Abstract
Emerging evidence suggests that circular RNA (circRNA) plays a fundamental role in tumorigenesis. However, its contribution to hepatocellular carcinoma (HCC) malignancy remains largely unknown. Here, we performed circRNA microarray expression profile in four paired HCC and normal tissues, and found that circ-ADD3, a novel circRNA derived from linear ADD3 exon 4 to exon 12, was significantly downregulated in HCC, which was further validated in 112 matched HCC and paracancerous tissues. High circ-ADD3 expression was negatively correlated with vascular invasion, intrahepatic metastasis as well as distant metastasis. Moreover, it was identified as an effective biomarker for diagnosis and prognosis of HCC. Functionally, exogenous expression of circ-ADD3 dramatically weakened HCC cell invasion and metastasis both in vitro and in vivo. Mechanistically, circ-ADD3 was capable of reinforcing the interaction between CDK1 and EZH2, resulting in increased EZH2 ubiquitination and subsequent degradation via phosphorylation at Thr-345 and Thr-487 sites. The decreased EZH2 markedly increased the expression of a cohort of anti-metastatic genes, including circ-ADD3, by reducing H3K27me3 levels on their promoter regions, which formed a regulatory circuit, thereby dampening HCC metastasis. Taken together, our findings unveil the essential role of circ-ADD3 in inhibiting HCC metastasis through regulation of EZH2 stability.
Keywords: Circular RNA, circ-ADD3, hepatocellular carcinoma, EZH2, biomarker
Introduction
Liver cancer is the sixth most common malignant tumor and the fourth leading cause of cancer-related death in the world, with an annual increase of 841,000 cases and 782,000 deaths [1]. Especially in China, known as the “leader in liver cancer”, it covers more than 50% of new and dead liver cancer patients worldwide [2]. Hepatocellular carcinoma (HCC) is the main histological subtype of liver cancer, accounting for 75%-85% of primary liver cancer. The prognosis of HCC is extremely dismal, especially in metastatic patients, with a five-year survival rate of less than 10% [3]. Therefore, there is an urgent need to elucidate the pathogenesis of HCC, especially metastasis, which will benefit HCC patients and improve their survival time.
As a new class of endogenous non-coding RNA, circular RNA (circRNA) is becoming a research hotspot in the field of RNA, attracting a lot of attention [4]. Unlike the traditional linear RNA, circRNA has a covalently closed ring structure lacking 5’-caps and 3’-poly(A) tails, which makes it scarcely unaffected by RNA exonuclease and is thus very stable [5]. CircRNA was initially recognized to be the ‘noise’ of transcription without important biological functions. Up to now, high-throughput sequencing results have shown that there are thousands of conserved circRNAs in eukaryotes, and some of which are even more abundant than their linear isoforms [6]. Accumulating evidence suggest that circRNA is aberrantly expressed in cancer and is closely associated with cancer initiation, growth and metastasis [7,8]. Nevertheless, the research on the mechanism of action of circRNA is still in its infancy. Most studies have focused on its role as an efficient molecular sponge for microRNAs (miRNAs), such as CDR1as [9], circ-HIPK3 [10,11], circ-PDZD8 [12] and circ-ABCB10 [13]. Only a small proportion of studies have demonstrated that circRNA is capable of interacting with proteins. Fox example, Du et al reported that circ-Foxo3 could form ternary complexes with p21 and CDK2 to retard cell cycle progression [14]. Circ-Pan3 was shown to interact with KSRP to regulate the self-renewal of intestinal stem cells [15]. Huang et al proposed that circ-Nfix was able to enhance the interaction between Ybx1 and Nedd4l via directly binding to them, thus participating in the cardiac regeneration after myocardial infarction [16]. These studies reveal that the interaction between circRNA and protein is another very important manner in which circRNA functions in addition to ‘miRNA sponges’.
In the present study, we screened and identified a novel circRNA, circ-ADD3 (originating from linear ADD3 exon 4 to exon 12), which was lowly expressed in HCC tissues, cells as well as plasma. Further, we characterized the biological function and protein interaction of circ-ADD3 in HCC.
Materials and methods
Reagents and antibodies
Actinomycin D (Sigma-Aldrich, MO, USA) and RNase R (Epicentre, WI, USA) were used to test the stability of circ-ADD3. Cycloheximide (CHX) and MG132 were purchased from Sigma-Aldrich and Selleck (TX, USA) companies, respectively. The primary antibodies used in this study are as follows: anti-EZH2 (#5246, CST), anti-CDK1 (#ab18, Abcam), anti-Ubiquitin (#3933, CST), anti-p-EZH2 (T345) (#61241, Active Motif), anti-p-EZH2 (T487) (#MABS160, Millipore), anti-H3K27me3 (#61018, Active Motif), anti-GAPDH (#2118, CST).
Specimen collection
A total of 112 paired HCC and adjacent normal tissues were obtained from patients diagnosed with HCC at Henan Provincial People’s Hospital. Among them, four pairs of HCC and paracancerous samples were subjected to human circRNA microarray screening (KangChen Bio-Tech, Shanghai, China), and 25 paired specimens were used to validate the top two upregulated and downregulated circRNAs. In addition, we also collected 19 healthy controls and 31 HCC plasma samples to test the diagnostic utility of circ-ADD3 for HCC. Each subject provided a handwritten informed consent. This procedure was approved by the Ethics Committee of Henan Provincial People’s Hospital.
Quantitative reverse transcription PCR (qRT-PCR)
Total RNA was extracted from HCC cells, tissues and plasma using TakaRa MiniBEST Universal RNA Extraction Kit (Takara, Otsu, Japan) as per manufacturer’s protocol, and was dissolved into 30 µL DEPC water. After that, a total of 1 µg RNA was utilized for reverse transcription, followed by amplification and quantification using SYBR Green SuperMix Kit (Takara). Gene expression levels were normalized to GAPDH. The primer sequences used in this study are shown below: Circ-ADD3 (hsa_circ_0020007): (Forward) 5’-AAGATTCGGGAACAAAATCG-3’, (Reverse) 5’-AAGATTCGGGAACAAAATCG-3’; hsa_circ_0027345: (Forward) 5’-TCACTGGTTTGGATGCATTG-3’, (Reverse) 5’-AAGGTGGCTCATGGAACTTG-3’; hsa_circ_0021603: (Forward) 5’-TACCTCTGCAGGCAGGAACT-3’, (Reverse) 5’-TCTCCTGGCAAACCTTGAGT-3’; hsa_circ_0000416: (Forward) 5’-ATGAAAGCCTGGCTCTGTGT-3’, (Reverse) 5’-GTCCGATGATTCCTGCTGAT-3’; ADD3: (Forward) 5’-GGTTGACCAGGGAAGTACCA-3’, (Reverse) 5’-GCTGTTGCAAGGGTATGGAT-3’; EZH2: (Forward) 5’-AGGAGTTTGCTGCTGCTCTC-3’, (Reverse) 5’-TTTCAGTCCCTGCTTCCCTA-3’; GAPDH: (Forward) 5’-TCACCAGGGCTGCTTTTAAC-3’, (Reverse) 5’-GACAAGCTTCCCGTTCTCAG-3’.
