complications | 127 (25.1) |
| Fistulas | 88 (17.4) |
| Biliary | 68 |
| Bronchial | 12 |
| Pleural | 6 |
| Pericardial | 1 |
| Cutaneous | 1 |
| Compression | 28 (5.5) |
| Displacement of adjacent structures | 18 |
| Obstruction | 10 |
| Portal hypertension | 2 (0.4) |
| Cyst rupture | 15 (2.9) |
| Cyst rupture | 14 |
| Collapse middle lobe | 1 |
| Infectious complications | 90 (17.8) |
| Cholangitis or biliary sepsis | 50 |
| Superinfection of CE | 40 |
| Immunoallergic complications | 17 (3.3) |
| Urticarial angioedema, | 9 |
| Bronchial hyper-reactivity | 2 |
| Anaphylaxis | 6 |
The patients who had biliary fistulas manifested jaundice and abdominal pain (32/88, 36.7%). The patients with bronchial fistulas had vomiting (7/12, 58.3%), and the patients with bronchial, pleural, or pericardial fistulas presented thoracic pain (3) and dyspnea (4). Most patients with fistulas (84/88, 95.5%) were treated surgically, either by surgery alone (34/88, 38.6%) or surgery and drugs (50/88, 56.8%) (OR = 9.3, 95% CI, 3.3–26.1, P < 0.001]. Four patients decided to “watch and wait.” There were 8/88 (9.1%) deaths among patients with fistulas, and 4/88 (4.5%) deaths were caused by CE.
Twenty-eight (5.5%) patients had compression with displacement of adjacent structures (18/28, 64.3%) or obstruction (10/28, 35.7%). The cysts ruptured in 15 (2.9%) patients.
Two patients with liver CE presented with complications such as portal hypertension. Both patients presented with portal cavernomatosis, and they were free from other liver diseases. A total of two patients presented with variceal hemorrhage and one patient had ascites.
In the lungs, 1/71 (1.4%) patients showed a medium lobe collapse caused by CE displacement. One of two (50%) patients presented medullary section syndrome secondary to intramedullary CE.
Infectious complications.
Coinfections associated with CE were detected in 17.8% (90) of patients (Table 2); 28/90 patients had fistulas (OR = 2.6, 95% CI, 1.5–4.5, P < 0.001). The clinical symptoms in these patients were cholangitis or biliary sepsis in 50/90 cases (55.5%) and hepatic or thoracic abscesses in 40/90 cases (44.4%) (Table 3). Mycetoma was the final diagnosis in 3 (0.6%) cases. The final microbiological diagnosis of superinfection only was achieved in 25 (40.9%) superinfected hepatic cysts versus 4 (44.0%) superinfected thoracic cysts.
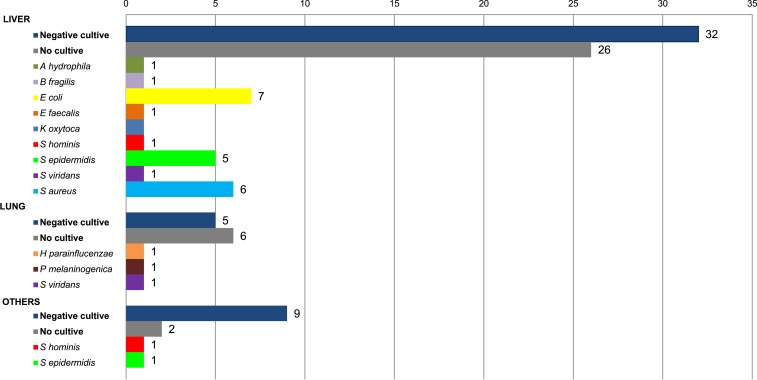
The isolated bacterial organisms are shown in Figure 4. The most frequently isolated microorganism was Escherichia coli (7), followed by Staphylococcus aureus (6) and Staphylococcus epidermidis (6). Polymicrobial infections were detected in 2 (6.8%) patients. Aspergillus fumigatus was found in the pathological anatomy of three patients with mycetoma. Antimicrobial and surgical agents were used in all patients, and surgical and/or radiological drainage was used in 10 patients (10/90, 11.1%).
Figure 4.
Microorganisms identified regarding the location of infection. This figure appears in color at www.ajtmh.org.
Immunoallergic complications.
