The synthesis, characterization and crystal structure of the title compounds are reported and the crystal supramolecularity is discussed.
Keywords: crystal structure, thiophene-based cyanoacrylates, polymorph, crystal supramolecularity
Abstract
The synthesis, crystal structure and structural motif of two thiophene-based cyanoacrylate derivatives, namely, ethyl (E)-2-cyano-3-(3-methylthiophen-2-yl)acrylate (1), C11H11NO2S, and ethyl (E)-2-cyano-3-(thiophen-2-yl)acrylate (2), C10H9NO2S, are reported. Derivative 1 crystallized with two independent molecules in the asymmetric unit, and derivative 2 represents a new monoclinic (C2/m) polymorph. The molecular conformations of 1 and the two polymorphs of 2 are very similar, as all non-H atoms are planar except for the methyl of the ethyl groups. The intermolecular interactions and crystal packing of 1 and 2 are described and compared with that of the reported monoclinic (C2/m) polymorph of derivative 2 [Castro Agudelo et al. (2017 ▸). Acta Cryst. E73, 1287–1289].
Chemical context
Cyanoacrylate derivatives are of industrial interest being subunits used to build many adhesives and polymeric materials (Faggi et al., 2019 ▸). They are also considered important intermediate precursors for the synthesis of different heterocyclic derivatives, see for example Qian et al. (2018 ▸), and as nitrile-activated species in bioreduction reactions (Brenna et al., 2013 ▸, 2015 ▸; Kong et al., 2016 ▸) among others. In addition, they show important practical properties, such as in organic dye-sensitized solar cells (DSSCs) (He et al., 2017 ▸; Zhou et al., 2015 ▸). Within these voltaic cells, cyanoacrylic acid is one of the most commonly employed acceptors. Thiophene and its derivatives, known to exhibit high charge mobility, serve as π-bridges (donor-π–acceptor structure) to provide conjugation and enhance light absorbance (Liu et al., 2012 ▸).
An understanding of the structure of thiophene-based acrylate subunits is necessary to benefit from their properties in photovoltaic cells. In a continuation of our work on the X-ray structural characterization of thiophene-containing derivatives (Ibrahim et al., 2019 ▸; Al-Refai et al., 2014 ▸, 2016 ▸), we report here the synthesis, characterization and crystal structures of two thiophene-based acrylate derivatives, namely, ethyl (E)-2-cyano-3-(3-methylthiophen-2-yl)acrylate (1) and ethyl (E)-2-cyano-3-(3-methylthiophen-2-yl)acrylate (2). Derivative 2 is a polymorph of a reported structure (Castro Agudelo et al., 2017 ▸), but with no disorder of the ethoxy group. The crystal supramolecularity of both compounds is also discussed.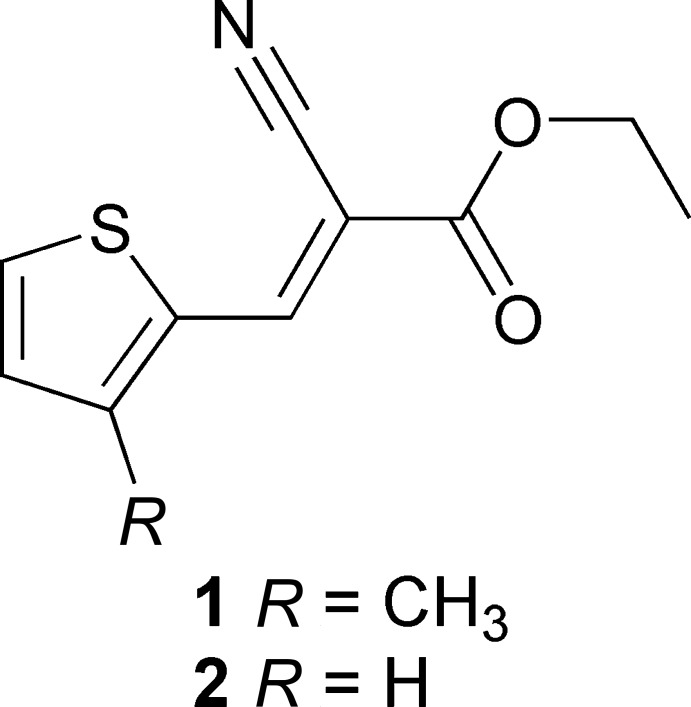
Structural commentary
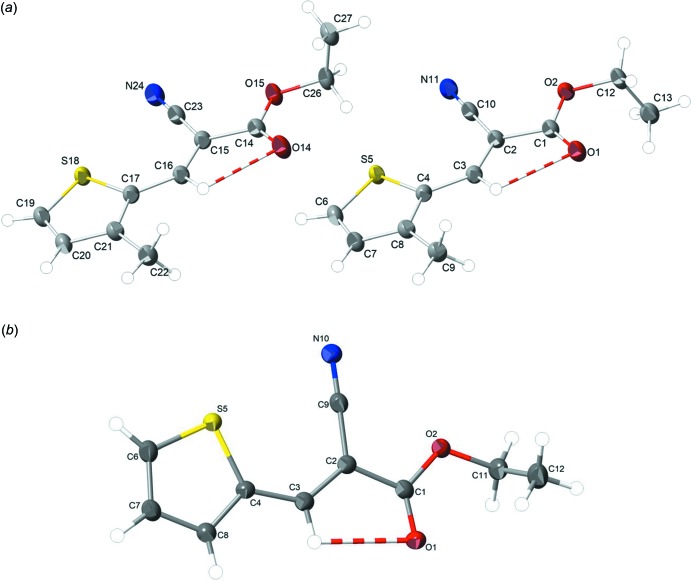
The molecular structures of the title compounds are depicted in Fig. 1 ▸. The asymmetric unit contains two independent molecules, A and B, in 1 and one molecule in 2. In these molecules, the bond distances and angles fall within similar ranges to those reported for similar compounds (Castro Agudelo et al., 2017 ▸; Xu et al., 2016 ▸). In both compounds, all non-hydrogen atoms, except for the methyl groups, lie nearly in the same planes. The differences in torsion angles [C1—C2—C3—C4 = −177.78 (14) and C1—O2—C12—C13 = 83.60 (15)° (molecule A), C14—C15—C16—C17 = 179.71 (15)° and C14—O15—C26—C27 = −88.66 (2)° (molecule B) in 1 and C1—C2—C3—C4 = −178.77 (11) and C1—O2—C11—C12 = −83.41 (13)° in 2] indicate an out-of-plane deviation of the methyl group. The planarity of the molecules allows intramolecular hydrogen bonds to occur [C3—H3⋯O1 (molecule A) and C16—H16⋯O14 (molecule B) in 1; C3—H3⋯O1 in 2] (Fig. 1 ▸ and Tables 1 ▸ and 2 ▸), forming an S(6) ring motif with the carbonyl O and cyano N atoms consequently exhibiting an anti-configuration to each other. The conformation of the ethene bond is always E [C2=C3 = 1.363 (2) Å (molecule A) and C15=C16 = 1.3625 (19) (molecule B) in 1; C2=C3 = 1.3592 (18) Å in 2].
Figure 1.
Molecular structures of compounds (a) 1 and (b) 2 with the atom-labelling scheme (displacement ellipsoids at 50% probability level). Intramolecular C—H⋯O interactions are presented as red–white multi-band cylinders.
