Abstract
Natural killer (NK) cells play a pivotal role in host immunity against different malignancies, including pancreatic ductal adenocarcinoma (PDAC). Our study aimed to evaluate the antitumor effects of NK cell-based adoptive transfer immunotherapy for PDAC in an orthotopic mouse model. Orthotopic KrasLSL-G12D p53LSL-R172H Pdx1-Cre (KPC) mice were used to evaluate the therapeutic efficacy. Mouse NK cells (LNK cells) (1×106) were intravenously injected to tumor-bearing mice once a week for 3 weeks. MRI measurements (tumor volume and apparent diffusion coefficient (ADC) values) and survival were compared between control and LNK treated tumors. Flow cytometry and enzyme-linked immunosorbent assay (ELISA) were used to determine LNK cells cytotoxicity and IFN-γ level, respectively. LNK cells can produce a higher level of IFN-γ and more effectively lyse PDAC cells compared with spleen NK cells in vitro. LNK-cell adoptive transfer therapy elicited potent in vivo antitumor activity, resulting in delayed tumor growth (P=0.033) in KPC mice. The ADC values at the last timepoint ((0.94±0.06)×10-3 mm2/s) were significantly higher than that at first timepoint ((0.75±0.04)×10-3 mm2/s) in treated tumors (P<0.001). ADC values were significantly different between control group and treated tumors at the last time point ((0.75±0.09)×10-3 mm2/s vs (0.94±0.06)×10-3 mm2/s, P=0.004) in KPC mice. Our data demonstrate the potential of NK cell-based adoptive transfer immunotherapy for PDAC treatment.
Keywords: Natural killer cell, immunotherapy, pancreatic cancer, magnetic resonance imaging
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal malignant diseases in Western countries. It is usually discovered at a late stage and has a poor prognosis [1,2]. Furthermore, PDAC displays rapid progression to late stage and has high resistance to chemo- and radiotherapy [1,3]. Despite advances in our understanding of PDAC biology and improved surgical techniques, the long-term survival of PDAC remains grim, with an overall 5-year survival rate of no more than 8% [3]. Thus, novel effective treatment strategies for PDAC remain an urgently needed clinical challenge.
Cancer immunotherapies have recently exhibited tremendous success in a range of malignancies [4,5]. Recently, natural killer (NK) cell-based adoptive transfer immunotherapy has evoked extensive interest and attention [4,6]. NK cells rapidly eliminate different types of malignant cells via different cytotoxicity mechanisms, including the death receptor pathway and the granule dependent pathway [7,8]. In addition to their direct anti-tumor activity, NK cells can induce adaptive immune responses and has immune regulatory functions [9]. These unique characteristics make NK cells promising agents for cancer immunotherapy. Several preclinical and clinical studies have demonstrated that increased infiltration of NK cells into tumors is associated with delayed tumor progression and improved prognosis in cancer types such as glioblastoma, solid lung, colorectal, head and neck cancers [5,8,10-12]. Furthermore, NK cell infusion is recognized as a safe and well-tolerated procedure [7]. However, few studies have focused specifically on NK cell-based adoptive transfer immunotherapy for PDAC [1,2].
The KrasLSL-G12D p53LSL-R172H Pdx1-Cre, termed KPC mouse, can develop PDAC and recapitulate the molecular and the pathophysiological characteristics of human PDAC [13,14]. Thus, KPC mice are one of the most relevant animal models for preclinical evaluation of potential PDAC treatments [15]. Magnetic resonance imaging (MRI) is regarded as one of the best imaging tools for preclinical and clinical studies that allows both monitoring of tumor progression and characterization of tumor fibrosis in subjects such as KPC mice [16,17]. In this study, we aimed to evaluate the efficacy of NK cell-based adoptive transfer immunotherapy for PDAC in KPC mouse model.
Materials and methods
All animal studies were performed in accordance with the institutional animal care and use committee of Northwestern University.
Cell lines and cell culture
The mouse Pan02 cell line is derived from pancreatic ductal adenocarcinoma in C57BL/6 mice and was obtained from the American Type Culture Collection (ATCC; Rockville, MD). The mouse KrasLSL-G12D p53LSL-R172H Pdx1-Cre (KPC) cell line was established in our laboratory using pancreatic cancers of genetically engineered KPC mice. Mouse NK cell line (LNK) was kindly provided by Stephen K. Anderson (National Cancer Institute, Frederick, MD).
