Abstract
Selective IgM deficiency (SIgMD) and IgA MGUS in a young woman are two rare disorders. IgA MGUS has not been described in patients with SIgMD. We present the first comprehensive analysis of various subsets of CD4+ T, CD8+ T cells, and B cells in a young woman with SIgMD and IgAλ MGUS. Analysis of B cell subsets revealed increased proportions of transitional B cells, germinal center (GC) B cells, B regulatory cells (Breg), and plasmablasts (PB), and decreased proportions of marginal zone (MZ) B cells. BAFF-R expression on both naïve and memory B cells was increased. CD4+ and CD8+ effector memory cells were decreased, whereas CD4+ and CD8+ naïve T cells were increased. These abnormalities in B cell subsets and plasmablasts are not observed in SIgMD, therefore appears be influenced by MGUS. No correlation was observed with changes in the levels of monoclonal IgA and serum IgM levels over nine years follow-up suggesting that SIgMD is likely to be primary rather than secondary to MGUS. These observations also suggest that IgAλ MGUS and perhaps other MGUS may occur at a young age in association with selective IgM deficiency. The abnormalities in B cell subsets may have a predictive value for progression to multiple myeloma.
Keywords: Germinal center B cells, transitional B cells, BAFF-R, breg, CD8 treg, CD4 treg
Introduction
Although John Hobbs and colleagues described selective IgM deficiency in 1967 [1], it took five decades for selective IgM deficiency (SIgMD) to be incorporated in IUIS classification of primary immunodeficiency diseases [2]. Its prevalence has been reported between 0.03% [3] and 1.68% [4]. More recently, in a screening of adult blood donors, the prevalence of SIgMD is reported to be 0.36% [5]. Serum IgA, IgG, T cells and T cell subsets, and T cell functions are relatively normal in SIgMD [6-10]. Although SIgMD has been associated with comorbid conditions, including allergic disease, autoimmune disease, and malignancies [6], to the best of our knowledge monoclonal gammopathy of undetermined significance (MGUS) has not been reported in patients with SIgMD.
Monoclonal gammopathy is an abnormal rapid multiplication of a single cell line of plasma cells due to a disturbance in immunoglobulin synthesis, resulting in a homogenous increase of a monoclonal peak. The definition of MGUS requires serum M-protein under 3 g/dl, less than 10% plasma cells in the bone marrow, absent or minimal M-proteinuria, and stability of the M-protein; as well as absent features of multiple myeloma including lytic lesions, anemia, hypercalcemia, renal insufficiency, or other lymphoproliferative disorders [11].
Since no detailed analysis of subsets of CD4+ T cells, CD8+ T cells and subsets of B cells have been reported in MGUS, a comprehensive immunological analysis of naïve (TN), central memory (TCM), and effector memory (TEM, TEMRA) subsets of CD4+ and CD8+ T cells, CD4+ Treg, CD8+ Treg, mature B cells, transitional B cells, IgM memory B cells, switched memory B cells, marginal zone B cells (MZ), germinal center (GC), plasmablast (PB), natural antibody producing B1 cells, CXCR3+ naïve and memory B cells, CD21low B cells, and regulatory B cells (Breg) was performed.
Case presentation
Patient was a 21 years young female referred to our clinic for leukopenia and recurrent urinary tract infections. She had no past medical history or relevant social history. Family history was positive for father with lymphoma. Her physical examination was unremarkable; there was no lymphadenopathy or hepatosplenomegaly. Immunological features are shown in Table 1. SIgMD is defined as serum IgM levels below 2 SD of mean for control and normal total IgG and IgA [12]. Specific antibody responses to Penumovax-23 vaccination, tetanus and diphtheria toxoid were normal. Immunofixation of the serum showed monoclonal IgAλ. Urine for light chains revealed normal ratio of κ to λ light chains.
Table 1.