Table 1. Hydrogen-bond geometry (Å, °) for 1 .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C3—H3⋯O1 | 0.95 | 2.43 | 2.8136 (19) | 104 |
| C16—H16⋯O14 | 0.95 | 2.38 | 2.7829 (19) | 105 |
| C19—H19⋯O1i | 0.95 | 2.34 | 3.2708 (19) | 165 |
| C22—H22A⋯O15ii | 0.98 | 2.59 | 3.292 (2) | 128 |
Symmetry codes: (i) 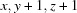 ; (ii)
; (ii) 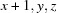 .
.
Table 2. Hydrogen-bond geometry (Å, °) for 2 .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C3—H3⋯O1 | 0.964 (19) | 2.444 (19) | 2.7998 (18) | 101.5 (14) |
| C3—H3⋯O1i | 0.964 (19) | 2.45 (2) | 3.3436 (18) | 153.4 (15) |
| C6—H6⋯N10ii | 0.99 (2) | 2.49 (2) | 3.465 (2) | 169.1 (18) |
| C8—H8⋯O1i | 0.94 (2) | 2.50 (2) | 3.3047 (19) | 143.6 (17) |
Symmetry codes: (i)  ; (ii)
; (ii) 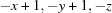 .
.
Derivative 2 is a polymorph of ethyl (E)-2-cyano-3-(thiophen-2-yl)acrylate (CSD refcode GEHYEA; Castro Agudelo et al., 2017 ▸). It shows a similar structure to 1, which has an extra methyl substituent on the thiophene ring. In compound 1 and the two polymorphs of 2, all thiophene-based cyanoacrylate non-H atoms, except for the ethyl group, lie in the same plane. It is also noteworthy that in the polymorph, the ethyl fragment occurs in more than one conformation, thus resulting in disorder, which is absent in 1 and 2.
Supramolecular features
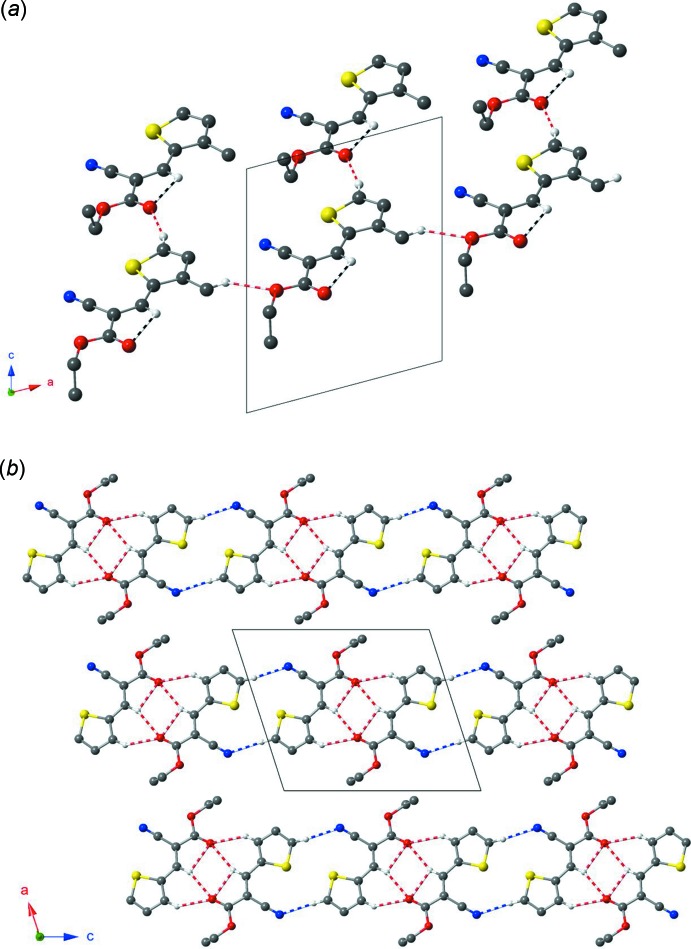
In the crystal of 1, the A and B molecules each form layers parallel to the ac plane, Fig. 2 ▸ a. The layers built up from chains of B molecules are connected via C—H⋯O hydrogen bonds along the a axis. These chains are further connected through C—H⋯O interactions with stacks of molecules A along the c axis. In the b-axis direction, interlayered interactions through van der Waals forces and/or weak dipolar interactions generate a three-dimensional network. In the crystal of 2, inversion dimers are assembled along the c axis through C—H⋯O interactions, Fig. 2 ▸ b. Adjacent dimers (along the c axis) are further connected through C—H⋯N interactions, leading to infinite chains propagating along the c-axis direction. The resulting chains interact via van der Waals forces to form sheets parallel to the ac plane (Fig. 2 ▸ b). The sheets are connected through van der Waals forces and/or weak dipolar interactions, thus consolidating the three-dimensional framework structure. Compounds 1, 2 and the polymorph of 2 (Castro Agudelo et al., 2017 ▸) show no apparent degree of π–π stacking.
Figure 2.
(a) Partial packing diagram for 1 showing layers of A and B molecules parallel to the ac plane, and connected via C—H⋯O intermolecular interactions (shown as multi-band cylinders). (b) The intramolecular (black and white) and intermolecular (red and white) interactions in 2 forming chains of dimeric species connected via C—H⋯O and C—H⋯N interactions. In both figures, hydrogen atoms not involved in interactions are omitted for clarity.
The polymorph of 2 shows a similar crystal packing arrangement, the molecules being connected via C—H⋯O/N interactions, generating centrosymmetric dimers. Chains of molecules are further connected by van der Waals forces into sheets.
Database survey
Castro Agudelo et al. (2017 ▸) reported a recent survey on the Cambridge Structural Database [CSD Version 5.37 with two updates; Groom et al., 2016 ▸] for hits containing the complete thiophene-based cyanoacrylate fragment, together with the possibility of other five-membered rings and/or the presence of a saturated chain longer than the ethyl fragment. They found three hits containing the main part of the title compounds, the thiophene-cyanoacrylate, with additional and/or longer substituents, namely ethyl-3-(3-chloro-4-cyano-5-{[4-(dimethylamino)phenyl]diazenyl}-2-thienyl)-2-cyanoacrylate (UMUYAE; Xu et al., 2016 ▸), octyl-2-cyano-3-(4,6-dibromo-7,7-dimethyl-7H-thieno[3′,4′:4,5]silolo[2,3-b]thiophen-2-yl)acrylate (QUSKAS; Liu et al., 2016 ▸) and ethyl-2-cyano-3-(3,3′′′-dihexyl-2,2′:5′,2′′:5′′,2′′′-quaterthiophen-5-yl)acrylate (AVUFON; Miyazaki et al., 2011 ▸). In all derivatives AVUFON, UMUYAE and QUSKAS, the non-H thiophene-based acrylate fragment is almost planar except for the methyl group (or the longer alkyl chain in QUSKAS) being slightly out of the plane. The crystal lattices of AVUFON, UMUYAE and QUSKAS are stabilized by C—H⋯O/S, C—H⋯O/N and C—H⋯N/S intermolecular interactions, respectively.