Cell culture, transient and stable transfection
The human normal liver LO2 cells and six HCC cells including SNU-423, HepG2, Hep3B, Huh7, SMMC-7721 and MHCC-97L were all obtained from ATCC and grown in RPMI 1640 or DMEM medium with 10% FBS. Cells were tested for mycoplasma before use. For transient transfection, si-EZH2 (5’-GCUGGAAUCAAAGGAUACA-3’), si-CDK1 (5’-CGGGAAAUUUCUCUAUUAA-3’), si-circ-ADD3#1 (5’-AGCCACCTTCTGTCTTGGCAT-3’), si-circ-ADD3#2 (5’-GATAAGCCACCTTCTGTCTTG-3’) (Gene-Pharma, Shanghai, China) and EZH2, ADD3 pcDNA 3.0 expression vector (Invitrogen, MA, USA) were respectively transfected into Hep3B and SMMC-7721 cells using Lipofectamine 2000 (Invitrogen) as per the standard protocol. The transfection efficiency was determined by qRT-PCR analysis after 48 hours of transfection. For stable transfection, circ-ADD3 PLCDH-ciR lentivirus expression vector was commercially purchased from Geneseed company (Guangzhou, China) and infected into Hep3B and SMMC-7721 cells, followed by selection using 0.8 μg/mL puromycin.
In vitro and in vivo metastasis assays
Transwell chambers coated without and with matrigel were employed to evaluate the migratory and invasive abilities of Hep3B and SMMC-7721 cells, respectively. In brief, 3×105 cells were plated on the upper surface of the chamber, and the lower surface of the chamber was immersed in DMEM medium with 10% FBS. After 16 hours, the migrated and invaded cells were fixed and stained. For the establishment of lung metastasis model, 1×106 SMMC-7721 cells with and without circ-ADD3 overexpression were tail vein injected into nude mice, respectively (n=10 per group). The IVIS Lumina II system was used to monitor lung metastasis and lung tissues were collected after six weeks of injection, followed by hematoxylin-eosin (H&E) staining.
The animal study was conducted with approval of the Animal Care Committee of Henan Provincial People’s Hospital.
Western blot and Co-immunoprecipitation (Co-IP)
Total protein form Hep3B and SMMC-7721 cells was collected by lysis buffer, separated by 10% SDS-PAGE gel, and transferred onto the PVDF membrane. Then, the membrane was incubated with corresponding primary and secondary antibodies, followed by detection for the signal using chemiluminescent reagent. For Co-IP assay, the lysis was incubated with anti-EZH2 or CDK1 primary antibody at 4°C overnight with gentle rotation, followed by addition with protein A/G agarose (#sc-2003, Santa Cruz Biotechnology, CA, USA) for two hours at 25°C. Then, the precipitated complex was subjected to western blot analysis. In particular, for detecting the ubiquitination level of EZH2, cells were treated with 5 μM MG132 for six hours before protein collection.
RNA pull-down assay and RNA immunoprecipitation (RIP)
For RNA pull-down assay, DNA probe targeting the junction site of circ-ADD3 was designed (5’-AGCCACCTTCTGTCTTGGCAT-3’) and synthesized by Sangon Biotech (Shanghai, China). The efficacy and specificity of circ-ADD3 probe were determined by qRT-PCR analysis. The lysates of control or circ-ADD3-overexpressing Hep3B and SMMC-7721 cells were incubated with control or circ-ADD3 probe at 4°C overnight with gentle rotation, followed by addition with dynabeads magnetic beads (Invitrogen) for three hours at room temperature and then 10 minutes at 60°C. Lastly, the precipitated complex was subjected to western blot analysis using anti-EZH2 or CDK1 primary antibody. For RIP assay, the EZ-Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (Millipore, MA, USA) was used based on manufacturer’s instruction.
Chromatin immunoprecipitation (ChIP)
ChIP assay was carried out using ChIP-IT® Express Chromatin Immunoprecipitation Kits (Active Motif) according to manufacturer’s manual with minor changes. Briefly, Hep3B and SMMC-7721 cells with 70-80% confluency were treated with with 37% formaldehyde and glycine in sequence. Then, the chromatin was sheared via using 200 U/ml Enzymatic Shearing Cocktail (Active Motif) at 37°C. 10 µL sheared chromatin was used as ‘input DNA’. Immunoprecipitation was performed at 4°C for four hours on an end-to-end rotator using 100 µL total volume reaction system containing 25 µL protein G magnetic beads, 10 µL ChIP buffer, 40 µL sheared chromatin, 1 µL protease inhibitor cocktail, 8 µL corresponding primary antibody, and 16 µL dH2O. Subsequently, the precipitated complex was incubated with 2 µL proteinase K at 37°C for 1 hour, followed by qPCR analysis for DNA expression.
Statistical analysis
All statistical results were two-tailed. Data were presented as mean ± SD of at least three independent experiments carried out in triplicate. Student’s t or Chi-square test was utilized to assess the difference between groups as appropriate. The survival curves were plotted by Kaplan-Meier method and determined by Log-rank test. ROC curve was used to detect the diagnostic utility of plasma circ-ADD3 for HCC. The relationship between circ-ADD3 and EZH2 expression in HCC tissues was measured using Pearson Correlation Coefficient. Statistically significant value was set to P < 0.05. *P < 0.05, **P < 0.01, ***P < 0.001.