Other complications detected in our cohort were immunological reactions attributable to CE in 17 (3.3%) patients (Table 2). Urticaria/angioedema was the most frequently detected complication, observed in 9/17 cases (52.9%), followed by anaphylaxis in 6/17 cases (35.3%) (Table 3). Patients with immunological reactions frequently presented with hyper-IgE (> 100 UI/mL), eosinophilia (> 450 eosinophils/mm3), and seropositivity (hemagglutination indirect title > 1/80) compared with the rest of the patients (P < 0.05). Surgical intervention for the cyst was performed in all 17 patients (100%) and in combination with antiparasitic drugs in 14 (82.3%) of them.
Disease mortality was higher in CE patients with mechanical complications (9.4%) than in patients with infectious complications (5.6%) and in patients with allergic complications (0%) (OR = 19.7, 95% CI, 4.3–89.1, P < 0.001) (Table 2).
DISCUSSION
Although treatment of echinococcosis is not well defined, antiparasitic drugs along with surgical procedures are usually the first line of treatment. Nevertheless, CE surgery is frequently associated with complications in clinical practice, in which certain patients, especially the elderly with other comorbidities, are treated based on “watch and wait.” In a recent study, this treatment strategy was associated with higher mortality attributable to CE complications.13
The spontaneous complications of CE have also been commonly referred to in the literature, although their frequency and risk factors involved have not been well established. We aimed to study the spontaneous complications of CE. Thus, we selected all the inpatients with a diagnosis of CE, and we studied the patients showing symptoms attributable to the complications of cysts during follow-up.
Because CE is usually an asymptomatic parasitic infection, we considered CE patient to have complications if the patient presented symptoms, clinical signs, or radiological findings attributable to CE. In our study, we excluded secondary complications due to a surgical procedure.
First, we detected a low complication rate, showing that CE complications are rare in our area, despite a high prevalence found in a previous report.15
Nevertheless, when we focused on people infected with CE, the complication rate was above 40%. These data are higher than those shown by other groups. Despite the selection bias, the results exclusively from inpatients suggest that patients with CE had a high risk of developing illnesses associated with CE.
In our work, we analyzed the variables associated with CE complications, and we found that younger patients presented a higher rate of complications than older patients did. This result could be due to a longer follow-up in younger people than in the elderly. Moreover, we detected that patients from rural areas had a higher rate of complications than did patients from urban areas. Patients in rural areas tend to have less access to health services than the urban population does and, consequently, rural residents may receive a late diagnosis when CE is diagnosed by a complication.
Regarding the characteristics of CE, we found a higher rate of CE complications located in the liver than in other locations. Other characteristics of CE, such as size or WHO stage, were not associated with a higher rate of CE complications. These results support the notion that the size and/or earlier WHO stage of CE is not useful for discriminating between patients who must undergo a surgical procedure and those who can wait.
The complications detected could be classified by their pathogenesis into the following: mechanical, which were caused by rupture (with frequent occurrence of fistula) or compression of the structures next to the cyst, superinfection, caused by involvement of microorganisms, and immunological, due to immunoallergic reactions attributable to echinococcosis.
Among all patients in our cohort, mechanical complications were the most frequently detected. The rupture of a liver or thoracic cyst, and consequently the occurrence of fistulas in the biliary tract, bronchial tree, or pleura, were detected in 88/506 (17.4%) patients. Postsurgery fistulas have been frequently described in the literature.16 In our study, we showed that this is the main spontaneous complication of echinococcosis. Other studies showed that the rupture of CE into the biliary tract occurs in 9% of operated patients because of hydatid cysts.17,18
Peritoneal, pleural, pericardial, and cutaneous fistulas have been described in the literature, although these are less frequent. We did not find an association between the presence of fistulas and patient characteristics, such as gender, age, and comorbidities.
