Abstract
Cancer is a diverse class of diseases characterized by uncontrolled cell growth with the potential to invade and spread to other parts of the body, and continues to be one of the leading causes of death worldwide. Conventional cancer treatment modalities include antitumor drugs, surgical resection, locally targeted therapies such as radiation therapy. Along with improved understanding of the molecular pathogenesis of various cancers, generation and the use of smart targeted anti-cancer drugs have been challenged. The need for novel therapeutic strategies remains paramount given the sustained development of drug resistance, tumor recurrence, and metastasis. Development of new strategies aimed at improving chemotherapy sensitivity and minimizing the adverse side effects is thus essential for obtaining satisfied therapeutic outcomes for patients and enhancing their quality of life. Emerging evidence has reported that many cancer patients use either herbs employed in complementary therapies or dietary agents that influence cellular signaling worldwide. Numerous components of edible plants, collectively termed phytochemicals that have beneficial effects for health, are being reported increasingly in the scientific literature. Of those, flavonoids have attracted much attention by virtue of its wide variety of biological functions including antioxidant, anti-inflammatory, and anticancer activity. In this review, we highlight the molecular mechanisms underlying its multiple pharmacological effects, especially focusing on cancer chemoprevention. We further discuss possible strategies to develop anticancer therapy by combining flavonoids nutraceuticals and conventional chemotherapeutic agents. We also highlight numerous pharmacokinetic challenges such as bioavailability, drug-drug interactions, which are still fundamental questions concerning its future clinical application.
Keywords: Cancer prevention, flavonoids, anticancer activity, combination treatment, immunosuppression, drug transporters, bioavailability, drug-drug interactions
Introduction
Cancer is a diverse class of diseases characterized by uncontrolled cell growth with the potential to invade and spread to other parts of the body, and continues to be one of the leading causes of death worldwide [1-3]. Previous reports have shown differences in cancer morbidity distribution between the developing and the developed world, and demonstrated that approximately 90-95% of all cancers are attributed to lifestyle factors, such as smoking, alcohol consumption, obesity, diet, physical inactivity, among other things, while the remaining 5-10% are due to inherited genes [2,4]. Conventional cancer treatment modalities include antitumor drugs, surgical resection, locally targeted therapies such as radiation, radiofrequency ablation, and photodynamic therapy [1,2,4]. In addition, along with notable advances in the understanding of the molecular pathogenesis of various cancers, generation and the use of smart targeted anti-cancer drugs have been challenged. For instance, tamoxifen (Nolvadex®) and trastuzumab (Herceptin®) have already been successfully applied to patients diagnosed with estrogen receptor (ER)-positive and human epidermal growth factor receptor-2 (HER2)-positive breast cancer, respectively [5]. Furthermore, arsenic trioxide has been approved in the USA and Europe under the brand name of Trisenox for ‘the induction of remission and consolidation in patients with acute promyelocytic leukemia (APL) whose conditions are refractory to, or who have relapsed from retinoid and anthracycline chemotherapy, and whose APL is characterized by the presence of the t(15;17) translocation or promyelocytic leukemia protein (PML)-retinoic acid receptor-α (RARα) fusion gene expression’ [6]. Despite advances in early detection, diagnosis, and targeted treatment options over the past two decades, the treatment modalities of cancer are still insufficient.
The development of new strategies aimed at improving chemotherapy sensitivity and minimizing the adverse side effects is essential for obtaining satisfied therapeutic outcomes for cancer patients and enhancing their quality of life. Emerging evidence has reported that many cancer patients use either herbs employed in complementary therapies or dietary agents that influence cellular signaling worldwide, especially in the areas of Asia [6-9]. Numerous components of edible plants, collectively termed phytochemicals that have beneficial effects for health, are being reported increasingly in the scientific literature [1-3,9]. These food-derived phytochemicals and their derivatives therefore represent a cornucopia of new anticancer compounds. Of those, flavonoids are phenolic phytochemicals with a wide range of structures consisting of two benzene rings designated as A and B, which are linked by three carbons and one oxygen atom to form the central pyrone ring, designated as C (C6-C3-C6 skeleton; Figure 1) [9,10]. According to the diversity in the structure, they are further categorized mainly into anthocyanins, flavones, flavonols, flavanones, flavanonols, flavanols, isoflavones and chalcones (Figure 1) [9-11]. Flavonoids exist either as glycoside derivatives or as free aglycones to form an integral part of the human diet as they are naturally present in fruits, vegetables, grain, tea and wines [9,10,12]. Flavonoids have attracted much attention by virtue of its wide variety of biological functions including antioxidant, anti-inflammatory, antibacterial, anti-angiogenic, anti-allergic and enzyme modulation [9-12]. Findings from the epidemiological studies suggest that higher dietary intake of flavonoids may be inversely associated with risk of mortality [7,13,14]. Many studies have also reported the remarkable and significant properties of flavonoids as anticancer and/or chemopreventive agents, suggesting a positive correlation between a lower risk of cancer and a flavonoid-rich diet [1,9,10,15,16]. Besides, it has been recently shown that flavonoids can affect immune system response and might have immune-modulator effects [8,16-18].
Figure 1.
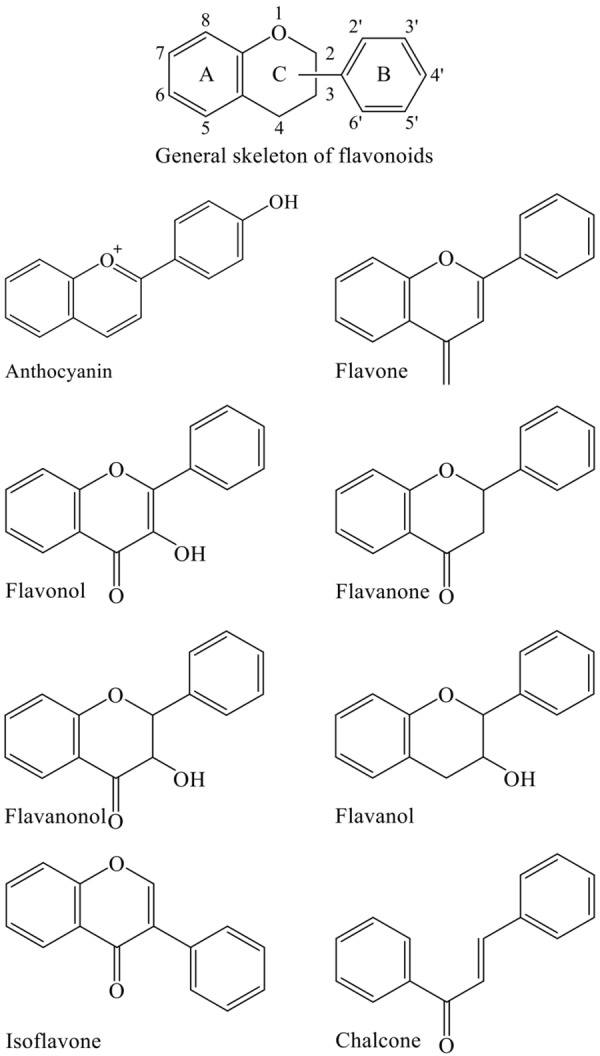
Basic backbone structure of flavonoids and flavonoid subclasses.
We have been being interested in the effects of naturally derived substances including flavonoids on different kinds of cancerous cells, including human colon carcinoma, breast cancer, glioblastoma and leukemic cells [6,19-32]. The promising anticancer activity of phytochemicals thus encourages us to focus on the development of novel anticancer therapy by combining flavonoids nutraceuticals and conventional chemotherapeutic agents. The aim of the present review is to review the progress of recent research and available data on the molecular mechanisms underlying the multiple pharmacological effects of flavonoids, especially focusing on cancer chemoprevention. We further discuss possible strategies to develop anticancer therapy by combining flavonoids nutraceuticals and conventional chemotherapeutic agents. We also highlight numerous pharmacokinetic challenges such as bioavailability, drug-drug interactions, which are still fundamental questions concerning its future clinical application.
Cancer preventive activities of the flavonoids
Contribution of apoptosis, necrosis, cell cycle arrest as well as autophagy to cancer preventive activities of flavonoids
Apoptosis is an essential part of the maintenance of tissue homeostasis, and is a tightly regulated process under the control of several signaling pathways [1,33]. Generally, apoptosis induction occurs through multiple pathways, and thus various stimuli activate different pathways [1,6,33]. So far, two principal signal pathways of apoptosis have been identified. The intrinsic mechanism of apoptosis involves a mitochondrial pathway. Apoptosis stimuli destruct mitochondrial membrane structure under the control of Bcl-2 (B-cell leukemia/lymphoma) family, resulting in the release of mitochondrial proteins including cytochrome c. Once cytochrome c is released it activates caspase-9 through interaction with Apaf-1 and dATP, and ultimately leads to caspase-3 and -7 activation [1,6,33]. On the other hand, the extrinsic pathway induced by death receptors, such as tumor necrosis factor receptor (TNFR) and Fas, which is responsible for the activation of caspase-8 and -10 accompanied by the activation of caspase-3 and -7 [1,6,33]. Caspase-3 and -7 are the final mediators in the two principal signal pathways that cleave substrates and lead to cell death.
Many diseases have been associated with aberrantly regulated apoptotic cell death, ultimately leads to inhibition of apoptosis and propagation of diseases such as cancer. Most of the anticancer therapies trigger apoptosis induction and related cell death networks to eliminate malignant cells [1,33]. On the other hand, cancer cells are often found to overexpress many of the proteins that play important roles in resisting the activation of apoptotic cascade, ultimately escape from apoptosis and lead to tumor development, progression and treatment resistance [33]. Bcl-2, a well-known antiapoptotic factor, functions through hetero-dimerization with proapoptotic members of the BH3 family to prevent mitochondrial pore formation and prevent cytochrome c release and initiation of apoptosis [34]. In addition, Bcl-2 has been suggested to play an oncogenic role through survival pathways other than its functions at the mitochondrial membrane [34]. Previous reports have shown that overexpression of Bcl-2 increases the activity of Akt and inhibitor of nuclear factor kappa B (NF-κB) kinase as well as NF-κB transcriptional activity in cancer [33]. Moreover, knock-down of Bcl-2 by antisense oligodeoxynucleotides induces downregulation of carbonic anhydrase IX (CA9) (an important pH regulator for the cancer cell microenvironment acidification), vascular endothelial growth factor (VEGF), and phosphorylation of Akt as well as radiation sensitization in prostate cancer cell lines in vitro and in vivo [35]. These studies provide evidence in support for a multi-functional role of Bcl-2 in cancer cells resistant to a variety of chemotherapeutic agents. Flavonoids have been reported to tip the cellular balance in favor of apoptosis induction by modulating Bcl-2 and its prosurvival relatives. For instance, casticin, a flavonoid isolated from Vitex species and widely used as an anti-inflammatory agent in Chinese traditional medicine, dose-dependently induced apoptosis along with the activation of intrinsic pathway accompanied by downregulation of Bcl-2, Bcl-xL, survivin, and upregulation of Bax in multiple cancer cells including esophageal cancer, gallbladder cancer, glioblastoma, colon cancer and leukemia [24,36-39]. Similarly, vitexin, a naturally-derived flavonoid compound found in the traditional Chinese herb Crataegus pinnatifida, reduced the Bcl-2/Bax ratio and caused the release of cytochrome c from mitochondria to cytosol, which further led to the cleavage of caspase-3 in human non-small cell lung cancer A549 cells [40]. Quercetin, one of the most abundant flavonoids present in variety of vegetables such as onions and broccoli, has also been demonstrate to inhibit the growth of human metastatic ovarian cancer cell PA-1 cells by downregulating the expression of antiapoptotic molecules such as Bcl-2, Bcl-xL, and upregulating the expression of proapoptotic molecules such as caspase-3, caspase-9, Bid, Bad, Bax and cytochrome c [41]. Recently, Verma et al. reported that polyphenolic compounds including apigenin, fisetin, galangin and luteolin bind to the hydrophobic groove of Bcl-2, and suggested that these polyphenols have the potential to be used as lead molecules for Bcl-2 inhibition [42]. Moreover, kaempferol, a natural dietary flavonoid, suppressed 17β-estradiolinduced Bcl-2 and survivin expression and caused apoptotic cell death in endometrial cancer [43].