A further search of the CSD for other five-membered rings instead of thiophene provided six hits. Of them, the following three are very similar to the title compounds: ethyl-(2E)-2-cyano-3-(1-methyl-1H-pyrrol-2-yl)prop-2-enoate (AYUGEH; Asiri et al., 2011 ▸), (E)-ethyl-2-cyano-3-(1H-pyrrol-2-yl)acrylate (EVIZEP; Yuvaraj et al., 2011 ▸) and (E)-ethyl-2-cyano-3-(furan-2-yl)acrylate (ZAQKIN; Kalkhambkar et al., 2012 ▸). In both AYUGEH and EVIZEP, all the non-H atoms are nearly in the same plane, while in ZAQKIN the furan-based cyanoacrylate moiety lies in the same plane except for the methyl groups, which are slightly out of plane. As far as crystal packing is concerned, the molecules in EVIZEP and ZAQKIN are linked into dimers via N—H⋯O and C—H⋯O hydrogen bonds, respectively, while in AYUGEH the molecules are linked into tapes via both C—H⋯O and C—H⋯N interactions. The tapes are further interconnected by C—H⋯π interactions into a three-dimensional structure.
Synthesis and crystallization
All reagents and solvent were purchased from Aldrich and used without further purifications. The title compounds were synthesized as outlined in Fig. 3 ▸.
Figure 3.
Syntheis of the title compounds.
In a 250 ml round-bottom flask connected with a condenser, a mixture of the corresponding thiophene-2-carboxaldehyde (1 mmol), ethylcyanoacetate (1.1 mmol) and ammonium acetate (8 mmol) in absolute ethanol was refluxed for 6 h. The reaction was monitored using thin layer chromatography (TLC plates coated with silica gel). After completion, the reaction mixture was cooled to room temperature, and the obtained yellowish-brown precipitate was filtered off, washed with cooled water, dried and recrystallized from ethanol solution to give the final products as pale-yellow crystals (90% yield for both 1 and 2).
Ethyl (E)-2-cyano-3-(3-methylthiophen-2-yl)acrylate (1): m.p. 381–382 K, 1H NMR (CD2Cl2, 300 MHz): δ (ppm) = 1.39 (t, J = 7.12, 3H, CH2CH3), 2.48 (s, 3H, CH3-3′), 4.36 (q, J = 7.12, 2H, CH2CH3), 7.07 (d, J = 5.01,1H, H-4′), 7.74 (d, J = 5.01, 1H, H-5′), 8.46 (s, 1H, H-3). 13C NMR (CD2Cl2, 75 MHz) δ (ppm) = 14.4 (CH2CH3), 14.9 (CH3-3′), 62.7 (CH2CH3), 98.0 (C-2), 116.4 (CN), 131.2 (C-2′), 131.4 (C-4′), 134.3 (C-5′), 145.0 (C-3), 149.9 (C-3′), 163.4 (C-1). (+)-ESIMS m/z = 244 ([M + Na]+, 100%), 465 ([2M + Na]+, 16%).
Ethyl (E)-2-cyano-3-(thiophen-2-yl)acrylate (2): mp. 371–372 K, 1H NMR (CD2Cl2, 300 MHz): δ (ppm) = 1.39 (t, J = 7.12, 3H, CH2CH3), 4.37 (q, J = 7.12, 2H, CH2CH3), 7.27 (t, J = 4.44,1H, H-4′), 7.85 (d, J = 4.36, 2H, H-3′,5′), 8.38 (s, 1H, H-3). 13C NMR (CD2Cl2, 75 MHz) δ (ppm) = 14.4 (CH2CH3), 62.9 (CH2CH3), 99.8 (C-2), 116.1 (CN), 129.0 (C-4′), 135.5 (C-5′), 136.5 (C-2′), 137.8 (C-3′), 146.9 (C-3), 162.9 (C-1). (+)-ESIMS m/z = 230 ([M + Na]+, 100%), 237 ([2M + Na]+, 11%).
Refinement
Detailed crystal data and structure refinement for the title compounds are listed in Table 3 ▸. In 1, C-bound hydrogen atoms were included in calculated positions (0.95–0.99 Å) and refined using a riding model with U iso(H) = 1.2U eq(C) or 1.5U eq(C-methyl). Methyl groups were allowed to rotate to fit best the electron density. All hydrogen atoms in 2 were located in difference-Fourier maps and refined isotropically.
Table 3. Experimental details.
| 1 | 2 | |
|---|---|---|
| Crystal data | ||
| Chemical formula | C11H11NO2S | C10H9NO2S |
| M r | 221.27 | 207.24 |
| Crystal system, space group | Triclinic, P
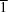
|
Monoclinic, P21/c |
| Temperature (K) | 100 | 100 |
| a, b, c (Å) | 9.2784 (2), 10.7925 (3), 11.6696 (2) | 11.5907 (3), 6.6883 (2), 13.4837 (3) |
| α, β, γ (°) | 74.464 (2), 74.179 (2), 85.073 (2) | 90, 107.859 (2), 90 |
| V (Å3) | 1083.11 (4) | 994.92 (5) |
| Z | 4 | 4 |
| Radiation type | Cu Kα | Cu Kα |
| μ (mm−1) | 2.49 | 2.68 |
| Crystal size (mm) | 0.26 × 0.24 × 0.11 | 0.32 × 0.20 × 0.20 |
| Data collection | ||
| Diffractometer | Stoe STADIVARI | Stoe STADIVARI |
| Absorption correction | Multi-scan (LANA; Stoe & Cie, 2016 ▸) | Multi-scan (LANA; Stoe & Cie, 2016 ▸) |
| T min, T max | 0.074, 0.546 | 0.051, 0.168 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 21624, 4399, 3911 | 10400, 2048, 1974 |
| R int | 0.032 | 0.026 |
| (sin θ/λ)max (Å−1) | 0.630 | 0.630 |
| Refinement | ||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.041, 0.122, 1.10 | 0.033, 0.100, 1.09 |
| No. of reflections | 4399 | 2048 |
| No. of parameters | 275 | 164 |