We analyzed the association between fistulas and infection rate. More than 30% of patients with fistulas were superinfected. It was difficult to determine which was the first pathogenic mechanism, fistula or superinfection. However, we observed an association with a larger size and a less advanced WHO stage. These results support the hypothesis that the ruptured cysts are frequently caused by high superficial tension in the wall of a large cyst; however, rupture has also been described after hard external traumatism.19
Other mechanical complications detected in our cohort were due to direct compression of the structures adjacent to CE cysts. The heart, central nervous system, or other main organs can become affected, especially in CE cysts of large size, causing arrhythmia and heart failure,20 headache, epilepsy, or paralysis.21
In our work, one patient had a spell of medullary syndrome caused by an intramedullary CE cyst. Because of slow growth in the liver, there are no complications until the cysts reach a large size. In our cohort, we detected two patients with portal hypertension in the clinical setting. The onset symptom in both patients was a variceal hemorrhage with portal vein cavernomatosis. Among the literature, only our study detected cases with these complications. It is possible that compression of the vena cava and portal vein thrombosis led to portal hypertension and secondary portal cavernomatosis, as presented in two previous patients.22
The second most frequent complication was superinfection of CE. In previous works, the rate of superinfection in hydatid cysts ranged from 1% to 8%, but these studies were specifically designed to evaluate this complication, and the inclusion criteria were not well defined.23–25 Our group conducted a retrospective study in 2009 that included patients with positive cultures or purulent drainage and included 37 cases in 12 years.26 Gram-positive cocci, Enterobacteriaceae, and Aspergillus spp. were the most prevalent pathogens in both hepatic and pulmonary cysts, but because of the characteristics of the study, we did not establish the frequency or the risk factors involved. Thus, in the present study with a cohort of inpatients, we detected more than 14% of patients with superinfection. Several bacterial or fungal superinfections were found in patients with CE from sites close to the hydatid cyst (e.g., biliary or bronchial tree). In these cases, a macroscopic or microscopic rupture of the wall cyst is involved in the pathogenesis. In this sense, above one-third of patients with superinfection presented with biliary or bronchial-associated fistulas. Moreover, it is possible that the cyst promotes superinfection by compression and distortion of the biliary tree.
On the other hand, microorganisms can be disseminated by bacteremia from a distant location causing CE infection. In our study, we detected six patients with superinfection by S. aureus, which colonizes the upper respiratory tract. The classic risk factors for bacteremia, such as diabetes mellitus, the use of steroids, renal failure, neoplasia, or HIV, were found in 23/204 patients; however, we did not detect statistically significant associations of these factors with superinfection.
The different types of microorganisms involved according to the location of the cyst and its clinical manifestations are another point of interest in our cohort. We found that although the patients with bacterial superinfection presented with fever, local pain, and other pulmonary cysts infected with Aspergillus spp., the patients in this study were asymptomatic at diagnosis. Kocer et al. described that 2% of patients with thoracic hydatidosis have a superinfection with Aspergillus spp. and present with symptoms of cough, hemoptysis, chest pain, and fever.27
Another classic complication is the immune or hypersensitive reaction28 with a wide variety of symptoms ranging from skin manifestations, such as urticaria/angioedema, or respiratory symptoms, such as asthma, to the most severe cases, such as anaphylactic shock. First, we found a low rate of this type of complication, with urticaria/angioedema being the most frequent, followed by anaphylactic shock and asthma. In vitro and in vivo studies have shown that protoscolex and somatic antigens can activate the alternative complement pathways C3a, C4a, and C5a, which bind to mast cell receptors causing degranulation. Consequently, histamine and other vasoactive amines are released, causing anaphylactic reactions.29,30 These reactions have also been described in other helminthic infections, such as canine dirofilariasis.31
On the other hand, it is possible that another IgE-based classic mechanism of allergy blinded to molecular EgEF-1 beta/delta is involved.32 In our cohort, patients with immunological reactions frequently displayed hyper-IgE eosinophilia and seropositivity. In previously published studies, a positive correlation was found between the IgE levels and disease severity and/or progression/regression after treatment.33,34
A limitation of this work is the selection bias because the cases included in the study were inpatient. Therefore, asymptomatic patients are usually diagnosed with incidentaloma. It is possible that the frequency of complications described in this manuscript is too minor to discuss. Therefore, it is very difficult to estimate the actual prevalence of the disease and the actual percentage of spontaneous complications, as many asymptomatic individuals will remain undetected.
In summary, there were frequent complications in the current cohort of patients with CE, especially in the liver cysts of younger patients, regardless of other variables, such as size or stage of the cyst. Mechanical complications and superinfection were the most frequent complications. Patients in endemic areas with urticarial and anaphylactic reactions of unknown cause should be tested for CE.
