Abstract
Due to their high heterogeneity and complex tumor microenvironment, the treatment of solid tumors by CAR-T cell technology is limited. This study developed bi-specific Trop2/PD-L1 specific third-generation CAR-T cells by lentiviral infection. The specific killing ability of the bi-specific CAR-T cells against Trop2+ and PD-L1+ expressed on the gastric cancer cell line by CCK-8 assay, was confirmed in vitro. The killing ability of bi-specific Trop2/PD-L1 CAR-T cells was higher than that of mono-specific CAR-T cells (Trop2 CAR-T and PD-L1 CAR-T) and the independent control group (CD19-CAR-T and CIK). The bi-specific Trop2/PD-L1 CAR-T cells produced IFN-γ and IL-2 in response to the overexpression of Trop2 and PD-L1 in gastric cancer cells through ELASA assay. The levels of cytokines (IFN-γ and IL-2) released by bi-specific Trop2/PD-L1 CAR-T cells were the highest among all other types of CAR-T cells and the independent control group. To further demonstrate the ability of bi-specific Trop2/PD-L1 CAR-T cells in vivo, this study testified to the anti-tumor effect of several types of CAR-T cells through a xenograft model bearing human gastric tumors. The results indicated that bi-specific Trop2/PD-L1 CAR-T cells can significantly reduce the tumor growth through intratumoral injection, with a higher inhibition effect than Trop2 specific CAR-T cells and the independent control group (CD19-CAR-T and untreated group). These results suggest that novel bi-specific Trop2/PD-L1 CAR-T cells are able to target Trop2/PD-L1 and checkpoint blockade, and reveal the killing effect on gastric cancer, therefore improving the killing effect of CAR-T cells in solid tumors.
Keywords: Gastric cancer, chimeric antigen receptors, Trop2, PD-L1, immune microenvironment
Introduction
Gastric cancer (GC) is one of the top five common cancers, and ranks as the third highest cause of cancer-related mortality worldwide [1,2]. GC is most prevalent in Asia, and the majority of GC patients are diagnosed at an advanced stage [3,4]. China is a country with a high incidence of GC, which ranks as the second highest in males and fourth in females [1,5]. In recent years, with improvements in dietary habits and improvements in people’s health the incidence of GC has experienced a downward trend, however owing to high metastasis and high recurrence, the 5-year survival rate of advanced-stage GC patients is less than 20% [6]. Traditional treatment methods for GC, such as surgery, radiotherapy and chemotherapy, do not significantly improve the prognosis of patients with GC. Therefore, innovative tumor therapy is urgently needed. Recently immunocyte therapy has attracted wide attention and has gradually become the fourth tumor therapy mode.
Chimeric antigen receptor T-cell immunotherapy (CAR-T) has made great progress, especially in hematological tumors, such as the molecular targeting of CD19 to treat Acute Lymphoid Leukemia [7,8]. This technology has also been named as the top ten technologies in the world by Science in 2013. The application of CAR-T cell technology in solid tumor therapy is one of the current research hotspots. Targeting therapeutic tumor markers, such as Her2, CEA and DF2, have been carried out in basic and clinical studies [9-11].
However, CAR-T cell technology still has many problems when treating solid tumors, such as off-target effects. This is mainly due to the improper choice of target antigens, and the tumor immune microenvironment’s influence on CAR-T cell function [11,12]. However, in 2016, Brown CE, et al. published a clinical medical case report in the New England Journal of Medicine concerning IL13Ra2 CAR-T cells used to treat recurrent, multifocal glioblastoma [13]. This important report demonstrated that CAR-T therapy has an effective role in the treatment of solid tumors. However at this early stage it is necessary to develop new types of CAR-T cells and make breakthroughs in the solid tumor treatment bottleneck.
Human trophoblast cell surface antigen 2 (TACSTD2/Trop2/M1S1/GA733-1) is a membrane surface antigen, which we have extensively studied previously [14]. Trop2 was first found on the surface of human trophoblast cells, and is mainly expressed on the epithelial cell membrane surface. Trop2 is a 36kDa single-pass transmembrane protein, which is divided into an extracellular, a transmembrane, and an intracellular domain, along with a cytoplasmic tail. Trop2 is lysed into the extracellular domain and intracellular domain by TNF-a conversion enzyme, and from the intracellular domain can enter the cytoplasm or nucleus, which once there plays a function. Trop2 is often expressed in different epithelium tissue tumors where it promotes their malignant biological behavior [15]. Previous experiments in our laboratory have shown that Trop2 highly expressed in breast cancer [14], pancreatic cancer [16], gastric cancer [1], could be related to differentiation, TNM stage, tumor size, lymph node metastasis, distant metastasis, and H. pylori infection. High Trop2 expression indicates a poorer prognosis for GC patients, so is therefore useful as a new target for GC.
Programmed death ligand 1 (PD-L1) is a member of the B7 family, and a ligand of PD-1. PD-L1 is a type I transmembrane protein with a total length of 290 amino acids, and is expressed on several types of tumor cells’ surface. Research has shown that PD-1 can trigger or inhibit signal which plays a key role in the tumor environment, through combining with PD-L1. This combination can not only block the activation of T cells by blocking the first and second T cell activation signal, but can also assist regulatory T cells (regulatory cell, Treg) to play an inhibitory function, and induce helper T cells (T helper cells, Th) convert to Treg. The widespread presence of PD-1/PD-L1 in a variety of solid tumors may be one of the main reasons for the poor effect of CAR-T technology in solid tumors [17-19]. The PD-1/PD-L1 monoclonal antibody has been very effective in clinical trials of solid tumors and Opdivo (anti-PD-1 monoclonal antibody) has already been approved for clinical treatment by the CFDA [20]. In our previous studies, results indicated that PD-L1 was significantly higher in GC tissues and the lymphoid tissues of GC metastasis, and was associated with a poor survival prognosis.
