Abstract
Currently, no definitive diagnostic tool is available to distinguish unifocal and multifocal papillary thyroid carcinoma (PTC). This study aims to identify potential diagnostic markers of multifocal PTC. In 471 Hashimoto’s thyroiditis (HT) patients, the significant difference was revealed in anti-thyroid peroxidase antibody (TPOAb) concentration, the cytokeratin-19 (CK-19) expression, the occurrence of the B-Raf proto-oncogene serine/threonine kinase (BRAF) mutations and the rearrangement in transformation (RET)/PTC. The patients’ samples were assayed for the expression of CK-19, cyclooxygenase-2 (COX-2), galectin-3, and the protein human bone marrow endothelial cell marker-1 (HBME-1) using immunohistochemistry. The BRAF gene mutation was detected using a sequencer. Differences were examined using the Kruskal-Wallis test and the Chi-squared and Fisher’s exact tests. The results showed that the elevated CK-19 expression, and the presence of BRAF mutations and RET/PTC rearrangements were indicators of multifocal PTC in HT, suggesting the need for total bilateral thyroidectomy. Among HT patients with TPOAb > 1300 IU/Ml, the occurrence of central lymph node metastasis is significantly higher in multi-focal PTC than single-focal PTC. Therefore, these markers may prove useful for discerning between uni- and multifocal PTC, thereby preventing unnecessary surgery in the treatment of unifocal PTC and promoting sufficient treatment of multifocal PTC.
Keywords: Hashimoto’s thyroiditis (HT), papillary thyroid carcinoma (PTC), biomarker, thyroidectomy, anti-thyroid peroxidase antibody (TPOAb)
Introduction
Hashimoto’s thyroiditis (HT) is an autoimmune condition that leads to the destruction of thyroid cells and is the leading cause of hypothyroidism worldwide. A growing body of evidence indicates that HT patients are at increased risk of developing papillary thyroid carcinoma (PTC) [1]. Although the extent of this relationship is still a matter of debate [2,3], two large-cohort meta-analyses of recent studies both conclude that HC is clearly associated with PTC [4,5]. Zhang et al. found that HT and PTC are significantly associated and concluded that long-term exposure to the elevated thyroid stimulating hormone (TSH) levels typical of HT likely predisposes HT patients to PTC [6].
PTC is most often unifocal, but multifocal PTC can arise from the spread of a single primary tumor or from multiple simultaneous primary tumors. Patients with multifocal PTC are at increased risk for lymph node metastasis, distant metastasis, local recurrence after initial treatment, and regional recurrence [7]. The rate of PTC multifocality is higher in HT patients [8]. The frequency of PTC metastasis to visceral lymph nodes is 4-fold higher in patients with HT than in those without [3]. Wang et al. found that bilateral PTC has greater invasiveness, faster progression, and a shorter 10-year disease-free survival compared to unilateral multifocal PTC. The increased incidence of lymph node metastasis in patients with bilateral PTC contributes to its poor prognosis [9].
The preferred treatment for PTC in HT is surgical resection followed by other treatment modalities. However, opinions vary as to the scope of surgical resection needed. Clearly, multifocal PTC in HT should be treated by bilateral thyroidectomy. After unilateral lobectomy, TC recurs in 2-9% of the contralateral lobes. Unilateral resection may leave behind remnant cancer, resulting in contralateral recurrence or even metastases after surgery. Thus, more extensive thyroidectomy is recommended [10]. However, patients with non-total resection have a lower incidence of postoperative complications such as hypothyroidism and hypocalcemia than do those who undergo total resection [11]. In addition, the prognosis of HT patients does not differ significantly between treatment with thyroidectomy and total resection. According to the 2015ATA thyroid cancer diagnosis and treatment guidelines [12], unilateral lobectomy is recommended for low-risk, unifocal PTC.
Unfortunately, no diagnostic tool is available for determining whether HT-associated PTC is uni- or multifocal. Intraoperative frozen section analysis examines only obvious nodules, often missing small cancerous lesions. Unilateral resection only examines the affected side and cannot detect the presence of cancerous lesions in the contralateral side. Pathological examination can only confirm a diagnosis of multifocal cancer after total bilateral resection. Thus, patients with unifocal cancer may be over-treated, exposing them to unnecessary risk of postoperative complications. Therefore, the identification of potential early indicators of multifocal cancer in HT is needed for guiding appropriate clinical treatment plans.
Current research in this area is focused on identifying specific biochemical and genetic markers to distinguish multifocal PTC. Azizi et al. found that elevated serum anti-thyroid peroxidase antibody (TPOAb), the key etiological autoantibody in HT, and TSH ≥ 1 μIU/mL are independent predictors of thyroid cancer in patients with thyroid nodules [13]. The expression of human bone marrow endothelial cell marker-1 (HBME-1), a membrane protein present in the microvilli of mesothelioma and follicular thyroid tumor cells, is significantly higher in PTC patients with malignant than with benign thyroid lesions [14]. Similarly, the expression of Galectin-3, a β-galactoside-binding protein involved in cell migration, adhesion, and apoptosis, is significantly higher in malignant than in benign thyroid lesions [14,15]. CK-19, a keratin family member responsible for the structural integrity of epithelial cells, is highly expressed in PTC follicular variants but not in benign lesions such as thyroid adenomas [16]. The expression of cyclooxygenase-2 (COX-2), which promotes cell proliferation and inhibits apoptosis by catalyzing prostaglandin synthesis, is elevated in many precancerous lesions and malignant tumors [17,18].