REFERENCES
- 1.Thompson RCA, McManus DP, 2002. Towards a taxonomic revision of the genus Echinococcus. Trends Parasitol 18: 452–457. [DOI] [PubMed] [Google Scholar]
- 2.Cappello E, Cacopardo B, Caltabiano E, Li Volsi S, Chiara R, Sapienza M, Nigro L, 2013. Epidemiology and clinical features of cystic hydatidosis in western Sicily: a ten-year review. World J Gastroenterol 19: 9351–9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmena D, Sánchez-Serrano LP, Barbero-Martínez I, 2008. Echinococcus granulosus infection in Spain. Zoonoses Public Health 55: 156–165. [DOI] [PubMed] [Google Scholar]
- 4.Rojo-Vazquez FA, Pardo-Lledias J, Francos-Von Hunefeld M, Cordero-Sánchez M, Alamo-Sanz R, Hernandez-Gonzalez A, Brunetti E, Siles-Lucas M, 2011. Cystic echinococcosis in Spain: current situation and relevance for other endemic areas in Europe. PLoS Negl Trop Dis 5: e893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hidalgo Pascual M, Barquet Esteve N, 1987. Hepatic hydatidosis. Study of a series of 7,435 cases. I: general aspects, epidemiology and diagnosis. Rev Esp Enferm Apar Dig 71: 1–6. [PubMed] [Google Scholar]
- 6.Pozo F, Fernández MJ, Suárez TV, Tojo S, Lamamie E, Rodrigo LR, 1987. Epidemiologic study of human hydatidosis in Asturias (1975–1984). Med Clín (Barc) 89: 773–777. [PubMed] [Google Scholar]
- 7.Romig T, Zeyhle E, Macpherson CN, Rees PH, Were JB, 1986. Cyst growth and spontaneous cure in hydatid disease. Lancet 1: 861. [DOI] [PubMed] [Google Scholar]
- 8.Armiñanzas C, Gutiérrez-Cuadra M, Fariñas MC, 2015. Hydatidosis: epidemiological, clinical, diagnostic and therapeutic aspects. Rev Esp Quimioter 28: 116–124. [PubMed] [Google Scholar]
- 9.Velasco-Tirado V, et al. 2017. Recurrence of cystic echinococcosis in an endemic area: a retrospective study. BMC Infect Dis 17: 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schenone H, 1989. Cysticercosis and hydatidosis do not always bring about detectable pathology in humans. Bol Chil Parasitol 44: 63–65. [PubMed] [Google Scholar]
- 11.Belhassen Garcia M, et al. 2014. Study of hydatidosis-attributed mortality in endemic area. PLoS One 9: e91342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akhan O, Salik AE, Ciftci T, Akinci D, Islim F, Akpinar B, 2017. Comparison of long-term results of percutaneous treatment techniques for hepatic cystic echinococcosis types 2 and 3b. AJR Am J Roentgenol 208: 878–884. [DOI] [PubMed] [Google Scholar]
- 13.Velasco-Tirado V, et al. 2018. Management of cystic echinococcosis in the last two decades: what have we learned? Trans R Soc Trop Med Hyg 112: 207–215. [DOI] [PubMed] [Google Scholar]
- 14.Ine.Es , 2015. Available at: http://www.ine.es. Accessed July 18, 2015.