In the present study, the authors prepared a bi-specific Trop2/PD-L1 CAR-T cell, which specifically targets Trop2 and PD-L1 tumor antigen and is also able block the PD-1/PD-L1 signaling pathway. The study also detected the killing effect of the bi-specific CAR-T cells towards GC cells in vitro and in vivo.
Materials and methods
Cell lines and T cells
BGC823, MGC803 cell line and 293T cell line were cultured in RPMI 1640 (Invitrogen, Grand Island, NY) supplemented with 10% FBS. Human T cells (1-2 × 106 cells/ml) were isolated from the peripheral blood of healthy donors. T cells were cultured in RBMI 1640 media.
Construction of Trop2/PD-L1 CAR recombinant lentiviral vector
The anti-Trop2 scFv and anti-PD-L1 scFv was derived from Fab [14], which has the ability to bind to Trop2 or the PD-L1 extramenbrane domain. The DNA sequence was optimized and synthesized by Genescript. The optimized sequence contained Trop2 heavy chain variable (Trop2 VH) region-Linker (GGGS), 3-Trop2 light chain variable (Trop2 Vκ) region sequence-Linker (GGGS), 3-PD-L1 heavy chain variable (PD-L1 VH), region-Linker (GGGS) and 3-PD-L1 light chain (PD-L1 Vκ) variable region sequence. Amplification primers were designed and each fragment was amplified according to the design principle of in-fusion polymerse chain reaction (PCR). The fragments were connected to form the Trop2/PD-L1 scFv through Overlap PCR. Trop2/PD-L1 CAR contains genetic elements which codes the anti-Trop2 and PD-L1 scFv, CD8TM (transmembrane) hinge, transmembrane domains of CD28 (amino acids 93-136), CD137, and the cytoplasmic component of CD3ζ chain. The fragment encoding was generated by overlap PCR, using the primers in supplementary: Figure 1A. Control scFv, such as CD19, PD-L1, and Trop2 scFv, were constructed in the same way.
Figure 1.
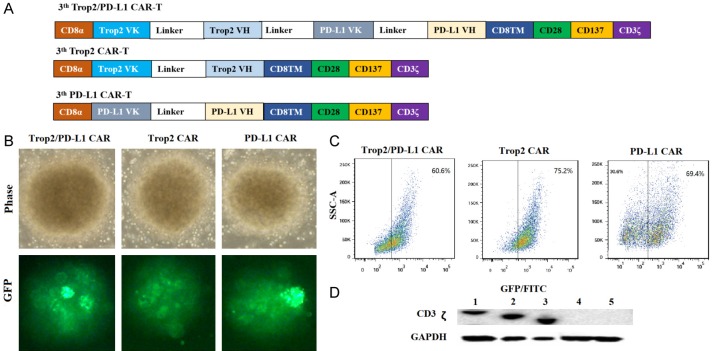
Characterization and expression of bi-specific Trop2/PD-L1 CAR-T cells. (A) Structure diagram of 3th bi-specific Trop2/PD-L1 CAR, 3th Trop2 CAR, 3th PD-L1 CAR. (B) Transduction efficiency of lentivirus in T cells by fluorescence microscope. T cells were transduced with bi-specific Trop2/PD-L1 CAR, Trop2 CAR and PD-L1 CAR. The images were taken under × 400 magnification. The GFP expression was also verified by FACS analysis (C). (D) Western blotting analysis of full length CAR expression in T cells using a CD3ζ antibody. Lane1 bi-specific Trop2/PD-L1 CAR-T cells; Lane2 Trop2 CAR-T cells; Lane3 PD-L1 CAR-T cells; Lane4 CIK T cells; Lane5 T cells.
Bi-specific Trop2/PD-L1 CAR lentivirus production and transduction
The target fragment was connected with lentiviral vector by in-fusion PCR, and then the connected product was transformed through DH5 alpha cells. Detection and identification of positive clones: The correctly sequenced original bacterial solution was transferred to the LB medium, cultured overnight, and the lentivirus vector plasmid was extracted through endotoxin free plasmid extraction kit and stored at -80°C. Bi-specific Trop2/PD-L1 CAR lentivirus plasmid were infected x-293T cells by polythylenimine (Invitrogen, Shanghai, China). The expression level of Trop2/PD-L1 CAR in 293T cells was detected by Western-blot. Control CAR, such as CD19, PD-L1, and Trop2 CAR, were constructed in the same way.