Results
Identification of circ-ADD3 in HCC
In an attempt to determine the dysregulated circRNAs in HCC, we performed circRNA microarray expression profile in four paired HCC and normal tissues (Figure 1A). We then selected the top two upregulated and downregulated circRNAs to verify in 25 paired HCC and paracancerous tissues, the qRT-PCR results were consistent with the microarray results (Figure 1B). Due to hsa_circ_0020007 had the greatest expression difference, we focused on it in the subsequent study. The sequence alignment results showed that hsa_circ_0020007 was derived from exon 4 to exon 12 of linear ADD3 (spliced mature full length is 1274 bp), we thus termed it as circ-ADD3 (Figure 1C). To test the stability of circ-ADD3, we treated LO2 cells with transcription inhibitor Actinomycin D, followed by qRT-PCR analysis. The results showed that circ-ADD3 had a half-life of more than 24 hours, whereas its linear isoform ADD3 mRNA had a half-life of less than 4 hours (Figure 1D). Similarly, the expression level of ADD3 mRNA rather than circ-ADD3 was substantially decreased after treatment with RNase R (Figure 1E). Overall, these results suggest that circ-ADD3 is a highly stable and dysregulated circRNA in HCC.
Figure 1.
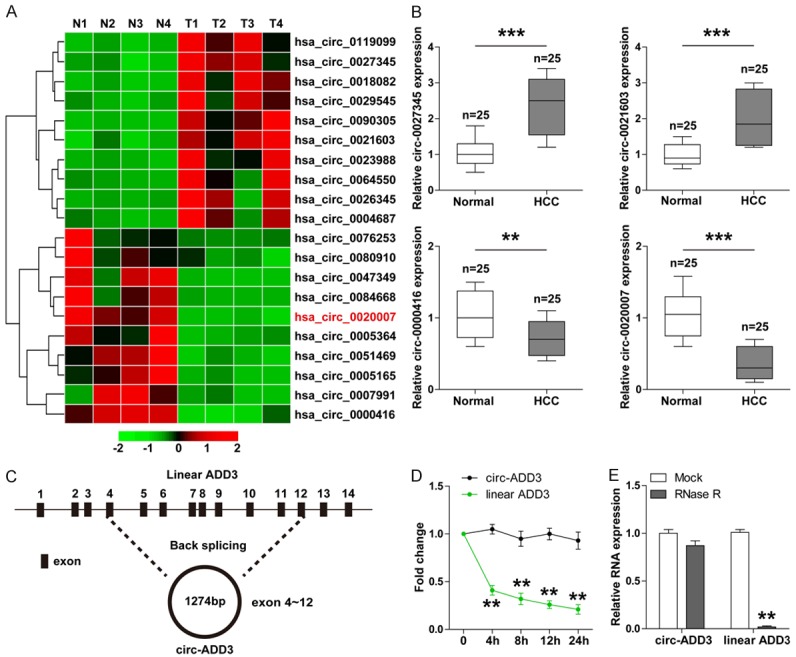
Identification of circ-ADD3 in HCC. A. The heap map showing top ten upregulated and downregulated circRNAs in 4 paired HCC and adjacent normal tissues. B. qRT-PCR analysis for the expression of top two upregulated and downregulated circRNAs in 25 paired HCC and adjacent normal tissues. C. The sketch showing the origination of circ-ADD3 and its spliced full length. D. qRT-PCR analysis for the expression of circ-ADD3 and ADD3 mRNA in LO2 cells at the indicated time after treatment with Actinomycin D (2 mg/ml). E. qRT-PCR analysis for the expression of circ-ADD3 and ADD3 mRNA in LO2 cells after treatment with 40U RNaseR. **P < 0.01, ***P < 0.001.
Circ-ADD3 is lowly expressed in HCC tissues, cells and plasma and may be an effective diagnostic and prognostic biomarker
To further confirm above results, we collected a total of 112 pairs of HCC and adjacent normal tissues to conduct qRT-PCR for circ-ADD3 expression. As expected, circ-ADD3 expression was dramatically reduced in HCC tissues in comparison to normal tissues (Figure 2A). The similar results were also observed in six HCC cell lines (Figure 2B). We then assessed the relationship between circ-ADD3 expression and clinicopathologic features of HCC patients. As shown in Table 1, patients with metastatic characteristics exhibited lower circ-ADD3 expression than patients without metastatic characteristics. Moreover, low circ-ADD3 expression was positively correlated with worse overall and progression-free survival (Figure 2C, 2D). Importantly, the expression level of circ-ADD3 was also significantly decreased in plasma samples from HCC patients compared with those form healthy controls (Figure 2E). We then further evaluated the diagnostic utility of plasma circ-ADD3 for HCC, the area under ROC curve (AUC) was 0.8878 (95% confidence interval: 0.8094 to 0.9662) (Figure 2F), implying that plasma circ-ADD3 level can fairly distinguish HCC patients from healthy controls. In all, these data indicate that circ-ADD3 may be an important circRNA that participates in the malignant behaviors of HCC.
Figure 2.
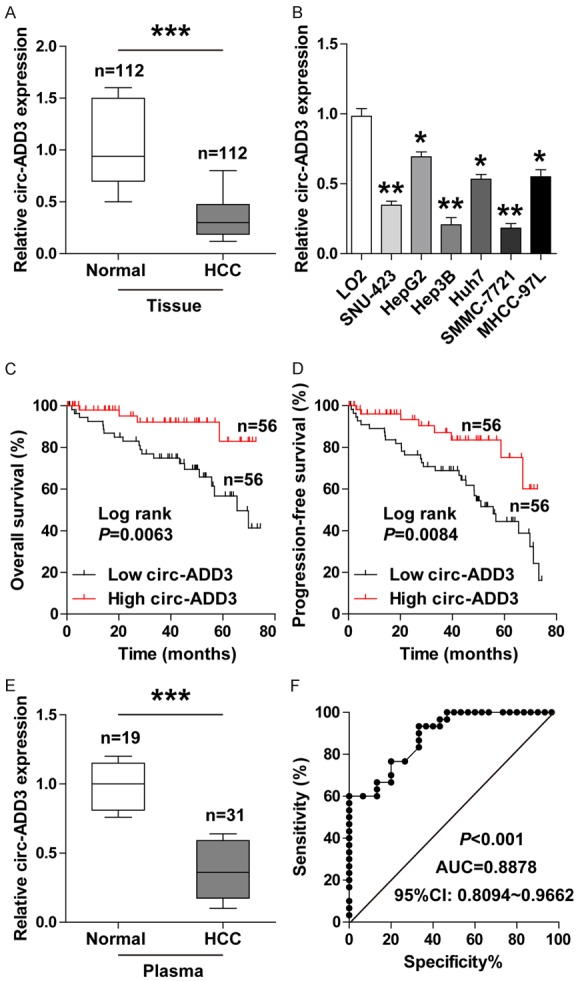
Circ-ADD3 is significantly decreased in HCC. A. qRT-PCR analysis for circ-ADD3 expression in 112 paired HCC and adjacent normal tissues. B. qRT-PCR analysis for circ-ADD3 expression in HCC cell lines. C, D. The overall and progression-free survival curves of HCC patients with low and high circ-ADD3 expression based on median circ-ADD3 value. E. qRT-PCR analysis for plasma circ-ADD3 expression in 19 healthy controls and 31 HCC patients. F. ROC curve analysis for the diagnostic utility of plasma circ-ADD3 for HCC. *P < 0.05, **P < 0.01, ***P < 0.001.