Intriguingly, the potency of flavonoids to exhibit cytotoxicity against cancer cells seems to depend on the presence of hydroxyl groups in the B-ring, which helps to counteract the presence/effect of the 3-hydroxyl group [10,16,44]. In agreement with these previous findings, we have recently reported that anthocyanidins, known as one of the flavonoids with a positive charge at the oxygen atom of the C-ring of basic flavonoid structure, show cytocidal effects against HL-60 cells in the order of delphinidin > malvidin > peonidin > cyanidin > pelargonidin, and suggested that the growth inhibitory activity of these anthocyanidins might be positively correlated with the presence of hydroxyl groups on ring B of the anthocyanidin molecule, although the structure-activity relationship is still controversial in different cell types [26].
Cell cycle arrest has also been viewed as another one of the major underlying mechanisms for the cytocidal effects of most chemotherapeutic drugs. The cell cycle is a complex process that is precisely regulated by vital molecules known as cyclin-dependent kinases (CDKs) and CDK inhibitors such as p21 and p27 [25,31,45,46]. In this regard, previous report has demonstrated that flavone, luteolin and apigenin induce forkhead box O3a (FOXO3a) expression by inhibiting phosphoinositide 3-kinase (PI3K) and Akt, subsequently elevate the expression of FOXO3a target genes including p21 and p27, ultimately inhibit breast cancer proliferation through Akt/FOXO3a-mediated cell cycle arrest and apoptosis [45]. Luteolin has also been demonstrated to induce cell cycle arrest in other cancer cell lines such as colon, pancreatic, lung, liver, among others, although different cell cycle arrest induction was observed in different types of cancer cell lines, suggesting its wide range of preventive and therapeutic options against various types of cancer [2]. In addition, casticin has been reported to suppress the phosphorylation of the FOXO3a protein, the expression of forkhead box M1 (FOXM1) as well as its downstream genes such as CDK1, cell division cycle 25B (cdc25B) and cyclin B, and increase the expression of p27, ultimately induce G2/M arrest in hepatocellular carcinoma cell lines [47]. Induction of cell cycle arrest was also observed in hematologic cancer cells treated with flavonoids. For instance, wogonin, a flavonoid derived from Scutellaria baicalensis Georgi, arrested cell cycle at G0/G1 phase along with the upregulation of p21 and downregulation of CDK4 as well as cyclin D1 in K562 and K562r (an imatinib-resistant cell line) [46]. The binding ability between GATA-binding factor 1 (GATA-1), a zinc finger transcription factor known to induce the differentiation of megakaryocytes and erythrocytes, and the promoter of p21 in both cells was also enhanced after treatment with wogonin for 48 h, suggesting that wogonin induced G0/G1 cell cycle by regulating GATA-1 associated cell cycle checkpoints in both cells [46]. Similarly, we also reported that Vitex, an extract from the ripe fruit of Vitex agnus-castus, induced a dose- and time-dependent decrease in cell viability associated with induction of apoptosis and G2/M cell cycle arrest in HL-60 cells [23]. In addition, necrosis is also involved in the antitumor activity of some flavonoids. Necrosis has been characterized as passive, accidental cell death resulting from environmental perturbations with uncontrolled release of inflammatory cellular contents [48]. The loss of structural integrity of the plasma membrane is a hallmark of necrosis and represents the common final endpoint at which a cell can no longer maintain its discrete identity from the environment [48]. Despite that apoptosis seems to be clearly advantageous for the organism, necrosis induction still plays critical role in proliferation inhibition of cancer cells, especially apoptosis-resistant cells [48-50]. Haghiac and colleagues have demonstrated that a major dietary flavonoid quercetin can inhibit oral cancer cell proliferation through induction of necrosis followed by apoptosis, and through S phase cell cycle arrest associated with the inhibition of the expression of thymidylate synthase, a key S-phase enzyme [50]. Furthermore, delphinidin has been reported to induce necrosis in hepatocellular carcinoma cells in the presence of 3-methyladenine, an autophagy inhibitor [49]. These results indicate the intricate interplay between apoptosis, necrosis as well as autophagy, and suggest that the different death routes may overlap, and several characteristics may be displayed at the same time.
Autophagy is a highly conserved catabolic process induced under various conditions of cellular stress, and known to positively regulate cellular processes for survival or death [25,31,48,51]. Induction of autophagy by various anticancer drugs has been suggested to be a potential therapeutic strategy for cancer [25,31,48,51]. Zhang and colleagues demonstrated that aqueous Allspice extract, which contains many different flavonoids, was able to activate autophagy signaling in breast cancer cells and induce cell death associated with the suppression of Akt/mammalian target of rapamycin (mTOR) signaling in vitro and in vivo [52]. Kaempferol has also been demonstrated to induce autophagy through adenosine monophosphate activated protein kinase (AMPK) and Akt signaling molecules, and cause G2/M arrest via downregulation of CDK1/cyclin B in SK-HEP-1 human hepatic cancer cells [53]. Moreover, induction of autophagy was involved in the chemopreventive/chemotherapeutic potential of genistein, a soy isoflavone, in multiple tumor types such as breast cancer, lung cancer and hepatoma [51,54]. The molecular details of the contribution of apoptosis, necrosis, cell cycle arrest as well as autophagy were summarized in Table 1.
Table 1.
The molecular details of the contribution of apoptosis, necrosis, cell cycle arrest as well as autophagy to cancer preventive activities of flavonoids
| Flavonoids | Mechanism of action (Cytotoxicity profiling) | Ref. |
|---|---|---|
| Casticin | Activation of caspase-9, -8, -3; p38 MAPK activation associated with histone H3 phosphorylation (Apoptosis induction, G2/M arrest) | [24] |
| Cytochrome C release, activation of caspase-9, -3 and PARP, activation of JNK; Decrease of mitochondria membrane potential associated with dysregulation of Bax/Bcl-2 pathway (Apoptosis induction, G2/M arrest) | [36] | |
| Activation of caspase-9, -3 and PARP, upregulation of Bax and p27; Inhibition of Bcl-2, cyclin D1/CDK4, Akt (Apoptosis induction; G0/G1 arrest) | [37] | |
| Upregulation of p53, Bax, caspase-3 and p21; Inhibition of polymerization of tubulin, cyclin B1 and CDK1 (Apoptosis induction, G2/M arrest) | [38] | |
| Induction of death receptor 5 and Bax, activation of caspase-3 and PARP; Downregulation of Bcl-xL, Bcl-2, survivin, cFLIP, XIAP, pro-form Bid (Apoptosis induction) | [39] | |
| Activation of FOXO3a along with downregulation of FOXM1, CDK1, cdc25B, cyclin B1 and upregulation of p27 (G2/M arrest) | [47] | |
| Vitexin | Activation of intrinsic apoptotic pathway associated with decrease of Bcl-2/Bax ratio, mitochondria membrane potential, cytochrome c release and activation of caspase-3; Inhibition of PI3K/Akt/mTOR (Apoptosis induction) | [40] |
| Quercetin | Activation of intrinsic apoptotic pathway associated with upregulation of Bax, Bad, Bid, caspase-9, -3, downregulation of Bcl-2; Bcl-xL, and cytochrome c release (Apoptosis induction) | [41] |
| Activation of caspase-3; Inhibition of thymidylate synthase (Necrosis and apoptosis induction, S phase arrest) | [50] | |
| Kaempferol | Upregulation of p53 and cleaved-PARP; Inhibition of ERα, Bcl-2, survivin (Apoptosis induction; G2/M arrest) | [43] |
| Upregulation of phosphorylated AMPK, Atg-5, Atg-7, Atg-12, Beclin 1 and LC3-II; Downregulation of CDK1/cyclin B, phosphorylation of Akt and mTOR (G2/M arrest, autophagy induction) | [53] | |
| Flavone; apigenin; luteolin | Activation of FOXO3a along with upregulation of p27, p21, and downregulation of cyclin B; cyclin D1, induction of p53, cleaved-PARP, cytochrome C; Inhibition of PI3K/Akt pathway (G1, S, and/or G2/M arrest) | [45] |
| Wogonin | Upregulation of GATA-1 and p21; Downregulation of CDK4, cyclin D1, MEK and ERK (G0/G1 arrest) | [46] |
| Delphinidin; Cyanidin-3-rutinoside | Induction of cellular vacuolization and LC3-II (Apoptosis, autophagy and necrosis) | [49] |
Abbreviations: AMPK, adenosine monophosphate activated protein kinase; CDK4, cyclin-dependent kinase 4; cFLIP, cellular FLICE-like inhibitory protein; ERα, estrogen receptor alpha; ERK, extracellular signal regulated kinase; FOXM1, forkhead box M1; FOXO3a, forkhead box O3a; GATA-1, GATA-binding factor 1; JNK, Jun-N-terminal kinase; MAPK, mitogen-activated protein kinase; MEK, mitogen-activated protein kinase kinase; mTOR, mammalian target of rapamycin; PARP, poly ADP-ribose polymerase; PI3K, phosphoinositide 3-kinase; XIAP, X-linked inhibitor of apoptosis protein.
Anti-angiogenic and anti-metastatic properties of flavonoids
Angiogenesis is the process which forms new blood vessels and occurs in many physiological and pathological processes such as reproduce in adults, wound healing, tumor development and some inflammatory diseases [1,55,56]. It is a process that is tightly controlled by a wide range of angiogenic inducers such as VEGF and adhesion molecules as well as various endogenous angiogenesis inhibitors including angiostatin and thrombospondin. It can also be stimulated by many inflammatory factors, which contribute to the pathology of inflammation and cancer, indicating angiogenesis, inflammation and cancer are closely related [1,55,56]. Uncontrolled angiogenesis is considered as a key step in cancer growth, invasion and metastasis, a great deal of attention has thus been paid to develop potent inhibitors of angiogenesis. In fact, a number of anti-angiogenesis drugs have been approved by FDA and are being used in cancer treatment [57]. Despite this, researchers never stop exploring novel candidates of angiogenesis inhibitors due to side effects of these drugs [57]. It has been demonstrated that wogonin inhibits LPS-induced tumor angiogenesis via suppressing PI3K/Akt/NF-κB signaling in breast cancer cell lines in vitro and in vivo [56]. Orientin (luteolin 8-C-β-D-glucopyranoside), a glycosyl dietary flavonoid, also inhibited invasion by suppressing matrix metalloproteinase 9 (MMP-9) and interleukin-8 (IL-8) expression via the intervention of protein kinase C alpha (PKCα)/extracellular signal regulated kinase (ERK)/nuclear translocations of activator protein-1 (AP-1)/signal transducer and activator of transcription 3 (STAT3)-mediated signaling pathways in 12-O-tetradecanoylphorbol-13-acetate (TPA)-treated MCF-7 breast cancer cells [58]. Genistein has been found to inhibit angiogenesis through manipulating the expression of VEGF, MMPs, epidermal growth factor receptor (EGFR) as well as NF-κB, PI3-K/Akt, ERK1/2 signaling pathways, ultimately causes strong anti-angiogenic effects [55]. Kaempferol also inhibits angiogenic ability by targeting VEGF receptor-2 and downregulating the PI3K/Akt, mitogen-activated protein kinase kinase (MEK) and ERK pathways in VEGF-stimulated human umbilical vein endothelial cells (HUVECs) [59]. In addition, quercetin has been demonstrated to possess antimetastatic effects in gastric cancer cells via the interruption of urokinase plasminogen activator (uPA)/uPA receptor (uPAR) function by modulating NF-κB, PKC-δ, ERK1/2, and AMPK [60]. More recently, Yao et al. demonstrated that luteolin significantly inhibited the proliferation, migration and invasion of A375 human melanoma cells, and induced a dose-dependent apoptosis along with the inhibition of the phosphorylation of Akt and PI3K [61]. They further demonstrated that luteolin reduced the expressions of MMP-2 and MMP-9 and increased the expression of tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2, and that luteolin significantly inhibited the tumor growth of A375 cells in a xenograft mouse model, suggesting that luteolin exhibited its antitumor activities by suppressing the expressions of MMP-2 and MMP-9 through the manipulation of PI3K/Akt pathway [61]. The molecular details of the anti-angiogenic and anti-metastatic properties of flavonoids were summarized in Table 2.