Bi-specific Trop2/PD-L1 CAR recombinant lentivirus infects human T cells
Non-tissue culture treated 24 well (BD Bioscience, USA) plates were coated with RetroNectin (20 ug/mL) (Takara, Japan), and then bi-specific Trop2/PD-L1 CAR and control lentiviral supernatants were thawed, and diluted quickly; after that CAR recombinant lentivirus were added to each well and centrifuged at 3800 rpm for 2 hours at 32°C. Peripheral blood Mononuclear cells (PBMCs) were isolated by Ficoll density gradient (Fresenius, Norway) separation and subsequently activated with anti-CD3/CD28 antibodies (eBioscience, USA). The CAR lentivirus were seeded in wells with 1 × 106 activated T cells at 1000 g for 10 minutes at 32°C. The transduction wells were incubated overnight at 37°C. The infection efficiency was detected through fluorescence microscope and western blot assay. Control CAR-T cells, such as CD19-T cells, PD-L1-T cells, and Trop2 CAR-T cells, were constructed in the same way.
Cytotoxicity assay
Before 24 hours, lymphocytes in wells were replaced with a culture medium with L-12 removed, and cultured overnight. The concentration of Target cell (Trop2+/PD-L1+ BGC823 means Trop2 and PD-L1 high expressed on the surface of BGC823 cells, Trop2-/PD-L1+ BGC823 means shRNA-Trop2 in BGC823 cells and Trop2+/PD-L1- BGC823 means shRNA-PD-L1 in BGC823 cells, Trop2-/PD-L1- BGC823 means shRNA-Trop2 and shRNA-PD-L1 in BGC823 cells) were adjusted to 5 × 105 cell/ml, 2 × 105 cell/ml, 1 × 105 cell/ml, 5 × 104 cell/ml. The effect cell’s concentration (Trop2/PD-L1 CAR-T cells, Trop2 CAR-T cells, PD-L1 CAR-T cells, CD19 CAR-T cells and CIK cells) were adjusted to 1 × 106 cell/ml. The ratio of effect: target (E:T) were set at 20:1, 10:1, 5:1 and 2:1. Each group was provided with 3 multiple holes. Cells were mixed and added in 96 well plates respectively, and then cultured at 37°C in a humidified atmosphere with 5% CO2. Cell growth viability was measured with a Cell Counting Kit 8 (Beyotime, Shanghai, China), following the manufacturer’s instructions. Absorbance was then recorded at 450 nm using Elx800 Reader (BioTECH Instruments Inc., Winooski, VT, USA).
ELISA analysis
According to an E:T ratio of 10:1, effect cells and target cells were added to a 24-well plate. Cells were cultured at 37°C in a humidified atmosphere with 5% CO2. The assay was performed in triplicate. The plates were incubated at 37°C for 24 hours. The supernatant concentration of IFN-γ and IF-2 was determined by ELISA according to the manufacturer’s instructions (eBioscience, USA).
Xenograft model
Animal experiments were performed with the approval of the Institutional Committee for Animal Research and in conformity with national guidelines for the care and use of laboratory animals. 1 × 106 prepared GC cell lines were obtained, and subcutaneously injected into two sides of the chest of immunodeficiency nude mice. When the tumor burden reached about 80 mm3 (approximately 10 days after tumor cell inoculation) the mice were randomly assigned to four different groups (n=5/group). The animals were injected with 1 × 107 T cells/100 ul on day 14, 18, 22 and 26. Group A acted as the untreated group, control group B received CD19 CAR-T cells, (independent control) group C received Trop2 CAR-T cells, and group D received Trop2/PD-L1 CAR-T cells. Prior to the collection of images in vivo, fluorescein potassium dissolved by 5% glucose was injected intraperitoneally (100 ul/each). After 5 weeks, the animals were sacrificed and the tumors were dissected. Tumor growth was monitored by caliper measurement, and tumor volume was calculated using the formula: 1/2 × length × (width)2.
Statistical analysis
The SPSS18.0 statistical software package (SPSS Inc., Chicago, IL) was used for general statistical analysis. The differences between groups were estimated by unpaired Student’s t-test. Values of P less than 0.05 were considered statistically significant.
Results
Construction and identification of Trop2/PD-L1 CAR lentivirus vector
A third-generation CAR, consisting of bi-specific Trop2 and PD-L1 scFv linked to CD8TM hinge, transmembrane domains of CD28 (amino acids 93-136) and CD137 cytoplasmic components of the CD3ζ chain (amino acids 52-163), was constructed and inserted into a lentivirusvector system with green fluorescence protein (GFP) (Figure 1A). T cells activated with anti-CD3 and anti-CD28 advanced antibodies were transduced with Trop2/PD-L1 CAR, Trop2 CAR, PD-L1 CAR and empty lentiviral vector, respectively. As shown in Figure 1B, 1C, following the different kinds of transduced T cells, the proportion of GFP-positive cells in bi-Trop2/PD-L1 CAR-, Trop2 CAR-, and PD-L1 CAR-T cells all exceeded 60%. To further evaluate the expression of Trop2 and PD-L1 CAR in T cells, the author performed western blot assay to recognize the portion of human CD3ζ chain. As shown in Figure 1D, the CAR lentiviral transduced T cells had 78 kDa, consistent with the calculated size of CAR vector protein.
