Abstract
Human gingiva-derived mesenchymal stem cells (GMSCs) have been considered to be a better source of MSCs for cell therapy in some immunological diseases. We describe a protocol for isolation and culture of mesenchymal stem cells (MSCs) from human gingival tissue in detail, which provides a methodology to help clinical researches and clinical trial. GMSCs are generally isolated from a remnant or discarded tissue following a routine dental procedure, then cultured in complete culture medium at 37°C in a humidified tissue culture incubator with 5% CO2 and 95% O2. Non-adherent cells are removed after 48~72 h and the fresh medium is replaced. When primary cultures become 80%~90% confluent, the plastic-adherent cells are treated with 0.25% trypsin-EDTA and subcultured. A purified population of GMSCs can be obtained 2-3 weeks after the initiation of culture.
Keywords: Human gingiva-derived mesenchymal stem cells, protocol, isolation and preparation, autoimmune and inflammatory diseases
Introduction
Mesenchymal stem cells (MSCs), also referred to as multipotent stromal cells, are a cluster of well-established cells characterized by non-hematopoietic abilities of self-renewal and multipotent differentiation [1,2]. Due to their multiple differentiation capabilities, inflammatory and immune modulation function, MSCs have been explored as an attractive therapeutic agent in various diseases including allergic conjunctivitis, acute lung injury, myocardial infarction, allergic rhinitis, Alzheimer’s disease, and type 1 diabetes [3-9]. MSCs can be isolated from different tissues, including bone marrow, umbilical cord, placenta, adipose tissue, and human gingiva. Human gingiva-derived mesenchymal stem cells (GMSCs) are a member of MSCs and have been considered as a better source of MSCs for their ease of isolation, homogeny, faster proliferation, stable characteristics, and stable karyotype. Recently, GMSCs have been increasingly appreciated for their anti-inflammatory and immunomodulatory effects in autoimmune and inflammatory diseases [10-12]. The main mechanisms involved in the immunomodulatory effects of GMSCs include the secretion of anti-inflammatory factors such as IDO, IL-10, PGE2, COX-2, adenosine and inducible nitric oxide synthase (iNOS) [13-15] and the interactions of GMSCs with immune cells including T cells, B cells, macrophages, and dendritic cells [7,14]. GMSCs treatment mitigated local inflammation by suppressing infiltration of inflammatory cells and production of inflammatory cytokines, promoting the induction of CD4+FoxP3+ T regulatory cell (Treg) or elicit M2 polarization of macrophages [10,12,16]. It is well recognized that inflammatory cells and cytokines production are crucially involved in the pathogenesis of autoimmune inflammatory diseases [17-21]. Treg cells also play a crucial role in preventing autoimmune diseases and treating inflammatory diseases [22-27]. Future study needs to determine whether GMSCs promote Treg induction, expansion or stability since these parameters all affect Treg biological features [28-30]. It is still unclear whether the therapeutic effect of GMSCs on autoimmune inflammatory diseases completely or partially depends upon Treg cells.
In addition, GMSCs infusion modulates cell growth by modulating the expression of apoptosis and proliferation-related genes such as p-JNK, cleaved PARP, cleaved caspase-3, p-ERK1/2, Bcl-2 [31]. GMSCs also inhibited human immune responses via CD39/CD73/adenosine and IDO signals [7,11,15]. These studies have an important implication on clinical setting. Indeed, recent safety study has highlighted the clinical feasibility and safety of GMSCs [32]. However, the depth mechanism(s) by which GMSCs display their immune modulatory function need to further be investigated. For this reason, a detailed protocol including GMSCs isolation, culture and characterization for researchers is required, especially for those who never previously study GMSCs.
For the past several years, our group has been focusing on the isolation, characterization, and function of GMSCs in different autoimmune diseases. We describe here a detailed protocol for uncomplicated isolation of GMSCs from human gingival tissues. This protocol is primarily derived from the original description by Le AD et al [13]. A purified population of GMSCs with spindle-shaped morphology is obtained in the first passage (about 2 weeks after the initiation of culture). Moreover, no additional growth factors are required for this protocol, which is advantageous because many growth factors and related products can modify protein synthesis and intracellular trafficking that will indirectly affect the biological behaviors of GMSCs.
Reagents and equipment
MEM Alpha(1X) + GlutaMAXTM-I (gibco, #32561-037), 0.25% Trypsin-EDTA (Gibco, #27250-018); Fetal Bovine Serum (gibco, #10270-106), Penicillin-Streptomycin (gibco, #5140122), Collagenase IV (sigma, #C5138), Dispase II (sigma, #D4693); Hood for cell culture with vertical laminar flow and equipped with UV light for decontamination; Centrifuge.