Table 1.
The correlation between circ-ADD3 expression and clinicopathologic features of HCC patients (n=112)
| Features | circ-ADD3 expression | P Value | |
|---|---|---|---|
|
| |||
| Low (n=56) | High (n=56) | ||
| Age | 0.571 | ||
| ≤ 50 | 26 | 29 | |
| > 50 | 30 | 27 | |
| Gender | 0.483 | ||
| Male | 46 | 43 | |
| Female | 10 | 13 | |
| AFP (ng/ml) | 0.393 | ||
| ≤ 400 | 17 | 13 | |
| > 400 | 39 | 43 | |
| HBV | 0.249 | ||
| Positive | 36 | 30 | |
| Negative | 20 | 26 | |
| Liver cirrhosis | 0.393 | ||
| Yes | 39 | 43 | |
| No | 17 | 13 | |
| Tumor diameter (cm) | 0.088 | ||
| ≤ 5 | 26 | 35 | |
| > 5 | 30 | 21 | |
| Vascular invasion | 0.023 | ||
| Yes | 33 | 21 | |
| No | 23 | 35 | |
| Intrahepatic metastasis | 0.018 | ||
| Yes | 20 | 9 | |
| No | 36 | 47 | |
| Distant metastasis | 0.007 | ||
| Yes | 13 | 3 | |
| No | 43 | 53 | |
| Edmondson | 0.200 | ||
| I-II | 18 | 12 | |
| III-IV | 38 | 44 | |
Circ-ADD3 inhibits HCC cell migration and invasion in vitro as well as lung metastasis in vivo
To determine the biological function of circ-ADD3 in HCC, we first established the stably circ-ADD3-overexpressing Hep3B and SMMC-7721 cells through PLCDH-ciR lentivirus vector. As shown in Figure 3A, the expression of circ-ADD3, but not ADD3 mRNA, was about 40-fold upregulated in cells infected with circ-ADD3 lentivirus vector as compared with cells infected with control empty vector. Given that circ-ADD3 was closely associated with metastatic clinicopathologic features of HCC patients (Table 1), we then tested whether circ-ADD3 affected the metastatic abilities of HCC cells. The results of transwell assays showed that overexpression of circ-ADD3 significantly weakened the migratory and invasive capabilities of Hep3B and SMMC-7721 cells (Figure 3B, 3C), and silencing of circ-ADD3 displayed the opposite trend (Supplementary Figure 1A-C). Besides, exogenous ADD3 expression could not block the enhanced aggressive phenotype following circ-ADD3 depletion (Supplementary Figure 1C). Next, we established the lung metastasis model via tail vein injection of SMMC-7721 cells into nude mice to determine whether circ-ADD3 also functioned in vivo. As expected, an average of approximately 25 lung metastasis nodules were observed in control group, whereas only an average of approximately 5 lung metastatic nodules were observed in circ-ADD3-overexpressing group (Figure 3D, 3E). Altogether, these in vitro and in vivo results reveal that circ-ADD3 is a negative regulator of HCC metastasis.
Figure 3.
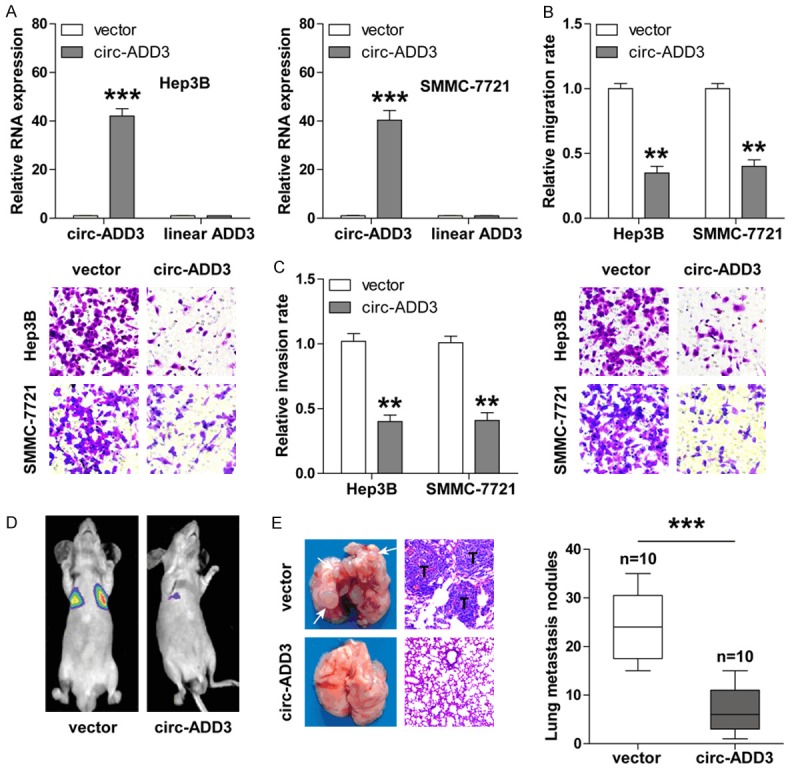
Overexpression of circ-ADD3 evidently inhibits HCC invasion and metastasis. A. qRT-PCR analysis for the expression of circ-ADD3 and ADD3 mRNA in Hep3B and SMMC-7721 cells after circ-ADD3 overexpression. B, C. Transwell migration (without matrigel) and invasion (with matrigel) assays in Hep3B and SMMC-7721 cells after circ-ADD3 overexpression. D. The IVIS Lumina II system showing the lung metastasis in nude mice tail-vein injected with control and circ-ADD3-overexpressing SMMC-7721 cells. E. The representative images of lung tissues and H&E staining in the indicated two groups. “T” denotes metastatic tumor. **P < 0.01, ***P < 0.001.