Genetic markers under investigation for discerning between metastatic and benign cancers include BRAF gene mutations and rearranged in transformation (RET)/PTC oncogene rearrangements. Both of these genetic alterations are common in thyroid cancer. Mutations in BRAF, a serine/threonine kinase, increase its kinase activity, resulting in the activation of ERK. RET/PTC oncogene rearrangements involve the fusion of the tyrosine kinase domain of RET to the 5’ region of unrelated genes, creating dominantly transforming oncogenes [3].
This retrospective study aims to identify markers that distinguish between unifocal and multifocal PTC in HT patients. Blood chemistry parameters, thyroid function assays, and TPOAb levels were compared between HT patients with unifocal PTC, multifocal PTC, and no PTC. These groups were also compared with respect to immunohistochemical analysis of tissue samples for CK-19, COX-2, Galectin-3, and HBME-1 expression and the presence of BRAF mutations and RET/PTC oncogene rearrangements.
Materials and methods
HT patients
This study is a retrospective analysis of 471 patients with PTC and HT who underwent thyroid surgery from January 2013 to January 2015, including 183 patients in group A (HT with multifocal PTC), 187 in group B (HT with single PTC), and 101 in group C (HT alone). All patients were diagnosed with thyroid nodules due to suspected malignancy or large nodules. Inclusion criteria included thyroid surgery indications and postoperative pathology confirmed as Hashimoto’s thyroiditis with papillary thyroid carcinoma or Hashimoto’s thyroiditis. Exclusion criteria included hyperthyroidism, history of subacute thyroiditis, history of tuberculosis or other malignant tumors, low immune function, and a history of other major medical conditions. Patients who underwent chemotherapy, radiotherapy, or immunotherapy before surgery were excluded. This study was approved by the ethics committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (No. 2016-119).
Clinical data analysis
Blood was collected from all patients before surgery. The following serum indicators were assayed: preoperative T3, T4, TSH, thyroglobulin (TG), calcitonin, and parathyroid hormone; tumor markers, including carcinoembryonic antigen (CEA), carbohydrate antigen CA199, CA125, alpha-fetoprotein (AFP), ferritin, blood sugar, and blood lipids. The preoperative TPOAb concentration was also determined. Routine pathology results were compared between groups to determine their correlations with unifocal cancer, multifocal cancer, and positive central lymph node metastasis.
Immunohistochemistry
The formalin-fixed, paraffin-embedded samples of all patients were assayed for the expression of cytokeratin-19 (CK-19), cyclooxygenase-2 (COX-2), Galectin-3, and HBME-1 using immunohistochemistry. Paraffin sections were prepared by dewaxing hydration, endogenous peroxidase blocking, non-specific protein blocking, incubation with primary, secondary, and streptavidin-HRP antibodies, DAB color development, hematoxylin counterstaining, and dehydration. The slides were photographed using a microscopic imaging system and carried out quantitative analysis of average optical density using internationally accepted Image Pro Plus software (Media Cybernetics, Rockville, MD, USA). The primary antibodies, all purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) were as follows: (1) rabbit anti-CK-19 antibody, (2) rabbit anti-COX-2 antibody, (3) rabbit anti-Galectin-3 antibody, and (4) rabbit anti-HBME-1 antibody.
Detection of the BRAF mutation
Genomic DNA was extracted from resected specimens from all patients. PCR was performed to amplify the target gene fragments, and the BRAF gene mutation was detected using a sequencer. PCR primers were designed based on the BRAF genomic DNA sequence using Primer5 online software, and PCR amplification was performed to amplify exon 15 of the BRAF gene. The primer sequences were as follows:
Forward primer: 5’-TCATAATGCTTGCTCTGATAGGA-3’; Reverse primer: 5’-GGCCAAAAATTTAATCAGTGGA-3’. The length of the amplified fragment was 224 bp, and the primer was synthesized by Shenggong Bioengineering Co., Ltd (Shanghai, China). The PCR reaction conditions were as follows: Pre-change at 94°C for 5 min, denaturation at 94°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 30 s, 35 cycles in total, extension at 72°C for 5 min, ending at 4°C. The PCR products were sequencing using the Sanger Chain Termination method.
RNA extraction
The tissue was placed in a 1-mL Trizol (Invitrogen, Carlsbad, CA, USA) homogenate tube, homogenized in a homogenizer for 20 sec, and incubated for 5 min. After centrifugation, the supernatants were added to 200 μL chloroform, shake well, and stand at room temperature for 2 min. After centrifugation, the supernatants were added to 600 μL isopropanol, mixed well, and left at room temperature for 15 min. After centrifugation, the pellets were washed with 1 ml 75% absolute ethanol and dried at room temperature for 10 min. To the samples was added 40 μL DEPC water to dissolve the RNA, and the solutions were stored at -80°C for later use.