Pan02 cells and KPC cells were cultured in RPMI 1640 (Gibco, Waltham, MA) supplemented with L-glutamine (2 mmol/L, Life Technologies, Carlsbad, CA), pyruvate (1 mmol/L, Sigma-Aldrich, St. Louis, Mo), penicillin and streptomycin (100 IU/mL, Sigma-Aldrich, St. Louis, Mo), and 10% fetal bovine serum (FBS; Gibco, Waltham, MA). The LNK cell line was cultured in RPMI 1640 containing 10% FBS, penicillin and streptomycin (100 IU/mL), pyruvate (1.5 g/L), L-glutamine (2 mmol/L), and IL-2 (8000 IU/mL). Cell cultures were maintained at 37°C in a humid atmosphere containing 5% CO2 and 95% air. Trypan blue (Sigma-Aldrich, St. Louis, MO) staining was performed before each administration to verify >90% cell viability.
In vitro cytotoxicity assay
NK cells were purified from mice spleens by negative selection with NK Cell Isolation Kit II (Miltenyi Biotec, Germany). Remaining cells were incubated at 37°C in complete RPMI 1640 with mouse IL-2 for 2 days. Target KPC cells were labeled with CellTrace CFSE (Thermo Fisher Scientific, Rockford, IL), washed, and co-incubated with spleen NK cells and LNK cells respectively at effector to target (E/T) ratio 1:10 for 4 hours at 37°C. After co-culture, cells were centrifuged, and supernatant was removed. The cells were resuspended in 200 µL of 1 µg/mL propidium iodide (PI) solution for flow cytometry. Dead target cells were labeled as CFSE and PI double positive. Spontaneous target cell lysis in the absence of effector cells was determined in samples only containing labeled target cells and subtracted to calculate specific cytotoxicity.
Establishment of orthotopic PDAC model
For orthotopic tumor implantation, female C57BL/6 mice (8-10 weeks) were anesthetized using a mixture of 2% isoflurane in oxygen at a rate of 1 L/min. After shaving and sterilizing, the skin and peritoneum were opened by a 1.5-cm incision along the left flank under strict aseptic conditions. 50 µL of the KPC cell stock solution (1×107 cells/mL) was slowly injected into the parenchyma of pancreatic tail using a 10-µL glass syringe with a 26 s-gauge needle (Hamilton). After replacement of the pancreas into the abdominal cavity, the incision was closed in two layers using a 4-0 polydioxanone suture for the peritoneum (Patterson Veterinary, Devens, MA) and 4.0-suture for the skin (Veterinary Products Laboratories, Phoenix, USA). Twelve KPC tumor-bearing mice were divided into two groups (treated group and control group) randomly. Four days after tumor cell inoculation, tumor-bearing mice were treated by intravenous injection of 1×106 LNK cells once a week for 3 weeks. No signs of toxicity or weight loss were observed during the LNK treatment. The other six mice were classified as control group and did not undergo any procedure. Endpoints were scored when mice displayed >15% loss of body weight, >1.8 cm tumor diameter, decreased mobility, extreme lethargy, or absolute survival event.
In vivo MRI scan and imaging analysis
MRI scans were performed using a Bruker 7.0 T preclinical scanner (Clinscan, Bruker BioSpin, Ettlinggen, Germany) with a commercial mouse coil (Clinscan, Bruker). The tumor-implanted mice were in the supine position and anesthetized using a mixture of 2-3% isoflurane in oxygen at a rate of 1 L/min via an automatic delivery system (Isoflurane Vaporizer, Vaporizer Sales and Services, Rockmart, GA). Body temperature was continuously monitored using a thermometer and controlled using a water-bed heating system (SA Instruments, Stony Brook, NY). Each mouse underwent imaging with a protocol including the described sequences (all acquired with free breathing of the animal), which are summarized in Table 1.
Table 1.