| H-atom treatment | H-atom parameters constrained | All H-atom parameters refined |
| Δρmax, Δρmin (e Å−3) | 0.37, −0.49 | 0.29, −0.28 |
Supplementary Material
Crystal structure: contains datablock(s) 1, 2, New_Global_Publ_Block. DOI: 10.1107/S2056989019011435/tx2012sup1.cif
Structure factors: contains datablock(s) 1. DOI: 10.1107/S2056989019011435/tx20121sup2.hkl
Structure factors: contains datablock(s) 2. DOI: 10.1107/S2056989019011435/tx20122sup3.hkl
Supporting information file. DOI: 10.1107/S2056989019011435/tx20121sup4.cml
Supporting information file. DOI: 10.1107/S2056989019011435/tx20122sup5.cml
Supporting information file. DOI: 10.1107/S2056989019011435/tx2012sup6.pdf
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
Ethyl (E)-2-cyano-3-(3-methylthiophen-2-yl)acrylate (1) . Crystal data
| C11H11NO2S | Z = 4 |
| Mr = 221.27 | F(000) = 464 |
| Triclinic, P1 | Dx = 1.357 Mg m−3 |
| a = 9.2784 (2) Å | Cu Kα radiation, λ = 1.54186 Å |
| b = 10.7925 (3) Å | Cell parameters from 24144 reflections |
| c = 11.6696 (2) Å | θ = 4.3–76.6° |
| α = 74.464 (2)° | µ = 2.49 mm−1 |
| β = 74.179 (2)° | T = 100 K |
| γ = 85.073 (2)° | Plate, colourless |
| V = 1083.11 (4) Å3 | 0.26 × 0.24 × 0.11 mm |
Ethyl (E)-2-cyano-3-(3-methylthiophen-2-yl)acrylate (1) . Data collection
| Stoe STADIVARI diffractometer | 4399 independent reflections |
| Radiation source: GeniX 3D HF Cu | 3911 reflections with I > 2σ(I) |
| Detector resolution: 5.81 pixels mm-1 | Rint = 0.032 |
| rotation method, ω scans | θmax = 76.1°, θmin = 4.3° |
| Absorption correction: multi-scan (LANA; Stoe & Cie, 2016) | h = −11→11 |
| Tmin = 0.074, Tmax = 0.546 | k = −9→13 |
| 21624 measured reflections | l = −14→14 |
Ethyl (E)-2-cyano-3-(3-methylthiophen-2-yl)acrylate (1) . Refinement
| Refinement on F2 | Primary atom site location: dual |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.041 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.122 | H-atom parameters constrained |
| S = 1.10 | w = 1/[σ2(Fo2) + (0.0848P)2 + 0.1177P] where P = (Fo2 + 2Fc2)/3 |
| 4399 reflections | (Δ/σ)max = 0.001 |
| 275 parameters | Δρmax = 0.37 e Å−3 |
| 0 restraints | Δρmin = −0.49 e Å−3 |
Ethyl (E)-2-cyano-3-(3-methylthiophen-2-yl)acrylate (1) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Ethyl (E)-2-cyano-3-(3-methylthiophen-2-yl)acrylate (1) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.50743 (12) | 0.18612 (11) | −0.05309 (9) | 0.0282 (2) | |
| C1 | 0.41104 (16) | 0.24976 (14) | −0.00171 (13) | 0.0244 (3) | |
| O2 | 0.26787 (11) | 0.25753 (11) | −0.00683 (9) | 0.0272 (2) | |
| C2 | 0.43611 (16) | 0.33079 (14) | 0.07627 (13) | 0.0241 (3) | |
| C3 | 0.57815 (16) | 0.33732 (14) | 0.08609 (13) | 0.0237 (3) | |
| H3 | 0.651544 | 0.290986 | 0.038820 | 0.028* | |
| C4 | 0.63437 (16) | 0.40276 (14) | 0.15613 (13) | 0.0237 (3) | |
| S5 | 0.52472 (4) | 0.48985 (3) | 0.25473 (3) | 0.02482 (13) | |
| C6 | 0.67767 (17) | 0.52373 (15) | 0.29500 (13) | 0.0279 (3) | |
| H6 | 0.673143 | 0.573149 | 0.352151 | 0.033* | |
| C7 | 0.80743 (16) | 0.47210 (15) | 0.23616 (13) | 0.0271 (3) | |
| H7 | 0.902616 | 0.481512 | 0.248382 | 0.033* | |
| C8 | 0.78481 (16) | 0.40304 (14) | 0.15496 (13) | 0.0253 (3) | |
| C9 | 0.90876 (16) | 0.33949 (15) | 0.07698 (14) | 0.0288 (3) | |
| H9A | 1.002445 | 0.344255 | 0.098973 | 0.043* | |
| H9B | 0.920518 | 0.383536 | −0.010108 | 0.043* | |
| H9C | 0.884318 | 0.249167 | 0.091128 | 0.043* | |
| C10 | 0.30968 (16) | 0.39583 (15) | 0.13711 (13) | 0.0260 (3) | |
| N11 | 0.20792 (14) | 0.44850 (13) | 0.18585 (12) | 0.0307 (3) | |
| C12 | 0.22775 (17) | 0.18030 (16) | −0.07799 (14) | 0.0293 (3) | |
| H12A | 0.311397 | 0.179597 | −0.151655 | 0.035* | |
| H12B | 0.138713 | 0.218888 | −0.106211 | 0.035* | |
| C13 | 0.19407 (17) | 0.04420 (16) | −0.00130 (15) | 0.0335 (3) | |
| H13A | 0.154960 | −0.003295 | −0.046871 | 0.050* | |
| H13B | 0.119282 | 0.045515 | 0.076064 | 0.050* | |
| H13C | 0.286108 | 0.002013 | 0.016692 | 0.050* | |
| O14 | 0.39990 (12) | 0.67602 (12) | 0.40403 (10) | 0.0326 (3) | |
| C14 | 0.29946 (16) | 0.72830 (15) | 0.46523 (13) | 0.0262 (3) | |
| O15 | 0.15438 (11) | 0.72446 (11) | 0.46857 (10) | 0.0285 (2) | |
| C15 | 0.32273 (16) | 0.80391 (14) | 0.54804 (13) | 0.0251 (3) | |
| C16 | 0.46568 (16) | 0.81134 (14) | 0.55532 (12) | 0.0246 (3) | |
| H16 | 0.538142 | 0.766223 | 0.506059 | 0.029* | |
| C17 | 0.52508 (16) | 0.87543 (14) | 0.62444 (13) | 0.0245 (3) | |