Detection of RET/PTC oncogene rearrangements
Specimens from all patients were selected and confirmed by pathological diagnosis. After total RNA extraction and cDNA synthesis, PCR amplification was carried out using the nested PCR method. The primer sequences were as follows: RET/PTC1 first round: F, 5’-GTCATCTCGCCGTTC-3’; R, 5’-CTTTCAGCATCTTCACGG-3’; RET/PTC1 second round: F, 5’-GCTGGAGACCTACAAACTGA-3’; R, 5’-CGTTGCCTTGACCACTTTTC-3’; RET/PTC3 first round: F, 5’-AAGCAAACCTGCCAGTGG-3’; R, 5’-CGTTGGCCTTGACCACTTTTC -3’; RET/PTC3 second round: F, 5’-CAAGCTCCTTACATACC-3’; R, 5’-CCTTCTCCTAGAGTTTTTCC-3’; 18S: F, 5’-CGACGACCCATTCGAACGTCT-3’; R, 5’-CTCTCCGGAATCGAACCCTGA-3’. PCR reaction conditions were as follows: pre-change for 5 min at 94°C, denaturation at 94°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 30 s, 35 cycles in total, extending at 72°C for 5 min, ending at 4°C. The product obtained by PCR amplification was subjected to gelatinization purification, and the base sequence was determined using a DNA sequencer. The sequence was compared to that of the genomic DNA to determine the presence or absence of the mutation and the mutation position.
Statistical analysis
Continuous variables were presented as the median with the interquartile range (IQR), and categorical variables were reported as the number with a percentage. Differences between groups were examined using the Kruskal-Wallis test for continuous variables and the Chi-squared and Fisher’s exact tests for categorical variables. Dunn’s test was implemented if a significant difference was obtained by the Kruskal-Wallis test. Logistic regression analysis was performed to identify predictors of multifocal PTC in HT, and all variables were evaluated by multivariate analysis with stepwise selection. Receiver operating characteristic (ROC) curves of CK-19 were generated to determine the optimal cut-off point and to distinguish between HT patients with multifocal and unifocal PTC. All p-values were two-sided, and P < 0.05 was considered statistically significant. All statistical analyses were performed using the statistical software package SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Demographic and clinical findings
Of the 471 HT patients enrolled in the study, 183 patients had HT with multifocal PTC (38.9%), 187 patients had HT with single PTC (39.7%), and 101 patients had HT alone (21.4%). The median patient age in these groups was 46, 41, and 46 years, respectively. More than 80% of the patients in all groups were female (86.9%, 87.2%, and 87.1%, respectively). Calcitonin and parathyroid hormone levels were normal in all patients. Only two patients with single PTC had tumor markers (CA125 and CA199). HT patients with multifocal (100.0%) or unifocal PTC (98.9%) underwent total thyroidectomy. However, two patients with single PTC underwent a thyroid lobectomy, and all of the patients with HT alone underwent subtotal thyroidectomy (Table 1).
Table 1.
Comparison of demographic and clinical data between groups
| HT with multifocal PTC | HT with single PTC | HT alone | P | |
|---|---|---|---|---|
| N = 183 | N = 187 | N = 101 | ||
| n (%) | n (%) | n (%) | ||
| Demographic | ||||
| Age | 46.0 (40.0, 51.0) | 41.0 (34.0, 54.0) | 46.0 (32.0, 51.0) | 0.989 |
| Female | 159 (86.9) | 163 (87.2) | 88 (87.1) | 0.996 |
| Clinical indicators | ||||
| TG (ng/mL) | 31.0 (21.0, 44.0) | 29.0 (22.0, 44.0) | 27.0 (21.0, 38.0) | 0.157 |
| Calcitonin = Normal | 183 (100.0) | 187 (100.0) | 101 (100.0) | NA |
| Tumor markers (Abnormal) | NA | |||
| AFP | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| CA125 | 0 (0.0) | 1 (0.6) | 0 (0.0) | |
| CA199 | 0 (0.0) | 1 (0.6) | 0 (0.0) | |
| CEA | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Ferritin | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| PTH = Normal | 183 (100.0) | 187 (100.0) | 101 (100.0) | NA |
| Blood sugar = Abnormal | 4 (2.2) | 4 (2.1) | 4 (4.0) | 0.651 |
| Blood lipid = Abnormal | 11 (6.0) | 12 (6.4) | 8 (7.9) | 0.819 |
| TPOAb > 1300 (IU/mL) | 130 (71.0) | 22 (11.8) | 48 (47.5) | < 0.001 |
| Central compartment of cervical lymph node metastasis = Positive | 124 (67.8) | 97 (51.9) | 0 (0.0) | 0.002* |
| TSH (μIU/mL) | 2.4 (1.6, 3.4)c | 2.3 (1.6, 2.7)c | 3.4 (2.6, 4.2)a,b | < 0.001 |
| Thyroid surgery | NA | |||
| LOBE | 0 (0.0) | 2 (1.1) | 0 (0.0) | |
| Subtotal | 0 (0.0) | 0 (0.0) | 101 (100.0) | |
| Total | 183 (100.0) | 185 (98.9) | 0 (0.0) | |
| Number of tumors | 2.0 (2.0, 2.0) | 1.0 (1.0, 1.0) | 1.0 (1.0, 1.0) | < 0.001 |
| Size of the largest tumor (cm) | 0.8 (0.6, 1.2) | 0.8 (0.5, 1.2) | 3.6 (3.3, 3.9) | < 0.001 |
HT, Hashimoto’s thyroiditis; PTC, papillary thyroid carcinoma; TG, thyroglobulin; AFP, alpha-fetoprotein; CEA, carcinoembryonic antigen; PTH, parathyroid hormone; TPOAb, thyroid peroxidase antibodies; TSH, thyroid-stimulating hormone; LOBE, thyroid lobectomy; Subtotal, subtotal/near-total thyroidectomy; Total, total thyroidectomy. Continuous variables were presented as median and IQR; categorical variables were presented as number and percentage.
HT with multifocal PTC vs. HT with single PTC.
Significant difference vs. HT with multifocal PTC, P < 0.05.