Imaging protocol
| Orientation | TR/TE (ms) | ST (mm) | FA (˚) | FOV (mm × mm) | |
|---|---|---|---|---|---|
| T1WI | Axial | 630/20 | 0.7 | 90 | 27×30 |
| T2WI | Axial | 1581/40 | 0.8 | 180 | 21×30 |
| T2WI | Coronal | 1600/40 | 0.5 | 180 | 40×30 |
| DWI* | Axial | 2700/40 | 1 | 90 | 24×30 |
T1WI: T1-weighted images; T2WI: T2-weighted images; DWI: diffusion weighted images; TR: repetition time; TE: echo time; ST: slice thickness, FA: flip angle; FOV: field of view.
b value=0, and 800 s/mm2.
Tumor volumes were measured on T2W images using ITK-SNAP software (v 3.6.0, www.itksnap.org) [18]. DW images were post-processed to generate apparent diffusion coefficient (ADC) maps in Matlab R2016b (Mathworks, Natick, MA), and ADC values were measured using ImageJ software (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, https://imagej.nih.gov/ij/, 1997-2016) [19]. The region of interest (ROI) was manually delineated encompassing the entire tumor using the same section of the T2W image as a reference.
IFN-γ release assay
The expression of IFN-γ was detected by enzyme-linked immunosorbent assay (ELISA). The concentrations of IFN-γ in mice serum were collected after 4 days of the last treatment. The cell culture supernatants (NK cells or LNK cells co-cultured with KPC cells) were determined by mouse IFN-γ kit (R&D bioscience, Minneapolis, MN) according to the manufacturer’s protocols. The absorbance was measured at 450 nm.
Histologic analysis
Mice were euthanized after experimental end points were reached including large tumor size, abdominal distension, reduced mobility, and/or other signs of distress. The tumor was harvested and fixed in 4% paraformaldehyde for further staining with hematoxylin-eosin (HE).
Statistical analysis
Data is presented as means ± standard deviations. Comparisons between groups were performed with the Student t test or by ANOVA with the Student-Newman-Keuls tests (for multiple comparisons). Log-rank test was used for survival curves. Statistical analysis was performed with software package (SPSS, version 19; Chicago, IL, USA). A P value of less than 0.05 was considered to be statistically significant.
Results
In vitro cytotoxicity and IFN-γ production
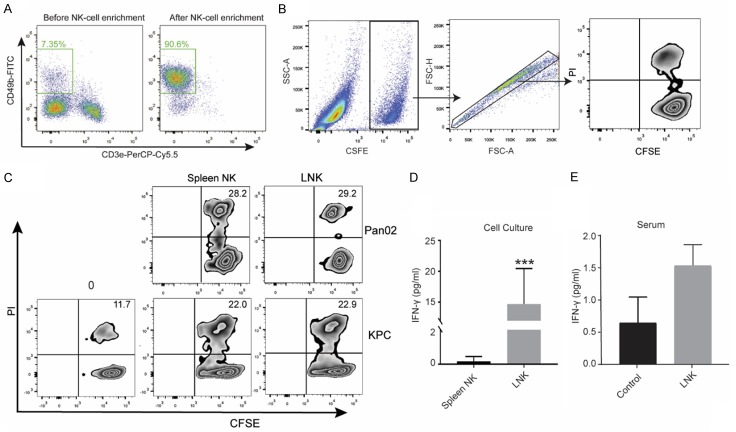
To investigate the cytotoxicity of LNK cells against the PDAC cells, LNK cells were co-incubated with CFSE labeled KPC cells and CFSE-labeled Pan02 cells respectively. Meanwhile, we further separated spleen NK cells from murine splenocytes (Figure 1A) and compared their cytotoxicity against PDAC cells with LNK cells. The flow cytometry gating strategy was shown in Figure 1B. Our results showed that LNK cells killed the KPC cell and Pan02 cell as efficiently as the spleen NK cells in vitro (Figure 1C). Furthermore, the co-culture supernatants were collected for determining IFN-γ secretion by ELISA. As shown in Figure 1D, LNK cells showed significantly increased secretion of IFN-γ in response to KPC cells compared with spleen NK cells (P<0.001). The mean supernatants levels of IFN-γ were 0.18±0.25 pg/ml for spleen NK cells and 14.68±5.27 pg/ml for LNK cells. Moreover, we collected the serum of KPC tumor-bearing mice in different groups after last treatment and evaluated the levels of IFN-γ. The mean serum IFN-γ levels were 1.54±0.72 pg/ml for the LNK cells treatment group and 0.65±0.80 pg/ml for the control group. There was no significant difference in serum IFN-γ level between the treatment and the control groups (P=0.12). (Figure 1E). Taken together, these findings suggested that the LNK cells could effectively lyse the PDAC cell and upregulate IFN-γ production.