| S18 | 0.42127 (4) | 0.96642 (3) | 0.72124 (3) | 0.02552 (13) | |
| C19 | 0.57711 (17) | 0.99953 (15) | 0.75821 (13) | 0.0282 (3) | |
| H19 | 0.575810 | 1.050830 | 0.813150 | 0.034* | |
| C20 | 0.70388 (17) | 0.94398 (15) | 0.69991 (13) | 0.0279 (3) | |
| H20 | 0.800387 | 0.952569 | 0.710077 | 0.033* | |
| C22 | 0.79644 (17) | 0.80089 (16) | 0.54916 (14) | 0.0306 (3) | |
| H22A | 0.894192 | 0.818438 | 0.558037 | 0.046* | |
| H22B | 0.796091 | 0.829397 | 0.462132 | 0.046* | |
| H22C | 0.777504 | 0.708354 | 0.579387 | 0.046* | |
| C21 | 0.67641 (16) | 0.87201 (15) | 0.62249 (13) | 0.0258 (3) | |
| C23 | 0.19658 (16) | 0.86371 (15) | 0.61513 (13) | 0.0271 (3) | |
| N24 | 0.09612 (15) | 0.91263 (14) | 0.67014 (12) | 0.0324 (3) | |
| C26 | 0.11875 (17) | 0.65600 (15) | 0.38777 (14) | 0.0300 (3) | |
| H26A | 0.017243 | 0.619428 | 0.424811 | 0.036* | |
| H26B | 0.191159 | 0.584110 | 0.378726 | 0.036* | |
| C27 | 0.12498 (19) | 0.74571 (17) | 0.26288 (14) | 0.0360 (4) | |
| H27A | 0.092643 | 0.700287 | 0.212052 | 0.054* | |
| H27B | 0.227878 | 0.775245 | 0.222919 | 0.054* | |
| H27C | 0.058482 | 0.819932 | 0.272496 | 0.054* |
Ethyl (E)-2-cyano-3-(3-methylthiophen-2-yl)acrylate (1) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0258 (5) | 0.0336 (6) | 0.0305 (5) | 0.0029 (4) | −0.0085 (4) | −0.0166 (4) |
| C1 | 0.0237 (7) | 0.0275 (8) | 0.0244 (6) | 0.0005 (5) | −0.0079 (5) | −0.0096 (6) |
| O2 | 0.0232 (5) | 0.0346 (6) | 0.0320 (5) | 0.0020 (4) | −0.0115 (4) | −0.0185 (4) |
| C2 | 0.0237 (7) | 0.0268 (8) | 0.0249 (6) | 0.0014 (5) | −0.0086 (5) | −0.0101 (6) |
| C3 | 0.0238 (7) | 0.0258 (7) | 0.0237 (6) | 0.0005 (5) | −0.0065 (5) | −0.0098 (5) |
| C4 | 0.0230 (7) | 0.0265 (8) | 0.0253 (6) | 0.0019 (5) | −0.0076 (5) | −0.0121 (5) |
| S5 | 0.0228 (2) | 0.0302 (2) | 0.0267 (2) | 0.00155 (14) | −0.00801 (14) | −0.01489 (15) |
| C6 | 0.0293 (7) | 0.0315 (8) | 0.0290 (7) | −0.0017 (6) | −0.0115 (6) | −0.0135 (6) |
| C7 | 0.0253 (7) | 0.0313 (8) | 0.0291 (7) | −0.0012 (6) | −0.0101 (6) | −0.0115 (6) |
| C8 | 0.0244 (7) | 0.0282 (8) | 0.0251 (6) | 0.0002 (5) | −0.0071 (5) | −0.0095 (6) |
| C9 | 0.0226 (7) | 0.0357 (9) | 0.0311 (7) | 0.0029 (6) | −0.0068 (6) | −0.0149 (6) |
| C10 | 0.0256 (7) | 0.0302 (8) | 0.0280 (6) | 0.0005 (6) | −0.0117 (5) | −0.0126 (6) |
| N11 | 0.0263 (6) | 0.0384 (8) | 0.0342 (6) | 0.0030 (5) | −0.0103 (5) | −0.0190 (6) |
| C12 | 0.0282 (7) | 0.0361 (9) | 0.0325 (7) | 0.0023 (6) | −0.0134 (6) | −0.0188 (6) |
| C13 | 0.0303 (7) | 0.0382 (9) | 0.0393 (8) | −0.0019 (6) | −0.0111 (6) | −0.0194 (7) |
| O14 | 0.0289 (5) | 0.0416 (7) | 0.0359 (6) | 0.0049 (5) | −0.0102 (4) | −0.0239 (5) |
| C14 | 0.0258 (7) | 0.0296 (8) | 0.0264 (7) | 0.0007 (6) | −0.0088 (5) | −0.0105 (6) |
| O15 | 0.0249 (5) | 0.0360 (6) | 0.0312 (5) | −0.0003 (4) | −0.0092 (4) | −0.0175 (4) |
| C15 | 0.0252 (7) | 0.0285 (8) | 0.0248 (6) | 0.0017 (5) | −0.0076 (5) | −0.0116 (5) |
| C16 | 0.0255 (7) | 0.0277 (8) | 0.0232 (6) | 0.0005 (5) | −0.0068 (5) | −0.0106 (5) |
| C17 | 0.0247 (7) | 0.0278 (8) | 0.0242 (6) | 0.0014 (5) | −0.0068 (5) | −0.0121 (5) |
| S18 | 0.0248 (2) | 0.0310 (2) | 0.0255 (2) | 0.00185 (14) | −0.00731 (14) | −0.01494 (15) |
| C19 | 0.0319 (7) | 0.0306 (8) | 0.0273 (7) | −0.0015 (6) | −0.0111 (6) | −0.0124 (6) |
| C20 | 0.0281 (7) | 0.0322 (8) | 0.0283 (7) | −0.0023 (6) | −0.0108 (6) | −0.0119 (6) |
| C22 | 0.0261 (7) | 0.0364 (9) | 0.0325 (7) | 0.0043 (6) | −0.0077 (6) | −0.0158 (6) |
| C21 | 0.0245 (7) | 0.0294 (8) | 0.0259 (7) | 0.0005 (6) | −0.0073 (5) | −0.0105 (6) |
| C23 | 0.0268 (7) | 0.0312 (8) | 0.0278 (7) | 0.0002 (6) | −0.0105 (6) | −0.0117 (6) |
| N24 | 0.0272 (6) | 0.0403 (8) | 0.0342 (7) | 0.0025 (5) | −0.0078 (5) | −0.0178 (6) |
| C26 | 0.0300 (7) | 0.0338 (9) | 0.0337 (8) | −0.0012 (6) | −0.0112 (6) | −0.0179 (6) |
| C27 | 0.0377 (8) | 0.0441 (10) | 0.0341 (8) | −0.0029 (7) | −0.0139 (6) | −0.0179 (7) |
Ethyl (E)-2-cyano-3-(3-methylthiophen-2-yl)acrylate (1) . Geometric parameters (Å, º)
| O1—C1 | 1.2057 (17) | O14—C14 | 1.2081 (18) |
| C1—O2 | 1.3404 (17) | C14—O15 | 1.3399 (17) |
| C1—C2 | 1.4912 (19) | C14—C15 | 1.4876 (19) |
| O2—C12 | 1.4545 (16) | O15—C26 | 1.4593 (16) |
| C2—C3 | 1.363 (2) | C15—C16 | 1.3625 (19) |
| C2—C10 | 1.4298 (19) | C15—C23 | 1.428 (2) |
| C3—C4 | 1.4296 (19) | C16—C17 | 1.4282 (19) |
| C3—H3 | 0.9500 | C16—H16 | 0.9500 |
| C4—C8 | 1.3925 (19) | C17—C21 | 1.3955 (19) |
| C4—S5 | 1.7375 (14) | C17—S18 | 1.7339 (14) |