Immunological Features of Patient with MGUS and SIgMD*
| Test | Results | Reference ranges |
|---|---|---|
| Serum Immunoglobulins (mg/dl) | ||
| IgM | 34 | 65-263 |
| IgA | 1,090 | 68-378 |
| IgG | 1,570 | 694-1,618 |
| IgG1 | 1,000 | 342-1,117 |
| IgG2 | 586 | 147-524 |
| IgG3 | 192 | 21-114 |
| IgG4 | 20 | 7-88 |
| IgE (IU/ml) | 316 | 10-150 |
| Autoantibodies | ||
| ANA (EIA units) | 28.4 | 0.2-12.0 |
| cANCA | Negative | Negative |
| dsDNA | Negative | Negative |
| Smith (ENA) | Negative | Negative |
| Thyroglobulin (U/ml) | 73.3 | 0.0-60.0 |
| Specific antibodies | ||
| Diphtheria Toxoid IgG (IU/ml) | 0.288 | >0.099 |
| Tetanus Toxoid IgG (IU/ml) | 0.728 | >0.150 |
| Strep. pneumoniae type 1 (ug/ml) | >20.0 | >1.0 |
| Strep. pneumoniae type 3 (ug/ml) | 5.5 | >1.0 |
| Strep. pneumoniae type 4 (ug/ml) | 12.9 | >1.0 |
| Strep. pneumoniae type 5 (ug/ml) | 0.8 | >1.0 |
| Strep. pneumoniae type 6B (ug/ml) | 1.0 | >1.0 |
| Strep. pneumoniae type 7F (ug/ml) | 13.8 | >1.0 |
| Strep. pneumoniae type 8 (ug/ml) | 6.8 | >1.0 |
| Strep. pneumoniae type 9N (ug/ml) | 3.5 | >1.0 |
| Strep. pneumoniae type 9V (ug/ml) | 1.2 | >1.0 |
| Strep. pneumoniae type 12F (ug/ml) | <0.2 | >1.0 |
| Strep. pneumoniae type 14 (ug/ml) | >20.0 | >1.0 |
| Strep. pneumoniae type 18C (ug/ml) | 16.8 | >1.0 |
| Strep. pneumoniae type 19F (ug/ml) | >20.0 | >1.0 |
| Strep. pneumoniae type 23F (ug/ml) | 1.7 | >1.0 |
| Antimicrobial antibodies (IgG) | ||
| H. pylori | Positive | Negative |
| Antistreptolysin O (IU/ml) | 323.0 | <200 |
| Complement | ||
| CH50 (U/ml) | 52.0 | 22-60 |
| C2 (mg/L) | 14.4 | 6.0-32.5 |
| C4 (mg/dl) | 15.0 | 15.0-45.0 |
| Immunofixation | IgAλ | None |
| Urine free light chains | ||
| Free κ light chain (mg/dl) | 11.90 | 1.35-24.19 |
| Free λ light chain (mg/dl) | 1.51 | 0.24-5.66 |
| κ/λ ratio | 7.89 | 2.04-10.37 |
At the initial diagnosis in 2008.
No lytic lesions were observed on axial skeletal survey. Bone marrow aspiration was normal. Serum total proteins, serum calcium, and alkaline phosphatase levels were with in normal range. Therefore, excluding any possibility of multiple myeloma. Urine cultures repeatedly grew Streptococcus hemolyticus group B (>100,000 colonies). These were successfully treated with appropriate antibiotics.
This patient was followed for the last 9 years for serum immunoglobulins by nephelometry and salient clinical features. These included culture positive vaginitis (PCR positive for Gardnerella), rapid strep screen positibe pharyngitis, and recurrent abdominal cramps and nausea characterized by serum specific IgG for H. pylori. In the 9 years of follow-up, IgA has ranged between 602 mg/dl-1090 mg/dl, and IgM between 35-45 mg/dl. No correlation was observed between changes in serum IgA and serum IgM over these 9 years period.
Materials and methods
Subject: Peripheral blood mononuclear cells from the patient and age- and gender-matched healthy controls were separated by density gradient using Lymphocyte Separation Medium. Institutional Review Board (Human) Committee of the University of California at Irvine approved the protocol. A signed consent was obtained from the subjects.
Analysis of subsets of B cells and CD4+ and CD8+ T cells
Analyses of T cells, B cells, various subsets of B cells (naïve, IgM and switched memory, transitional B cells, MZ B cells, GC B cells, CD21low B cells, B1 cells, CXCR3+ naïve and memory B cells, and plasmablasts), several subsets of CD4+ and CD8+ T cells (naive, central memory, effector memory), and CD4 Treg, CD8 Treg, and Breg cells were performed by multicolor flow cytometry, using various monoclonal antibodies and isotype controls. Data were analyzed by Flow Jo software.
Antibodies and reagents
Antibodies for B cell subsets
The following anti-human antibodies were used to identify various subsets of B cells: CD19 PerCP, CD27 FITC, CD38 FITC, CD21 PE, CD70 PE, CD27 APC, CD24 FITC, CD38 PE, CD183 PE, anti-IgM APC, and anti-IgD PE; all from BD Pharmingen, San Jose, California. CD43 APC was purchased from Biolegand, San Diego, California.
Antibodies for T cell subsets
The following monoclonal antibodies and isotype controls were used for the analysis of subsets of CD4+ and CD8+ T cells: CD4 PerCP, CD8 PerCP, CD45RA APC, CCR7 FITC, CD3 PerCP, and CD278 (ICOS) PE. All antibodies were purchased from BD Parmingen, San Jose, California.