Figure 1.
NK cells were enriched from splenocytes by magic bead-based sorting and the purity of NK cells (CD3ε- CD49b+) was analyzed by flow cytometry (A). Gating strategy of flow cytometry of in vitro cytotoxic assay (B). Flow cytometric analysis of apoptosis in KPC cells and Pan02 cells treated with LNK cells or spleen NK cells in vitro (C), and KPC cell death at time 0 was shown in left panel. KPC cells were respectively cultured with spleen NK cells and LNK cells. Culture supernatants were harvested at 24 hours and analyzed for IFN-γ by Enzyme-linked immunosorbent assay (ELISA) (P<0.001) (D). After the last treatment, the serum of KPC tumor-bearing mice were collected to analyzed for INF-γ by ELISA (E).
In vivo tumor size measurement on MR images
For in vivo study, representative MR images of pancreatic KPC tumors from LNK cells treatment mice and control mice were shown in Figure 2A-F. At 1 w, 2 w, 3 w, and 4 w after enrollment, the mean tumor volumes were respectively 49.79±23.87 mm3, 84.82±33.39 mm3, 158.14±73.09 mm3, and 277.33±139.28 mm3 for the treatment group and 63.25±47.92 mm3, 157.99±91.95 mm3, 355.25±254.23 mm3, and 374.25±308.86 mm3 for the control group. Although the mean tumor volumes in both groups continued to increase after enrollment, LNK cells therapy inhibited tumor growth effectively (P=0.033) (Figure 2G). These results together suggested that LNK cell treatment could delay tumor growth.
Figure 2.
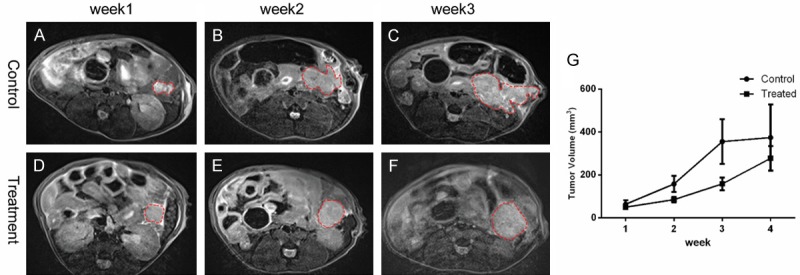
Representative axial T2W images in control group (A-C) and treated tumor (D-F) from week 1 to week 3. The tumor size was increased along with time. The tumor growth was inhibited effectively by LNK cells therapy (P=0.033) (G).
ADC measurement
The ADC maps of LNK treated mice and control mice at two time points (one week after enrollment and end timepoint) were shown in Figure 3A-D. The mean ADC values of the tumors at two time points were summarized in Figure 3E. The mean ADC values were increased from (0.75±0.04)×10-3 mm2/s at one week after enrollment to (0.94±0.06)×10-3 mm2/s at end timepoint in LNK cells treated group (P<0.001). There also showed significant differences in ADC values between control group ((0.75±0.09)×10-3 mm2/s) and treated group ((0.94±0.06)×10-3 mm2/s) at end timepoint (P=0.004).
Figure 3.