Table 2.
The molecular details of anti-angiogenic and anti-metastatic properties of flavonoids
| Flavonoids | Mechanism of action (Cytotoxicity profiling) | Ref. |
|---|---|---|
| Wogonin | Inhibition of PI3K/Akt/NF-κB pathway and TLR4 pathway, inhibition of VEGF secretion from cancer cells, inhibition of tube formation of HUVECs induced by LPS-treated MCF-7 cells and vascularization in mice (Angiogenesis suppression without apoptosis induction) | [56] |
| Orientin | Downregulation of MMP-9 and IL-8 via inhibition of PKCα/ERK/AP-1/STAT3 signaling pathway (Inhibition of migration and invasion) | [58] |
| Kaempferol | Inhibition of VEGF/VEGFR-2 and its downstream signaling cascades PI3K/Akt; MEK; ERK (Inhibition of migration, invasion, and tube formation) | [59] |
| Quercetin | Inhibition of NF-κB, PKC-δ, ERK1/2 along with AMPK activation, and downregulation of uPA/uPAR, MMP-9 and -2 (Inhibition of migration and invasion) | [60] |
| Luteolin | Upregulation of TIMP-1 and TIMP-2; Inhibition of PI3K/Akt pathway along with downregulation of MMP-9 and -2 (Apoptosis induction; inhibition of migration and invasion) | [61] |
Abbreviations: AMPK, adenosine monophosphate activated protein kinase alpha; AP-1, nuclear translocations of activator protein-1; ERK, extracellular signal regulated kinase; HUVECs, human umbilical vein endothelial cells; IL-8, interleukin-8; LPS, lipopolysaccharide; MEK, mitogen-activated protein kinase kinase; MMP-9, matrix metalloproteinase-9; NF-κB, nuclear factor kappa B; PKCα, protein kinase C alpha; PKC-δ, protein kinase C delta; PI3K, phosphoinositide 3-kinase; STAT3, signal transducer and activator of transcription 3; TIMP-1, tissue inhibitor of metalloproteinase 1;TLR4, Toll-like receptor 4; uPA, urokinase plasminogen activator; uPAR, uPA receptor; VEGF, vascular endothelial growth factor; VEGFR-2, VEGF receptor 2.
Immunomodulatory effects of flavonoids
It has been known that flavonoids such as quercetin, fisetin, luteolin and kaempferol have specific immunomodulatory effects that are might be linked to their anti-allergic activities and beneficial effects against autoimmune diseases [8,16-18]. Previous studies on the effects of quercetin on the immune system showed its inhibitory effects on cytotoxic lymphocyte function, and clarified that quercetin can affect the balance of Th1/Th2 in a murine model of asthma [17]. Fisetin also significantly inhibited Th1 and Th2 cytokine production, cell cycle and the ratio of T CD4+/CD8+ cells in vitro through the suppression of NF-κB and nuclear factor of activated T cells (NFAT) signaling pathway [62]. Furthermore, kaempferol has been recently reported to enhance the function of CD4+CD25+Foxp3+ regulatory T (Treg) cells by inhibiting FOXP3 phosphorylation [63]. In fact, Treg cells have received considerable attention due to their immunosuppressive properties in vitro and in vivo [64,65]. These previous findings thus support the immunosuppressive effects of flavonoids. On the other hand, immunosuppression has been widely recognized in cancer patients due to immune tolerance induced by malignant tumor cells and/or undesirable side-effects of many types of chemotherapeutic drugs [66,67]. Accumulating evidence has shown an increased number and function of Treg cells in patients with solid tumors and hematologic malignancies, suggesting its critical role in limiting antitumor immune response and promoting immunological ignorance of cancer cells [64,65,67].
Of note, based on the fact that cancer cells and activated Th cells use glycolysis to supply their energy needs, and that the PI3K/Akt/mTOR pathway plays a crucial role in both cells, a recent review article focusing on the effects of flavonoids on the immune system and their impact on the mTOR pathway demonstrated that flavonoids can suppress mTOR activity and are consequently able to induce the Treg cell subset [17]. Akt/PI3K/mTOR axis is known as one of the most important signaling pathway linked to the proliferation of various cancer cells, and has been considered to be a therapeutic target for cancer [1,16,17,25,31,33,55]. These previous findings thus provide evidence for the pleiotropic effects of flavonoids, and suggest that much attention should be paid to both the immunosuppressive effects of flavonoids and their anticancer efficacy, especially when combined them with the conventional anticancer drugs.
Effects of flavonoids on drug transporters and metabolic enzymes
Since drug action usually requires uptake of the drug, it was considered that intracellular drug concentrations might determine the efficacy of respective drug. Cancer cells usually express a high protein level of ATP binding cassette (ABC) transporters that can attenuate the efficacy of treatment by actively pumping drugs out of the cells, leading to the multidrug resistance phenotype [6,68]. It has been established that multidrug resistance-associated protein 1 (MRP1)/MRP2, P-glycoprotein (P-gp), multidrug resistance 1 (MDR1) and breast cancer resistance protein (BCRP), which belong to the ABC transporter superfamily, play a prominent role in the chemoresistance to various anticancer drugs [6,25,32,68-72]. Fortunately, flavonoids have been demonstrated to serve as modulators of drug transporters and metabolic enzymes, consequently exhibit their cancer chemopreventive activity [68-73]. Recently, novel flavone derivatives have been demonstrated to serve as selective and dual inhibitors of the transporters P-gp and BCRP [72]. Nobiletin, a major flavonoid compound from oranges (Citrus sinensis) peel, has been shown to inhibit MRP1, resulting in the accumulation of intracellular adriamycin (ADR) in A549 human non-small-cell lung cancer (NSCLC) cells, ultimately enhance chemosensitivity to ADR [70]. Flavonoids including genistein, quercetin, wogonin were found to downregulate MRP1 in resistant human tumor cell lines, such as pancreatic adenocarcinoma cells (Panc-1) and chronic myelogenous leukemia (CML) cells (K562/A02), suggesting their MDR reversal potential [68]. Previous reports further demonstrated that flavonoids might inhibit MRP1 by binding to certain regions of the transporter (substrate-binding site, nucleotide-binding domains) or depleting intracellular glutathione [68,69]. Structure-activity relationships seem to be linked with the inhibition activity against MRP1, suggesting that the degrees of hydroxylation and methoxylation, as well as 2,3-double bonds, play important roles in MRP1 inhibition [68]. Regarding P-gp, Mohana et al. recently reported that flavonoids such as quercetin, rutin, epicatechin 3 gallate significantly decreased the expression of Wnt and glycogen synthase kinase 3beta (GSK-3β) in multidrug resistant KBCHR8-5 cells and subsequently downregulated P-gp overexpression in the cells [74]. Similarly, quercetin has been demonstrated to reverse MDR to conventional anticancer drugs including doxorubicin and paclitaxel in breast cancer cells through downregulating P-gp expression and eliminating cancer stem cells mediated by Y-box binding protein 1 nuclear translocation [75]. Intriguingly, opposite effects of flavonoids on the expression of P-gp was also observed in quercetin and other flavonoids [68,76], indicating much more complex P-gp-flavonoid interactions.
It is well known that cytochrome 450 (CYP450) system, one of the most important phase I drug-metabolizing enzymes, is involved in the oxidative biotransformation of numerous xenobiotics and endogenous compounds [68,73,77]. Previous report has clarified that P-gp and CYP3A4, a major P450 in humans, share common regulation pathways and substrates, and act synergistically in the intestine to limit the bioavailability of their substrates [68]. A great deal of attention has been recently paid to herb-drug interactions since its high risk mediated by the herbal medicines and dietary supplements containing abundant flavonoids had become more and more frequent in our daily life [68,73]. Li and colleagues recently demonstrated that among 44 different structures of flavonoids, several compounds exhibited the selective inhibition toward CYP3A4 [73]. They further verified drug-drug interaction between some flavonoids and clinical drug diazepam in human liver microsomes, providing useful models for early predicting the inhibitory effects of different structures of flavonoids toward CYP3A4 [73]. Surprisingly, the oral bioavailability of other P-gp/CYP3A4 substrates (tamoxifen, cyclosporine) was reduced when coadministered with biochanin A, quercetin, known as P-gp/CYP3A4 inhibitors in rats [68,78]. These unexpected decrease in cyclosporine bioavailability might be attributed, at least in part, to CYP3A4 activation by quercetin serum metabolites [78], although more preclinical and clinical investigation of these interactions are needed to verify. These findings thus suggest that systematic clinical monitoring and evaluation of intracellular concentrations of conventional anticancer drugs are needed for cancer patients when coadministered with herbal medicines containing flavonoid components.
Differentiation-inducing activity of flavonoids in cancer cells
The aim of differentiation therapy is to induce the differentiation of malignant cells, consequently cause them to cease proliferation, ultimately control their tumorigenic and malignant potential [79,80]. Differentiation therapy arises from the fact that leukemic cells have lost their ability to differentiate and eventually become malignant [80]. Use of all-trans retinoic acid (ATRA) and/or arsenic trioxide in the treatment of APL has acquired a therapeutic niche, represented as one of most successful model of differentiation therapy [6,81-84]. Differentiation therapy possesses the obvious characteristics of relatively low toxicity compared with conventional chemotherapy [79,80]. Therefore, there is an urgent need to develop novel agents with potent differentiation-inducing activity and less toxicity for differentiation therapy due to poor cellular differentiation of cancer cells and their acquired resistance to differentiation agents.
A previous review [10] highlighting the therapeutic potential of natural and synthetic flavonoids as anticancer agents in leukemia treatment with respect to the structure-activity relationships and their molecular mechanisms demonstrated that flavones apigenin and luteolin induced HL-60 cells differentiation into granulocytes, whereas flavonol quercetin induced their differentiation into monocytes rather than granulocytes. Interestingly, flavonols galangin and kaempferol and the flavanone naringenin did not induce the differentiation of HL-60 cells [10]. These findings concluded that the flavone structure might be crucial for the induction of differentiation of HL-60 cells into granulocytes [10]. More recently, Moradzadeh et al. demonstrated that green tea polyphenol epigallocatechin gallate (EGCG) enhanced differentiation of APL cells including HL-60 and NB4 towards granulocytic pattern in a similar manner to that observed for ATRA [85]. They further demonstrated that EGCG suppressed the expression of clinical marker PML-RARα in NB4 cells and reduced the expression of histone deacetylase 1 (HDAC1) in both HL-60 and NB4 cells [85]. In addition, wogonin has been demonstrated to induce differentiation of K562, imatinib-resistant K562, and primary patient-derived CML cells, along with the upregulation of transcription factor GATA-1 and the enhancement of the binding between GATA-1 and transcriptional coactivator FOG-1 [46]. Flavonoids have also recently been demonstrated to induce differentiation in cancer cells derived from various types of solid tumors such as breast cancer, liver cancer, malignant melanoma glioma [86-89]. Especially, the differentiation-inducing activity of genistein [88] and isoliquiritigenin [87], a member of the flavonoids isolated from licorice (Glycyrrhizae radix), have been reported in breast cancer stem/progenitor cells through the interaction with ER+ cancer cells by a paracrine mechanism, and the downregulation of the Notch1 signaling pathway, respectively. These findings thus provide evidence supporting the potential application of flavonoids in the treatment of patients with different types of cancer. The molecule details of the differentiation-inducing activity were summarized in Table 3.