| S5—C6 | 1.7068 (14) | S18—C19 | 1.7076 (15) |
| C6—C7 | 1.368 (2) | C19—C20 | 1.366 (2) |
| C6—H6 | 0.9500 | C19—H19 | 0.9500 |
| C7—C8 | 1.4181 (19) | C20—C21 | 1.421 (2) |
| C7—H7 | 0.9500 | C20—H20 | 0.9500 |
| C8—C9 | 1.4997 (19) | C22—C21 | 1.499 (2) |
| C9—H9A | 0.9800 | C22—H22A | 0.9800 |
| C9—H9B | 0.9800 | C22—H22B | 0.9800 |
| C9—H9C | 0.9800 | C22—H22C | 0.9800 |
| C10—N11 | 1.1517 (19) | C23—N24 | 1.153 (2) |
| C12—C13 | 1.509 (2) | C26—C27 | 1.508 (2) |
| C12—H12A | 0.9900 | C26—H26A | 0.9900 |
| C12—H12B | 0.9900 | C26—H26B | 0.9900 |
| C13—H13A | 0.9800 | C27—H27A | 0.9800 |
| C13—H13B | 0.9800 | C27—H27B | 0.9800 |
| C13—H13C | 0.9800 | C27—H27C | 0.9800 |
| O1—C1—O2 | 124.91 (13) | O14—C14—O15 | 124.76 (13) |
| O1—C1—C2 | 124.13 (13) | O14—C14—C15 | 123.55 (13) |
| O2—C1—C2 | 110.96 (12) | O15—C14—C15 | 111.68 (12) |
| C1—O2—C12 | 116.44 (11) | C14—O15—C26 | 116.44 (11) |
| C3—C2—C10 | 123.99 (13) | C16—C15—C23 | 123.75 (13) |
| C3—C2—C1 | 117.90 (13) | C16—C15—C14 | 117.06 (13) |
| C10—C2—C1 | 118.10 (12) | C23—C15—C14 | 119.19 (12) |
| C2—C3—C4 | 130.33 (14) | C15—C16—C17 | 130.94 (14) |
| C2—C3—H3 | 114.8 | C15—C16—H16 | 114.5 |
| C4—C3—H3 | 114.8 | C17—C16—H16 | 114.5 |
| C8—C4—C3 | 123.93 (13) | C21—C17—C16 | 123.66 (14) |
| C8—C4—S5 | 111.36 (10) | C21—C17—S18 | 111.12 (11) |
| C3—C4—S5 | 124.71 (11) | C16—C17—S18 | 125.22 (11) |
| C6—S5—C4 | 91.34 (7) | C19—S18—C17 | 91.77 (7) |
| C7—C6—S5 | 112.77 (11) | C20—C19—S18 | 112.44 (11) |
| C7—C6—H6 | 123.6 | C20—C19—H19 | 123.8 |
| S5—C6—H6 | 123.6 | S18—C19—H19 | 123.8 |
| C6—C7—C8 | 112.84 (13) | C19—C20—C21 | 113.02 (13) |
| C6—C7—H7 | 123.6 | C19—C20—H20 | 123.5 |
| C8—C7—H7 | 123.6 | C21—C20—H20 | 123.5 |
| C4—C8—C7 | 111.68 (13) | C21—C22—H22A | 109.5 |
| C4—C8—C9 | 124.57 (13) | C21—C22—H22B | 109.5 |
| C7—C8—C9 | 123.75 (13) | H22A—C22—H22B | 109.5 |
| C8—C9—H9A | 109.5 | C21—C22—H22C | 109.5 |
| C8—C9—H9B | 109.5 | H22A—C22—H22C | 109.5 |
| H9A—C9—H9B | 109.5 | H22B—C22—H22C | 109.5 |
| C8—C9—H9C | 109.5 | C17—C21—C20 | 111.66 (13) |
| H9A—C9—H9C | 109.5 | C17—C21—C22 | 124.81 (13) |
| H9B—C9—H9C | 109.5 | C20—C21—C22 | 123.53 (13) |
| N11—C10—C2 | 179.8 (2) | N24—C23—C15 | 178.96 (15) |
| O2—C12—C13 | 110.63 (12) | O15—C26—C27 | 110.46 (12) |
| O2—C12—H12A | 109.5 | O15—C26—H26A | 109.6 |
| C13—C12—H12A | 109.5 | C27—C26—H26A | 109.6 |
| O2—C12—H12B | 109.5 | O15—C26—H26B | 109.6 |
| C13—C12—H12B | 109.5 | C27—C26—H26B | 109.6 |
| H12A—C12—H12B | 108.1 | H26A—C26—H26B | 108.1 |
| C12—C13—H13A | 109.5 | C26—C27—H27A | 109.5 |
| C12—C13—H13B | 109.5 | C26—C27—H27B | 109.5 |
| H13A—C13—H13B | 109.5 | H27A—C27—H27B | 109.5 |
| C12—C13—H13C | 109.5 | C26—C27—H27C | 109.5 |
| H13A—C13—H13C | 109.5 | H27A—C27—H27C | 109.5 |
| H13B—C13—H13C | 109.5 | H27B—C27—H27C | 109.5 |
| O1—C1—O2—C12 | 1.4 (2) | O14—C14—O15—C26 | −2.6 (2) |
| C2—C1—O2—C12 | −178.63 (12) | C15—C14—O15—C26 | 178.16 (12) |
| O1—C1—C2—C3 | 2.7 (2) | O14—C14—C15—C16 | −1.2 (2) |
| O2—C1—C2—C3 | −177.20 (13) | O15—C14—C15—C16 | 178.04 (13) |
| O1—C1—C2—C10 | −176.67 (14) | O14—C14—C15—C23 | 179.03 (15) |
| O2—C1—C2—C10 | 3.39 (18) | O15—C14—C15—C23 | −1.8 (2) |
| C10—C2—C3—C4 | 1.6 (3) | C23—C15—C16—C17 | −0.5 (3) |
| C1—C2—C3—C4 | −177.78 (14) | C14—C15—C16—C17 | 179.71 (15) |
| C2—C3—C4—C8 | −178.52 (15) | C15—C16—C17—C21 | 179.19 (16) |
| C2—C3—C4—S5 | 2.4 (2) | C15—C16—C17—S18 | −0.6 (2) |
| C8—C4—S5—C6 | −0.81 (12) | C21—C17—S18—C19 | 0.10 (12) |
| C3—C4—S5—C6 | 178.37 (14) | C16—C17—S18—C19 | 179.92 (14) |
| C4—S5—C6—C7 | 0.29 (12) | C17—S18—C19—C20 | −0.07 (13) |
| S5—C6—C7—C8 | 0.30 (17) | S18—C19—C20—C21 | 0.02 (18) |
| C3—C4—C8—C7 | −178.06 (14) | C16—C17—C21—C20 | −179.93 (14) |
| S5—C4—C8—C7 | 1.12 (16) | S18—C17—C21—C20 | −0.11 (17) |
| C3—C4—C8—C9 | 2.5 (2) | C16—C17—C21—C22 | −0.4 (2) |
| S5—C4—C8—C9 | −178.36 (12) | S18—C17—C21—C22 | 179.43 (12) |
| C6—C7—C8—C4 | −0.93 (19) | C19—C20—C21—C17 | 0.1 (2) |
| C6—C7—C8—C9 | 178.56 (14) | C19—C20—C21—C22 | −179.49 (14) |
| C1—O2—C12—C13 | 83.60 (15) | C14—O15—C26—C27 | −88.66 (16) |
Ethyl (E)-2-cyano-3-(3-methylthiophen-2-yl)acrylate (1) . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C3—H3···O1 | 0.95 | 2.43 | 2.8136 (19) | 104 |
| C16—H16···O14 | 0.95 | 2.38 | 2.7829 (19) | 105 |
| C19—H19···O1i | 0.95 | 2.34 | 3.2708 (19) | 165 |
| C22—H22A···O15ii | 0.98 | 2.59 | 3.292 (2) | 128 |
Symmetry codes: (i) x, y+1, z+1; (ii) x+1, y, z.