Bi-specific Trop2/PD-L1 CAR-T cells specifically kill Trop2+ and PD-L1+ gastric cancer cells by CCK-8 assay in vitro
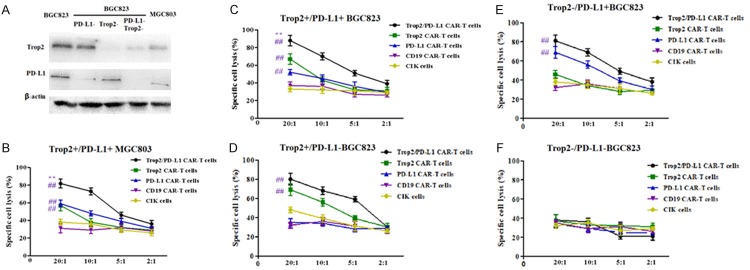
To assess the functionality of the bi-specific Trop2/PD-L1 CAR-T cells and other kinds of CAR-T cells, we investigated whether they could recognize and kill GC cell lines. We select several types of GC cell lines, according to different expression levels of Trop2 and PD-L1 (Figure 2A). Trop2+/PD-L1+ BGC823/MGC803, Trop2-/PD-L1 BGC823, Trop2+/PD-L1- BGC823 and Trop2-/PD-L1- BGC823 cells were used as target cells to determine the cytotoxic potential of 20:1, 10:1, 5:1 and 2:1 transfected T cells. The Trop2/PD-L1 CAR-T cells, Trop2 CAR-T cells, PD-L1 CAR-T cells, CD19 CAR-T cells (as an independent control) and CIK cells were co-cultured with cancer cells for 24 h respectively. As shown in Figure 2, bi-specific Trop2/PD-L1 CAR-T cells showed strongest killing activity against Trop2+ and PD-L1+ expression BGC823, compared with other types of CAR-T cells. Furthermore, control groups, such as CD19 CAR-T cells and CIK cells showed the weakest killing activity. These results demonstrated that bi-specific Trop2/PD-L1 CAR-T cells could recognize and kill specific Trop2+/PD-L1+ GC cells (Figure 2B-F).
Figure 2.
Cytotoxic activity of bi-specific Trop2/PD-L1 CAR-T cells against GC cells by CCK-8. Transduced cells were transferred into free- IL-12 medium for 24 h, several types of CAR-T cells were incubated with Trop2+ and PD-L1+ expression BGC823, MGC803, BGC823 shRNA-Trop2 and shRNA-PD-L1, BGC823 shRNA-Trop2, BGC823 shRNA-PD-L1 cells independently at the indicated E:T ratio for 4 h. Mean values ± SEM calculated from three independent experiments. A. Difference expression of Trop2 and PD-L1 protein between BGC823, BGC823 shRNA-Trop2, shRNA-PD-L1, shRNA-PD-L1/Trop2 and MGC803. B. MGC803 that expressed Trop2 and PD-L1 cells were used as target cells for CCK-8 assay. Effector cells were added at the ratio indicated on the x-axis. The differences between several different groups are significant at each E:T ratio. C. BGC823 that expressed Trop2 and PD-L1 cells were used as target cells for CCK-8 assay. Effector cells were added at the ratio indicated on the x-axis. The differences between several different groups are significant at each E:T ratio. D. Trop2+/PD-L1- BGC823 cells were used as target cells for CCK-8 assay. Effector cells were added at the ratio indicated on the x-axis. E. Trop2-/PD-L1+ BGC823 cells were used as target cells for CCK-8 assay. Effector cells were added at the ratio indicated on the x-axis. F. Trop2-/PD-L1- BGC823 cells were used as target cells for CCK-8 assay. Effector cells were added at the ratio indicated on the x-axis. None type of T cells showed specific cytotoxicity. **means compared with solo specific CAR-T group, ##means compared with CIK group, P<0.001.
Trop2/PD-L1 CAR-T cells produced IFN-γ and IL-2 in a Trop2 and PD-L1 specific manner
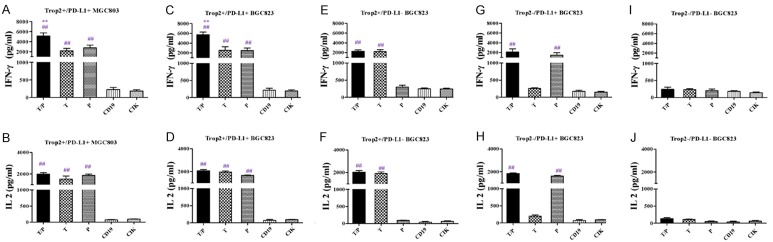
To investigate whether the CAR-T cells could be activated by GC cells, the transfected T cells and target cells were co-cultured for 24 h at a 10:1 ratio. IFN-γ and IL-2 were detected in the supernatants by ELISA. After incubation, the levels of cytokines released by CAR-T cells were elevated in the supernatants, when co-cultured with Trop2+ PD-L1+ BGC823/MGC803 cell lines, the levels of cytokines released by Trop2/PD-L1 CAR-T cells were the highest of all other types of CAR-T cells. These results indicated that the bi-specific Trop2/PD-L1 CAR-T cells, compared to solo CAR-T cells (Trop2 CAR-T and PD-L1 CAR-T) and control effector cells (CD19 CAR-T cells and T cells) produced more cytokine in response to Trop2+ PD-L1+ BGC823 cell lines (Figure 3).
Figure 3.