Surface markers for B cell subsets
B cell and B cell subsets were identified by following cell surface markers: naïve B cells-CD19+/CD27-/IgD+/IgM+, transitional B cells-CD19+/CD38+/IgM++, MZ B cells-CD19+/CD27+/IgD+/IgM+, IgM memory B cells-CD19+/CD27+/IgM+, GC B cells-CD19+/IgD-/CD27+/CD38+, Class switch memory B cells-CD19+/CD27+/IgD-/IgM, plasmablasts-CD19+/CD38++/IgM-, mature B cells-CD21high/CD19+/CD38-, CD21Low cells CD19+/CD38-/CD21low, B1 cells-CD20+/CD70/CD27+/CD43+, CXCR3+ B cells-CD19+/CD27/CD183+, and Breg-CD19+/CD24+/CD38+.
Surface markers of T cell subsets
Following cell surface phenotype identified subsets of CD4 T cells and CD8+ T cells: naïve (TN)-CD4+/CD8+CD45RA+CCR7+, central memory (TCM)-CD4+/CD8+CD45RA-CCR7+, effector memory (TEM)-CD4+/CD8+CD45RA-CCR7-, CD45RA+ effector memory (TEMRA) or terminally differentiated effector memory-CD4+/CD8+CD45RA+CCR7-, CD8 Treg-CD8+CD183+CCR7+CD45RA-.
Analysis of regulator lymphocytes
For CD4 Treg, MNCs were stained with PerCP-labeled anti-CD4 and FITC-labeled anti-CD25 monoclonal antibodies and isotype controls, followed by Foxp3 intracellular staining with APC-labeled anti-Foxp3 antibody and isotype control. Staining procedures were performed according to the manufacturer’s recommendation. In the population of CD4 cells, Treg cells were identified as CD4+CD25high Foxp3+ cells.
For CD8 Treg, MNCs were stained with PerCP-labeled anti-CD8, PE-labeled anti-CD183, and FITC-labeled anti-CD25, followed by Foxp3 intracellular staining with APC-labeled anti-Foxp3 antibody and isotype control (Mouse IgG1κ-APC). Staining procedures were performed according to the manufacturer’s recommendations. In the population of CD8+ T cells, CD8 Treg cells were identified as CD8+CD183+CD25high Foxp3+ cells.
For Breg, MNCs were stained with PerCP-labeled CD19, FITC-labeled CD24, and APC-labeled CD38 monoclonal antibodies and isotype controls. Breg were identified as CD19+CD24+CD38+.
Results
B cell subsets are altered in MGUS with SIgMD
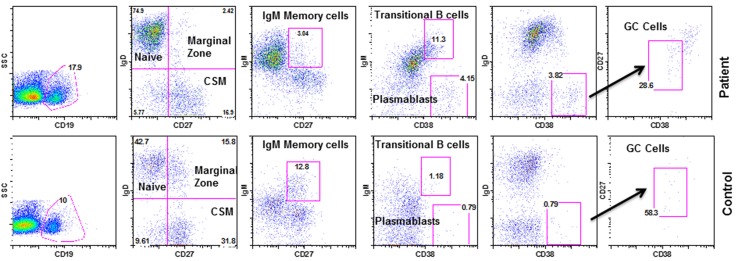
B cell subsets in the patient and age and gender-matched healthy control were analyzed using monoclonal antibodies and isotype controls with FACSCalibur. Figure 1 shows increased transitional B cells, GC B cells, and PB, and decreased MZ B cells in the patient as compared to control.
Figure 1.
Subsets of B cells. Identified by various markers: Naïve B cells-CD19+/CD27-/IgD+/IgM+, transitional B cells-CD19+/CD38++/IgM++, MZ B cells-CD19+/CD27+/IgD+/IgM+, IgM memory-CD19+/CD27+/IgM+, GC B cells-CD19+/IgD-/CD27+/CD38+, Class switch memory-CD19+/CD27+/IgD-/IgM-, plasmablasts-CD19+/CD38++/IgM-.
BAFF-R expression on B cells is increased in MGUS with SIgMD
The B cell activating factor (BAFF) and a proliferative-inducing ligand (APIL) provide B cell survival signal via BAFF-R and TACI [13]. Therefore, we examined the expression of BAFF-R and TACI on naïve (CD19+CD27-) and memory (CD19+CD27+) B cells. Figure 2 shows an increased expression of BAFF-R in naïve B cells. BAFF-R on memory B cells displayed two peaks; one with increased and other with lower expression of BAFF-R in the patient as compared to single peak in the control. TACI expression in the patient was comparable to controls.