Significant difference vs. single PTC, P < 0.05.
Significant different vs. HT alone, P < 0.05.
Groups differed significantly with respect to the concentration of TPOAb, positive central lymph node metastasis, TSH level, number of tumors, and size of the largest tumor (all P < 0.001). More HT patients with multifocal PTC had TPOAb > 1300 IU/mL (71.0%) and were positive for central cervical lymph node metastasis (67.8%) than were patients in other groups (Table 1).
In addition, the status of central lymph node metastasis differed with the level of TPOAb between HT patients with multifocal and unifocal PTC (P < 0.001). Among HT patients with multifocal PTC and TPOAb concentration > 1300 IU/mL, most were positive for central lymph node metastasis (43.7%); among unifocal patients with TPOAb < 1300 IU/mL, most were negative for central lymph node metastasis (45.5%) (Supplementary Table 1).
Immunohistochemistry results
The expression of CK-19, COX-2, Galectin-3, and HBME-1 differed significantly between tissue samples from different groups (all Ps ≤ 0.045) (Figure 2 and Table 2). However, Dunn’s multiple comparisons test showed that only the median optical density of CK-19 differed significantly between PTC patients with multifocal and unifocal PTC (0.011 vs. 0.006, respectively) (Table 2).
Figure 2.
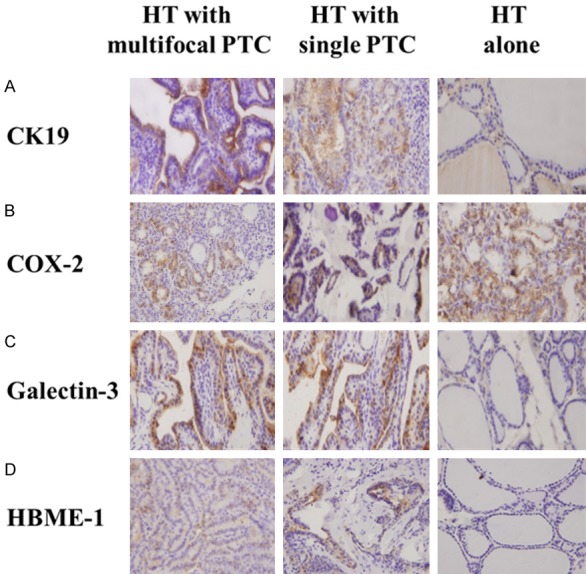
Immunohistochemistry results of CK-19, COX-2, Galetin-3 and HBME-1 in each group. The protein expression of (A) CK-19, (B) COX-2, (C) Galectin-3, and (D) HBME-1 was detected in HT patients with multifocal PTC, with single PTC, and without PTC. Fields at 40× magnification were shown, and 1 representative from triplicates was shown for each staining.
Table 2.
Comparison of tissue immunohistochemical data between groups
| HT with multifocal PTC | HT with single PTC | HT alone | P | |
|---|---|---|---|---|
| N = 183 | N = 187 | N = 101 | ||
| n (%) | n (%) | n (%) | ||
| Immunohistochemistry | ||||
| CK19 | 0.011 (0.007, 0.013)b,c | 0.006 (0.002, 0.011)a,c | 0.003 (0.001, 0.004)a,b | < 0.001 |
| COX-2 | 0.005 (0.003, 0.009)c | 0.004 (0.002, 0.008)c | 0.002 (0.001, 0.004)a,b | < 0.001 |
| Galectin-3 | 0.009 (0.005, 0.021)c | 0.014 (0.009, 0.021)c | 0.003 (0.002, 0.004)a,b | < 0.001 |
| HBME-1 | 0.006 (0.004, 0.008)c | 0.006 (0.004, 0.007) | 0.006 (0.003, 0.006)a | 0.045 |
HT, Hashimoto’s thyroiditis; PTC, papillary thyroid carcinoma; CK19, cytokeratin 19; COX-2, cyclooxygenase 2; HBME-1, mouse anti mesothelioma. Continuous variables are presented as the median and IQR.
Significant difference vs. HT with multifocal PTC, P < 0.05.
Significant difference vs. HT with single PTC, P < 0.05.
Significant different vs. patients with HT alone, P < 0.05.
We determined the optimal cutoff point in the optical density of CK-19 to determine the relationship between CK-19 expression and multifocal vs. unifocal PTC. ROC analysis indicated that CK-19 optical density had acceptable discrimination for distinguishing between multifocal and unifocal PTC in HT patients (AUC, 0.749; median optimal cutoff point, 0.008) (Figure 1).
Figure 1.
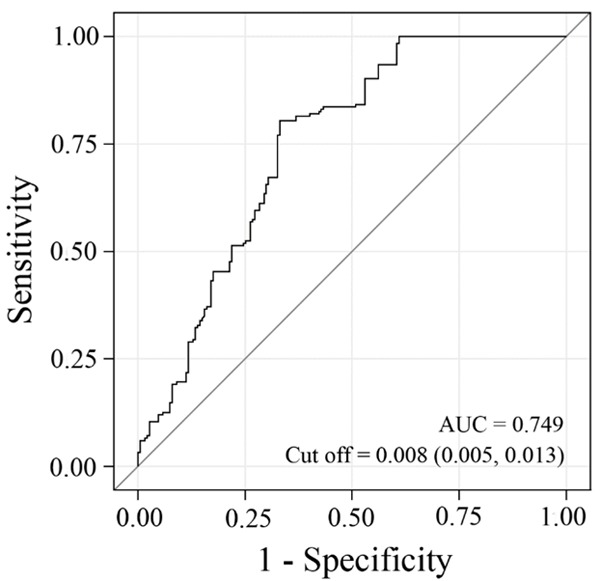
ROC curves to detect the optimal cutoff of CK-19 expression for multifocal PTC in HT.