Ethyl (E)-2-cyano-3-(thiophen-2-yl)acrylate (2) . Crystal data
| C10H9NO2S | F(000) = 432 |
| Mr = 207.24 | Dx = 1.384 Mg m−3 |
| Monoclinic, P21/c | Cu Kα radiation, λ = 1.54186 Å |
| a = 11.5907 (3) Å | Cell parameters from 15380 reflections |
| b = 6.6883 (2) Å | θ = 3.5–76.4° |
| c = 13.4837 (3) Å | µ = 2.68 mm−1 |
| β = 107.859 (2)° | T = 100 K |
| V = 994.92 (5) Å3 | Block, colourless |
| Z = 4 | 0.32 × 0.20 × 0.20 mm |
Ethyl (E)-2-cyano-3-(thiophen-2-yl)acrylate (2) . Data collection
| Stoe STADIVARI diffractometer | 2048 independent reflections |
| Radiation source: GeniX 3D HF Cu | 1974 reflections with I > 2σ(I) |
| Detector resolution: 5.81 pixels mm-1 | Rint = 0.026 |
| rotation method, ω scans | θmax = 76.3°, θmin = 6.8° |
| Absorption correction: multi-scan (LANA; Stoe & Cie, 2016) | h = −14→14 |
| Tmin = 0.051, Tmax = 0.168 | k = −8→8 |
| 10400 measured reflections | l = −16→10 |
Ethyl (E)-2-cyano-3-(thiophen-2-yl)acrylate (2) . Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.033 | All H-atom parameters refined |
| wR(F2) = 0.100 | w = 1/[σ2(Fo2) + (0.0688P)2 + 0.2209P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.09 | (Δ/σ)max < 0.001 |
| 2048 reflections | Δρmax = 0.29 e Å−3 |
| 164 parameters | Δρmin = −0.28 e Å−3 |
| 0 restraints | Extinction correction: SHELXL-2018/3 (Sheldrick 2015), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: dual | Extinction coefficient: 0.0030 (6) |
Ethyl (E)-2-cyano-3-(thiophen-2-yl)acrylate (2) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Ethyl (E)-2-cyano-3-(thiophen-2-yl)acrylate (2) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.67411 (9) | 0.47068 (14) | 0.54042 (8) | 0.0266 (3) | |
| C1 | 0.72087 (12) | 0.46539 (17) | 0.47233 (11) | 0.0214 (3) | |
| O2 | 0.84079 (8) | 0.45263 (13) | 0.48771 (8) | 0.0238 (2) | |
| C2 | 0.65241 (12) | 0.46897 (17) | 0.35943 (11) | 0.0211 (3) | |
| C3 | 0.52935 (12) | 0.47114 (18) | 0.33171 (11) | 0.0215 (3) | |
| H3 | 0.4938 (17) | 0.475 (2) | 0.3876 (14) | 0.025 (4)* | |
| C4 | 0.44161 (12) | 0.47115 (17) | 0.23001 (11) | 0.0210 (3) | |
| S5 | 0.47197 (3) | 0.46815 (5) | 0.11198 (2) | 0.02218 (16) | |
| C6 | 0.32131 (13) | 0.47018 (18) | 0.04368 (12) | 0.0250 (3) | |
| H6 | 0.301 (2) | 0.470 (3) | −0.0329 (19) | 0.044 (6)* | |
| C7 | 0.24983 (13) | 0.47201 (19) | 0.10750 (12) | 0.0256 (3) | |
| H7 | 0.158 (2) | 0.472 (3) | 0.0817 (19) | 0.053 (6)* | |
| C8 | 0.31729 (13) | 0.47269 (18) | 0.21394 (12) | 0.0229 (3) | |
| H8 | 0.2819 (18) | 0.476 (2) | 0.2678 (16) | 0.031 (5)* | |
| C9 | 0.71814 (12) | 0.46836 (18) | 0.28517 (11) | 0.0223 (3) | |
| H9 | 0.8744 (14) | 0.354 (3) | 0.6320 (12) | 0.026 (4)* | |
| N10 | 0.76940 (11) | 0.46866 (17) | 0.22440 (10) | 0.0272 (3) | |
| H10 | 0.9901 (15) | 0.379 (3) | 0.5923 (13) | 0.029 (4)* | |
| C11 | 0.91574 (13) | 0.4415 (2) | 0.59654 (12) | 0.0277 (3) | |
| H11 | 0.8636 (16) | 0.712 (3) | 0.6428 (13) | 0.039 (5)* | |
| C12 | 0.94012 (13) | 0.6466 (3) | 0.64456 (12) | 0.0355 (3) | |
| H12 | 0.9946 (17) | 0.630 (3) | 0.7176 (16) | 0.051 (5)* | |
| H13 | 0.9802 (15) | 0.731 (3) | 0.6063 (14) | 0.044 (5)* |
Ethyl (E)-2-cyano-3-(thiophen-2-yl)acrylate (2) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0208 (5) | 0.0392 (6) | 0.0210 (5) | 0.0009 (4) | 0.0081 (4) | 0.0005 (4) |
| C1 | 0.0179 (6) | 0.0224 (6) | 0.0235 (7) | −0.0005 (4) | 0.0055 (5) | −0.0001 (4) |
| O2 | 0.0170 (5) | 0.0334 (5) | 0.0205 (5) | 0.0013 (3) | 0.0050 (4) | 0.0002 (3) |
| C2 | 0.0196 (6) | 0.0236 (6) | 0.0203 (7) | 0.0004 (4) | 0.0062 (5) | 0.0002 (4) |
| C3 | 0.0205 (7) | 0.0220 (7) | 0.0225 (7) | 0.0002 (4) | 0.0071 (5) | −0.0001 (4) |
| C4 | 0.0202 (7) | 0.0236 (6) | 0.0203 (7) | −0.0006 (4) | 0.0077 (5) | −0.0004 (4) |
| S5 | 0.0193 (2) | 0.0285 (2) | 0.0190 (2) | −0.00064 (10) | 0.00624 (14) | −0.00010 (10) |
| C6 | 0.0221 (6) | 0.0269 (7) | 0.0234 (7) | −0.0009 (5) | 0.0033 (5) | 0.0002 (5) |
| C7 | 0.0196 (6) | 0.0290 (7) | 0.0264 (8) | −0.0006 (5) | 0.0046 (5) | 0.0004 (5) |
| C8 | 0.0210 (7) | 0.0240 (6) | 0.0236 (7) | −0.0003 (4) | 0.0068 (5) | −0.0001 (4) |
| C9 | 0.0178 (6) | 0.0245 (7) | 0.0231 (7) | 0.0000 (4) | 0.0040 (5) | 0.0003 (4) |
| N10 | 0.0213 (6) | 0.0371 (7) | 0.0236 (6) | 0.0006 (4) | 0.0073 (5) | 0.0007 (4) |
| C11 | 0.0195 (6) | 0.0398 (7) | 0.0212 (7) | 0.0042 (5) | 0.0024 (5) | 0.0014 (5) |
| C12 | 0.0229 (6) | 0.0484 (9) | 0.0318 (8) | 0.0018 (6) | 0.0032 (5) | −0.0091 (7) |
Ethyl (E)-2-cyano-3-(thiophen-2-yl)acrylate (2) . Geometric parameters (Å, º)
| O1—C1 | 1.2012 (18) | C6—H6 | 0.99 (2) |
| C1—O2 | 1.3438 (15) | C7—C8 | 1.408 (2) |
| C1—C2 | 1.4855 (19) | C7—H7 | 1.01 (2) |
| O2—C11 | 1.4600 (16) | C8—H8 | 0.938 (19) |
| C2—C3 | 1.3592 (18) | C9—N10 | 1.1501 (19) |
| C2—C9 | 1.4322 (18) | C11—C12 | 1.506 (2) |
| C3—C4 | 1.4350 (19) | C11—H9 | 0.969 (16) |
| C3—H3 | 0.964 (18) | C11—H10 | 0.976 (16) |
| C4—C8 | 1.3899 (19) | C12—H11 | 0.982 (18) |
| C4—S5 | 1.7323 (14) | C12—H12 | 1.00 (2) |
| S5—C6 | 1.7062 (15) | C12—H13 | 0.974 (19) |
| C6—C7 | 1.365 (2) | ||
| O1—C1—O2 | 124.87 (13) | C6—C7—H7 | 124.0 (14) |
| O1—C1—C2 | 123.94 (12) | C8—C7—H7 | 123.2 (14) |