Table 3.
The molecular details of the differentiation-inducing activity of flavonoids
| Flavonoids | Mechanism of action (Cytotoxicity profiling) | Ref. |
|---|---|---|
| Wogonin | Upregulation of GATA-1, glycophorin A, CD71, enhancement of the binding between GATA and FOG-1 (Erythroid differentiation induction) | [46] |
| EGCG | Upregulation of Bax/Bcl-2 ratio, caspase-8, -3, p-53, PTEN, p21; Inhibition of PML-RARα and HDAC1, downregulation of P-gp and MRP1 (Induction of differentiation and apoptosis) | [85] |
| Kaempferol, genistein, 3’3-diindolylmethane | Activation of ROS-p38-p53 signaling pathway and ER stress, upregulation of p21 and Bax; Downregulation of Bcl-2, Cyclin E, cyclin B (Apoptosis and differentiation induction) | [86] |
| Isoliquiritigenin | Upregulation of GFAP and β III tubulin; Downregulation of Notch1 and Hes1 (Growth inhibition; differentiation induction) | [87] |
| Genistein | Upregulation of E-cadherin, α-SMA, Claudin-1, activation of PI3K/Akt and MEK/ERK pathway; Inhibition of cell population with CD44+/CD24-/ESA+, downregulation of fibronectin, slug, snail (Mammosphere formation inhibition, differentiation induction of stem/progenitor cells) | [88] |
| Oroxylin A | Upregulation of PKM1/PKM2 ratio and ALB, activation of HNF-4α/HNF-1α, enhancement of binding between PKM1 and HNF-4α; Downregulation of cyclin A, cyclin B1, AFP, PTB (Proliferation inhibition associated with G2/M arrest, differentiation induction, tumor growth inhibition of HepG2 xenograft model and PDTX model) | [89] |
Abbreviations: AFP, α-fetoprotein; ALB, serum albumin; α-SMA, α-smooth muscle actin; ER stress, endoplasmic reticulum stress; ERK, extracellular signal regulated kinase; GATA-1, GATA-binding factor 1; GFAP, glial fibrillary acidic protein; HDAC1, histone deacetylase 1; HNF-1α, hepatocyte nuclear factor 1 alpha; HNF-4α, hepatocyte nuclear factor 4 alpha; MEK, mitogen-activated protein kinase kinase; MRP1, multidrug resistance-associated protein 1; PKM1, pyruvate kinase M 1; PKM2, pyruvate kinase M 2; P-gp, P-glycoprotein; PI3K, phosphoinositide 3-kinase; PML-RARα, promyelocytic leukemia protein-retinoic acid receptor-α; PTB, polypyrimidine tract-binding protein; PTEN, phosphatase and tensin homolog deleted on chromosome 10; ROS, reactive oxygen species.
Bioavailability of flavonoids
Low bioavailability of flavonoids has been a concern as it can limit or even hinder their health effects [6,12,90]. Flavonoids are present in food products mostly in the form of glycosides that are generally hydrolyzed, consequently converted to their respective aglycones by intestinal or colon microflora prior to absorption in the gastrointestinal tract, followed by biotransformation to various metabolites which enter bloodstream [1,9,90,91]. Previous pharmacokinetic data indicate that the absorption of anthocyanins into the bloodstream of rodents and humans is minimal, suggesting that they may have little efficacy in tissues other than the gastrointestinal tract and skin, where they can be absorbed locally [6,9,90]. Therefore, a number of formulation strategies including liposomes, nanoparticles, nanoemulsions and mucoadhesive buccal films have been developed in recent years in order to maximize the bioavailability of flavonoids [1,90,92,93]. More recently, Deepika and colleagues developed a novel therapeutic polymeric complex of rutin (a hydrophobic polyphenolic flavonoid phytochemical) and fucoidan (a well-known sulfated polysaccharide of brown seaweed), which aimed to overcoming the limitations of bioavailability of rutin, and showed that the rutin-fucoidan complex induced G0/G1 and S phase cell cycle arrest, and has the ability to induce apoptosis via reactive oxygen species generation and mitochondrial potential loss in cervical cancer cell, but was biocompatible on normal cells [94]. Of note, Chen et al. [95] have recently expressed concerns about the potential toxic effect of some polyphenol including apigenin against non-transformed cells when used at high concentrations, markedly higher than that assumed with diet, suggesting higher concentrations of flavonoids could be toxic and therefore more toxicological studies should be warranted.
Anticancer activity of combination treatment of flavonoids and conventional chemotherapeutic drugs
Combination treatments, which aims to improve overall clinical efficacy, are widely accepted as safe and effective approach in cancer therapy [6,96-99]. Due to multidrug resistance and tumor recurrence, the development of new strategies aimed at improving chemotherapy sensitivity and minimizing the adverse side effects is still urgently needed. In this regard, flavonoids have been considered to be one of most promising candidates by virtue of its diverse biological properties such as anticancer activity [1,2,68]. We have previously provided evidence for the potential combination of arsenite and natural product including delphinidin, one of anthocyanin compounds, against human APL cells NB4 and HL-60, in which delphinidin sensitized leukemia cells to arsenite by strengthening intrinsic/extrinsic pathway-mediated apoptosis induction, modulating the amount of intracellular glutathione and NF-κB binding activity [6,26,30]. We further demonstrated that the combination treatment strongly preferred to selectively enhance the cytotoxicity of arsenite against cancer cells rather than human peripheral blood mononuclear cells [26,30]. In agreement with our findings, a comprehensive review paper recently summarized the detailed chemomodulating effects of flavonoids in human leukemia cells, and further demonstrated that the secondary metabolites of flavonoids can also sensitize malignant cells to conventional chemotherapeutic drugs and could be considered as potential adjunctive agents in cancer treatment [3]. Despite a wide clinical application of ATRA and its successful clinical efficacy in the treatment of APL patient, continuous effects have been made to explore novel promising candidate aiming to improve the effectiveness of ATRA and overcome clinical problems such as resistance. In this regard, He and colleagues recently demonstrated that dihydromyricetin (DMY), one of flavonoid bioactive compound extracted from Ampelopsis grossedentata, exhibited a strong synergy with ATRA to promote NB4 cells differentiation [100]. They further clarified that DMY sensitized the NB4 cells to ATRA-induced cell growth inhibition, CD11b expression, nitrobluetetrazolium (NBT) reduction and myeloid regulator expression, all of which seemed to be dependent on the activation of p38-STAT1 signaling pathway, providing new opportunities for the combination of DMY and ATRA as a promising approach for future differentiation therapy [100].
The beneficial effects of combination treatment are also observed in various types of solid tumor cancer cells. Quercetin has been demonstrated to sensitize human glioblastoma U87 and U251 cells to temozolomide, an oral alkylating chemotherapeutic agent, in vitro via inhibition of heat-shock protein 27 [101]. In fact, flavonoids including quercetin have been clarified to be able to enter the brain to influence brain function by modulating the activity of gamma-aminobutyric acid A (GABAA)-receptor and monoamine oxidase A/B [102,103]. Similarly, the anticancer potential of combination of isoflavone biochanin A and temozolomide against glioblastoma U87 and T98G cells was reported to be linked to enhanced expression of p-p53, and inhibition of cell viability, expression of cell survival proteins EGFR, p-ERK, p-Akt, c-myc and membrane-type-MMP1 [104]. The combination treatment also induced G1 arrest, and a shift in the metabolic phenotype from glycolytic to oxidative phosphorylation in cancer cells [104]. Casticin has been demonstrated to potentiate TNF-related apoptosis-inducing ligand-induced apoptosis in colon cancer cells through downregulation of survival proteins such as Bcl-2, Bcl-xL, survivin, X-linked inhibitor of apoptosis protein (XIAP) and cellular FLICE-like inhibitory protein (cFLIP) and upregulation of death receptor 5 [39]. Palko-Łabuz et al. recently demonstrated that combined use of statins, inhibitors of the key enzyme of mevalonate pathway 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase, and flavonoids such as baicalein strengthened cell growth inhibition and apoptosis induction as compared to the application of statins alone to a human colorectal adenocarcinoma cell line LoVo [71]. They further demonstrated that in doxorubicin-resistant cell line LoVo/Dx, a stronger decrease of resistance to doxorubicin was observed in the presence of statins in combination with flavones in comparison with the effect observed in the presence of statins alone [71]. EGCG was recently suggested to serve as a novel chemo-sensitizer to enhance the sensitivity of cancer cells to 5-fluorouracil (5-FU) by inhibiting glucose-regulated protein 78 (GRP78)/NF-κB/miR-155-5p/MDR1 pathway, and the IC50 values of 5-FU in the presence of EGCG were approximately 8-fold and 10-fold lower in comparison to the IC50 values for 5-FU alone in human colon carcinoma cell line-HCT-116 and DLD1, respectively [105].
A previous clinical study has demonstrated that breast cancer patients who are given radiotherapy plus oral administration of EGCG show significantly lower serum levels of VEGF, hepatocyte growth factor (HGF), and reduced activation of MMP9/MMP2, raising the possibility that this tea polyphenol has potential to be a therapeutic adjuvant against human metastatic breast cancer [106]. Furthermore, luteolin has been demonstrated to sensitize human breast cancer MDA-MB-231 cells to doxorubicin [107] and paclitaxel [108] by suppressing nuclear factor erythroid 2-related factor 2 (Nrf2) mediated signaling and blocking STAT3, respectively. In addition, it has been recently demonstrated that a flavonoid compound glabridin can decrease the half maximal inhibitory concentration of paclitaxel and doxorubicin in breast cancer cells like MDA-MB-231/MDR1 cells (P-gp overexpressed) and MCF-7/ADR cells (P-gp overexpressed and MRP2 expressed) [109]. The sensitizing effect of glabridin could be explained that it increased the accumulation of doxorubicin in MDA-MB-231/MDR1 cells by suppressing the expression of P-gp and competitively inhibiting the P-gp efflux pump, and enhanced doxorubicin-mediated apoptosis of the cells [109]. Interestingly, Kundur et al. recently demonstrated that the combination treatment of quercetin and curcumin acted synergistically to induce anticancer activity against triple-negative breast cancer cell (TNBC) lines including MDA-MB-231 by enhancing the expression of breast cancer type 1 susceptibility protein, which is mutated in a majority of TNBC and cause the loss of function of this tumor suppressor gene [110].