Figure 2.
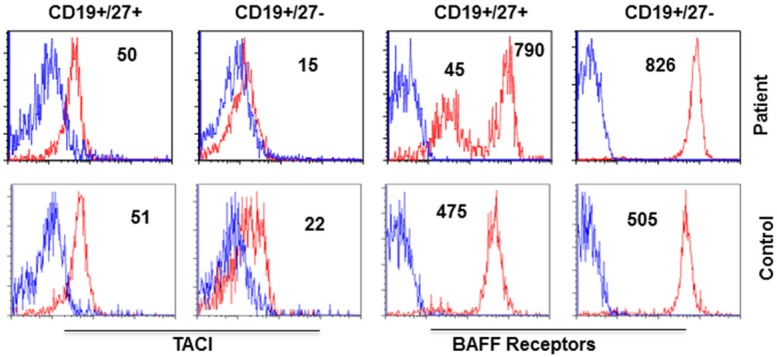
Expression of TACI and BAFF-R on naïve and memory B cells. BAFF-R on naïve (CD19+CD27-) and one peak of memory B cells (CD19+CD27+) were increased in the patient.
CXCR3+ naïve and memory B cells are decreased in MGUS with SIgMD
A role of CXCR3 in T cell trafficking is well established [14]. However, its role in B cell migration has recently been explored. Figure 3 shows that CXCR3 expression on naïve and memory B cells was decreased. No difference was observed in CD21low B cells and natural antibody producing B1 cells between the patient and control.
Figure 3.
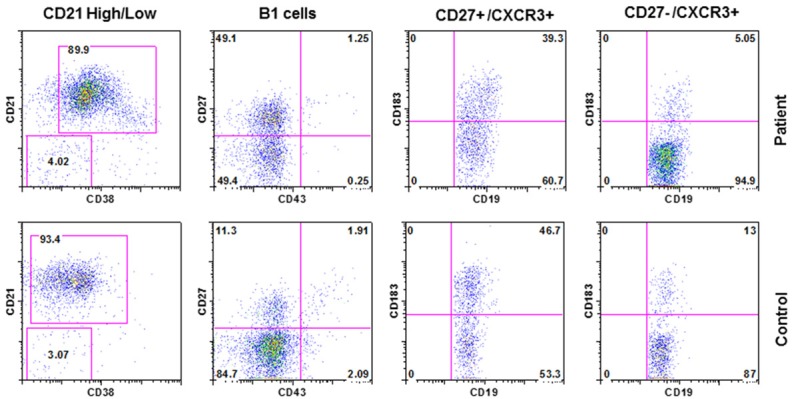
CD21+ B cells, B1 cells, and CXCR3+ B cells. Identified by CD21low (CD19+/CD38-/CD21low), CD21high (CD21high CD19+/CD38-/CD21++), B1 (CD20+/CD70/CD27+/CD43+), CXCR3+ naïve (CD19+CD27-CXCR3+) and memory (CD19+CD27+CXCR3+) B cells. CXCR3+ B cells were decreased.
CD4+ and CD8+ naïve and memory subsets are altered in MGUS with SIgMD
Both CD4 and CD8 T cells, based upon their functions and migration characteristics, have been divided into nave (TN), central memory (TCM), and effector memory (TEM, TEMRA) subsets [15-17]. Therefore, we examined these subsets of CD4+ and CD8+ T cells in the patient and control. CD8+ TCM and CD8+ TEM were decreased, whereas CD8+ TN cells were increased (Figure 4). CD4+ TN cells were also increased, and CD4+ TEMRA cells were markedly decreased as comparable to control.
Figure 4.
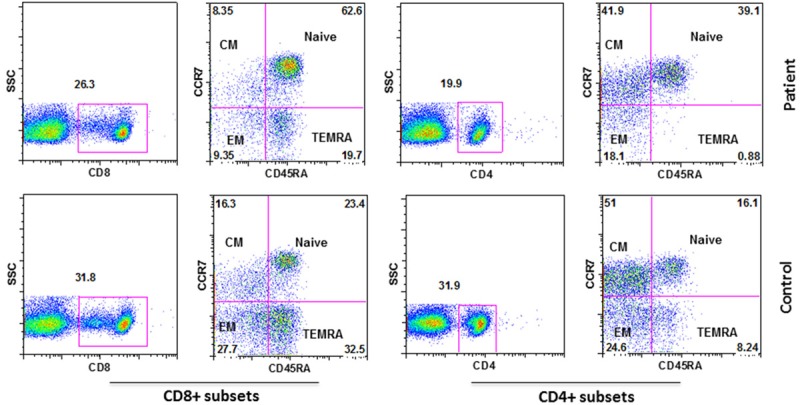
Subsets of CD4+ and CD8+ T cells. Identified by, naïve (TN)-CD4+/CD8+CD45RA+CCR7+, central memory (TCM)-CD4+/CD8+CD45RA-CCR7+, effector memory (TEM)-CD4+/CD8+CD45RA-CCR7-, CD45RA+ effector memory (TEMRA) or terminally differentiated effector memory-CD4+/CD8+CD45RA+CCR7-.