Genetic findings
The occurrence of BRAF and RET/PTC in HT patients differed significantly between those with multifocal and unifocal PTC (all P < 0.001). A greater fraction of HT patients with multifocal PTC had the BRAF mutation (41.5%) or positivity for RET/PTC (56.8%) than did those with unifocal PTC (Table 3).
Table 3.
Comparison of genetic alterations between groups
| HT with multifocal PTC | HT with unifocal PTC | HT alone | P | |
|---|---|---|---|---|
| N = 183 | N = 187 | N = 10 | ||
| n (%) | n (%) | n (%) | ||
| Gene detection | ||||
| BRAF Mutation | 76 (41.5) | 18 (9.6) | 0 (0.0) | < 0.001* |
| RET/PTC-positive | 104 (56.8) | 39 (20.9) | 0 (0.0) | < 0.001* |
HT, Hashimoto’s thyroiditis; PTC, papillary thyroid carcinoma; BRAF, B-type Raf kinase; RET/PTC, rearranged in transformation/papillary thyroid carcinoma. Categorical variables are presented as count and percentage.
Multifocal PTC vs. unifocal PTC.
Risk factors for multifocal PTC in HT patients
The results of comparisons between HT with multifocal PTC patients and HT single PTC patients in associated factors are shown in Supplementary Table 2. Univariate regression analysis revealed significant differences between HT patients with multifocal and unifocal PTC with respect to TPOAb concentration, central lymph node metastasis, tissue CK-19 optical density, and the presence of BRAF and RET/PTC. After stepwise selection, the concentration of TPOAb and presence of BRAF and RET/PTC were further investigated using multivariate regression analysis. The results show that elevated TPOAb (aOR, 83.11; 95% CI, 33.96-203.44; P < 0.001), BRAF mutation (aOR, 10.92; 95% CI, 4.64-25.72; P < 0.001), and positivity for RET/PTC (aOR, 13.36; 95% CI, 5.81-30.73; P < 0.001) are associated with multifocal PTC (Supplementary Table 2).
Discussion
This retrospective study of 471 HT patients aims to identify potential diagnostic markers to discern between uni- and multifocal PTC. We observed a significant difference in TPOAb concentration, CK-19 expression, and the occurrence of BRAF mutations and RET/PTC rearrangements between patients with uni- and multifocal PTC. Our results indicate that TPOAb > 1300 IU/mL, elevated TSH only together with TPOAb > 1300 IU/mL, elevated CK-19 expression, and the presence of BRAF mutations and RET/PTC rearrangements are indicators of multifocal PTC in HT. Among HT patients with TPOAb > 1300 IU/Ml, the occurrence of central lymph node metastasis is significantly higher in those with multi-focal than single-focus PTC. These markers may prove useful for discerning between uni- and multifocal PTC, thereby preventing unnecessarily extensive surgery in the treatment of unifocal PTC and promoting sufficient treatment of multifocal PTC.
Thyroid peroxidase, a major component of thyroid microsomes, is a key enzyme in the synthesis and secretion of thyroxine. Autoimmune attack on this enzyme by TPOAb, the etiological agent of HT, causes thyroid cell destruction, inhibiting thyroxine production and resulting in a rise in thyroid stimulating hormone (TSH). The chronic inflammation induced by TPOAb is considered to be a risk factor for thyroid cancer [19,20]. The relationship between elevated TSH and TPOAb is closely related to the development of multifocal cancer. Our results indicate that TPOAb > 1300 IU/mL is a risk factor for HT with multifocal PTC. This finding supports the observation of Dong et al. that a high level of TPOAb (> 1300 IU/mL) is a definitive indicator of multifocal PTC in HT patients [21]. We also observed that elevated TSH accompanied by TPOAb > 1300 IU/mL is a risk factor for multifocal PTC in HT. In such cases, we recommend bilateral thyroidectomy. In contrast, elevated TSH with mild to moderate elevation of TPOAb (< 1300 IU/mL) did not correlate with multifocal PTC in HT, demonstrating that elevated TSH cannot be used as a sole indicator of multifocal PTC.
Studies suggest that central lymph node dissection should be standard treatment for thyroid cancer patients with central lymph node metastasis [22,23]. Our results showed that positivity for central lymph node metastasis in HT was significantly higher in multi-focal PTC with TPOAb > 1300 IU/Ml than in unifocal PTC. Therefore, we recommend central lymph node dissection for HT patients with high-level expression of TPOAb.