Moon and colleagues recently reported that nobiletin treatment downregulated the expression of a neuroblastoma-derived MYC (MYCN), MRP1, Akt, GSK-3β, β-catenin, and enhanced the accumulation of intracellular adriamycin (ADR), ultimately enhanced chemosensitivity of ADR-resistant a human NSCLC cell line A549/ADR to ADR [70]. Moreover, it has been recently demonstrated that apigenin combined with gefitinib, one of EGFR tyrosine kinase inhibitors, inhibits multiple oncogenic drivers such as c-Myc, hypoxia-inducible factor 1 alpha (HIF-1α) as well as EGFR, and damages the glucose uptake and utilization by suppressing glucose transporter 1 protein expression on EGFR mutant-resistant NSCLC cells, suggesting apigenin plus gefitinib is a very clinically promising combination use [111]. Naringin, a polyphenolic flavonoid derive from grapefruit and other citrus fruits, can act as a chemosensitizer to synergistically strength the cytotoxic effect of paclitaxel in human prostate cancer cells regardless of androgen dependence by inducing the activation of intrinsic apoptosis pathway, G1 phase arrest as well as the expression of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) (a major negative regulator of the PI3K/Akt signaling pathway), and suppressing cell migration along with the downregulation of NF-κB, Snail, Twist and c-Myc mRNA expression [112]. These results suggest that naringin in combination with paclitaxel may be useful in the treatment of prostate cancer although more detailed evaluations of the mechanism underlying the combination action in vivo are obviously needed. The molecule details of the enhanced anticancer activity of their combination treatment were summarized in Table 4.
Table 4.
The molecular details of the enhanced anticancer activity of combination treatment of flavonoids and conventional chemotherapeutic drugs
| Flavonoids | Combined drugs | Mechanism of action (Combinatorial effect) | Ref. |
|---|---|---|---|
| Delphinidin | Arsenite | Activation of Caspase-8, -9, -3 and Bid; Decrease of GSH and inhibition of NFκB (Enhancement of cytotoxicity of arsenite along with apoptosis induction) | [26] |
| Arsenite | Activation of caspase-8, -9, -3 and Bid; Downregulation of mitochondrial membrane potential (Enhancement of cytotoxicity) | [30] | |
| Casticin | TRAIL | Activation of caspase-3/PARP, induction of DR5 mediated by ROS; Downregulation of Bcl-2, Bcl-xL, survivin, XIAP, cFLIP (Enhancement of apoptosis induction) | [39] |
| Baicalein, 6-hydroxyflavone, 7-hydroxyflavone | Dox/Sim or Mev | Activation of caspase-3 along with DNA fragmentation; Downregulation of proliferating cell nuclear antigen (Synergistic effect on growth inhibition along with apoptosis induction) | [71] |
| Dihydromyricetin | ATRA | Upregulation of CD11b, PU.1, C/EBPβ, activation of p-38-STAT1 pathway (Synergistic effect on cell differentiation) | [100] |
| Quercetin | TMZ | Upregulation of Hsp27 phosphorylation, and caspase-3 activity (Enhancement of apoptosis induction) | [101] |
| Biochanin A | TMZ | Activation of p53; Downregulation of EGFR, phosphorylation of Akt and ERK, c-myc, MT-MMP1, MMP-2, and inhibition of complex IV activity (Enhancement of cell viability inhibition associated with G1 phase arrest) | [104] |
| EGCG | 5-FU | Activation of caspase-3/PARP, and upregulation of Bad; Inhibition of GRP78/ NF-κB/miR-155-5p/MDR1 pathway, downregulation of Bcl-2 (Enhancement of cytotoxicity of 5-FU along with apoptosis induction) | [105] |
| RT | Upregulation of Bax; Downregulation of serum levels of VEGF, HGF, MMP-9/MMP-2 in vivo, downregulation of Bcl-2, inhibition of c-Met, NF-κB, Akt in vitro (Enhancement of cell growth/invasion inhibition and apoptosis induction) | [106] | |
| Luteolin | Dox | Inhibition of Nrf2 mediated signaling (Enhancement of sensitivity to Dox) | [107] |
| PTX | Activation of caspase-8, -3, PARP along with upregulation of Fas; Downregulation of Bcl-xL and inhibition of STAT3 (Enhancement of apoptosis induction and tumor growth inhibition) | [108] | |
| Glabridin | Dox | Inhibition of P-gp expression and function (Increased accumulation of doxorubicin, enhancement of apoptosis induction and S phase arrest) | [109] |
| Quercetin | Curcumin | Induction of BRCA1 expression, and E-cadherin; Downregulation of MMP-9 (Synergistic effect on cell viability and invasion inhibition) | [110] |
| Naringin | PTX/Dox | Upregulation of PTEN; Inhibition of NF-κB signaling, and downregulation of survivin, c-myc (Synergistic effect on cytotoxicity of drugs associated with apoptosis induction, G1 phase arrest and migration inhibition) | [112] |
Abbreviations: 5-FU, 5-fluorouracil; BRCA1, breast cancer type 1 susceptibility protein; C/EBPβ, CCAAT/enhancer-binding protein beta; cFLIP, cellular FLICE-like inhibitory protein; Dox, doxorubicin; DR5, death receptor 5; EGFR, epidermal growth factor receptor; ERK, extracellular signal regulated kinase; GSH, glutathione; HGF, hepatocyte growth factor; Hsp27, heat shock protein 27; MDR1, multidrug resistance 1; Mev, mevastatin; MMP-2, matrix metalloproteinase-2; MMP-9, matrix metalloproteinase-9; MT-MMP1, membrane-type matrix metalloproteinase 1; NF-κB, nuclear factor kappa B; Nrf2, nuclear factor erythroid 2-related factor 2; PARP, poly ADP-ribose polymerase; P-gp, P-glycoprotein; PTEN, phosphatase and tensin homolog deleted on chromosome 10; PTX, paclitaxel, ROS, reactive oxygen species; RT, radiotherapy; Sim, simvastatin; STAT1, signal transducer and activator of transcription 1; STAT3, signal transducer and activator of transcription 3; TMZ, temozolomide; VEGF, vascular endothelial growth factor; XIAP, X-linked inhibitor of apoptosis protein.
Conclusions
Given the importance of flavonoids to cancer prevention/therapy as potential adjunctive agents and/or chemosensitizer, a long-term effort to explore the effects and mechanisms of action of flavonoids whatever they are used alone or in combination is likely to be continued. Especially, more clinical trials are obviously needed to validate the usefulness of flavonoid in patients with different characteristics such as age, gender, the type and stage of a disease, previous treatment history, and other medical conditions. Considering the critical role of flavonoids in drug-drug interactions and novel approach for improving its bioavailability, enhanced clinical efficacy of a combinatorial treatment regimens of conventional anticancer and flavonoids might reasonably be expected. Paradoxically, unwanted side effects might occur due to enhanced intracellular drug accumulation, suggesting that detailed monitoring of clinical efficacy along with the concentrations of drugs and their metabolites should be diligently performed. Overall, further basic and clinical researches should be warranted in order to provide satisfied chemotherapeutic regimens for cancer patients in terms of bioavailability, efficacy, safety and tolerance.
Acknowledgements
This work was partially supported by The Japan Society for the Promotion of Science (JSPS) KAKENHI Grant to Bo Yuan (Grant Numbers 26460233) (Grant Numbers 17K08465).
Disclosure of conflict of interest
None.
Abbreviations
- 5-FU
5-fluorouracil
- ABC transporters
ATP binding cassette transporters
- ADR
adriamycin
- AMPK
adenosine monophosphate activated protein kinase alpha
- AP-1
nuclear translocations of activator protein-1
- APL
acute promyelocytic leukemia
- ATRA
all-trans retinoic acid
- BCRP
breast cancer resistance protein
- CDKs
cyclin-dependent kinases
- CML
chronic myelogenous leukemia
- CYP450
cytochrome P450
- EGCG
epigallocatechin gallate
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal-regulated kinase
- FOXO3a
forkhead box O3a
- GATA-1
GATA-binding factor 1
- GSK-3β
glycogen synthase kinase 3beta
- HIF-1α
hypoxia-inducible factor 1 alpha
- HNF-4α
hepatocyte nuclear factor 4 alpha
- MDR1
multidrug resistance 1
- MMP-9
matrix metalloproteinase-9
- MRPs
multidrug resistance-associated proteins
- mTOR
mammalian target of rapamycin
- NF-κB
nuclear factor kappa B
- NSCLC
non-small-cell lung cancer
- PKC
protein kinase C
- P-gp
P-glycoprotein
- PI3K
phosphoinositide 3-kinase
- PML
promyelocytic leukemia
- RARα
retinoic acid receptor alpha
- STAT
signal transducer and activator of transcription
- TNBC cells lines
triple-negative breast cancer cell lines
- Treg cells
CD4+CD25+Foxp3+ regulatory T cells
- VEGF
vascular endothelial growth factor
References
- 1.Abotaleb M, Samuel SM, Varghese E, Varghese S, Kubatka P, Liskova A, Busselberg D. Flavonoids in cancer and apoptosis. Cancers (Basel) 2018;11 doi: 10.3390/cancers11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imran M, Rauf A, Abu-Izneid T, Nadeem M, Shariati MA, Khan IA, Imran A, Orhan IE, Rizwan M, Atif M, Gondal TA, Mubarak MS. Luteolin, a flavonoid, as an anticancer agent: a review. Biomed Pharmacother. 2019;112:108612. doi: 10.1016/j.biopha.2019.108612. [DOI] [PubMed] [Google Scholar]
- 3.Sak K, Everaus H. Chemomodulating effects of flavonoids in human leukemia cells. Anticancer Agents Med Chem. 2015;15:1112–1126. doi: 10.2174/1871520615666150519112513. [DOI] [PubMed] [Google Scholar]
- 4.Seyed MA, Jantan I, Bukhari SN, Vijayaraghavan K. A comprehensive review on the chemotherapeutic potential of piceatannol for cancer treatment, with mechanistic insights. J Agric Food Chem. 2016;64:725–737. doi: 10.1021/acs.jafc.5b05993. [DOI] [PubMed] [Google Scholar]
- 5.Sutherland S, Miles D, Makris A. Use of maintenance endocrine therapy after chemotherapy in metastatic breast cancer. Eur J Cancer. 2016;69:216–222. doi: 10.1016/j.ejca.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Yuan B, Yoshino Y, Kaise T, Toyoda H. Application of Arsenic Trioxide Therapy for Patients with Leukaemia. In: Sun H, editor. Biological Chemistry of Arsenic, Antimony and Bismuth. Chichester: John Wiley Sons, Ltd.; 2010. pp. 263–292. [Google Scholar]
- 7.Grosso G, Micek A, Godos J, Pajak A, Sciacca S, Galvano F, Giovannucci EL. Dietary flavonoid and lignan intake and mortality in prospective cohort studies: systematic review and dose-response meta-analysis. Am J Epidemiol. 2017;185:1304–1316. doi: 10.1093/aje/kww207. [DOI] [PubMed] [Google Scholar]