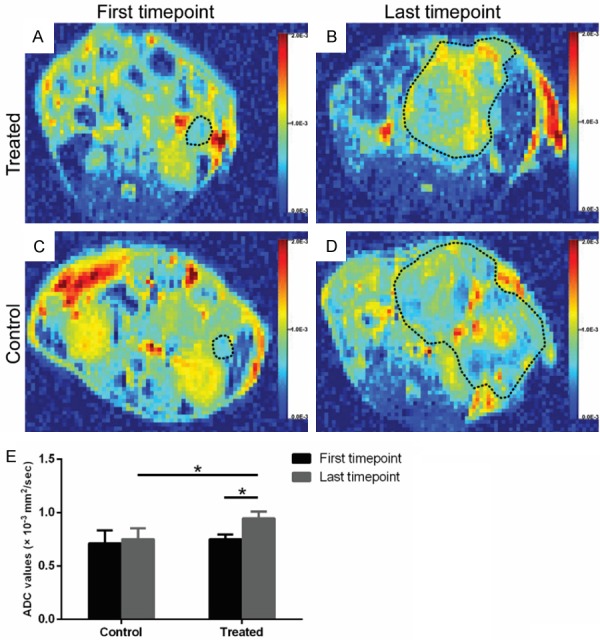
The colored ADC maps of LNK treated mice (A, B) and control mice (C, D) at the first timepoint and last timepoint. The bar chart (E) showed a significant difference in ADC values between control group and treated tumors at last timepoint (P=0.004), and between first timepoint and last timepoint in treated group (P<0.001).
LNK cells suppress PDAC progression in orthotopic KPC mouse model
The KPC tumors in different groups were detected at end timepoint for histological analysis. H&E staining of the KPC tumors showed increased necrosis area in LNK treated mice (Figure 4A), while the tumors in control group showed dense atypical cells and absence of normal-looking tissue (Figure 4B). For overall survival, mice treated with LNK cells (54.0 days) presented a relatively prolonged median survival than untreated mice (26.5 days), but without significant difference between the control and treated group (P=0.2324) (Figure 5).
Figure 4.
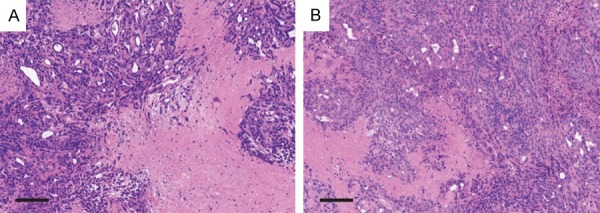
Representative photomicrographs (H&E stained) of treated (A) and untreated (B) tumor (scale bar=0.2 mm). Treated tumor showed larger percentages of necrotic area than untreated tumor.
Figure 5.
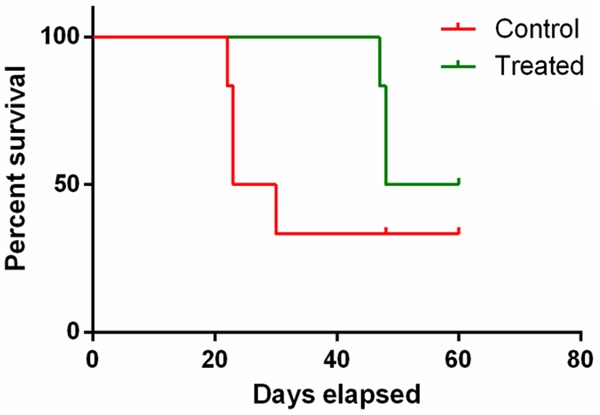
Survival curves of orthotopic KPC mice. Kaplan-Meier analysis of survival showed that the mice treated with LNK cells presented a relatively prolonged median survival than untreated mice.
Discussion
In this study, we demonstrated the in vivo and in vitro efficacy of NK cell-based adoptive transfer immunotherapy against PDAC. Reduced tumor burden and improved survival were observed in an allogeneic tumor setting using orthotopic KPC mouse model.
NK cells are a subset of innate lymphocytes that comprise about 5-15% of the circulating cell population [1] and can kill cancer cells via induction of programmed cell death [3]. Patients with high circulating NK cells had a reduced risk of malignancy, while low peripheral NK cell is usually correlated with poorer prognosis in malignant patients [3,8,20,21]. Moreover, the cytotoxic capacity of circulating NK cells is decreased in PDAC patients [1], which may be a key factor in cancer immune evasion and progression [22]. The function and positive effect of NK cells are suboptimal because of the negative influence of the tumor and its immunosuppressive microenvironment [1]. NK-cell adoptive transfer immunotherapy provides large amounts of activated NK cells to directly supplement or replace malfunctioning NK cells in cancer patients [21]. Here, we tried to explore the therapeutic efficacy of NK cells in an established murine PDAC model.