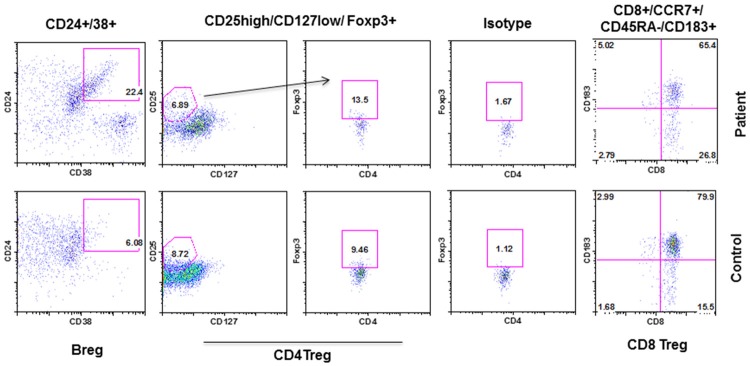
Regulatory B lymphocytes are increased in MGUS with SIgMD
There are three major types of regulatory lymphocytes; the CD4+ Treg, CD8 Treg, and Breg. They play an important role in tolerance and autoimmune diseases [18-23]. Figure 5 shows that Breg are increased in our patient as compared to control; however, CD4 Treg and CD8 Treg were comparable to controls.
Figure 5.
Regulatory lymphocytes. CD8+ Treg were identified by CD8+CD183+CCR7+CD45RA-, CD4+ Treg by CD4+CD25highCD127lowFoxP3+, and Breg by CD19+/CD24+/CD38+.
Discussion
Here we report a comprehensive immunological analysis in a 21 years young woman with SIgMD and IgAλ MGUS. MGUS is a disorder of older subjects with an average age at diagnosis of 72 years, of which only 2% are diagnosed under the age 40, and virtually none in their 20’s. MGUS is more common in men than women [24]. The most common manifestations of MGUS are peripheral neuropathy or unexplained bone loss [25]. IgG and IgA MGUS are known precursor conditions for multiple myeloma with a risk of progression of 1% per year [26]. To the best of our knowledge our patient is the first case of IgAλ MGUS in a 21 years young women associated with selective SIgMD.
Patients with SIgMD commonly present with infections, especially of upper and lower respiratory tract. Infections may be caused by a variety of extracellular and intracellular bacteria, protozoa, viruses, and fungi [6,8,10,19,27,28]. Our patient presented with recurrent group B streptococcus urinary tract infections, which is not a common infectious presentation in SIgMD or MGUS. Approximately 50% of patients have impaired specific antibody response to pneumococcal polysaccharide [8]. However, our patient had normal IgG specific antibody response to Pneumovax-23 vaccine, as well as diphtheria and tetanus toxoid. Yel et al. [8] also reported a subset of symptomatic patients with SIgMD with normal IgG anti-pneumococcal response, which may suggest that other contributing factors, including a deficiency of specific IgM antibodies, may be responsible for recurrent infections in SIgMD.
A number of autoimmune manifestations are associated with SIgMD [6]. Our patient also had positive ANA and thyroglobulin antibodies.
A number of malignant disorders have been reported in SIgMD [6]. To the best of our knowledge, our patient is the first case of MGUS in SIgMD. However, it is unclear if the prevalence of malignancy is increased in SIgMD. Although comprehensive analyses of lymphocyte subset have been reported in patients with SIgMD [8,29], no such studies of subsets of B cells and subsets of CD4+ and CD8+ T cells have been reported in MGUS.
CD3+ T cell, CD4+ T cell, and CD8+ T cell numbers and T cell functions are preserved in majority of patients with SIgMD [7-10]. Our patient also has normal CD3+, CD4+, and CD8+ T cells. CD4+ and CD8+ T cells have been further classified into TN, TCM, TEM, and TEMRA subpopulation [15-17]. Patients with SIgMD have been reported to display normal distribution of TN, TCM, TEM, and TEMRA subsets of CD4+ and CD8+ T cells [29]. However, in the present case abnormalities in subsets of both CD4+ and CD8+ T cells were observed. Therefore, these changes likely are likely to be influenced by MGUS. A role of changes in various subsets of CD4+ and CD8+ T cells in the pathogenesis of MGUS is unclear.