- 8.Mohamed SIA, Jantan I, Haque MA. Naturally occurring immunomodulators with antitumor activity: an insight on their mechanisms of action. Int Immunopharmacol. 2017;50:291–304. doi: 10.1016/j.intimp.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Sak K. Intake of individual flavonoids and risk of carcinogenesis: overview of epidemiological evidence. Nutr Cancer. 2017;69:1119–1150. doi: 10.1080/01635581.2017.1367934. [DOI] [PubMed] [Google Scholar]
- 10.Menezes JC, Orlikova B, Morceau F, Diederich M. Natural and synthetic flavonoids: structure-activity relationship and chemotherapeutic potential for the treatment of leukemia. Crit Rev Food Sci Nutr. 2016;56(Suppl 1):S4–S28. doi: 10.1080/10408398.2015.1074532. [DOI] [PubMed] [Google Scholar]
- 11.Saraei R, Marofi F, Naimi A, Talebi M, Ghaebi M, Javan N, Salimi O, Hassanzadeh A. Leukemia therapy by flavonoids: future and involved mechanisms. J Cell Physiol. 2019;234:8203–8220. doi: 10.1002/jcp.27628. [DOI] [PubMed] [Google Scholar]
- 12.Amawi H, Ashby CR Jr, Tiwari AK. Cancer chemoprevention through dietary flavonoids: what’s limiting? Chin J Cancer. 2017;36:50. doi: 10.1186/s40880-017-0217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godos J, Castellano S, Ray S, Grosso G, Galvano F. Dietary polyphenol intake and depression: results from the mediterranean healthy eating, lifestyle and aging (MEAL) study. Molecules. 2018;23 doi: 10.3390/molecules23050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang SC, Cassidy A, Willett WC, Rimm EB, O’Reilly EJ, Okereke OI. Dietary flavonoid intake and risk of incident depression in midlife and older women. Am J Clin Nutr. 2016;104:704–714. doi: 10.3945/ajcn.115.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paller CJ, Rudek MA, Zhou XC, Wagner WD, Hudson TS, Anders N, Hammers HJ, Dowling D, King S, Antonarakis ES, Drake CG, Eisenberger MA, Denmeade SR, Rosner GL, Carducci MA. A phase I study of muscadine grape skin extract in men with biochemically recurrent prostate cancer: safety, tolerability, and dose determination. Prostate. 2015;75:1518–1525. doi: 10.1002/pros.23024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imran M, Rauf A, Shah ZA, Saeed F, Imran A, Arshad MU, Ahmad B, Bawazeer S, Atif M, Peters DG, Mubarak MS. Chemo-preventive and therapeutic effect of the dietary flavonoid kaempferol: a comprehensive review. Phytother Res. 2019;33:263–275. doi: 10.1002/ptr.6227. [DOI] [PubMed] [Google Scholar]
- 17.Hosseinzade A, Sadeghi O, Naghdipour Biregani A, Soukhtehzari S, Brandt GS, Esmaillzadeh A. Immunomodulatory effects of flavonoids: possible induction of T CD4+ regulatory cells through suppression of mTOR pathway signaling activity. Front Immunol. 2019;10:51. doi: 10.3389/fimmu.2019.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rengasamy KRR, Khan H, Gowrishankar S, Lagoa RJL, Mahomoodally FM, Khan Z, Suroowan S, Tewari D, Zengin G, Hassan STS, Pandian SK. The role of flavonoids in autoimmune diseases: therapeutic updates. Pharmacol Ther. 2019;194:107–131. doi: 10.1016/j.pharmthera.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Han L, Yuan B, Shimada R, Hayashi H, Si N, Zhao HY, Bian B, Takagi N. Cytocidal effects of arenobufagin and hellebrigenin, two active bufadienolide compounds, against human glioblastoma cell line U-87. Int J Oncol. 2018;53:2488–2502. doi: 10.3892/ijo.2018.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imai M, Kikuchi H, Yuan B, Aihara Y, Mizokuchi A, Ohyama K, Hirobe C, Toyoda H. Enhanced growth inhibitory effect of 5-fluorouracil in combination with Vitex agnus-castus fruits extract against a human colon adenocarcinoma cell line, COLO 201. Jorunal of Chinese Clinical Medicine. 2011;6:14–19. [Google Scholar]
- 21.Imai M, Yuan B, Kikuchi H, Saito M, Ohyama K, Hirobe C, Oshima T, Hosoya T, Morita H, Toyoda H. Growth inhibition of a human colon carcinoma cell, COLO 201, by a natural product, Vitex agnus-castus fruits extract, in vivo and in vitro. Adv Biol Chem. 2012;2:20–28. [Google Scholar]
- 22.Kikuchi H, Yuan B, Nishimura Y, Imai M, Furutani R, Kamoi S, Seno M, Fukushima S, Hazama S, Hirobe C, Ohyama K, Hu XM, Takagi N, Hirano T, Toyoda H. Cytotoxicity of Vitex agnus-castus fruit extract and its major component, casticin, correlates with differentiation status in leukemia cell lines. Int J Oncol. 2013;43:1976–1984. doi: 10.3892/ijo.2013.2133. [DOI] [PubMed] [Google Scholar]
- 23.Kikuchi H, Yuan B, Yuhara E, Imai M, Furutani R, Fukushima S, Hazama S, Hirobe C, Ohyama K, Takagi N, Toyoda H. Involvement of histone H3 phosphorylation via the activation of p38 MAPK pathway and intracellular redox status in cytotoxicity of HL-60 cells induced by Vitex agnus-castus fruit extract. Int J Oncol. 2014;45:843–852. doi: 10.3892/ijo.2014.2454. [DOI] [PubMed] [Google Scholar]
- 24.Kikuchi H, Yuan B, Yuhara E, Takagi N, Toyoda H. Involvement of histone H3 phosphorylation through p38 MAPK pathway activation in casticin-induced cytocidal effects against the human promyelocytic cell line HL-60. Int J Oncol. 2013;43:2046–2056. doi: 10.3892/ijo.2013.2106. [DOI] [PubMed] [Google Scholar]
- 25.Yao M, Yuan B, Wang X, Sato A, Sakuma K, Kaneko K, Komuro H, Okazaki A, Hayashi H, Toyoda H, Pei X, Hu X, Hirano T, Takagi N. Synergistic cytotoxic effects of arsenite and tetrandrine in human breast cancer cell line MCF-7. Int J Oncol. 2017;51:587–598. doi: 10.3892/ijo.2017.4052. [DOI] [PubMed] [Google Scholar]
- 26.Yoshino Y, Yuan B, Okusumi S, Aoyama R, Murota R, Kikuchi H, Takagi N, Toyoda H. Enhanced cytotoxic effects of arsenite in combination with anthocyanidin compound, delphinidin, against a human leukemia cell line, HL-60. Chem Biol Interact. 2018;294:9–17. doi: 10.1016/j.cbi.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Yuan B, He J, Kisoh K, Hayashi H, Tanaka S, Si N, Zhao HY, Hirano T, Bian B, Takagi N. Effects of active bufadienolide compounds on human cancer cells and CD4+CD25+Foxp3+ regulatory T cells in mitogen-activated human peripheral blood mononuclear cells. Oncol Rep. 2016;36:1377–1384. doi: 10.3892/or.2016.4946. [DOI] [PubMed] [Google Scholar]
- 28.Yuan B, Imai M, Kikuchi H, Fukushima S, Hazama S, Akaike T, Yoshino Y, Ohyama K, Hu X, Pei X, Toyoda H. Cytocidal Effects of Polyphenolic Compounds, Alone or in Combination with, Anticancer Drugs Against Cancer Cells: Potential Future Application of the Combinatory Therapy. In: TM N, editor. Apoptosis and Medicine. InTech; 2012. pp. 155–174. [Google Scholar]
- 29.Yuan B, Iriyama N, Hu XM, Hirano T, Toyoda H, Takagi N. Perspective on Therapeutic Strategies of Leukemia Treatment-Focus on Arsenic Compounds, Leukemias - Updates and New Insights. In: Guenova M, editor. Leukemias - Updates and New Insights. InTech; 2015. pp. 191–218. [Google Scholar]
- 30.Yuan B, Okusumi S, Yoshino Y, Moriyama C, Tanaka S, Hirano T, Takagi N, Toyoda H. Delphinidin induces cytotoxicity and potentiates cytocidal effect in combination with arsenite in an acute promyelocytic leukemia NB4 cell line. Oncol Rep. 2015;34:431–438. doi: 10.3892/or.2015.3963. [DOI] [PubMed] [Google Scholar]
- 31.Yuan B, Yao M, Wang X, Sato A, Okazaki A, Komuro H, Hayashi H, Toyoda H, Pei X, Hu X, Hirano T, Takagi N. Antitumor activity of arsenite in combination with tetrandrine against human breast cancer cell line MDA-MB-231 in vitro and in vivo. Cancer Cell Int. 2018;18:113. doi: 10.1186/s12935-018-0613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan B, Yoshino Y, Fukushima H, Markova S, Takagi N, Toyoda H, Kroetz DL. Multidrug resistance-associated protein 4 is a determinant of arsenite resistance. Oncol Rep. 2016;35:147–54. doi: 10.3892/or.2015.4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohammad RM, Muqbil I, Lowe L, Yedjou C, Hsu HY, Lin LT, Siegelin MD, Fimognari C, Kumar NB, Dou QP, Yang H, Samadi AK, Russo GL, Spagnuolo C, Ray SK, Chakrabarti M, Morre JD, Coley HM, Honoki K, Fujii H, Georgakilas AG, Amedei A, Niccolai E, Amin A, Ashraf SS, Helferich WG, Yang X, Boosani CS, Guha G, Bhakta D, Ciriolo MR, Aquilano K, Chen S, Mohammed SI, Keith WN, Bilsland A, Halicka D, Nowsheen S, Azmi AS. Broad targeting of resistance to apoptosis in cancer. Semin Cancer Biol. 2015;35(Suppl):S78–S103. doi: 10.1016/j.semcancer.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 35.Anai S, Shiverick K, Medrano T, Nakamura K, Goodison S, Brown BD, Rosser CJ. Downregulation of BCL-2 induces downregulation of carbonic anhydrase IX, vascular endothelial growth factor, and pAkt and induces radiation sensitization. Urology. 2007;70:832–837. doi: 10.1016/j.urology.2007.06.1118. [DOI] [PubMed] [Google Scholar]
- 36.Qiao Z, Cheng Y, Liu S, Ma Z, Li S, Zhang W. Casticin inhibits esophageal cancer cell proliferation and promotes apoptosis by regulating mitochondrial apoptotic and JNK signaling pathways. Naunyn Schmiedebergs Arch Pharmacol. 2019;392:177–187. doi: 10.1007/s00210-018-1574-5. [DOI] [PubMed] [Google Scholar]
- 37.Song XL, Zhang YJ, Wang XF, Zhang WJ, Wang Z, Zhang F, Zhang YJ, Lu JH, Mei JW, Hu YP, Chen L, Li HF, Ye YY, Liu YB, Gu J. Casticin induces apoptosis and G0/G1 cell cycle arrest in gallbladder cancer cells. Cancer Cell Int. 2017;17:9. doi: 10.1186/s12935-016-0377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu E, Kuang Y, He W, Xing X, Gu J. Casticin induces human glioma cell death through apoptosis and mitotic arrest. Cell Physiol Biochem. 2013;31:805–814. doi: 10.1159/000350098. [DOI] [PubMed] [Google Scholar]
- 39.Tang SY, Zhong MZ, Yuan GJ, Hou SP, Yin LL, Jiang H, Yu ZY. Casticin, a flavonoid, potentiates TRAIL-induced apoptosis through modulation of anti-apoptotic proteins and death receptor 5 in colon cancer cells. Oncol Rep. 2013;29:474–80. doi: 10.3892/or.2012.2127. [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Jiang Q, Liu H, Luo S. Vitexin induces apoptosis through mitochondrial pathway and PI3K/Akt/mTOR signaling in human non-small cell lung cancer A549 cells. Biol Res. 2019;52:7. doi: 10.1186/s40659-019-0214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teekaraman D, Elayapillai SP, Viswanathan MP, Jagadeesan A. Quercetin inhibits human metastatic ovarian cancer cell growth and modulates components of the intrinsic apoptotic pathway in PA-1cell line. Chem Biol Interact. 2019;300:91–100. doi: 10.1016/j.cbi.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 42.Verma S, Singh A, Kumari A, Tyagi C, Goyal S, Jamal S, Grover A. Natural polyphenolic inhibitors against the antiapoptotic BCL-2. J Recept Signal Transduct Res. 2017;37:391–400. doi: 10.1080/10799893.2017.1298129. [DOI] [PubMed] [Google Scholar]