We investigated whether CK-19, COX-2, Galectin-3, and HBME-1 expression can distinguish between uni- and multifocal PTC in HT. These proteins were chosen because of their known patterns of expression and potential specificity as indicated by previous studies. CK-19 and HBME-1 were the two most commonly used immunological markers for PTC diagnosis. These markers could be a useful complement to conventional histological diagnostic methods [14]. Cochand-Priollet et al. found that diagnostic CK-19 and HBME-1 antibody staining had a lower rate of false positive and false negative results in PTC diagnosis compared to morphological methods, thereby improving the accuracy of diagnosis and reducing unnecessary surgical treatment [24]. Liu et al. found that the identification of up-regulated CK-19 (or HBME-1) expression together with down-regulated TPO expression improved the specificity of PTC diagnosis [25]. CK-19 is present in tumors of normal epithelium and epithelial origin and is used to diagnose adenocarcinoma. CK-19 also is expressed in thyroid cancer [18], with high expression in PTC follicular variants but no expression in benign lesions such as thyroid adenomas [16]. Baloch et al. found that analysis of CK-19 expression was very helpful for PTC diagnosis [26]. Guyetant et al. [27] reported that CK-19 was expressed in all PTCs, with 100% sensitivity and 82.15% specificity, and was useful for detecting micrometastases in thyroid tissue. Compared with these findings, however, we observed significantly higher expression in multifocal than in unifocal PTC for CK-19 but not HBME-1. In addition, ROC analysis indicated that CK-19 optical density had acceptable discrimination for distinguishing between multifocal and unifocal PTC in HT patients. Thus, in intraoperative frozen section analysis of CK-19, a mean optical density > 0.008 indicates multifocal cancer, strongly suggesting that the best treatment choice is total thyroidectomy.
Galectin-3 is a β-galactoside-binding protein involved in cell growth, adhesion, inflammatory responses, immune regulation, and apoptosis. Studies have found that Galectin-3 is expressed in breast cancer, gastric cancer, colon cancer, and other tumors [28], but its expression in thyroid cancer is still uncertain. Galectin-3 is thought to be expressed in malignant tumors, particularly PTC, but not in benign lesions and normal thyroid tissue. Studies have shown that Galectin-3 is valuable for the differential diagnosis of PTC and benign thyroid lesions [29]. Galectin-3 expression in benign lesions may suggest that the lesion has the potential to differentiate into cancer [30]. We observed that Galectin-3 is positively expressed in HT with PTC and weakly positively expressed in HT alone. No significant difference was observed between uni- and multifocal cancer in HT. Therefore, we conclude that Galectin-3 cannot be used as an indicator to distinguish between uni- and multifocal PTC and can be used only as an auxiliary indicator for judging whether PTC is present in HT.
COX-2 catalyzes the conversion of arachidonic acid to prostaglandin, which promotes cell proliferation and inhibits apoptosis. COX-2 expression is induced by growth factors and cytokines and is elevated in many precancerous lesions and malignant tumors. COX-2 expression correlates closely with tumorigenesis, invasion, and metastasis, as it degrades the extracellular matrix and promotes tumor angiogenesis [17,18]. Studies have shown that tissue COX-2 expression is significantly elevated in patients with PTC compared to those with HT or simple goiter [31]. Similarly, our immunohistochemical analysis revealed significantly higher COX-2 expression in PTC with HT as compared to HT alone. However, we observed no significant difference in COX-2 expression between uni-and multifocal PTC, precluding its use as a marker for multifocal PTC.
The RET/PTC-RAS-BRAF-MEK-ERK pathway (mitogen-activated protein kinase pathway or MAPK), which plays a fundamental role in proliferation, differentiation, apoptosis, and survival, is a key intracellular signaling pathway involved in thyroid carcinogenesis [32]. BRAF gene mutations and RET/PTC oncogene rearrangements are common in thyroid cancer. Studies suggest that thyroid carcinogenesis in HT is associated with the combined elevation in TSH and RET/PTC oncogene rearrangement. Rhoden et al. [33] detected RET/PTC oncogene rearrangements in HT and concluded that HT and PTC are closely related, possibly via HT-induced inflammation. The BRAF V600E mutation results in constitutive activation of BRAF [34,35] and has been associated with aggressive PTC subtypes [32]. We observed a significant difference in the presence of the RET/PTC oncogene rearrangement between HT patients with uni- and multifocal PTC (20.9% vs. 56.8%, respectively; P < 0.001). The presence of the BRAF mutation also differed significantly between these groups (9.6% vs. 41.5%, respectively; P < 0.001). These promising results suggested that the presence of the BRAF mutation or RET/PTC oncogene rearrangement is an indicator of multifocal PTC. For patients with such test results, we recommend total bilateral thyroidectomy.
In conclusions, TPOAb > 1300 IU/mL, elevated TSH (only together with TPOAb > 1300 IU/mL), elevated CK-19 expression, and the presence of BRAF mutations and RET/PTC rearrangements are markers of multifocal PTC in HT and indicate the need for total bilateral thyroidectomy. Among HT patients with TPOAb > 1300 IU/Ml, the occurrence of central lymph node metastasis is significantly higher in those with multi-focal than single-focus PTC, indicating the need for central lymph node dissection. These markers may prove useful for discerning between uni- and multifocal PTC, thereby preventing unnecessarily extensive surgery in the treatment of unifocal PTC and promoting sufficient treatment of multifocal PTC.
If the HT of patients are diagnosed to be accompanied with PTC using the preoperative B-ultrasound and fine needle aspiration (FNA), it suggests that the HT is likely to be accompanied with multi-focal PTC once one of the following conditions is fit: (1) TPOAb > 1300 IU/ml. (2) CK-19 immunohistochemical average optical density > 0.008 (3) BRAF gene mutation. (4) RET/PTC oncogene activation rearrangement is positive. Thus, it is recommended to perform total thyroidectomy combining the intraoperative frozen section at this time.
If the average optical density of CK-19 immunohistochemistry is more than 0.008, it is more likely to be HT multifocal cancer once one of the following conditions is fit: (1) TPOAb > 1300 IU/ml. (2) BRAF gene mutation. (3) RET/PTC oncogene activation rearrangement is positive. It is strongly recommended to perform total thyroidectomy.