Procedure
1. Human gingival tissues are obtained as a remnant or discarded tissue following a routine dental procedure and the donor should have no history of periodontal diseases and relatively healthy periodontium.
2. The collected tissue will be soaked in MEM Alpha medium supplemented with 2% fetal bovine serum and 100 ug/mL penicillin/streptomycin and stored at 4°C refrigerator or brought to the lab immediately.
3. Gingival tissues will be washed with washing buffer (PBS supplemented with 2% fetal bovine serum and 100 ug/mL penicillin/streptomycin) for 3~5 times and incubated overnight at 4°C with dispase II (2 mg/mL in wash buffer) and then washed with washing buffer for one time.
4. Gingival tissues will be minced into 1-3 mm2 fragments and digested with collagenase IV (4 mg/mL in wash buffer) at 37°C for 2 h.
5. The digested tissue suspension will be filtered through a 40-μm cell strainer (Falcon) and centrifuged at 300 g for 5 minutes.
6. Abandon supernatant, and add 1 mL complete culture medium (MEM Alpha medium supplemented with 10% fetal bovine serum, 100 ug/mL penicillin/streptomycin and 10 mM Hepes) to resuspend dissociated cells and then plate on a 10 cm petri-dish with 10 mL complete culture medium and culture at 37°C in a humidified tissue culture incubator with 5% CO2 and 95% O2.
7. After being cultured for 48 h to 72 h, the non-adherent cells will be removed and fresh complete culture medium will be added. Change medium every two days.
8. Adherent Cells will be digested with 1.5 mL/dish of 0.25% trypsin-EDTA when they reached to an 80-90% confluent density and subcultured at a ratio of 1 to 4 in complete culture medium. Cells from passages 3 to 5 will be used for the experiments.
Anticipated results
1. After 72 h of initiation culture, one can observe several plastic-adherent cells in a spindle fibroblast-like shape (Figure 1).
Figure 1.
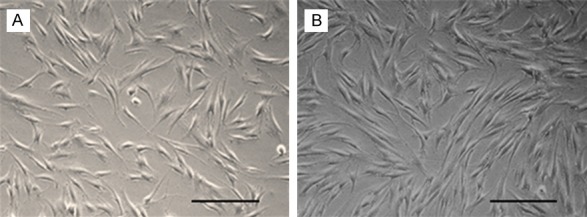
Morphological features of GMSCs. A. Spindle-shaped morphology of GMSC at day 7 after cell culturing. B. More confluent GMSCs in spiral shape at 10-14 days of cell culturing. Scale: 200 um.
2. At about two weeks later, cells will reach an 80-90% confluent density, one can achieve about 1-2 × 106 GMSCs from one donor (passage 0).
3. GMSCs characterization: GMSCs are positive for CD90, CD29, CD73, CD105, HLA-A, B, C, CD44 and negative for CD45, CD80, CD86, CD31, CD11b and HLA-DR (Figure 2). These cells can be readily differentiated into osteocyte, adipocyte and chondrocyte cells by culturing in appropriate induction media [33] (Figure 3).
Figure 2.