Circ-ADD3 reduces EZH2 protein expression via directly interaction with it
Given that histone methyltransferase EZH2 is a key metastasis-related gene that can physically bind to a large amount of non-coding RNAs, [17] we then investigated whether there was also an interaction between circ-ADD3 and EZH2. First, we predicted the interaction probability between them using RPISeq online tool (http://pridb.gdcb.iastate.edu/). The results showed that circ-ADD3 and EZH2 were likely to interact each other (RF classifier =0.8, SVM classifier =0.96) (Figure 4A) (Supplementary Figure 2). RNA pull-down assay was carried out to verify this prediction, as shown in Figure 4B, EZH2 was abundantly enriched by circ-ADD3 probe, especially in circ-ADD3-overexpressing Hep3B and SMMC-7721 cells. Likewise, RIP results showed that circ-ADD3 was abundantly immunoprecipitated by EZH2 antibody (Figure 4C), suggesting that circ-ADD3 is able to bind to EZH2 in HCC cells. In light of the interaction between them, we then assessed whether circ-ADD3 altered EZH2 expression levels. The results showed that enforced expression of circ-ADD3 had no effect on EZH2 mRNA expression (Figure 4D), but dramatically reduced EZH2 protein expression both in Hep3B and SMMC-7721 cells (Figure 4E). Next, we performed IHC staining for EZH2 in 50 paired HCC and normal tissues, the results displayed that EZH2 was notably upregulated in HCC (Figure 4F), and importantly, there was a strong negative correlation between EZH2 and circ-ADD3 expression (Figure 4G). In addition, we found that the attenuated migratory and invasive capacities of Hep3B and SMMC-7721 cells caused by circ-ADD3 overexpression were significantly rescued by exogenous EZH2 expression (Figure 4H). Collectively, these above findings demonstrate that EZH2 is involved in the function of circ-ADD3 in HCC.
Figure 4.
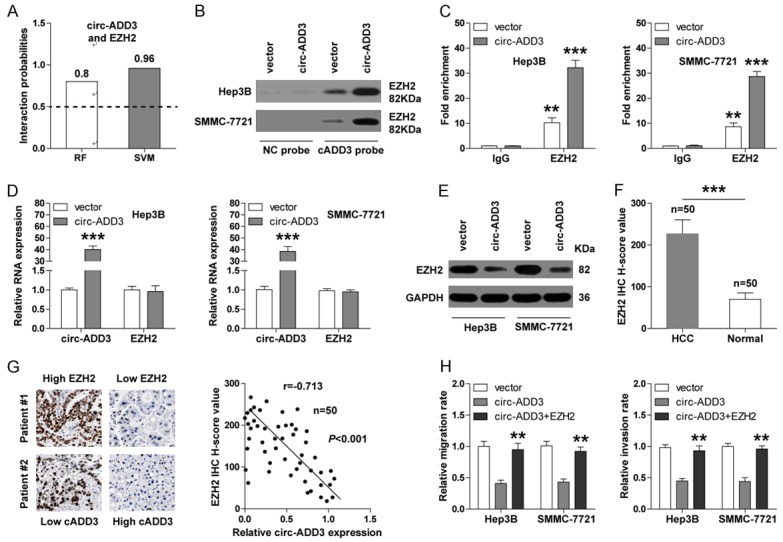
Circ-ADD3 binds to EZH2 and reduces its expression. A. The interaction probability between circ-ADD3 and EZH2 predicted by RPISeq online software based on RF and SVM classifiers (probabilities > 0.5 were considered “positive”). B. RNA pull-down assay in control and circ-ADD3-overexpressing Hep3B and SMMC-7721 cells, followed by western blot analysis for EZH2 expression. C. RNA immunocoprecipitation (RIP) assay in control and circ-ADD3-overexpressing Hep3B and SMMC-7721 cells with EZH2 antibody, followed by qRT-PCR analysis for circ-ADD3 expression. D. qRT-PCR analysis for the expression of circ-ADD3 and EZH2 in control and circ-ADD3-overexpressing Hep3B and SMMC-7721 cells. E. Western blot analysis for EZH2 expression in control and circ-ADD3-overexpressing Hep3B and SMMC-7721 cells. F. The H-score values of EZH2 IHC staining in 50 pairs of normal and HCC tissues. G. The correlation between circ-ADD3 and EZH2 expression in 50 HCC tissues. H. Transwell migration (without matrigel) and invasion (with matrigel) assays in circ-ADD3-overexpressing Hep3B and SMMC-7721 cells after exogenous EZH2 expression. **P < 0.01, ***P < 0.001.
Circ-ADD3 destabilizes EZH2 via potentiating CDK1-mediated EZH2 ubiquitination
It was shown that circ-ADD3 only altered the expression of EZH2 protein, but not EZH2 mRNA, implying that circ-ADD3 regulates EZH2 expression at the post-transcriptional level. We then tested the stability of EZH2 protein by using protein synthesis inhibitor cycloheximide (CHX). As shown in Figure 5A, ectopic expression of circ-ADD3 significantly shortened the half-life of EZH2. Consistently, the ubiquitination level of EZH2 was remarkably increased after circ-ADD3 overexpression (Figure 5B), suggesting circ-ADD3 affects EZH2 protein stability through ubiquitination. It has been proposed that CDK1, a cyclin-dependent kinase, is an important regulator for EZH2 ubiquitination and subsequent proteasomal degradation via phosphorylation at Thr-345 and Thr-487 sites of EZH2 [18]. We thus inferred that CDK1 might be involved in circ-ADD3-mediated EZH2 ubiquitination. To verify this hypothesis, we first tested whether circ-ADD3 could interact with CDK1. By using RPISeq online tool, we found that circ-ADD3 and CDK1 were likely to bind each other (RF classifier =0.85, SVM classifier =0.96) (Figure 5C) (Supplementary Figure 3). Subsequent RNA pull-down and RIP assays both confirmed this prediction (Figure 5D, 5E). Importantly, Co-IP assay results showed that more CDK1 was precipitated by EZH2 antibody in circ-ADD3-overexpressing Hep3B and SMMC-7721 cells in comparison to control cells (Figure 5F), revealing that circ-ADD3 enhances the interaction between CDK1 and EZH2. Concordantly, the phosphorylation levels at Thr-345 and Thr-487 sites of EZH2 were notably increased after circ-ADD3 overexpression, but these effects were effectively blocked by CDK1 knockdown (Figure 5G). Taken together, these results suggest that CDK1 is required for circ-ADD3-mediated EZH2 ubiquitination and degradation.