Specific cytokine released by bi-specific Trop2/PD-L1 CAR-T cells against GC cells. A. IFN-γ released by bi-specific Trop2/PD-L1 CAR-T cells, were measured by enzyme-linked immunosorbent assay (ELISA) after 24 h incubation with Trop2+/PD-L1+ MGC803 cells at an E:T ratio of 10:1, compared with other types of CAR-T cells. B. IL-2 released by bi-specific Trop2/PD-L1 CAR-T cells, were measured by ELISA after 24 h incubation with Trop2+/PD-L1+ MGC803 cells at an E:T ratio of 10:1, compared with other types of CAR-T cells. C. IFN-γ released by bi-specific Trop2/PD-L1 CAR-T cells, were measured by enzyme-linked immunosorbent assay (ELISA) after 24 h incubation with Trop2+/PD-L1+ BGC823 cells at an E:T ratio of 10:1, compared with other types of CAR-T cells. D. IL-2 released by bi-specific Trop2/PD-L1 CAR-T cells, were measured by ELISA after 24 h incubation with Trop2+/PD-L1+ BGC823 cells at an E:T ratio of 10:1, compared with other types of CAR-T cells. E. IFN-γ released by bi-specific Trop2/PD-L1 CAR-T cells, were measured by ELISA after 24 h incubation with Trop2+/PD-L1- BGC823 cells at an E:T ratio of 10:1, compared with other types of CAR-T cells. F. IL-2 released by bi-specific Trop2/PD-L1 CAR-T cells, were measured by ELISA after 24 h incubation with Trop2+/PD-L1- BGC823 cells at an E:T ratio of 10:1, compared with other types of CAR-T cells. G. IFN-γ released by bi-specific Trop2/PD-L1 CAR-T cells, were measured by ELISA after 24 h incubation with Trop2-/PD-L1+ BGC823 cells at an E:T ratio of 10:1, compared with other types of CAR-T cells. H. IL-2 released by bi-specific Trop2/PD-L1 CAR-T cells, were measured by ELISA after 24 h incubation with Trop2-/PD-L1+ BGC823 cells at an E:T ratio of 10:1, compared with other types of CAR-T cells. I. IFN-γ released by bi-specific Trop2/PD-L1 CAR-T cells, were measured by ELISA after 24 h incubation with Trop2-/PD-L1- BGC823 cells at an E:T ratio of 10:1, compared with other types of CAR-T cells. J. IL-2 released by bi-specific Trop2/PD-L1 CAR-T cells, were measured by ELISA after 24 h incubation with Trop2-/PD-L1- BGC823 cells at an E:T ratio of 10:1, compared with other types of CAR-T cells. **means compared with solo specific CAR-T group, ##means compared with CIK group, P<0.001.
Trop2/PD-L1 CAR-T cells inhibited the growth of Trop2+ and PD-L1+ expressing BGC823 cells in vivo
To further demonstrate both the specificity and effector functions of bi-specific Trop2/PD-L1 CAR-T cells in response to GC cells, we confirmed the anti-tumor effect in vivo through a xenograft model bearing human gastric tumors. We expressed the luciferase gene in BGC823 cell lines, together with the puromycin-resistance gene. After puromycin selection, we injected 1 × 106 prepared GC cell lines into nude mice. When the mean tumor volume reached approximately 80 mm3, the animals were divided into four groups (Trop2/PD-L1 CAR-T group, Trop2 CAR-T group, CD19 CAR-T group and untreated group) then the tumor was injected with 1 × 107 T cells/100 ul at day 14, 18, 22 and 26. Luciferase expression was evaluated by bioluminescence imaging at day 14 and 30. As shown in Figure 4A, the rates of tumor growth were considerably inhibited by Trop2/PD-L1 CAR-T cells 16 days after injection, which was lower than that in the CD19 CAR-T cell group and untreated group, where the tumor grew rapidly. Furthermore, statistical analysis of the tumor growth curves indicated that the tumor volume size in Trop2/PD-L1 CAR-T group was significantly smaller than for other groups (Figure 4B, 4C). These findings suggest that the inhibitory ability of bi-specific Trop2/PD-L1 CAR-T cells were significantly greater than that of the other types of CAR-T cells in the treatment of GC.
Figure 4.
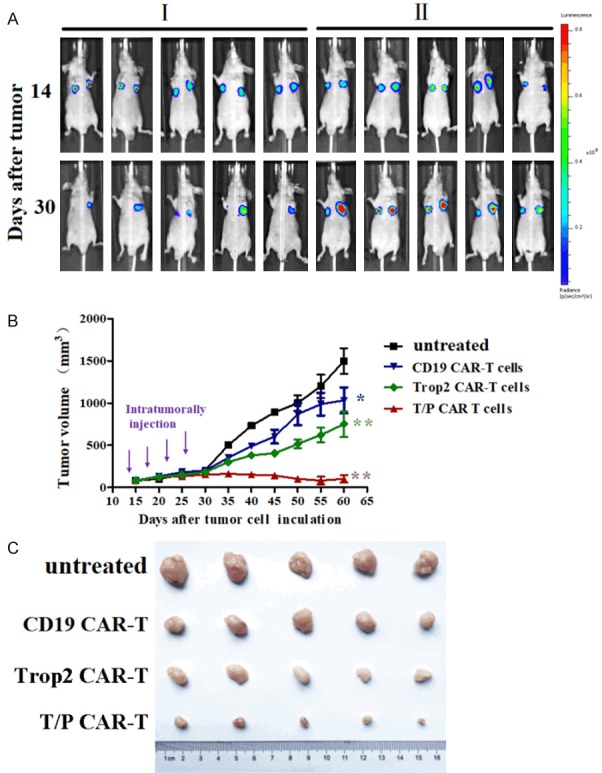
Anti-tumor activity of bi-specific Trop2/PD-L1 CAR-T cells against human gastric cancer xenografts established with BGC823 cells. Nude mice were inculated with BGC823 cells over 10 days. The mice were then randomly assigned to four groups. A. Schematic diagram showing the treatment program of the mice. I, bi-specific Trop2/PD-L1 CAR-T cells inject in the left tumor of the mice, CD19 CAR-T cells injected in the right tumor of the mice. II, Trop2 CAR-T cells inject in the left tumor of the mice, the right tumor of the mice was untreated. The results are expressed as the mean tumor volume (mm3 ± SD) for the four groups (N=5 per group). The standard deviation (SD) is represented by error bars, *indicate P<0.05. B. The tumor growth curves during the experiment. C. Tumor volume from nude mice 60 d after inoculation.