CD4 Treg, Breg, and more recently described CD8+ Treg cells have shown to play an important role in immune tolerance and autoimmune diseases [18-23]. In SIgMD, CD8+ Treg and Breg are increased; whereas CD4 Treg are decreased [29]. In our patient, CD4 Treg and CD8 Treg are found to be normal.
Various B cell subsets have been studied in patients with SIgMD [29,30]. Louis et al. [29] observed decreased GC B cells, whereas Mensen et al. [27] reported increased transitional and MZ B cells in a subset of patients with SIGMD. In our patient, transitional B cells, GC B cells, and PB are increased, whereas MZ B cells are decreased, suggesting that changes in B cell subsets may be influenced by MGUS.
A major population of transitional B cells migrates and differentiates into mature follicular B cells and a minor population into mature MZ B cells. MZ B cells differentiate into plasmablasts that produce large amounts of IgM and IgG and IgA via class switch recombination [31]. Marginal zone B cells generally differentiate into short-lived plasma cells, whereas GC B cells differentiate into long-lived memory cells, and plasma cells that migrate to the bone marrow [32]. In our patient, both GC B cells and PB are increased.
In humans, MZ-derived plasmablasts receive additional maturation and survival signals from various cytokines and chemokines including CXCL10 [33]. CXCL10 is a CXCR3 ligand that stimulates B cells. Since our patient has B cells with decreased expression of CXCR3, and decreased MZ B cells, increased plasmablasts may be derived from GC B cells, and CXCR3 may not be playing a role in the survival of PB in our patient. In patients with SIgMD, GC are decreased [29]. Therefore, an increased GC B cells may be an association with MGUS.
The B-cell activating factor (BAFF) and APRIL (a proliferation-inducing ligand) plays a critical role in B cell survival [13]. BAFF and APRIL both bind to transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI), and BAFF binds to BAFF receptor (BAFF-R). BAFF-R and TACI are mainly expressed on B cells. BAFF-R is receptors are expressed on all stages of B cells including plasmablasts except plasma cells [34]. BAFF is also involved in the survival of plasmablasts and GC B cells. In our patient, BAFF-R expression was increased in both naïve and a subset of memory B cells (displayed two distinct peaks, one with increased expression). Therefore, increased expression of BAFF-R may play a role in increased GC cells and plasmablasts in our patient. However, It is unclear if increased BAFF-R expression plays a role in the pathogenesis of MGUS. Studies of expression of BAFF-R and TACI on B cells have not been reported in MGUS or SIgMD.
B1 cells that spontaneously produce natural antibodies, predominantly IgM isotype of low affinity and polyspecificity [35] were comparable to control. B1 cell numbers are also normal in SIgMD patients [29].
CD21low B cells are innate-like B cells that respond poorly to polysaccharide antigens [36]. Lau et al. [37] have reported that CD21low cells are recent GC graduates that are refractory to GC reentry, and are predisposed to differentiate into long-lived plasma cells. CD21low B cells are increased in common variable immunodeficiency [36] and SIgMD [29] that are associated impaired response to pneumococcal polysaccharides. In the present case, both CD21low B cells and response to pneumococcal polysaccharide were normal.
Regulatory B cells (Breg) regulate various immune responses and play an important role in inflammatory, autoimmune diseases, and cancer [23,24,38]. Breg cells inhibit apoptosis [39]. In our patient, Breg cell are increased. Increased Breg are also observed in SIgMD without MGUS [29]. Therefore, a role of Breg in the development of MGUS is unclear.
Since the changes in various subsets of lymphocytes in our patient are distinct from those reported for SIgMD without MGUS [29,30], it suggests that changes in both subsets of B cells (GC B cells and PB) and subsets of CD4+ and CD8+ T cells (TN, TCM, TEM and TEMRA) are likely to be influenced by or associated with IgA MGUS. It remains to be determined whether some of these abnormalities in subsets of B cells and CD8+ T cells may have a predictive value for progression to multiple myeloma.
In summary, IgAλ MGUS associated with SIgMD appears to occur at a young age, and is characterized by abnormalities predominantly in various B cell subsets, including plasmablasts. These abnormalities may have a predictive value for progression to multiple myeloma. However, this would require a comprehensive analysis of B cell subsets in large cohort of patients with MGUS with a long follow-up.
Acknowledgements
We thank Dr. Sharon Williams for retrieving clinical data from patient’s electronic medical record.
Disclosure of conflict of interest
None.