Figure 5.
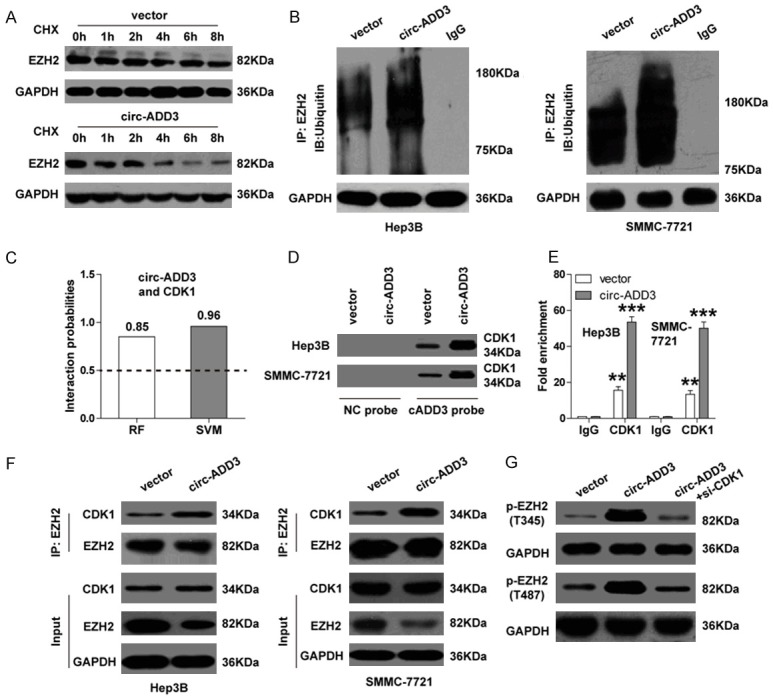
Circ-ADD3 potentiates CDK1-mediated EZH2 ubiquitination and degradation. A. Western blot analysis for EZH2 expression in Hep3B cells at the indicated time after treatment with protein synthesis inhibitor CHX (100 μg/ml). B. Co-IP assay in control and circ-ADD3-overexpressing Hep3B and SMMC-7721 cells using EZH2 antibody, followed by western blot analysis for ubiquitin expression. C. The interaction probability between circ-ADD3 and CDK1 predicted by RPISeq online software. D. RNA pull-down assay in control and circ-ADD3-overexpressing Hep3B and SMMC-7721 cells, followed by western blot analysis for CDK1 expression. E. RIP assay in control and circ-ADD3-overexpressing Hep3B and SMMC-7721 cells with CDK1 antibody, followed by qRT-PCR analysis for circ-ADD3 expression. F. Co-IP assay in control and circ-ADD3-overexpressing Hep3B and SMMC-7721 cells using EZH2 antibody, followed by western blot analysis for CDK1 and EZH2 expression. G. Western blot analysis for p-EZH2 (T345 and T487) expression in circ-ADD3-overexpressing Hep3B cells after silencing of CDK1. **P < 0.01, ***P < 0.001.
Circ-ADD3 elevates a cohort of metastasis suppressors via reduction of EZH2-mediated H3K27me3 levels
Given that EZH2 is a well-known metastasis driver that can epigenetically inhibit a large number of metastasis suppressors through catalyzing H3K27me3 on their promoter regions [19], we then wondered whether circ-ADD3 also affected their expression levels. Six anti-metastatic genes including CDX1, FOXC1, HOXA10, CDH1, DAB2IP and LATS2 were selected for verification according to previous literatures. As expected, exogenous expression of circ-ADD3 pervasively upregulated these genes, whereas these upregulation effects were significantly abrogated by EZH2 overexpression (Figure 6A). Consistently, ChIP assay results showed that the occupations of EZH2 as well as H3K27me3 on the promoter regions of above anti-metastatic genes were dramatically decreased after circ-ADD3 overexpression (Figure 6B, 6C). Intriguingly, we also found that a great quantity of EZH2 and H3K27me3 were enriched on circ-ADD3 promoter (Figure 6D), and overexpression of EZH2 decreased, while silencing of EZH2 increased the expression of circ-ADD3 both in Hep3B and SMMC-7721 cells (Figure 6E). Overall, these data indicate that circ-ADD3 epigenetically promotes the expression of several anti-metastatic genes through EZH2 in HCC.
Figure 6.