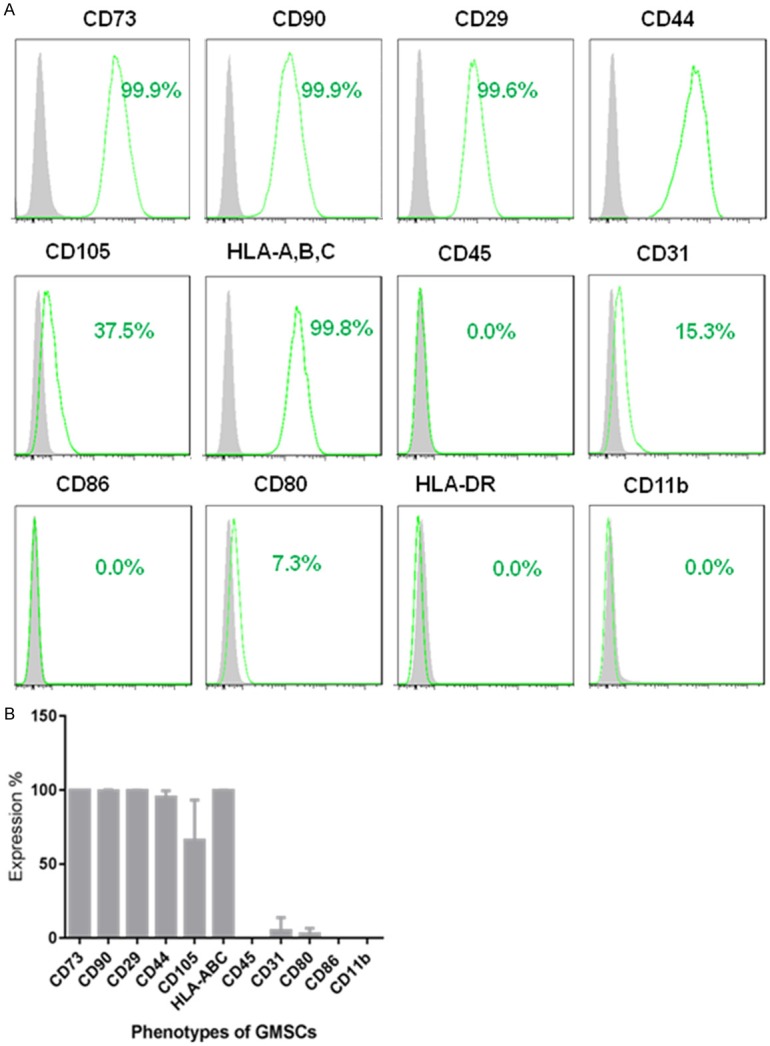
Expression of cell surface markers on GMSCs. A. Histogram analysis of cell surface markers of GMSCs (passage 2) determined by flow cytometry. B. Quantification of percentage of respective surface markers from 3 different gingiva tissue donors (mean ± SD).
Figure 3.
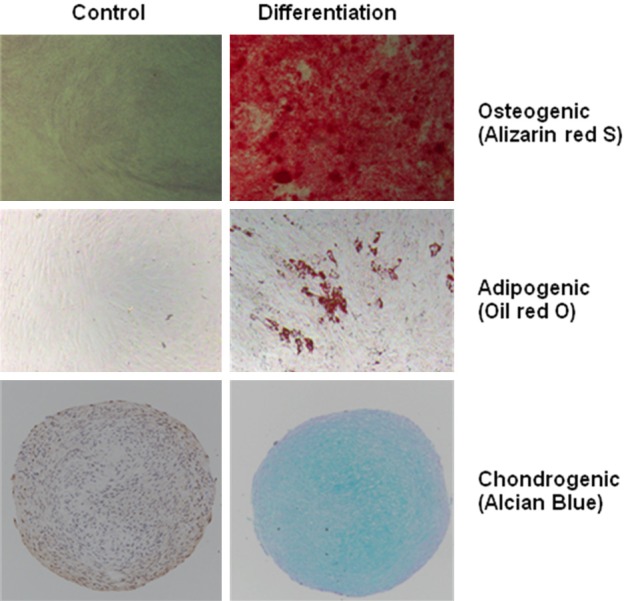
Multi-differentiation capacity of GMSCs. The cells differentiated into (up) mineralizing cells stained with Alizarin red S, (middle) adipocytes stained with Oil red O or (down) chondrocytic lineage cells stained with Alcian blue.
Acknowledgements
This research was supported by the National Key R&D Program of China (2017YFA0105800), Guangdong Natural Science Foundation (2014A030308005) and the Science and Technology Program of Guangzhou, China (Special Project on the Integration of Industry, Education and Research) (201508020060).
Disclosure of conflict of interest
None.