- 43.Chuwa AH, Sone K, Oda K, Tanikawa M, Kukita A, Kojima M, Oki S, Fukuda T, Takeuchi M, Miyasaka A, Kashiyama T, Ikeda Y, Nagasaka K, Mori-Uchino M, Matsumoto Y, Wada-Hiraike O, Kuramoto H, Kawana K, Osuga Y, Fujii T. Kaempferol, a natural dietary flavonoid, suppresses 17beta-estradiol-induced survivin expression and causes apoptotic cell death in endometrial cancer. Oncol Lett. 2018;16:6195–6201. doi: 10.3892/ol.2018.9340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Q, Kroon PA, Shao H, Needs PW, Yang X. Differential effects of quercetin and two of its derivatives, isorhamnetin and isorhamnetin-3-glucuronide, in inhibiting the proliferation of human breast-cancer MCF-7 cells. J Agric Food Chem. 2018;66:7181–7189. doi: 10.1021/acs.jafc.8b02420. [DOI] [PubMed] [Google Scholar]
- 45.Lin CH, Chang CY, Lee KR, Lin HJ, Chen TH, Wan L. Flavones inhibit breast cancer proliferation through the Akt/FOXO3a signaling pathway. BMC Cancer. 2015;15:958. doi: 10.1186/s12885-015-1965-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang H, Hui H, Wang Q, Li H, Zhao K, Zhou Y, Zhu Y, Wang X, You Q, Guo Q, Lu N. Wogonin induces cell cycle arrest and erythroid differentiation in imatinib-resistant K562 cells and primary CML cells. Oncotarget. 2014;5:8188–8201. doi: 10.18632/oncotarget.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.He L, Yang X, Cao X, Liu F, Quan M, Cao J. Casticin induces growth suppression and cell cycle arrest through activation of FOXO3a in hepatocellular carcinoma. Oncol Rep. 2013;29:103–8. doi: 10.3892/or.2012.2076. [DOI] [PubMed] [Google Scholar]
- 48.Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta. 2013;1833:3448–3459. doi: 10.1016/j.bbamcr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 49.Feng R, Wang SY, Shi YH, Fan J, Yin XM. Delphinidin induces necrosis in hepatocellular carcinoma cells in the presence of 3-methyladenine, an autophagy inhibitor. J Agric Food Chem. 2010;58:3957–64. doi: 10.1021/jf9025458. [DOI] [PubMed] [Google Scholar]
- 50.Haghiac M, Walle T. Quercetin induces necrosis and apoptosis in SCC-9 oral cancer cells. Nutr Cancer. 2005;53:220–231. doi: 10.1207/s15327914nc5302_11. [DOI] [PubMed] [Google Scholar]
- 51.Hasima N, Ozpolat B. Regulation of autophagy by polyphenolic compounds as a potential therapeutic strategy for cancer. Cell Death Dis. 2014;5:e1509. doi: 10.1038/cddis.2014.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang L, Shamaladevi N, Jayaprakasha GK, Patil BS, Lokeshwar BL. Polyphenol-rich extract of Pimenta dioica berries (Allspice) kills breast cancer cells by autophagy and delays growth of triple negative breast cancer in athymic mice. Oncotarget. 2015;6:16379–16395. doi: 10.18632/oncotarget.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang WW, Tsai SC, Peng SF, Lin MW, Chiang JH, Chiu YJ, Fushiya S, Tseng MT, Yang JS. Kaempferol induces autophagy through AMPK and AKT signaling molecules and causes G2/M arrest via downregulation of CDK1/cyclin B in SK-HEP-1 human hepatic cancer cells. Int J Oncol. 2013;42:2069–2077. doi: 10.3892/ijo.2013.1909. [DOI] [PubMed] [Google Scholar]
- 54.Prietsch RF, Monte LG, da Silva FA, Beira FT, Del Pino FA, Campos VF, Collares T, Pinto LS, Spanevello RM, Gamaro GD, Braganhol E. Genistein induces apoptosis and autophagy in human breast MCF-7 cells by modulating the expression of proapoptotic factors and oxidative stress enzymes. Mol Cell Biochem. 2014;390:235–242. doi: 10.1007/s11010-014-1974-x. [DOI] [PubMed] [Google Scholar]
- 55.Varinska L, Gal P, Mojzisova G, Mirossay L, Mojzis J. Soy and breast cancer: focus on angiogenesis. Int J Mol Sci. 2015;16:11728–11749. doi: 10.3390/ijms160511728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao K, Song X, Huang Y, Yao J, Zhou M, Li Z, You Q, Guo Q, Lu N. Wogonin inhibits LPS-induced tumor angiogenesis via suppressing PI3K/Akt/NF-kappaB signaling. Eur J Pharmacol. 2014;737:57–69. doi: 10.1016/j.ejphar.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 57.Rajabi M, Mousa SA. The role of angiogenesis in cancer treatment. Biomedicines. 2017;5 doi: 10.3390/biomedicines5020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim SJ, Pham TH, Bak Y, Ryu HW, Oh SR, Yoon DY. Orientin inhibits invasion by suppressing MMP-9 and IL-8 expression via the PKCalpha/ ERK/AP-1/STAT3-mediated signaling pathways in TPA-treated MCF-7 breast cancer cells. Phytomedicine. 2018;50:35–42. doi: 10.1016/j.phymed.2018.09.172. [DOI] [PubMed] [Google Scholar]
- 59.Chin HK, Horng CT, Liu YS, Lu CC, Su CY, Chen PS, Chiu HY, Tsai FJ, Shieh PC, Yang JS. Kaempferol inhibits angiogenic ability by targeting VEGF receptor-2 and downregulating the PI3K/AKT, MEK and ERK pathways in VEGF-stimulated human umbilical vein endothelial cells. Oncol Rep. 2018;39:2351–2357. doi: 10.3892/or.2018.6312. [DOI] [PubMed] [Google Scholar]
- 60.Li H, Chen C. Quercetin has antimetastatic effects on gastric cancer cells via the interruption of uPA/uPAR function by modulating NF-kappab, PKC-delta, ERK1/2, and AMPKalpha. Integr Cancer Ther. 2018;17:511–523. doi: 10.1177/1534735417696702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao X, Jiang W, Yu D, Yan Z. Luteolin inhibits proliferation and induces apoptosis of human melanoma cells in vivo and in vitro by suppressing MMP-2 and MMP-9 through the PI3K/AKT pathway. Food Funct. 2019;10:703–712. doi: 10.1039/c8fo02013b. [DOI] [PubMed] [Google Scholar]
- 62.Song B, Guan S, Lu J, Chen Z, Huang G, Li G, Xiong Y, Zhang S, Yue Z, Deng X. Suppressive effects of fisetin on mice T lymphocytes in vitro and in vivo. J Surg Res. 2013;185:399–409. doi: 10.1016/j.jss.2013.05.093. [DOI] [PubMed] [Google Scholar]
- 63.Lin F, Luo X, Tsun A, Li Z, Li D, Li B. Kaempferol enhances the suppressive function of Treg cells by inhibiting FOXP3 phosphorylation. Int Immunopharmacol. 2015;28:859–865. doi: 10.1016/j.intimp.2015.03.044. [DOI] [PubMed] [Google Scholar]
- 64.Facciabene A, Motz GT, Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72:2162–2171. doi: 10.1158/0008-5472.CAN-11-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maruyama T, Kono K, Mizukami Y, Kawaguchi Y, Mimura K, Watanabe M, Izawa S, Fujii H. Distribution of Th17 cells and FoxP3(+) regulatory T cells in tumor-infiltrating lymphocytes, tumor-draining lymph nodes and peripheral blood lymphocytes in patients with gastric cancer. Cancer Sci. 2010;101:1947–1954. doi: 10.1111/j.1349-7006.2010.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fadul CE, Fisher JL, Gui J, Hampton TH, Cote AL, Ernstoff MS. Immune modulation effects of concomitant temozolomide and radiation therapy on peripheral blood mononuclear cells in patients with glioblastoma multiforme. Neuro Oncol. 2011;13:393–400. doi: 10.1093/neuonc/noq204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 68.Miron A, Aprotosoaie AC, Trifan A, Xiao J. Flavonoids as modulators of metabolic enzymes and drug transporters. Ann N Y Acad Sci. 2017;1398:152–167. doi: 10.1111/nyas.13384. [DOI] [PubMed] [Google Scholar]
- 69.Dury L, Nasr R, Lorendeau D, Comsa E, Wong I, Zhu X, Chan KF, Chan TH, Chow L, Falson P, Di Pietro A, Baubichon-Cortay H. Flavonoid dimers are highly potent killers of multidrug resistant cancer cells overexpressing MRP1. Biochem Pharmacol. 2017;124:10–18. doi: 10.1016/j.bcp.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 70.Moon JY, Manh Hung LV, Unno T, Cho SK. Nobiletin Enhances Chemosensitivity to Adriamycin through Modulation of the Akt/GSK3beta/beta(-)Catenin/MYCN/MRP1 Signaling Pathway in A549 Human Non-Small-Cell Lung Cancer Cells. Nutrients. 2018;10 doi: 10.3390/nu10121829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Palko-Labuz A, Sroda-Pomianek K, Wesolowska O, Kostrzewa-Suslow E, Uryga A, Michalak K. MDR reversal and pro-apoptotic effects of statins and statins combined with flavonoids in colon cancer cells. Biomed Pharmacother. 2019;109:1511–1522. doi: 10.1016/j.biopha.2018.10.169. [DOI] [PubMed] [Google Scholar]
- 72.Silbermann K, Shah CP, Sahu NU, Juvale K, Stefan SM, Kharkar PS, Wiese M. Novel chalcone and flavone derivatives as selective and dual inhibitors of the transport proteins ABCB1 and ABCG2. Eur J Med Chem. 2019;164:193–213. doi: 10.1016/j.ejmech.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 73.Li Y, Ning J, Wang Y, Wang C, Sun C, Huo X, Yu Z, Feng L, Zhang B, Tian X, Ma X. Drug interaction study of flavonoids toward CYP3A4 and their quantitative structure activity relationship (QSAR) analysis for predicting potential effects. Toxicol Lett. 2018;294:27–36. doi: 10.1016/j.toxlet.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 74.Mohana S, Ganesan M, Rajendra Prasad N, Ananthakrishnan D, Velmurugan D. Flavonoids modulate multidrug resistance through wnt signaling in P-glycoprotein overexpressing cell lines. BMC Cancer. 2018;18:1168. doi: 10.1186/s12885-018-5103-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 75.Li S, Zhao Q, Wang B, Yuan S, Wang X, Li K. Quercetin reversed MDR in breast cancer cells through down-regulating P-gp expression and eliminating cancer stem cells mediated by YB-1 nuclear translocation. Phytother Res. 2018;32:1530–1536. doi: 10.1002/ptr.6081. [DOI] [PubMed] [Google Scholar]
- 76.Lou Y, Guo Z, Zhu Y, Zhang G, Wang Y, Qi X, Lu L, Liu Z, Wu J. Astragali radix and its main bioactive compounds activate the Nrf2-mediated signaling pathway to induce P-glycoprotein and breast cancer resistance protein. J Ethnopharmacol. 2019;228:82–91. doi: 10.1016/j.jep.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 77.Choi JS, Piao YJ, Kang KW. Effects of quercetin on the bioavailability of doxorubicin in rats: role of CYP3A4 and P-gp inhibition by quercetin. Arch Pharm Res. 2011;34:607–613. doi: 10.1007/s12272-011-0411-x. [DOI] [PubMed] [Google Scholar]