Abbreviations
- MGUS
monoclonal gammopathy of undetermined significance
- MZ
Marginal zone
- GC
germinal center
- PB
plasmablasts
- SIgMD
selective IgM deficiency
References
- 1.Hobbs JR, Milner RD, Watt PJ. Gamma-M deficiency predisposing to meningococcal septicaemia. Br Med J. 1967;4:583–586. doi: 10.1136/bmj.4.5579.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bousfiha A, Jeddane L, Picard C, Ailal F, Bobby Gaspar H, Al-Herz W, Chatila T, Crow YJ, Cunningham-Rundles C, Etzioni A, Franco JL, Holland SM, Klein C, Morio T, Ochs HD, Oksenhendler E, Puck J, Tang MLK, Tangye SG, Torgerson TR, Casanova JL, Sullivan KE. The 2017 IUIS phenotypic classification for primary immunodeficiencies. J Clin Immunol. 2018;38:129–143. doi: 10.1007/s10875-017-0465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cassidy JT, Nordby GL. Human serum immunoglobulin concentrations: prevalence of immunoglobulin deficiencies. J Allergy Clin Immunol. 1975;55:35–48. doi: 10.1016/s0091-6749(75)80006-6. [DOI] [PubMed] [Google Scholar]
- 4.Faulk WP, Kiyasu WS, Cooper MD, Fudenberg HH. Deficiency of IgM. Pediatrics. 1971;47:399–404. [PubMed] [Google Scholar]
- 5.Entezari N, Adab Z, Zeydi M, Saghafi S, Jamali M, Kardar GA, Pourpak Z. The prevalence of selective immunoglobulin M deficiency (SIgMD) in Iranian volunteer blood donors. Hum Immunol. 2016;77:7–11. doi: 10.1016/j.humimm.2015.09.051. [DOI] [PubMed] [Google Scholar]
- 6.Louis AG, Gupta S. Primary selective IgM deficiency: an ignored immunodeficiency. Clin Rev Allergy Immunol. 2014;46:104–111. doi: 10.1007/s12016-013-8375-x. [DOI] [PubMed] [Google Scholar]
- 7.Ohno T, Inaba M, Kuribayashi K, Masuda T, Kanoh T, Uchino H. Selective IgM deficiency in adults: phenotypically and functionally altered profiles of peripheral blood lymphocytes. Clin Exp Immunol. 1987;68:630–637. [PMC free article] [PubMed] [Google Scholar]
- 8.Yel L, Ramanuja S, Gupta S. Clinical and immunological features in IgM deficiency. Int Arch Allergy Immunol. 2009;150:291–298. doi: 10.1159/000222682. [DOI] [PubMed] [Google Scholar]
- 9.Inoue T, Okumura Y, Shirama M, Ishibashi H, Kashiwagi S, Okubo H. Selective partial IgM deficiency: functional assessment of T and B lymphocytes in vitro. J Clin Immunol. 1986;6:130–135. doi: 10.1007/BF00918745. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein MF, Goldstein AL, Dunsky EH, Dvorin DJ, Belecanech GA, Shamir K. Selective IgM immunodeficiency: retrospective analysis of 36 adult patients with review of the literature. Ann Allergy Asthma Immunol. 2006;97:717–730. doi: 10.1016/S1081-1206(10)60962-3. [DOI] [PubMed] [Google Scholar]
- 11.Kyle RA, Rajkumar SV. Monoclonal gammopathies of undetermined significance. Hematol Oncol Clin North Am. 1999;13:1181–1202. doi: 10.1016/s0889-8588(05)70120-9. [DOI] [PubMed] [Google Scholar]
- 12.Gupta S, Gupta A. Defining selective IgM deficiency. J Clin Immunol. 2019;39:350–352. doi: 10.1007/s10875-019-00641-4. [DOI] [PubMed] [Google Scholar]
- 13.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 14.Groom JR, Luster AD. CXCR3 in T cell function. Exp Ceell Res. 2011;317:620–631. doi: 10.1016/j.yexcr.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 16.Gupta S. Molecular mechanisms of apoptosis in the cells of the immune system in human aging. Immunol Rev. 2005;205:114–29. doi: 10.1111/j.0105-2896.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 17.Gupta S, Bi R, Su K, Yel L, Chiplunkar S, Gollapudi S. Characterization of naïve, memory, and effector CD8+ T cells: effect of age. Exp Gerontol. 2004;39:545–550. doi: 10.1016/j.exger.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Sakaguchi S. Regulatory T cells: key controllers of immunological self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 19.Shevach EM. Foxp3+ T regulatory cells: still many unanswered questions- a perspective after 20 years of study. Front Immunol. 2018;9:1048. doi: 10.3389/fimmu.2018.01048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang YM, Alexander SI. CD8 regulatory T cells: what’s old is now new. Immunol Cell Biol. 2009;87:192–193. doi: 10.1038/icb.2009.8. [DOI] [PubMed] [Google Scholar]