| O2—C1—C2 | 111.18 (11) | C4—C8—C7 | 112.58 (13) |
| C1—O2—C11 | 115.27 (10) | C4—C8—H8 | 123.9 (13) |
| C3—C2—C9 | 123.10 (13) | C7—C8—H8 | 123.5 (13) |
| C3—C2—C1 | 117.88 (12) | N10—C9—C2 | 178.99 (14) |
| C9—C2—C1 | 119.01 (11) | O2—C11—C12 | 111.16 (12) |
| C2—C3—C4 | 129.73 (13) | O2—C11—H9 | 107.1 (10) |
| C2—C3—H3 | 116.7 (11) | C12—C11—H9 | 113.1 (10) |
| C4—C3—H3 | 113.5 (11) | O2—C11—H10 | 103.1 (10) |
| C8—C4—C3 | 123.09 (13) | C12—C11—H10 | 111.3 (10) |
| C8—C4—S5 | 110.48 (11) | H9—C11—H10 | 110.5 (13) |
| C3—C4—S5 | 126.43 (10) | C11—C12—H11 | 110.2 (11) |
| C6—S5—C4 | 91.90 (7) | C11—C12—H12 | 107.6 (13) |
| C7—C6—S5 | 112.23 (11) | H11—C12—H12 | 111.4 (15) |
| C7—C6—H6 | 131.8 (13) | C11—C12—H13 | 111.0 (11) |
| S5—C6—H6 | 116.0 (13) | H11—C12—H13 | 107.7 (15) |
| C6—C7—C8 | 112.81 (12) | H12—C12—H13 | 109.0 (15) |
| O1—C1—O2—C11 | 1.03 (17) | C2—C3—C4—S5 | 0.03 (19) |
| C2—C1—O2—C11 | −178.07 (10) | C8—C4—S5—C6 | 0.27 (9) |
| O1—C1—C2—C3 | −2.45 (17) | C3—C4—S5—C6 | 179.99 (11) |
| O2—C1—C2—C3 | 176.65 (10) | C4—S5—C6—C7 | −0.31 (10) |
| O1—C1—C2—C9 | 178.04 (11) | S5—C6—C7—C8 | 0.27 (14) |
| O2—C1—C2—C9 | −2.85 (15) | C3—C4—C8—C7 | −179.90 (11) |
| C9—C2—C3—C4 | 0.7 (2) | S5—C4—C8—C7 | −0.17 (13) |
| C1—C2—C3—C4 | −178.77 (11) | C6—C7—C8—C4 | −0.06 (16) |
| C2—C3—C4—C8 | 179.71 (12) | C1—O2—C11—C12 | −83.41 (13) |
Ethyl (E)-2-cyano-3-(thiophen-2-yl)acrylate (2) . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C3—H3···O1 | 0.964 (19) | 2.444 (19) | 2.7998 (18) | 101.5 (14) |
| C3—H3···O1i | 0.964 (19) | 2.45 (2) | 3.3436 (18) | 153.4 (15) |
| C6—H6···N10ii | 0.99 (2) | 2.49 (2) | 3.465 (2) | 169.1 (18) |
| C8—H8···O1i | 0.94 (2) | 2.50 (2) | 3.3047 (19) | 143.6 (17) |
Symmetry codes: (i) −x+1, −y+1, −z+1; (ii) −x+1, −y+1, −z.
Funding Statement
This work was funded by Al al-Bayt University grant . Deutsche Forschungsgemeinschaft grant .
References
- Al-Refai, M., Geyer, A., Marsch, M. & Ali, B. F. (2014). J. Chem. Crystallogr. 44, 407–414.
- Al-Refai, M., Ibrahim, M. M., Geyer, A., Marsch, M. & Ali, B. F. (2016). J. Chem. Crystallogr. 46, 331–340.
- Asiri, A. M., Al-Youbi, A. O., Alamry, K. A., Faidallah, H. M., Ng, S. W. & Tiekink, E. R. T. (2011). Acta Cryst. E67, o2315. [DOI] [PMC free article] [PubMed]
- Brenna, E., Crotti, M., Gatti, F. G., Monti, D., Parmeggiani, F., Powell, R. W. III, Santangelo, S. & Stewart, J. D. (2015). Adv. Synth. Catal. 357, 1849–1860.
- Brenna, E., Gatti, F. G., Manfredi, A., Monti, D. & Parmeggiani, F. (2013). Catal. Sci. Technol. 3, 1136–1146.
- Castro Agudelo, B., Cárdenas, J. C., Macías, M. A., Ochoa-Puentes, C. & Sierra, C. A. (2017). Acta Cryst. E73, 1287–1289. [DOI] [PMC free article] [PubMed]
- Crystal Impact (2014). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Faggi, E., Aguilera, J., Sáez, R., Pujol, F., Marquet, J., Hernando, J. & Sebastián, R. M. (2019). Macromolecules, 52, 2329–2339.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- He, J., Liu, Y., Gao, J. & Han, L. (2017). Photochem. Photobiol. Sci. 16, 1049–1056. [DOI] [PubMed]
- Ibrahim, M. M., Al-Refai, M., Ali, B. F., Geyer, A., Harms, K. & Marsch, M. (2019). IUCrData, 4, x191046.
- Kalkhambkar, R. G., Gayathri, D., Gupta, V. K., Kant, R. & Jeong, Y. T. (2012). Acta Cryst. E68, o1482. [DOI] [PMC free article] [PubMed]
- Kong, D., Li, M., Wang, R., Zi, G. & Hou, G. (2016). Org. Biomol. Chem. 14, 1216–1220. [DOI] [PubMed]
- Liu, L., Song, J., Lu, H., Wang, H. & Bo, Z. (2016). Polym. Chem. 7, 319–329.
- Liu, Q., Kong, F.-T., Okujima, T., Yamada, H., Dai, S.-Y., Uno, H., Ono, N., You, X.-Z. & Shen, Z. (2012). Tetrahedron Lett. 53, 3264–3267.
- Miyazaki, E., Okanishi, T., Suzuki, Y., Ishine, N., Mori, H., Takimiya, K. & Harima, Y. (2011). Bull. Chem. Soc. Jpn, 84, 459–465.
- Qian, S., Xie, Z., Liu, J., Li, M., Wang, S., Luo, N. & Wang, C. (2018). J. Org. Chem. 83, 14768–14776. [DOI] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Stoe & Cie (2016). X-AREA and LANA. Stoe & Cie, Darmstadt, Germany.
- Xu, D., Li, Z., Peng, Y.-X., Geng, J., Qian, H.-F. & Huang, W. (2016). Dyes Pigments, 133, 143–152.
- Yuvaraj, H., Gayathri, D., Kalkhambkar, R. G., Gupta, V. K. & Rajnikant (2011). Acta Cryst. E67, o2135. [DOI] [PMC free article] [PubMed]
- Zhou, N., Prabakaran, K., Lee, B., Chang, S. H., Harutyunyan, B., Guo, P., Butler, M. R., Timalsina, A., Bedzyk, M. J., Ratner, M. A., Vegiraju, S., Yau, S., Wu, C.-G., Chang, R. P. H., Facchetti, A., Chen, M.-C. & Marks, T. J. (2015). J. Am. Chem. Soc. 137, 4414–4423. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) 1, 2, New_Global_Publ_Block. DOI: 10.1107/S2056989019011435/tx2012sup1.cif
Structure factors: contains datablock(s) 1. DOI: 10.1107/S2056989019011435/tx20121sup2.hkl
Structure factors: contains datablock(s) 2. DOI: 10.1107/S2056989019011435/tx20122sup3.hkl
Supporting information file. DOI: 10.1107/S2056989019011435/tx20121sup4.cml
Supporting information file. DOI: 10.1107/S2056989019011435/tx20122sup5.cml
Supporting information file. DOI: 10.1107/S2056989019011435/tx2012sup6.pdf
Additional supporting information: crystallographic information; 3D view; checkCIF report