The loss of NK cells occurs at the pre-malignant stage of PDAC is associated with KRAS mutation [23]. NK-cell defect induced by environmental and genetic factors at the pre-malignant stage of PDAC may contribute to the establishment and progression of pancreatic cancer [23,24]. Hence, the KPC mouse model that completely recapitulates the human PDAC at the pathophysiologic and biologic level was used to study NK cell adoptive therapy in this study. PDAC was implemented by orthotopic inoculation of KPC tumor cells derived from a transgenic mouse that was generated in our lab.
NK cell-based adoptive transfer immunotherapy has several advantages. NK cells can efficiently kill tumor cells and rapidly secrete a vast number of chemokines and cytokines that can influence the adaptive immune response. Moreover, NK cells can be directly activated without antigen presentation via antigen-presenting cell [25]. NK cell lines are one of the primary sources of NK cells for adoptive transfer, which had the advantages of stability and better therapeutic cell quality [21]. LNK cells were selected as the practical cell line in the current study. LNK cells could produce similar levels of IFN-γ and show effective in vitro cytotoxicity against PDAC cells that is similar to autologous spleen NK cells. These results indicated that LNK cells can elicit antitumor response in PDAC tumor. IFN-γ secreted by NK cells is one of the most potent effector cytokines and has been reported to augment NK cell cytolytic activity against tumor cells [25,26]. NK cells may substantially contribute to the immunotherapy of solid tumor through IFN-γ and consequently augment the anti-tumor immunity [26]. IFN-γ can activate dendritic cells and macrophages and has pleiotropic effects on the adaptive immune response [27]. Moreover, the results of our study also showed that LNK cells treatment suppressed tumor growth and increased tumor necrosis area, which further support the potential therapeutic role of NK cell in PDAC.
MRI is recognized as a valuable tool for both preclinical and clinical research due to several combined advantages [16]. For instance, MRI is capable of multi-faceted and multi-sequence imaging and provides better resolution and discrimination of soft tissues [28,29]. Furthermore, tumor ADC has been described as a sensitive imaging biomarker for evaluating treatment response in various tumors [30]. Accordingly, our results revealed that therapeutic response of NK cells adoptive treatment in PDAC murine model could be detected both by tumor volume and ADC values. Tumor growth inhibition can be exhibited via tumor size based on MR images. The increased ADC values may be explained by more freedom of the water molecules due to overall tumor cell loss, a low cell density and an associated increase in the extracellular space [31,32]. These results further indicated that ADC measurements can provide a valuable imaging biomarker of therapeutic response in PDAC.
However, there are several limitations in this study. Firstly, adoptively transferred NK cells had a shorter lifespan in vivo which may hinder their efficacy [33]. In addition, NK cells migration and their ability to penetrate into tumor tissues have been described to be inferior to that of T cells, which may affect their application in solid tumors [27]. Therefore, it is essential to develop more efficacious therapeutic strategies to fully exploit the potential of NK cells to enhance antitumor effect.
In summary, this study demonstrated NK cell-based adoptive transfer immunotherapy can effectively elicit antitumor response and prolong the median survival in KPC mice.
Acknowledgements
This study was supported by the National Cancer Institute (grants R01CA209886, R01CA196967) and 2019 Harold E. Eisenberg Foundation Scholar Award. We also gratefully acknowledge Matteo Figini for assistance with the MR imaging studies.
Disclosure of conflict of interest
None.