- 15.Lopez-Bernus A, Belhassen Garcia M, Alonso-Sardón M, Carpio-Perez A, Velasco-Tirado V, Romero-Alegria A, Muro A, Cordero-Sánchez M, Pardo-Lledias J, 2015. Surveillance of human echinococcosis in Castilla-Leon (Spain) between 2000–2012. PLoS Negl Trop Dis 9: e0004154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stamm B, Fejgl M, Hueber C, 2008. Satellite cysts and biliary fistulas in hydatid liver disease. A retrospective study of 17 liver resections. Hum Pathol 39: 231–235. [DOI] [PubMed] [Google Scholar]
- 17.Köksal N, Müftüoglu T, Günerhan Y, Uzun MA, Kurt R, 2001. Management of intrabiliary ruptured hydatid disease of the liver. Hepatogastroenterology 48: 1094–1096. [PubMed] [Google Scholar]
- 18.Paksoy M, Karahasanoglu T, Carkman S, Giray S, Senturk H, Ozcelik F, Ergüney S, 1998. Rupture of the hydatid disease of the liver into the biliary tracts. Dig Surg 15: 25–29. [DOI] [PubMed] [Google Scholar]
- 19.Yilmaz M, Akbulut S, Kahraman A, Yilmaz S, 2012. Liver hydatid cyst rupture into the peritoneal cavity after abdominal trauma: case report and literature review. Int Surg 97: 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oraha AY, Faqe DA, Kadoura M, Kakamad FH, Yaldo FF, Aziz SQ, 2018. Cardiac hydatid cysts; presentation and management. A case series. Ann Med Surg (Lond) 30: 18–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nourbakhsh A, Vannemreddy P, Minagar A, Toledo EG, Palacios E, Nanda A, 2010. Hydatid disease of the central nervous system: a review of literature with an emphasis on Latin American countries. Neurol Res 32: 245–251. [DOI] [PubMed] [Google Scholar]
- 22.Kirmizi S, Kayaalp C, Yilmaz S, 2016. Hydatid liver cyst causing portal vein thrombosis and cavernous transformation: a case report and literature review. Gastroenterol Hepatol Bed Bench 9: 331–335. [PMC free article] [PubMed] [Google Scholar]
- 23.Pedrosa I, Saíz A, Arrazola J, Ferreirós J, Pedrosa CS, 2000. Hydatid disease: radiologic and pathologic features and complications. Radiographics 20: 795–817. [DOI] [PubMed] [Google Scholar]
- 24.Symeonidis N, Pavlidis T, Baltatzis M, Ballas K, Psarras K, Marakis G, Sakantamis A, 2013. Complicated liver echinococcosis: 30 years of experience from an endemic area. Scand J Surg 102: 171–177. [DOI] [PubMed] [Google Scholar]
- 25.Alexiou K, Mitsos S, Fotopoulos A, Karanikas I, Tavernaraki K, Konstantinidis F, Antonopoulos P, Ekonomou N, 2012. Complications of hydatid cysts of the liver: spiral computed tomography findings. Gastroenterology Res 5: 139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García MB, Lledías JP, Pérez IG, Tirado VV, Pardo LF, Bellvís LM, Varela G, Sánchez MC, 2010. Primary super-infection of hydatid cyst—clinical setting and microbiology in 37 cases. Am J Trop Med Hyg 82: 376–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koçer NE, Kibar Y, Güldür ME, Deniz H, Bakir K, 2008. A retrospective study on the coexistence of hydatid cyst and aspergillosis. Int J Infect Dis 12: 248–251. [DOI] [PubMed] [Google Scholar]
- 28.Vuitton DA, 2004. Echinococcosis and allergy. Clin Rev Allergy Immunol 26: 93–104. [DOI] [PubMed] [Google Scholar]
- 29.Perrigone R, Fontana L, De Carolis C, Ottaviani P, 1980. Activation of alternative complement pathway by fluid from hydatid cysts. N Engl J Med 302: 808–809. [DOI] [PubMed] [Google Scholar]
- 30.Diáz A, Ferreira AM, Nieto A, 1995. Echinococcus granulosus: interactions with host complement in secondary infection in mice. Exp Parasitol 80: 473–482. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton RG, Wagner E, April M, Winkelstein JA, Sobotka AK, Bleeker E, Adkinson NF, 1986. Dirofilaria immitis: diethylcarbamazine-induced anaphylactoid reactions in infected dogs. Exp Parasitol 61: 405–420. [DOI] [PubMed] [Google Scholar]
- 32.Ortona E, Margutti P, Vaccari S, Riganò R, Profumo E, Buttari B, Chersi A, Teggi A, Siracusano A, 2001. Elongation factor 1 beta/delta of Echinococcus granulosus and allergic manifestations in human cystic echinococcosis. Clin Exp Immunol 125: 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riganò R, Profumo E, Teggi A, Siracusano A, 1996. Production of IL-5 and IL-6 by peripheral blood mononuclear cells (PBMC) from patients with Echinococcus granulosus infection. Clin Exp Immunol 105: 456–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torcal J, Navarro-Zorraquino M, Lozano R, Larrad L, Salinas JC, Ferrer J, Roman J, Pastor C, 1996. Immune response and in vivo production of cytokines in patients with liver hydatidosis. Clin Exp Immunol 106: 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]