Discussion
In recent years, the treatment of tumor immunocytes with CAR-T cell immunotherapy has attracted wide attention in academic circles, with the hope of creating a new breakthrough in gastric cancer treatment. Chimeric antigen receptor (CAR) is known to identify the single-chain antibody fragment (scFv) of the tumor associated antigen (tumor associated antigen, TAA) and intracellular signal domain “immune receptor tyrosine activation motif (ITAM)” through restructuring in vitro [21,22]. The structure of CAR has been developed as far as the fifth generation since the first generation appeared in 1989. The signal peptide zone of the first generation CARs consisted only of: T cell (antigen) receptor (TCR)/CD3ζ chain, or a single signaling molecule of the immunoglobulin Fc receptor FcεRIγ chain, which is capable of providing a comparable stimulatory signal. However, this type of CARs is insufficient to induce resting T cell proliferation and cytokine production, and inhibit sustained antitumor responses in vivo [23]. For the purpose of improving the activation effect, the second-generation CAR’s signal peptide added a coordinated stimulus molecule (mainly CD28), which could better provide an activation signal for CAR-T cells through the promotion of interlrukin-2 (IL-2) secretion and improved T cell activity [24]. The third-generation CAR’s signal peptide consisted of the integration of two coordinated stimulus molecules (CD28, CD137), which aimed to further enhance signaling capacity and antitumor responses. The fourth-generation CAR added IL-12 on the basis of second-generation structures, which are known as T cells redirected for universal cytokine-mediated killing (TRUCKs) [25].
Although there have been major breakthroughs in the treatment of acute leukemia by CD19 CAR-T cells, many problems still remain in the treatment of solid tumors, for example, lack of tumor-specific antigen which causes the “off-target effect”-negative immune regulation in the tumor microenvironment (TME) which inhibits the killing effect of CAR-T cells [17]. Owing to these, it is necessary to adopt appropriate methods and strategies to overcome the difficulties encountered in tumor immunotherapy. Therefore, we speculate that the screening and verification of more appropriate target antigen and a reduction of the effect of TME immune inhibition are two major factors in CAR-T cell therapy towards solid tumors.
In previous studies, our results demonstrated that the anti-tumor activity of a CD28 co-stimulated LMP1 CAR in nasopharyngeal cancer [26], further study indicated that the CAR-T cells, possess the CD28 and CD137 co-stimulation signaling domain, have the highest anti-tumor and persistence ability than that in the only have CD28 signaling domain’s CAR-T cells [27]. In the present study, we selected Trop2 as the target antigen, which is only expressed on the membrane surface of GC cells and is either absent or lowly expressed in normal cells [28]. As an important consequence, off-target toxicity can be reduced when applying CAR-T cell technology to solid tumors.
In addition to this, negative immune regulation in the tumor microenvironment is also an important role in the application of CAR-T cell technology to combat solid tumors [29], meaning that the bi-specific target CAR-T cells are able to target specific Trop2 and PD-L1 tumor antigen, while blocking the PD-1/PD-L1 signaling pathway. During in vitro experiments, bi-specific Trop2/PD-L1 CAR-T cells showed the strongest killing activity against Trop2+ and PD-L1+ expression BGC823, when compared with sole specific CAR-T cells and the T cell control group. Meanwhile, bi-specificTrop2/PD-L1 CAR-T cells produced more IFN-γ and IL-2 in response to the expression of Trop2 and PD-L1 in tumor cells. This is most likely the result of the efficient killing ability of the bi-specific Trop2/PD-L1 CAR-T cells.
In 2018, Rafiq et al. constructed CAR-T cells that secret a PD-1 blocking scFv, which was found to enhance the survival of PD-L1+ tumor bearing mice. In their study, the author demonstrated CAR-T cells can be deliver immune regulatory scFv to the tumor micro-environment [30]. In our present study, we hoped to combine two targets with a checkpoint blockade, and further demonstrate the anti-tumor advantage of bi-specific Trop2/PD-L1 CAR-T cells in vivo. To compare the anti-tumor effect of several CAR-T cells, the study used a xenograft model bearing human gastric tumors and found bi-specific Trop2/PD-L1 CAR-T cells were able to specifically control Trop2+ and PD-L1+ BGC823 cells.