Acknowledgements
This study was supported by Foundation of Zhejiang Science and Technology Committee (2014C33194).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Zhang Y, Dai J, Wu T, Yang N, Yin Z. The study of the coexistence of Hashimoto’s thyroiditis with papillary thyroid carcinoma. J Cancer Res Clin Oncol. 2014;140:1021–1026. doi: 10.1007/s00432-014-1629-z. [DOI] [PubMed] [Google Scholar]
- 2.Anand A, Singh KR, Kushwaha JK, Hussain N, Sonkar AA. Papillary thyroid cancer and hashimoto’s thyroiditis: an association less understood. Indian J Surg Oncol. 2014;5:199–204. doi: 10.1007/s13193-014-0325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Konturek A, Barczynski M, Wierzchowski W, Stopa M, Nowak W. Coexistence of papillary thyroid cancer with Hashimoto thyroiditis. Langenbecks Arch Surg. 2013;398:389–394. doi: 10.1007/s00423-012-1021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai X, Xia Y, Zhang B, Li J, Jiang Y. A meta-analysis of Hashimoto’s thyroiditis and papillary thyroid carcinoma risk. Oncotarget. 2017;8:62414–62424. doi: 10.18632/oncotarget.18620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Resende de Paiva C, Gronhoj C, Feldt-Rasmussen U, von Buchwald C. Association between Hashimoto’s thyroiditis and thyroid cancer in 64,628 patients. Front Oncol. 2017;7:53. doi: 10.3389/fonc.2017.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L, Li H, Ji QH, Zhu YX, Wang ZY, Wang Y, Huang CP, Shen Q, Li DS, Wu Y. The clinical features of papillary thyroid cancer in Hashimoto’s thyroiditis patients from an area with a high prevalence of Hashimoto’s disease. BMC Cancer. 2012;12:610. doi: 10.1186/1471-2407-12-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.So YK, Kim MW, Son YI. Multifocality and bilaterality of papillary thyroid microcarcinoma. Clin Exp Otorhinolaryngol. 2015;8:174–178. doi: 10.3342/ceo.2015.8.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang J, Zeng W, Fang F, Yu T, Zhao Y, Fan X, Guo N, Gao X. Clinical analysis of Hashimoto thyroiditis coexistent with papillary thyroid cancer in 1392 patients. Acta Otorhinolaryngol Ital. 2017;37:393–400. doi: 10.14639/0392-100X-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W, Su X, He K, Wang Y, Wang H, Wang H, Zhao Y, Zhao W, Zarnegar R, Fahey TJ 3rd, Teng X, Teng L. Comparison of the clinicopathologic features and prognosis of bilateral versus unilateral multifocal papillary thyroid cancer: an updated study with more than 2000 consecutive patients. Cancer. 2016;122:198–206. doi: 10.1002/cncr.29689. [DOI] [PubMed] [Google Scholar]
- 10.Hay ID, McConahey WM, Goellner JR. Managing patients with papillary thyroid carcinoma: insights gained from the Mayo Clinic’s experience of treating 2,512 consecutive patients during 1940 through 2000. Trans Am Clin Climatol Assoc. 2002;113:241–260. [PMC free article] [PubMed] [Google Scholar]
- 11.Haigh PI, Urbach DR, Rotstein LE. Extent of thyroidectomy is not a major determinant of survival in low- or high-risk papillary thyroid cancer. Ann Surg Oncol. 2005;12:81–89. doi: 10.1007/s10434-004-1165-1. [DOI] [PubMed] [Google Scholar]
- 12.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azizi G, Keller JM, Lewis M, Piper K, Puett D, Rivenbark KM, Malchoff CD. Association of Hashimoto’s thyroiditis with thyroid cancer. Endocr Relat Cancer. 2014;21:845–852. doi: 10.1530/ERC-14-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abd-El Raouf SM, Ibrahim TR. Immunohistochemical expression of HBME-1 and galectin-3 in the differential diagnosis of follicular-derived thyroid nodules. Pathol Res Pract. 2014;210:971–978. doi: 10.1016/j.prp.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Sumana BS, Shashidhar S, Shivarudrappa AS. Galectin-3 immunohistochemical expression in thyroid neoplasms. J Clin Diagn Res. 2015;9:EC07–11. doi: 10.7860/JCDR/2015/16277.6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srivastava S, Banerjee H, Chaudhry A, Khare A, Sarin A, George A, Bal V, Durdik JM, Rath S. Apoptosis-inducing factor regulates death in peripheral T cells. J Immunol. 2007;179:797–803. doi: 10.4049/jimmunol.179.2.797. [DOI] [PubMed] [Google Scholar]
- 17.Uefuji K, Ichikura T, Mochizuki H, Shinomiya N. Expression of cyclooxygenase-2 protein in gastric adenocarcinoma. J Surg Oncol. 1998;69:168–172. doi: 10.1002/(sici)1096-9098(199811)69:3<168::aid-jso9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schror K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198–204. [PubMed] [Google Scholar]