- 21.Lu L, Cantor H. Generation and regulation of CD8 (+) regulatory Tcells. Cell Mol Immunol. 2008;5:401–6. doi: 10.1038/cmi.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mauri C, Blair PA. Regulatory B cells in autoimmunity: developments and controversies. Nat Rev Rheumatol. 2013;6:636–643. doi: 10.1038/nrrheum.2010.140. [DOI] [PubMed] [Google Scholar]
- 23.Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176:705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 24.Therneau TM, Kyle RA, Melton LJ, Larson DR, Benson JT, Colby CL, Dispenzieri A, Kumar S, Katzmann JA, Cerhan JR, Rajkumar SV. Incidence of monoclonal gammopathy of undetermined significance and estimation of duration before first clinical recognition. Mayo Clin Proc. 2012;87:1071–1079. doi: 10.1016/j.mayocp.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bladé J. Clinical practice. Monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;355:2765–2770. doi: 10.1056/NEJMcp052790. [DOI] [PubMed] [Google Scholar]
- 26.Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, Melton LJ 3rd. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–569. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 27.Hong R, Gupta S. Selective immunoglobulin M deficiency in an adult with streptococcus pneumoniae sepsis and invasive aspergillosis. J Investig Allergol Clin Immunol. 2008;18:214–218. [PubMed] [Google Scholar]
- 28.Gupta S, Gupta A. Selective IgM deficiencyan understimated primary immunodeficiency. Front Immunol. 2017;8:1056. doi: 10.3389/fimmu.2017.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Louis AG, Agrawal S, Gupta S. Analysis of subsets of B cells, Breg, CD4 Treg and CD8 Treg cells in adult patients with primary selective IgM deficiency. Am J Clin Exp Immunol. 2016;5:21–32. [PMC free article] [PubMed] [Google Scholar]
- 30.Mensen A, Krause T, Hanitsch LG, Meisel C, Kleint ME, Volk HD, Na IK, Scheibenbogen C. Altered B-cell subsets and functional B-cell defects in selective IgM deficiency. Clin Immunol. 2015;161:96–102. doi: 10.1016/j.clim.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 31.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Ann Rev Immunol. 2009;27:267–285. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- 32.Martin F, Kearney JF. Marginal zone B cells. Nat Rev Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 33.Xu W, Joo H, Clayton S, Dullaers M, Herve MC, Blankenship D, De La Morena MT, Balderas R, Picard C, Casanova JL, Pascual V, Oh S, Banchereau J. Macrophage-induced differentiation of plasma cells though CXCL10/IP10. J Exp Med. 2012;209:1813–1823. doi: 10.1084/jem.20112142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darce JR, Arendt BK, Wu X, Jelinek DF. Regulated expression of BAFF-binding receptors during B cell differentiation. J Immunol. 2007;179:7276–7286. doi: 10.4049/jimmunol.179.11.7276. [DOI] [PubMed] [Google Scholar]
- 35.Rothchild TL, Griffin DO, Holodick NE, Quach TD, Kaku H. Human B1 cells take the stage. N Y Acad Sci. 2013;1285:97–114. doi: 10.1111/nyas.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rakhmanov M, Keller B, Gutenberger S, Foerster C, Hoenig M, Drissen G, van der Burg M, van Dongen JJ, Wiech E, Visentini M, Quinti I, Prasse A, Voelxen N, Salzer U, Goldacker S, Fisch P, Eibel H, Schwarz K, Peter HH, Warnatz K. Circulating CD21low B cells in common variable immunodeficiency resembles tissue homing, innate-like B cells. Proc Natl Acad Sci U S A. 2010;106:13451–13456. doi: 10.1073/pnas.0901984106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lau D, Lan LY, Andrews SF, Henry C, Rojas KT, Neu KE, Huang M, Huang Y, DeKosky B, Palm AE, Ippolito GC, Georgiou G, Wilson PC. Low CD21 expression defines a population of recent germinal center graduates primed for plasma cell differentiation. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aai8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding T, Yan F, Cao S, Ren X. Regulatory B cells: new member of immunosuppressive cell club. Hum Immunol. 2015;76:615–621. doi: 10.1016/j.humimm.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 39.Shao Y, Lo CM, Ling CC, Liu XB, Ng KT, Chu AC, Ma YY, Li CX, Fan ST, Man K. Regulatory B cells accelerate hepatocellular carcinoma progression via CD40/CD154 signaling pathway. Cancer Lett. 2014;355:264–72. doi: 10.1016/j.canlet.2014.09.026. [DOI] [PubMed] [Google Scholar]