References
- 1.Van Audenaerde JRM, Roeyen G, Darcy PK, Kershaw MH, Peeters M, Smits ELJ. Natural killer cells and their therapeutic role in pancreatic cancer: a systematic review. Pharmacol Ther. 2018;189:31–44. doi: 10.1016/j.pharmthera.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Lin M, Liang S, Wang X, Liang Y, Zhang M, Chen J, Niu L, Xu K. Percutaneous irreversible electroporation combined with allogeneic natural killer cell immunotherapy for patients with unresectable (stage III/IV) pancreatic cancer: a promising treatment. J Cancer Res Clin Oncol. 2017;143:2607–2618. doi: 10.1007/s00432-017-2513-4. [DOI] [PubMed] [Google Scholar]
- 3.Xu JW, Wang L, Cheng YG, Zhang GY, Hu SY, Zhou B, Zhan HX. Immunotherapy for pancreatic cancer: a long and hopeful journey. Cancer Lett. 2018;425:143–151. doi: 10.1016/j.canlet.2018.03.040. [DOI] [PubMed] [Google Scholar]
- 4.Shook DR, Leung W. Natural killer cell therapy for cancer: delivering on a promise. Transfusion. 2013;53:245–248. doi: 10.1111/trf.12091. [DOI] [PubMed] [Google Scholar]
- 5.Gras Navarro A, Bjorklund AT, Chekenya M. Therapeutic potential and challenges of natural killer cells in treatment of solid tumors. Front Immunol. 2015;6:202. doi: 10.3389/fimmu.2015.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galli F, Rapisarda AS, Stabile H, Malviya G, Manni I, Bonanno E, Piaggio G, Gismondi A, Santoni A, Signore A. In vivo imaging of natural killer cell trafficking in tumors. J Nucl Med. 2015;56:1575–1580. doi: 10.2967/jnumed.114.152918. [DOI] [PubMed] [Google Scholar]
- 7.Martin-Antonio B, Sune G, Perez-Amill L, Castella M, Urbano-Ispizua A. Natural killer cells: angels and devils for immunotherapy. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18091868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malmberg KJ, Carlsten M, Bjorklund A, Sohlberg E, Bryceson YT, Ljunggren HG. Natural killer cell-mediated immunosurveillance of human cancer. Semin Immunol. 2017;31:20–29. doi: 10.1016/j.smim.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Pahl JHW, Koch J, Gotz JJ, Arnold A, Reusch U, Gantke T, Rajkovic E, Treder M, Cerwenka A. CD16A activation of NK cells promotes NK cell proliferation and memory-like cytotoxicity against cancer cells. Cancer Immunol Res. 2018;6:517–527. doi: 10.1158/2326-6066.CIR-17-0550. [DOI] [PubMed] [Google Scholar]
- 10.Rezvani K, Rouce RH. The application of natural killer cell immunotherapy for the treatment of cancer. Front Immunol. 2015;6:578. doi: 10.3389/fimmu.2015.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahlberg CI, Sarhan D, Chrobok M, Duru AD, Alici E. Natural killer cell-based therapies targeting cancer: possible strategies to gain and sustain anti-tumor activity. Front Immunol. 2015;6:605. doi: 10.3389/fimmu.2015.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bottcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, Rogers NC, Sahai E, Zelenay S, Reis e Sousa C. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172:1022–1037. e14. doi: 10.1016/j.cell.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Courtin A, Richards FM, Bapiro TE, Bramhall JL, Neesse A, Cook N, Krippendorff BF, Tuveson DA, Jodrell DI. Anti-tumour efficacy of capecitabine in a genetically engineered mouse model of pancreatic cancer. PLoS One. 2013;8:e67330. doi: 10.1371/journal.pone.0067330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Ruckert F, Grutzmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liby KT, Royce DB, Risingsong R, Williams CR, Maitra A, Hruban RH, Sporn MB. Synthetic triterpenoids prolong survival in a transgenic mouse model of pancreatic cancer. Cancer Prev Res (Phila) 2010;3:1427–1434. doi: 10.1158/1940-6207.CAPR-10-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Partecke IL, Kaeding A, Sendler M, Albers N, Kuhn JP, Speerforck S, Roese S, Seubert F, Diedrich S, Kuehn S, Weiss UF, Mayerle J, Lerch MM, Hadlich S, Hosten N, Heidecke CD, Puls R, von Bernstorff W. In vivo imaging of pancreatic tumours and liver metastases using 7 Tesla MRI in a murine orthotopic pancreatic cancer model and a liver metastases model. BMC Cancer. 