- 19.Kim ES, Lim DJ, Baek KH, Lee JM, Kim MK, Kwon HS, Song KH, Kang MI, Cha BY, Lee KW, Son HY. Thyroglobulin antibody is associated with increased cancer risk in thyroid nodules. Thyroid. 2010;20:885–891. doi: 10.1089/thy.2009.0384. [DOI] [PubMed] [Google Scholar]
- 20.Vasileiadis I, Boutzios G, Charitoudis G, Koukoulioti E, Karatzas T. Thyroglobulin antibodies could be a potential predictive marker for papillary thyroid carcinoma. Ann Surg Oncol. 2014;21:2725–2732. doi: 10.1245/s10434-014-3593-x. [DOI] [PubMed] [Google Scholar]
- 21.Dong S, Xia Q, Wu YJ. High TPOAb Levels (>1300 IU/mL) indicate multifocal PTC in Hashimoto’s thyroiditis patients and support total thyroidectomy. Otolaryngol Head Neck Surg. 2015;153:20–26. doi: 10.1177/0194599815581831. [DOI] [PubMed] [Google Scholar]
- 22.Wartofsky L. Highlights of the American Thyroid Association Guidelines for patients with thyroid nodules or differentiated thyroid carcinoma: the 2009 revision. Thyroid. 2009;19:1139–1143. doi: 10.1089/thy.2009.1599. [DOI] [PubMed] [Google Scholar]
- 23.American Thyroid Association Surgery Working Group; American Association of Endocrine Surgeons; American Academy of Otolaryngology-Head and Neck Surgery; American Head and Neck Society. Carty SE, Cooper DS, Doherty GM, Duh QY, Kloos RT, Mandel SJ, Randolph GW, Stack BC Jr, Steward DL, Terris DJ, Thompson GB, Tufano RP, Tuttle RM, Udelsman R. Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid. 2009;19:1153–1158. doi: 10.1089/thy.2009.0159. [DOI] [PubMed] [Google Scholar]
- 24.Cochand-Priollet B, Dahan H, Laloi-Michelin M, Polivka M, Saada M, Herman P, Guillausseau PJ, Hamzi L, Pote N, Sarfati E, Wassef M, Combe H, Raulic-Raimond D, Chedin P, Medeau V, Casanova D, Kania R. Immunocytochemistry with cytokeratin 19 and anti-human mesothelial cell antibody (HBME1) increases the diagnostic accuracy of thyroid fine-needle aspirations: preliminary report of 150 liquid-based fine-needle aspirations with histological control. Thyroid. 2011;21:1067–1073. doi: 10.1089/thy.2011.0014. [DOI] [PubMed] [Google Scholar]
- 25.Liu Z, Yu P, Xiong Y, Zeng W, Li X, Maiaiti Y, Wang S, Song H, Shi L, Liu C, Cheng B, Zhang B, Ming J, Dong F, Ge H, Nie X, Huang T. Significance of CK19, TPO, and HBME-1 expression for diagnosis of papillary thyroid carcinoma. Int J Clin Exp Med. 2015;8:4369–4374. [PMC free article] [PubMed] [Google Scholar]
- 26.Beesley MF, McLaren KM. Cytokeratin 19 and galectin-3 immunohistochemistry in the differential diagnosis of solitary thyroid nodules. Histopathology. 2002;41:236–243. doi: 10.1046/j.1365-2559.2002.01442.x. [DOI] [PubMed] [Google Scholar]
- 27.Baloch ZW, Abraham S, Roberts S, LiVolsi VA. Differential expression of cytokeratins in follicular variant of papillary carcinoma: an immunohistochemical study and its diagnostic utility. Hum Pathol. 1999;30:1166–1171. doi: 10.1016/s0046-8177(99)90033-3. [DOI] [PubMed] [Google Scholar]
- 28.Guyetant S, Michalak S, Valo I, Saint-Andre JP. Diagnosis of the follicular variant of papillary thyroid carcinoma. Significance of immunohistochemistry. Ann Pathol. 2003;23:11–20. [PubMed] [Google Scholar]
- 29.Eude-Le Parco I, Gendronneau G, Dang T, Delacour D, Thijssen VL, Edelmann W, Peuchmaur M, Poirier F. Genetic assessment of the importance of galectin-3 in cancer initiation, progression, and dissemination in mice. Glycobiology. 2009;19:68–75. doi: 10.1093/glycob/cwn105. [DOI] [PubMed] [Google Scholar]
- 30.Coli A, Bigotti G, Zucchetti F, Negro F, Massi G. Galectin-3, a marker of well-differentiated thyroid carcinoma, is expressed in thyroid nodules with cytological atypia. Histopathology. 2002;40:80–87. doi: 10.1046/j.1365-2559.2002.01304.x. [DOI] [PubMed] [Google Scholar]
- 31.Krawczyk-Rusiecka K, Wojciechowska-Durczynska K, Cyniak-Magierska A, Zygmunt A, Lewinski A. Assessment of cyclooxygenase-1 and 2 gene expression levels in chronic autoimmune thyroiditis, papillary thyroid carcinoma and nontoxic nodular goitre. Thyroid Res. 2014;7:10. doi: 10.1186/s13044-014-0010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 33.Rhoden KJ, Unger K, Salvatore G, Yilmaz Y, Vovk V, Chiappetta G, Qumsiyeh MB, Rothstein JL, Fusco A, Santoro M, Zitzelsberger H, Tallini G. RET/papillary thyroid cancer rearrangement in nonneoplastic thyrocytes: follicular cells of Hashimoto’s thyroiditis share low-level recombination events with a subset of papillary carcinoma. J Clin Endocrinol Metab. 2006;91:2414–2423. doi: 10.1210/jc.2006-0240. [DOI] [PubMed] [Google Scholar]
- 34.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 35.Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, Jones CM, Marshall CJ, Springer CJ, Barford D, Marais R Cancer Genome Project. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