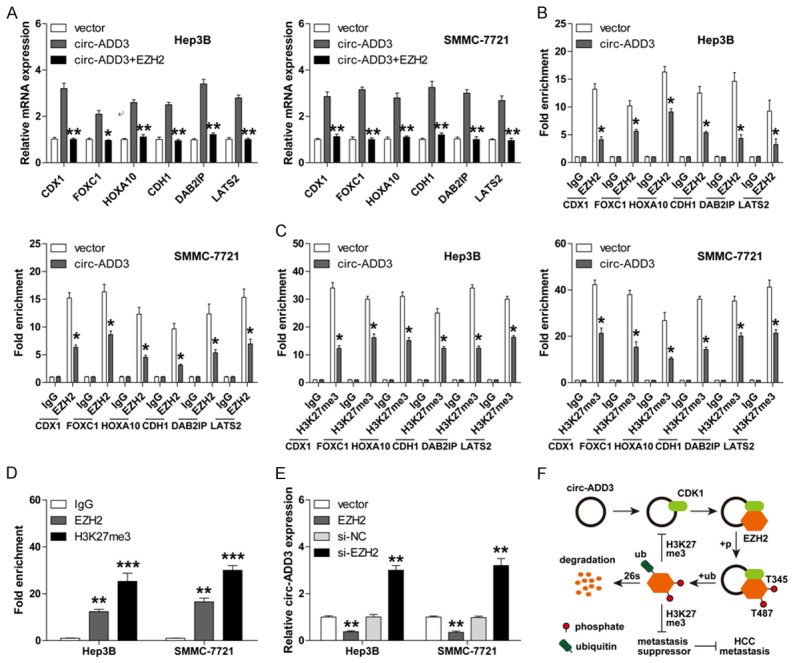
Circ-ADD3 elevates a set of anti-metastatic genes via interaction with EZH2. A. qRT-PCR analysis for the expression of the indicated six anti-metastatic genes in circ-ADD3-overexpressing Hep3B and SMMC-7721 cells after exogenous EZH2 expression. B, C. ChIP assay in circ-ADD3-overexpressing Hep3B and SMMC-7721 cells using EZH2 or H3K27me3 antibody, followed by qPCR analysis for the enriched promoter fractions of the indicated six anti-metastatic genes. D. ChIP assay in Hep3B and SMMC-7721 cells using EZH2 or H3K27me3 antibody, followed by qPCR analysis for the enriched promoter fractions of circ-ADD3. E. qRT-PCR analysis for circ-ADD3 expression in Hep3B and SMMC-7721 cells after EZH2 knockdown or overexpression. F. The diagrammatic sketch showing the anti-metastatic role of circ-ADD3 in HCC through modulation of EZH2 stability via CDK1. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
EZH2, a histone methyltransferase belonging to core subunits of polycomb repressive complex 2 (PRC2), is a well-known oncoprotein that participates in cancer malignant behaviors, especially metastasis [20]. Numerous studies have shown that non-coding RNAs, such as miRNAs and long non-coding RNAs (lncRNAs), can physically bind to EZH2 to affect tumorigenesis and aggressiveness [17]. However, whether circRNA, as a new and highly concerned non-coding RNA, is also able to directly interact with EZH2 is unknown. In the current study, we identified a EZH2-bound circRNA, circ-ADD3, which was significantly downregulated in HCC. Stepwise investigations revealed that circ-ADD3 could facilitate the binding of CDK1 and EZH2 by directly interacting with them, leading to increased EZH2 phosphorylation at threonines 345 and 487 and subsequent ubiquitination and degradation, ultimately restraining HCC metastasis (Figure 6F). Therefore, our study provides the first evidence that circRNA is capable of binding to EZH2, which advances the understanding of the regulation of EZH2 protein stability.
Emerging evidence shows that circRNA is abundant in eukaryotes and abnormally expressed in human cancers [21]. Here, by performing circRNA microarray expression profile in paired HCC and normal tissues, we found a large number of dysregulated circRNAs in HCC, suggesting circRNA may be an important previously ignored regulator for cancer development and progression. We then mainly focused on circ-ADD3 (a circRNA derived from linear ADD3 exon 4 to exon 12) due to the greatest expression difference. The sequence alignment results showed that there are 87% similarity between hsa-circ-ADD3 and homologous mus-circ-ADD3 (Supplementary Figure 4), indicating that circ-ADD3 is a highly conserved circRNA. Recent studies suggest that circRNA can be used as an efficacious biomarker for cancer due to its high stability [22,23]. For instance, circ-UVRAG [24], circ-ANKS1B [25] and circBACH2 [26] were reported to be diagnostic or prognostic biomarker for gastric cancer, breast cancer and papillary thyroid carcinoma, respectively. Likewise, we also identified that circ-ADD3 had high stability and was a promising diagnostic and prognostic biomarker for HCC. Further study is warranted to explore whether circ-ADD3 can also be detected in other body fluids, such as in sweat, urine, and even in exosomes, which will provide an early noninvasive diagnostic approach for HCC patients. In addition, whether there is also a dysregulation of circ-ADD3 in other cancers may be worth of in-depth investigation.
Multiple lines of evidence demonstrates that circRNA participates in tumorigenesis mainly through the following mechanisms: acting as ‘miRNA sponges’, binding to protein, regulation of mRNA stability, transcriptional regulation and translation of protein [27]. Of them, the mechanism of ‘miRNA sponges’ has been extensively studied [28]. However, little is known about the role of circRNA in interacting with protein. In this study, we found that circ-ADD3 could directly bind to EZH2 and inhibit its protein expression, but not mRNA expression. EZH2 is well recognized as a epigenetic metastasis driver that can catalyze H3K27me3 to transcriptionally silence its target metastasis suppressors [29]. In line with this notion, exogenous circ-ADD3 expression substantially decreased the occupations of EZH2 and H3K27me3 on the promoter regions of some anti-metastatic genes, suggesting EZH2 is important for the metastasis-inhibiting activity of circ-ADD3 in HCC. Of note, we also found that EZH2 was able to epigenetically regulate circ-ADD3 expression, thus a feedback loop is formed between them. Previous studies reported that EZH2 could be phosphorylated at Thr-345 and Thr-487 sites by CDK1, resulting in increased EZH2 ubiquitination and subsequent proteasomal degradation [18,30]. Herein, we found that circ-ADD3 potentiated the interaction between CDK1 and EZH2 via physically binding to them, implying that circ-ADD3 may be a scaffold for CDK1-mediated EZH2 ubiquitination. It is worthwhile to decipher which structures or sequences of circ-ADD3 are necessary for the formation of this ternary complex.
Collectively, our findings indicate that circ-ADD3 functions as a novel metastasis suppressor in HCC through destabilizing EZH2 via CDK1, restoration of circ-ADD3 may be a potential therapeutic approach for patients with metastatic HCC.