2011;11:40. doi: 10.1186/1471-2407-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu S, Pan L, Shangguan J, Figini M, Eresen A, Sun C, Wang B, Ma Q, Hu C, Yaghmai V, Velichko Y, Yang J, Zhang Z. Non-invasive dynamic monitoring initiation and growth of pancreatic tumor in the LSL-Kras(G12D/+); LSL-Trp53(R172H/+); Pdx-1-Cre (KPC) transgenic mouse model. J Immunol Methods. 2019;465:1–6. doi: 10.1016/j.jim.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pampena MB, Levy EM. Natural killer cells as helper cells in dendritic cell cancer vaccines. Front Immunol. 2015;6:13. doi: 10.3389/fimmu.2015.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Sun R. Tumor immunotherapy: new aspects of natural killer cells. Chin J Cancer Res. 2018;30:173–196. doi: 10.21147/j.issn.1000-9604.2018.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lowry LE, Zehring WA. Potentiation of natural killer cells for cancer immunotherapy: a review of literature. Front Immunol. 2017;8:1061. doi: 10.3389/fimmu.2017.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaur K, Chang HH, Topchyan P, Cook JM, Barkhordarian A, Eibl G, Jewett A. Deficiencies in natural killer cell numbers, expansion, and function at the pre-neoplastic stage of pancreatic cancer by KRAS mutation in the pancreas of obese mice. Front Immunol. 2018;9:1229. doi: 10.3389/fimmu.2018.01229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaur K, Nanut MP, Ko MW, Safaie T, Kos J, Jewett A. Natural killer cells target and differentiate cancer stem-like cells/undifferentiated tumors: strategies to optimize their growth and expansion for effective cancer immunotherapy. Curr Opin Immunol. 2018;51:170–180. doi: 10.1016/j.coi.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 25.Paul S, Lal G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front Immunol. 2017;8:1124. doi: 10.3389/fimmu.2017.01124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang F, Xiao W, Tian Z. Challenges of NK cell-based immunotherapy in the new era. Front Med. 2018;12:440–450. doi: 10.1007/s11684-018-0653-9. [DOI] [PubMed] [Google Scholar]
- 27.Daher M, Rezvani K. Next generation natural killer cells for cancer immunotherapy: The promise of genetic engineering. Curr Opin Immunol. 2018;51:146–153. doi: 10.1016/j.coi.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang R, Guo Q, Chen Y, Gao Y, Wu L, Hu B, Jiang L. Efficacy of sub-threshold focused ultrasound irradiation against pancreatic cancer xenografts evaluated using magnetic resonance imaging. Oncotarget. 2017;8:80453–80460. doi: 10.18632/oncotarget.19241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmid A, Braumuller H, Wehrl HF, Rocken M, Pichler BJ. Non-invasive monitoring of pancreatic tumor progression in the RIP1-Tag2 mouse by magnetic resonance imaging. Mol Imaging Biol. 2013;15:186–193. doi: 10.1007/s11307-012-0548-0. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Wang Y, Tang W, Jiang M, Li K, Tao X. Multiparametric MR imaging detects therapy efficacy of radioactive seeds brachytherapy in pancreatic ductal adenocarcinoma xenografts. Radiol Med. 2018;123:481–488. doi: 10.1007/s11547-018-0867-6. [DOI] [PubMed] [Google Scholar]
- 31.Kobes JE, Daryaei I, Howison CM, Bontrager JG, Sirianni RW, Meuillet EJ, Pagel MD. Improved treatment of pancreatic cancer with drug delivery nanoparticles loaded with a novel AKT/PDK1 inhibitor. Pancreas. 2016;45:1158–1166. doi: 10.1097/MPA.0000000000000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang T, Zhang F, Meng Y, Wang H, Le T, Wei B, Lee D, Willis P, Shen B, Yang X. Diffusion-weighted MRI monitoring of pancreatic cancer response to radiofrequency heat-enhanced intratumor chemotherapy. NMR Biomed. 2013;26:1762–1767. doi: 10.1002/nbm.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicholson SE, Keating N, Belz GT. Natural killer cells and anti-tumor immunity. Mol Immunol. 2017;110:40–47. doi: 10.1016/j.molimm.2017.12.002. [DOI] [PubMed] [Google Scholar]