References
- 1.Zhang X, Huang F, Chen Y, Qian X, Zheng SG. Progress and prospect of mesenchymal stem cell-based therapy in atherosclerosis. Am J Transl Res. 2016;8:4017–4024. [PMC free article] [PubMed] [Google Scholar]
- 2.Huang F, Liu ZM, Zheng SG. Updates on GMSCs treatment for autoimmune diseases. Curr Stem Cell Res Ther. 2018;13:345–349. doi: 10.2174/1574888X13666180220141114. [DOI] [PubMed] [Google Scholar]
- 3.Su W, Wan Q, Huang J, Han L, Chen X, Chen G, Olsen N, Zheng SG, Liang D. Culture medium from TNF-alpha-stimulated mesenchymal stem cells attenuates allergic conjunctivitis through multiple antiallergic mechanisms. J Allergy Clin Immunol. 2015;136:423–432. e428. doi: 10.1016/j.jaci.2014.12.1926. [DOI] [PubMed] [Google Scholar]
- 4.Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q, Monsel A, Qu JM, Matthay MA, Lee JW. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32:116–125. doi: 10.1002/stem.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan XL, Zeng QX, Li X, Li CL, Xu ZB, Deng XQ, Shi J, Chen D, Zheng SG, Fu QL. Induced pluripotent stem cell-derived mesenchymal stem cells activate quiescent T cells and elevate regulatory T cell response via NF-kappaB in allergic rhinitis patients. Stem Cell Res Ther. 2018;9:170. doi: 10.1186/s13287-018-0896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W, Zhou L, Dang J, Zhang X, Wang J, Chen Y, Liang J, Li D, Ma J, Yuan J, Chen W, Zadeh HH, Olsen N, Zheng SG. Human gingiva-derived mesenchymal stem cells ameliorate streptozoticin-induced T1DM in mice via suppression of T effector cells and up-regulating treg subsets. Sci Rep. 2017;7:15249. doi: 10.1038/s41598-017-14979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su W, Li Z, Jia Y, Zhu Y, Cai W, Wan P, Zhang Y, Zheng SG, Zhuo Y. microRNA-21a-5p/PDCD4 axis regulates mesenchymal stem cell-induced neuroprotection in acute glaucoma. J Mol Cell Biol. 2017;9:289–301. doi: 10.1093/jmcb/mjx022. [DOI] [PubMed] [Google Scholar]
- 9.Shin JY, Park HJ, Kim HN, Oh SH, Bae JS, Ha HJ, Lee PH. Mesenchymal stem cells enhance autophagy and increase beta-amyloid clearance in Alzheimer disease models. Autophagy. 2014;10:32–44. doi: 10.4161/auto.26508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen M, Su W, Lin X, Guo Z, Wang J, Zhang Q, Brand D, Ryffel B, Huang J, Liu Z, He X, Le AD, Zheng SG. Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheum. 2013;65:1181–1193. doi: 10.1002/art.37894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang F, Chen M, Chen W, Gu J, Yuan J, Xue Y, Dang J, Su W, Wang J, Zadeh HH, He X, Rong L, Olsen N, Zheng SG. Human gingiva-derived mesenchymal stem cells inhibit xeno-graft-versus-host disease via CD39-CD73-adenosine and IDO signals. Front Immunol. 2017;8:68. doi: 10.3389/fimmu.2017.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Huang F, Li W, Dang JL, Yuan J, Wang J, Zeng DL, Sun CX, Liu YY, Ao Q, Tan H, Su W, Qian X, Olsen N, Zheng SG. Human gingiva-derived mesenchymal stem cells modulate monocytes/macrophages and alleviate atherosclerosis. Front Immunol. 2018;9:878. doi: 10.3389/fimmu.2018.00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, Le AD. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183:7787–7798. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Jiang CM, An S, Cheng Q, Huang YF, Wang YT, Gou YC, Xiao L, Yu WJ, Wang J. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells. Oral Dis. 2014;20:25–34. doi: 10.1111/odi.12086. [DOI] [PubMed] [Google Scholar]
- 15.Luo Y, Wu W, Gu J, Zhang X, Dang J, Wang J, Zheng Y, Huang F, Yuan J, Xue Y, Fu Q, Kandalam U, Colello J, Zheng SG. Human gingival tissue-derived MSC suppress osteoclastogenesis and bone erosion via CD39-adenosine signal pathway in autoimmune arthritis. EBioMedicine. 2019;43:620–631. doi: 10.1016/j.ebiom.2019.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang QZ, Su WR, Shi SH, Wilder-Smith P, Xiang AP, Wong A, Nguyen AL, Kwon CW, Le AD. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madouri F, Guillou N, Fauconnier L, Marchiol T, Rouxel N, Chenuet P, Ledru A, Apetoh L, Ghiringhelli F, Chamaillard M, Zheng SG, Trovero F, Quesniaux VF, Ryffel B, Togbe D. Caspase-1 activation by NLRP3 inflammasome dampens IL-33-dependent house dust mite-induced allergic lung inflammation. J Mol Cell Biol. 2015;7:351–365. doi: 10.1093/jmcb/mjv012. [DOI] [PubMed] [Google Scholar]