One limitation of our study however, was the use of a xenograft model, namely used in lymphocyte dysfunctional mice, therefore the study was not able to simulate the immune-suppressive microenvironment entirely [31]. These NSG mice, have a combined immunodeficiency which includes T, B and NK cells [32], so we speculated that NSG was not completely suitable. To counter this, in a future study we intend to use mice-derived CAR-T cells (in a mice model), so as to stimulate the human-like immune-suppressive microenvironment. In addition, because Trop2 and PD-L1 are antigens that are not only express on cancer cells, they also express in some normal epithelial and immune cells. Although there might be higher expression on tumor cells than in normal cells, it would still be necessary to test whether this bi-specific CAR can potentially target normal tissues to make sure it is safe to use. Therefore, we will construct different types of CAR-T cells through screen different affinities antibodies, and further compare the killing effects of these types of CAR-T cells towards cells with different expression levels antigen.
This study provided an effective strategy for the clinical application of CAR-T cell technology in solid tumors, and indications show that bi-specific Trop2/PD-L1 CAR-T cells have the potential for high therapeutic efficacy in treating GC.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (No. 81602119, 81773100).
Disclosure of conflict of interest
None.
References
- 1.Zhao W, Zhu H, Zhang S, Yong H, Wang W, Zhou Y, Wang B, Wen J, Qiu Z, Ding G, Feng Z, Zhu J. Trop2 is overexpressed in gastric cancer and predicts poor prognosis. Oncotarget. 2016;7:6136–45. doi: 10.18632/oncotarget.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song Y, Tong C, Wang Y, Gao Y, Dai H, Guo Y, Zhao X, Wang Y, Wang Z, Han W, Chen L. Effective and persistent antitumor activity of HER2-directed CAR-T cells against gastric cancer cells in vitro and xenotransplanted tumors in vivo. Protein Cell. 2017;9:867–878. doi: 10.1007/s13238-017-0384-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sue S, Shibata W, Maeda S. Helicobacter pylori-Induced signaling pathways contribute to intestinal metaplasia and gastric carcinogenesis. Biomed Res Int. 2015;2015:737621. doi: 10.1155/2015/737621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang G, Gu D, Zhao Q, Chu H, Xu Z, Wang M, Tang C, Wu D, Tong N, Gong W, Zhou J, Xu Y, Zhang Z, Chen J. Genetic variation in C12orf51 is associated with prognosis of intestinal-type gastric cancer in a Chinese population. Biomed Pharmacother. 2015;69:133–138. doi: 10.1016/j.biopha.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Lv ZD, Zhao WJ, Jin LY, Wang WJ, Dong Q, Li N, Xu HM, Wang HB. Blocking TGF-beta1 by P17 peptides attenuates gastric cancer cell induced peritoneal fibrosis and prevents peritoneal dissemination in vitro and in vivo. Biomed Pharmacother. 2017;88:27–33. doi: 10.1016/j.biopha.2017.01.039. [DOI] [PubMed] [Google Scholar]
- 6.Zhou ML, Wang L, Wang JZ, Yang W, Hu R, Li GC, Sheng WQ, Zhang Z. Validation of the memorial sloan kettering cancer center nomogram to predict disease-specific survival in a chinese gastric cancer population receiving postoperative chemoradiotherapy after an R0 resection. Oncotarget. 2016;7:64757–64765. doi: 10.18632/oncotarget.11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chimeric antigen Receptor-Modified t cells in chronic lymphoid leukemia; Chimeric antigen Receptor-Modified t cells for acute lymphoid leukemia; Chimeric antigen receptor t cells for sustained remissions in leukemia. N Engl J Med. 2016;374:998. doi: 10.1056/NEJMx160005. [DOI] [PubMed] [Google Scholar]
- 9.Sun M, Shi H, Liu C, Liu J, Liu X, Sun Y. Construction and evaluation of a novel humanized HER2-specific chimeric receptor. Breast Cancer Res. 2014;16:R61. doi: 10.1186/bcr3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craddock JA, Lu A, Bear A, Pule M, Brenner MK, Rooney CM, Foster AE. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J Immunother. 2010;33:780–788. doi: 10.1097/CJI.0b013e3181ee6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rainusso N, Brawley VS, Ghazi A, Hicks MJ, Gottschalk S, Rosen JM, Ahmed N. Immunotherapy targeting HER2 with genetically modified T cells eliminates tumor-initiating cells in osteosarcoma. Cancer Gene Ther. 2012;19:212–217. doi: 10.1038/cgt.2011.83. [DOI] [PubMed] [Google Scholar]
- 13.Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, Ostberg JR, Blanchard MS, Kilpatrick J, Simpson J, Kurien A, Priceman SJ, Wang X, Harshbarger TL, D’Apuzzo M, Ressler JA, Jensen MC, Barish ME, Chen M, Portnow J, Forman SJ, Badie B. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375:2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin H, Zhang H, Wang J, Lu M, Zheng F, Wang C, Tang X, Xu N, Chen R, Zhang D, Zhao P, Zhu J, Mao Y, Feng Z. A novel human Fab antibody for Trop2 inhibits breast cancer growth in vitro and in vivo. Int J Cancer. 2014;134:1239–1249. doi: 10.1002/ijc.28451. [DOI] [PubMed] [Google Scholar]