- 78.Ferreira A, Pousinho S, Fortuna A, Falcão A, Alves G. Flavonoid compounds as reversal agents of the P-glycoprotein-mediated multidrug resistance: biology, chemistry and pharmacology. Phytochemistry Reviews. 2014;14:233–272. [Google Scholar]
- 79.Talukdar S, Emdad L, Das SK, Sarkar D, Fisher PB. Evolving strategies for therapeutically targeting cancer stem cells. Adv Cancer Res. 2016;131:159–191. doi: 10.1016/bs.acr.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 80.de The H. Differentiation therapy revisited. Nat Rev Cancer. 2018;18:117–127. doi: 10.1038/nrc.2017.103. [DOI] [PubMed] [Google Scholar]
- 81.Iriyama N, Yuan B, Hatta Y, Horikoshi A, Yoshino Y, Toyoda H, Aizawa S, Takeuchi J. Granulocyte colony-stimulating factor potentiates differentiation induction by all-trans retinoic acid and arsenic trioxide and enhances arsenic uptake in the acute promyelocytic leukemia cell line HT93A. Oncol Rep. 2012;28:1875–82. doi: 10.3892/or.2012.2006. [DOI] [PubMed] [Google Scholar]
- 82.Iriyama N, Yuan B, Hatta Y, Takagi N, Takei M. Lyn, a tyrosine kinase closely linked to the differentiation status of primary acute myeloid leukemia blasts, associates with negative regulation of all-trans retinoic acid (ATRA) and dihydroxyvitamin D3 (VD3)-induced HL-60 cells differentiation. Cancer Cell Int. 2016;16:37. doi: 10.1186/s12935-016-0314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iriyama N, Yuan B, Yoshino Y, Hatta Y, Horikoshi A, Aizawa S, Takei M, Takeuchi J, Takagi N, Toyoda H. Enhancement of differentiation induction and upregulation of CCAAT/enhancer-binding proteins and PU. 1 in NB4 cells treated with combination of ATRA and valproic acid. Int J Oncol. 2014;44:865–73. doi: 10.3892/ijo.2013.2236. [DOI] [PubMed] [Google Scholar]
- 84.Iriyama N, Yuan B, Yoshino Y, Hatta Y, Horikoshi A, Aizawa S, Takeuchi J, Toyoda H. Aquaporin 9, a promising predictor for the cytocidal effects of arsenic trioxide in acute promyelocytic leukemia cell lines and primary blasts. Oncol Rep. 2013;29:2362–2368. doi: 10.3892/or.2013.2388. [DOI] [PubMed] [Google Scholar]
- 85.Moradzadeh M, Roustazadeh A, Tabarraei A, Erfanian S, Sahebkar A. Epigallocatechin-3-gallate enhances differentiation of acute promyelocytic leukemia cells via inhibition of PML-RARalpha and HDAC1. Phytother Res. 2018;32:471–479. doi: 10.1002/ptr.5990. [DOI] [PubMed] [Google Scholar]
- 86.Heo JR, Lee GA, Kim GS, Hwang KA, Choi KC. Phytochemical-induced reactive oxygen species and endoplasmic reticulum stress-mediated apoptosis and differentiation in malignant melanoma cells. Phytomedicine. 2018;39:100–110. doi: 10.1016/j.phymed.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 87.Lin Y, Sun H, Dang Y, Li Z. Isoliquiritigenin inhibits the proliferation and induces the differentiation of human glioma stem cells. Oncol Rep. 2018;39:687–694. doi: 10.3892/or.2017.6154. [DOI] [PubMed] [Google Scholar]
- 88.Liu Y, Zou T, Wang S, Chen H, Su D, Fu X, Zhang Q, Kang X. Genistein-induced differentiation of breast cancer stem/progenitor cells through a paracrine mechanism. Int J Oncol. 2016;48:1063–1072. doi: 10.3892/ijo.2016.3351. [DOI] [PubMed] [Google Scholar]
- 89.Wei L, Dai Y, Zhou Y, He Z, Yao J, Zhao L, Guo Q, Yang L. Oroxylin A activates PKM1/HNF4 alpha to induce hepatoma differentiation and block cancer progression. Cell Death Dis. 2017;8:e2944. doi: 10.1038/cddis.2017.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thilakarathna SH, Rupasinghe HP. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients. 2013;5:3367–3387. doi: 10.3390/nu5093367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nimptsch K, Zhang X, Cassidy A, Song M, O’Reilly EJ, Lin JH, Pischon T, Rimm EB, Willett WC, Fuchs CS, Ogino S, Chan AT, Giovannucci EL, Wu K. Habitual intake of flavonoid subclasses and risk of colorectal cancer in 2 large prospective cohorts. Am J Clin Nutr. 2016;103:184–191. doi: 10.3945/ajcn.115.117507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nabavi SM, Habtemariam S, Daglia M, Nabavi SF. Apigenin and breast cancers: from chemistry to medicine. Anticancer Agents Med Chem. 2015;15:728–735. doi: 10.2174/1871520615666150304120643. [DOI] [PubMed] [Google Scholar]
- 93.Lagoa R, Silva J, Rodrigues JR, Bishayee A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnol Adv. 2019 doi: 10.1016/j.biotechadv.2019.04.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 94.Deepika MS, Thangam R, Sheena TS, Sasirekha R, Sivasubramanian S, Babu MD, Jeganathan K, Thirumurugan R. A novel rutin-fucoidan complex based phytotherapy for cervical cancer through achieving enhanced bioavailability and cancer cell apoptosis. Biomed Pharmacother. 2019;109:1181–1195. doi: 10.1016/j.biopha.2018.10.178. [DOI] [PubMed] [Google Scholar]
- 95.Chen R, Hollborn M, Grosche A, Reichenbach A, Wiedemann P, Bringmann A, Kohen L. Effects of the vegetable polyphenols epigallocatechin-3-gallate, luteolin, apigenin, myricetin, quercetin, and cyanidin in primary cultures of human retinal pigment epithelial cells. Mol Vis. 2014;20:242–258. [PMC free article] [PubMed] [Google Scholar]
- 96.Lee JG, Wu R. Erlotinib-cisplatin combination inhibits growth and angiogenesis through c-MYC and HIF-1alpha in EGFR-mutated lung cancer in vitro and in vivo. Neoplasia. 2015;17:190–200. doi: 10.1016/j.neo.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Iriyama N, Yoshino Y, Yuan B, Horikoshi A, Hirabayashi Y, Hatta Y, Toyoda H, Takeuchi J. Speciation of arsenic trioxide metabolites in peripheral blood and bone marrow from an acute promyelocytic leukemia patient. J Hematol Oncol. 2012;5:1. doi: 10.1186/1756-8722-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, Ozguroglu M, Ye D, Feyerabend S, Protheroe A, Sulur G, Luna Y, Li S, Mundle S, Chi KN. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20:686–700. doi: 10.1016/S1470-2045(19)30082-8. [DOI] [PubMed] [Google Scholar]
- 99.Looi CK, Chung FF, Leong CO, Wong SF, Rosli R, Mai CW. Therapeutic challenges and current immunomodulatory strategies in targeting the immunosuppressive pancreatic tumor microenvironment. J Exp Clin Cancer Res. 2019;38:162. doi: 10.1186/s13046-019-1153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.He MH, Zhang Q, Shu G, Lin JC, Zhao L, Liang XX, Yin L, Shi F, Fu HL, Yuan ZX. Dihydromyricetin sensitizes human acute myeloid leukemia cells to retinoic acid-induced myeloid differentiation by activating STAT1. Biochem Biophys Res Commun. 2018;495:1702–1707. doi: 10.1016/j.bbrc.2017.12.030. [DOI] [PubMed] [Google Scholar]
- 101.Sang DP, Li RJ, Lan Q. Quercetin sensitizes human glioblastoma cells to temozolomide in vitro via inhibition of Hsp27. Acta Pharmacol Sin. 2014;35:832–838. doi: 10.1038/aps.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jager AK, Saaby L. Flavonoids and the CNS. Molecules. 2011;16:1471–1485. doi: 10.3390/molecules16021471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Johnston GA. Flavonoid nutraceuticals and ionotropic receptors for the inhibitory neurotransmitter GABA. Neurochem Int. 2015;89:120–125. doi: 10.1016/j.neuint.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 104.Desai V, Jain A, Shaghaghi H, Summer R, Lai JCK, Bhushan A. Combination of biochanin a and temozolomide impairs tumor growth by modulating cell metabolism in glioblastoma multiforme. Anticancer Res. 2019;39:57–66. doi: 10.21873/anticanres.13079. [DOI] [PubMed] [Google Scholar]
- 105.La X, Zhang L, Li Z, Li H, Yang Y. (-)-Epigallocatechin gallate (EGCG) enhances the sensitivity of colorectal cancer cells to 5-FU by inhibiting GRP78/NF-kappaB/miR-155-5p/MDR1 pathway. J Agric Food Chem. 2019;67:2510–2518. doi: 10.1021/acs.jafc.8b06665. [DOI] [PubMed] [Google Scholar]
- 106.Zhang G, Wang Y, Zhang Y, Wan X, Li J, Liu K, Wang F, Liu K, Liu Q, Yang C, Yu P, Huang Y, Wang S, Jiang P, Qu Z, Luan J, Duan H, Zhang L, Hou A, Jin S, Hsieh TC, Wu E. Anti-cancer activities of tea epigallocatechin-3-gallate in breast cancer patients under radiotherapy. Curr Mol Med. 2012;12:163–176. doi: 10.2174/156652412798889063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sabzichi M, Hamishehkar H, Ramezani F, Sharifi S, Tabasinezhad M, Pirouzpanah M, Ghanbari P, Samadi N. Luteolin-loaded phytosomes sensitize human breast carcinoma MDA-MB 231 cells to doxorubicin by suppressing Nrf2 mediated signalling. Asian Pac J Cancer Prev. 2014;15:5311–5316. doi: 10.7314/apjcp.2014.15.13.5311. [DOI] [PubMed] [Google Scholar]
- 108.Yang MY, Wang CJ, Chen NF, Ho WH, Lu FJ, Tseng TH. Luteolin enhances paclitaxel-induced apoptosis in human breast cancer MDA-MB-231 cells by blocking STAT3. Chem Biol Interact. 2014;213:60–68. doi: 10.1016/j.cbi.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 109.Qian J, Xia M, Liu W, Li L, Yang J, Mei Y, Meng Q, Xie Y. Glabridin resensitizes p-glycoprotein-overexpressing multidrug-resistant cancer cells to conventional chemotherapeutic agents. Eur J Pharmacol. 2019;852:231–243. doi: 10.1016/j.ejphar.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 110.Kundur S, Prayag A, Selvakumar P, Nguyen H, McKee L, Cruz C, Srinivasan A, Shoyele S, Lakshmikuttyamma A. Synergistic anticancer action of quercetin and curcumin against triple-negative breast cancer cell lines. J Cell Physiol. 2019;234:11103–11118. doi: 10.1002/jcp.27761. [DOI] [PubMed] [Google Scholar]
- 111.Chen Z, Tian D, Liao X, Zhang Y, Xiao J, Chen W, Liu Q, Chen Y, Li D, Zhu L, Cai S. Apigenin combined with gefitinib blocks autophagy flux and induces apoptotic cell death through inhibition of HIF-1alpha, c-Myc, p-EGFR, and glucose metabolism in EGFR L858R+T790M-mutated H1975 cells. Front Pharmacol. 2019;10:260. doi: 10.3389/fphar.2019.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Erdogan S, Doganlar O, Doganlar ZB, Turkekul K. Naringin sensitizes human prostate cancer cells to paclitaxel therapy. Prostate Int. 2018;6:126–135. doi: 10.1016/j.prnil.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