- 18.Tan Z, Jiang R, Wang X, Wang Y, Lu L, Liu Q, Zheng SG, Sun B, Ryffel B. RORgammat+IL-17+ neutrophils play a critical role in hepatic ischemia-reperfusion injury. J Mol Cell Biol. 2013;5:143–146. doi: 10.1093/jmcb/mjs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li N, Wang JC, Liang TH, Zhu MH, Wang JY, Fu XL, Zhou JR, Zheng SG, Chan P, Han J. Pathologic finding of increased expression of interleukin-17 in the synovial tissue of rheumatoid arthritis patients. Int J Clin Exp Pathol. 2013;6:1375–1379. [PMC free article] [PubMed] [Google Scholar]
- 20.Pan HF, Leng RX, Feng CC, Li XP, Chen GM, Li BZ, Xu WD, Zheng SG, Ye DQ. Expression profiles of Th17 pathway related genes in human systemic lupus erythematosus. Mol Biol Rep. 2013;40:391–399. doi: 10.1007/s11033-012-2073-2. [DOI] [PubMed] [Google Scholar]
- 21.Pan HF, Leng RX, Li XP, Zheng SG, Ye DQ. Targeting T-helper 9 cells and interleukin-9 in autoimmune diseases. Cytokine Growth Factor Rev. 2013;24:515–522. doi: 10.1016/j.cytogfr.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25- precursors. J Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 23.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 24.Liu ZM, Wang KP, Ma J, Guo Zheng S. The role of all-trans retinoic acid in the biology of Foxp3+ regulatory T cells. Cell Mol Immunol. 2015;12:553–557. doi: 10.1038/cmi.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Lin F, Zhuo C, Deng G, Chen Z, Yin S, Gao Z, Piccioni M, Tsun A, Cai S, Zheng SG, Zhang Y, Li B. PIM1 kinase phosphorylates the human transcription factor FOXP3 at serine 422 to negatively regulate its activity under inflammation. J Biol Chem. 2014;289:26872–26881. doi: 10.1074/jbc.M114.586651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Lan Q, Lu L, Chen M, Xia Z, Ma J, Wang J, Fan H, Shen Y, Ryffel B, Brand D, Quismorio F, Liu Z, Horwitz DA, Xu A, Zheng SG. Phenotypic and functional characteristic of a newly identified CD8+ Foxp3- CD103+ regulatory T cells. J Mol Cell Biol. 2014;6:81–92. doi: 10.1093/jmcb/mjt026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao Y, Tang J, Chen W, Li Q, Nie J, Lin F, Wu Q, Chen Z, Gao Z, Fan H, Tsun A, Shen J, Chen G, Liu Z, Lou Z, Olsen NJ, Zheng SG, Li B. Inflammation negatively regulates FOXP3 and regulatory T-cell function via DBC1. Proc Natl Acad Sci U S A. 2015;112:E3246–3254. doi: 10.1073/pnas.1421463112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 29.Zhou X, Kong N, Wang J, Fan H, Zou H, Horwitz D, Brand D, Liu Z, Zheng SG. Cutting edge: all-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. J Immunol. 2010;185:2675–2679. doi: 10.4049/jimmunol.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu L, Lan Q, Li Z, Zhou X, Gu J, Li Q, Wang J, Chen M, Liu Y, Shen Y, Brand DD, Ryffel B, Horwitz DA, Quismorio FP, Liu Z, Li B, Olsen NJ, Zheng SG. Critical role of all-trans retinoic acid in stabilizing human natural regulatory T cells under inflammatory conditions. Proc Natl Acad Sci U S A. 2014;111:E3432–3440. doi: 10.1073/pnas.1408780111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji X, Zhang Z, Han Y, Song J, Xu X, Jin J, Su S, Mu D, Liu X, Xu S, Cui H, Zhao Z, Wang Y, Liu H. Mesenchymal stem cells derived from normal gingival tissue inhibit the proliferation of oral cancer cells in vitro and in vivo. Int J Oncol. 2016;49:2011–2022. doi: 10.3892/ijo.2016.3715. [DOI] [PubMed] [Google Scholar]
- 32.Zhao J, Wang J, Dang J, Zhu W, Chen Y, Zhang X, Xie J, Hu B, Huang F, Sun B, Bellanti JA, Zheng SG. A preclinical study-systemic evaluation of safety on mesenchymal stem cells derived from human gingiva tissue. Stem Cell Res Ther. 2019;10:165. doi: 10.1186/s13287-019-1262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomar GB, Srivastava RK, Gupta N, Barhanpurkar AP, Pote ST, Jhaveri HM, Mishra GC, Wani MR. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem Biophys Res Commun. 2010;393:377–383. doi: 10.1016/j.bbrc.2010.01.126. [DOI] [PubMed] [Google Scholar]