- 15.Shvartsur A, Bonavida B. Trop2 and its overexpression in cancers: regulation and clinical/therapeutic implications. Genes Cancer. 2015;6:84–105. doi: 10.18632/genesandcancer.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Liu QQ, Tang XJ, Xu X, Chu C, Xiong SP, Zheng F, Tong H, Zhu J, Feng ZQ, Lin H. Eukaryotic expression of human anti-TROP2 antibody IgG and its inhibitory effect on cell. Acta Universitatis Medicinalis Nanjing. 2014:863–869. [Google Scholar]
- 17.Weinstock M, McDermott D. Targeting PD-1/PD-L1 in the treatment of metastatic renal cell carcinoma. Ther Adv Urol. 2015;7:365–377. doi: 10.1177/1756287215597647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varki V, Ioffe OB, Bentzen SM, Heath J, Cellini A, Feliciano J, Zandberg DP. PD-L1, B7-H3, and PD-1 expression in immunocompetent vs. Immunosuppressed patients with cutaneous squamous cell carcinoma. Cancer Immunol Immunother. 2018;67:805–814. doi: 10.1007/s00262-018-2138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singer M, Wang C, Cong L, Marjanovic ND, Kowalczyk MS, Zhang H, Nyman J, Sakuishi K, Kurtulus S, Gennert D, Xia J, Kwon J, Nevin J, Herbst RH, Yanai I, Rozenblatt-Rosen O, Kuchroo VK, Regev A, Anderson AC. A distinct gene module for dysfunction uncoupled from activation in Tumor-Infiltrating t cells. Cell. 2017;171:1221–1223. doi: 10.1016/j.cell.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ivashko IN, Kolesar JM. Pembrolizumab and nivolumab: PD-1 inhibitors for advanced melanoma. Am J Health Syst Pharm. 2016;73:193–201. doi: 10.2146/ajhp140768. [DOI] [PubMed] [Google Scholar]
- 21.Turtle CJ, Hudecek M, Jensen MC, Riddell SR. Engineered T cells for anti-cancer therapy. Curr Opin Immunol. 2012;24:633–639. doi: 10.1016/j.coi.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melenhorst JJ, Levine BL. Innovation and opportunity for chimeric antigen receptor targeted T cells. Cytotherapy. 2013;15:1046–1053. doi: 10.1016/j.jcyt.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Brocker T. Chimeric Fv-zeta or Fv-epsilon receptors are not sufficient to induce activation or cytokine production in peripheral T cells. Blood. 2000;96:1999–2001. [PubMed] [Google Scholar]
- 24.Ramos CA, Dotti G. Chimeric antigen receptor (CAR)-engineered lymphocytes for cancer therapy. Expert Opin Biol Ther. 2011;11:855–873. doi: 10.1517/14712598.2011.573476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pegram HJ, Purdon TJ, van Leeuwen DG, Curran KJ, Giralt SA, Barker JN, Brentjens RJ. IL-12-secreting CD19-targeted cord blood-derived T cells for the immunotherapy of B-cell acute lymphoblastic leukemia. Leukemia. 2015;29:415–422. doi: 10.1038/leu.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang X, Zhou Y, Li W, Tang Q, Chen R, Zhu J, Feng Z. T cells expressing a LMP1-specific chimeric antigen receptor mediate antitumor effects against LMP1-positive nasopharyngeal carcinoma cells in vitro and in vivo. J Biomed Res. 2014;28:468–475. doi: 10.7555/JBR.28.20140066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu YR, Zhou Y, Tang Q, Liu ZY, Huang XC, Yang TT, Zhang HL, Zhao W, Kuai XW, Qiu ZN, Zhu J, Feng ZQ. Construction of Trop-2-targeted chimeric antigen receptor-modified T cells and their effects on the proliferation of ovarian cancer cells in vitro. Acta Universitatis Medicinalis Nanjing. 2017;37:653–658. [Google Scholar]
- 28.Ju X, Jiao X, Ertel A, Casimiro MC, Di Sante G, Deng S, Li Z, Di Rocco A, Zhan T, Hawkins A, Stoyanova T, Ando S, Fatatis A, Lisanti MP, Gomella LG, Languino LR, Pestell RG. V-Src oncogene induces trop2 proteolytic activation via cyclin D1. Cancer Res. 2016;76:6723–6734. doi: 10.1158/0008-5472.CAN-15-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long AH, Highfill SL, Cui Y, Smith JP, Walker AJ, Ramakrishna S, El-Etriby R, Galli S, Tsokos MG, Orentas RJ, Mackall CL. Reduction of MDSCs with all-trans retinoic acid improves CAR-Therapy efficacy for sarcomas. Cancer Immunol Res. 2016;4:869–880. doi: 10.1158/2326-6066.CIR-15-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rafiq S, Yeku OO, Jackson HJ, Purdon TJ, van Leeuwen DG, Drakes DJ, Song M, Miele MM, Li Z, Wang P, Yan S, Xiang J, Ma X, Seshan VE, Hendrickson RC, Liu C, Brentjens RJ. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol. 2018;36:847–856. doi: 10.1038/nbt.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krenciute G, Prinzing BL, Yi Z, Wu MF, Liu H, Dotti G, Balyasnikova IV, Gottschalk S. Transgenic expression of IL15 improves antiglioma activity of IL13Ralpha2-CAR-T cells but results in antigen loss variants. Cancer Immunol Res. 2017;5:571–581. doi: 10.1158/2326-6066.CIR-16-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griessinger E, Andreeff M. NSG-S mice for acute myeloid leukemia, yes. For myelodysplastic syndrome, no. Haematologica. 2018;103:921–923. doi: 10.3324/haematol.2018.193847. [DOI] [PMC free article] [PubMed] [Google Scholar]