Acknowledgements
This work was financially supported by grants from the National Natural Science Foundation of China (ZC20170062).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Xiao J, Wang F, Wong NK, He J, Zhang R, Sun R, Xu Y, Liu Y, Li W, Koike K, He W, You H, Miao Y, Liu X, Meng M, Gao B, Wang H, Li C. Global liver disease burdens and research trends: analysis from a china perspective. J Hepatol. 2019;71:212–221. doi: 10.1016/j.jhep.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Finn RS, Zhu AX, Farah W, Almasri J, Zaiem F, Prokop LJ, Murad MH, Mohammed K. Therapies for advanced stage hepatocellular carcinoma with macrovascular invasion or metastatic disease: a systematic review and meta-analysis. Hepatology. 2018;67:422–35. doi: 10.1002/hep.29486. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Yang L, Chen LL. The biogenesis, functions, and challenges of circular RNAs. Mol Cell. 2018;71:428–42. doi: 10.1016/j.molcel.2018.06.034. [DOI] [PubMed] [Google Scholar]
- 5.Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–57. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One. 2012;7:e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng WL, Mohd MT, Shukla K. Functional role of circular RNAs in cancer development and progression. RNA Biol. 2018;15:995–1005. doi: 10.1080/15476286.2018.1486659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen B, Wang Z, Li Z, Song H, Ding X. Circular RNAs: an emerging landscape in tumor metastasis. Am J Cancer Res. 2019;9:630–43. [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–8. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 10.Zeng K, Chen X, Xu M, Liu X, Hu X, Xu T, Sun H, Pan Y, He B, Wang S. CircHIPK3 promotes colorectal cancer growth and metastasis by sponging miR-7. Cell Death Dis. 2018;9:417. doi: 10.1038/s41419-018-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Zheng Q, Bao C, Guo W, Li S, Chen J, Chen B, Luo Y, Lyu D, Li Y, Shi G, Liang L, Gu J, He X, Huang S. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun. 2016;7:11215. doi: 10.1038/ncomms11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qu D, Yan B, Xin R, Ma T. A novel circular RNA hsa_circ_0020123 exerts oncogenic properties through suppression of miR-144 in non-small cell lung cancer. Am J Cancer Res. 2018;8:1387–402. [PMC free article] [PubMed] [Google Scholar]
- 13.Liang HF, Zhang XZ, Liu BG, Jia GT, Li WL. Circular RNA circ-ABCB10 promotes breast cancer proliferation and progression through sponging miR-1271. Am J Cancer Res. 2017;7:1566–76. [PMC free article] [PubMed] [Google Scholar]
- 14.Du WW, Yang W, Liu E, Yang Z, Dhaliwal P, Yang BB. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016;44:2846–58. doi: 10.1093/nar/gkw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu P, Zhu X, Wu J, He L, Lu T, Wang Y, Liu B, Ye B, Sun L, Fan D, Wang J, Yang L, Qin X, Du Y, Li C, He L, Ren W, Wu X, Tian Y, Fan Z. IL-13 secreted by ILC2s promotes the self-renewal of intestinal stem cells through circular RNA circPan3. Nat Immunol. 2019;20:183–94. doi: 10.1038/s41590-018-0297-6. [DOI] [PubMed] [Google Scholar]
- 16.Huang S, Li X, Zheng H, Si X, Li B, Wei G, Li C, Chen Y, Chen Y, Liao W, Liao Y, Bin J. Loss of super-enhancer-regulated CircRNA nfix induces cardiac regeneration after myocardial infarction in adult mice. Circulation. 2019;139:2857–2876. doi: 10.1161/CIRCULATIONAHA.118.038361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benetatos L, Voulgaris E, Vartholomatos G, Hatzimichael E. Non-coding RNAs and EZH2 interactions in cancer: long and short tales from the transcriptome. Int J Cancer. 2013;133:267–74. doi: 10.1002/ijc.27859. [DOI] [PubMed] [Google Scholar]
- 18.Wu SC, Zhang Y. Cyclin-dependent kinase 1 (CDK1)-mediated phosphorylation of enhancer of zeste 2 (Ezh2) regulates its stability. J Biol Chem. 2011;286:28511–9. doi: 10.1074/jbc.M111.240515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim KH, Roberts CW. Targeting EZH2 in cancer. Nat Med. 2016;22:128–34. doi: 10.1038/nm.4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laugesen A, Hojfeldt JW, Helin K. Molecular mechanisms directing PRC2 recruitment and H3K27 methylation. Mol Cell. 2019;74:8–18. doi: 10.1016/j.molcel.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shang Q, Yang Z, Jia R, Ge S. The novel roles of circRNAs in human cancer. Mol Cancer. 2019;18:6. doi: 10.1186/s12943-018-0934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei B, Tian Z, Fan W, Ni B. Circular RNA: a novel biomarker and therapeutic target for human cancers. Int J Med Sci. 2019;16:292–301. doi: 10.7150/ijms.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheng JQ, Liu L, Wang MR, Li PY. Circular RNAs in digestive system cancer: potential biomarkers and therapeutic targets. Am J Cancer Res. 2018;8:1142–56. [PMC free article] [PubMed] [Google Scholar]
- 24.Yang C, Wu S, Wu X, Zhou X, Jin S, Jiang H. Silencing circular RNA UVRAG inhibits bladder cancer growth and metastasis by targeting the microRNA-223/fibroblast growth factor receptor 2 axis. Cancer Sci. 2019;110:99–106. doi: 10.1111/cas.13857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng K, He B, Yang BB, Xu T, Chen X, Xu M, Liu X, Sun H, Pan Y, Wang S. The pro-metastasis effect of circANKS1B in breast cancer. Mol Cancer. 2018;17:160. doi: 10.1186/s12943-018-0914-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai X, Zhao Z, Dong J, Lv Q, Yun B, Liu J, Shen Y, Kang J, Li J. Circular RNA circBACH2 plays a role in papillary thyroid carcinoma by sponging miR-139-5p and regulating LMO4 expression. Cell Death Dis. 2019;10:184. doi: 10.1038/s41419-019-1439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristensen LS, Hansen TB, Veno MT, Kjems J. Circular RNAs in cancer: opportunities and challenges in the field. Oncogene. 2018;37:555–65. doi: 10.1038/onc.2017.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verduci L, Strano S, Yarden Y, Blandino G. The circRNA-microRNA code: emerging implications for cancer diagnosis and treatment. Mol Oncol. 2019;13:669–80. doi: 10.1002/1878-0261.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao R, Zhang Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev. 2004;14:155–64. doi: 10.1016/j.gde.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Wei Y, Chen YH, Li LY, Lang J, Yeh SP, Shi B, Yang CC, Yang JY, Lin CY, Lai CC, Hung MC. CDK1-dependent phosphorylation of EZH2 suppresses methylation of H3K27 and promotes osteogenic differentiation of human mesenchymal stem cells. Nat Cell Biol. 2011;13:87–94. doi: 10.1038/ncb2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


