Abstract
Over the last decade, interest in the therapeutic potential of cannabis and its constituents (e.g. cannabidiol) in the management of inflammatory bowel diseases (IBD) has escalated. Cannabis has been increasingly approved for a variety of medical conditions in several jurisdictions around the world. In animal models, cannabinoids have been shown to improve intestinal inflammation in experimental models of IBD through their interaction with the endocannabinoid system. However, the few randomized controlled trials of cannabis or cannabidiol in patients with IBD have not demonstrated efficacy in modulating inflammatory disease activity. Cannabis may be effective in the symptomatic management of IBD. Given the increasing utilization and cultural acceptance of cannabis, physicians need to be aware of its safety and efficacy in order to better counsel patients. The aim of this review is to provide an overview of the role of cannabis in the management of patients with IBD.
Keywords: cannabis, cannabidiol, Crohn’s, inflammatory bowel disease, ulcerative colitis
Introduction
The inflammatory bowel diseases (IBD), which comprise Crohn’s disease (CD) and ulcerative colitis (UC), are chronic immune-mediated diseases of the gastrointestinal tract.1 IBD is characterized by periods of inflammatory flares, quiescence, and relapse, which places a substantial psychologic, emotional, and symptomatic burden on affected individuals.2 The incidence and prevalence of IBD globally continues to rise.3–7 The current therapeutic goals in managing patients with IBD are: a reduction in inflammation, elimination of symptoms, improvement in quality of life, and the prevention of complications.8 Advances in the understanding of the immunopathological mechanisms behind the disease have led to the development of a number of new effective therapies used in the induction and maintenance of remission of disease activity.9 These have reduced the need for surgery and hospitalizations, and have led to improved patient quality of life.10 Current therapies, however, are not effective in all patients, and patients that do respond often lose response over time11,12 Some patients also complain of symptoms even when inflammation is controlled and in remission. Cannabis may be an adjunct to medications in controlling inflammation, as well as improving a patient’s symptoms and quality of life.13
Cannabis, a drug made up of the flowers and buds of the Cannabis sativa plant, has been used therapeutically for centuries.14,15 Ancient Chinese cultures have reported use in their medical practices, dating back as early as 2700 BC.15 Although widely used recreationally during the 19th and 20th centuries, the use of medical cannabis has exploded over the last decade, as a result of mainstream cultural acceptance and legalization in several countries around the world.
Cannabis contains over 100 different constituents, with Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) being the most prominent and best characterized.16 These act on the endocannabinoid system (ECS),17 and have demonstrated analgesic and antinociceptive activity in several animal and human models.18–21 The primary clinical use of cannabis has been in the management of acute and chronic pain. It may be useful in reducing the use and dependence on opioids in the management of pain. The constituents of cannabis, however, also act on a number of other central and peripheral receptors, and can impact cytokine and immunoglobulin production as well as control immune cell migration.22–24 Cannabis has been demonstrated to have anti-inflammatory effects, and therefore may be useful in the treatment of a number of chronic inflammatory conditions including IBD.25,26
Patients will often turn to complementary medications, including cannabis, in the management of their IBD. Many physicians are unaware of the potential therapeutic role of cannabis, due to its relatively recent emergence and legalization for medical use.27,28 They should, however, be familiar with the its current use, evidence for a role in symptom management and disease control, as well as potential adverse effects, in order to safely advise patients. The aim of this review is to provide an overview of the role of cannabis in the management of patients with IBD. Relevant manuscripts were included after a comprehensive abstract review of the PubMed library and the Cochrane database using the keywords ‘cannabis, cannabidiol, inflammatory bowel disease, Crohn’s disease and ulcerative colitis’.
Cannabis: mechanistic considerations
Cannabis contains a number of chemically active compounds, including cannabinoids, terpenoids, flavonoids, and alkaloids.29 The most active constituent is the cannabinoid, THC, which is responsible for the well-characterized psychotropic effects associated with cannabis use. There are, however, over 100 other active cannabinoids that have been identified, each which modulates one or more components of the body’s ECS.30 A basic knowledge of the ECS is essential to understand how cannabis may be beneficial in patients with IBD.
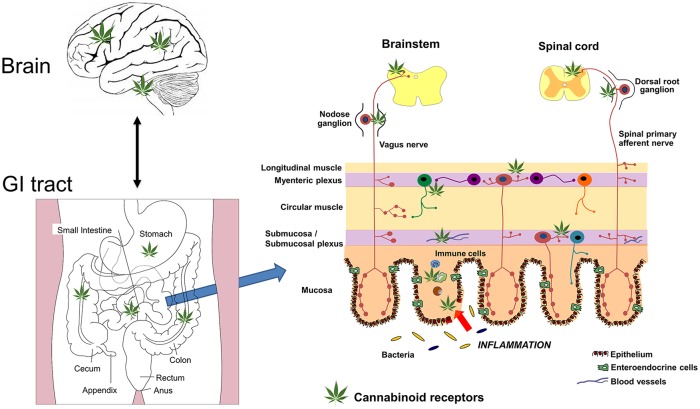
The ECS consists of cannabinoid receptors, endogenous ligands for these receptors, and the enzymes for their synthesis and degradation.17,30 The human body naturally produces endocannbinoids, which are lipid-mediators that bind to cannabinoid receptors, found throughout the body (Figure 1). This complex system is involved in regulating a number of cognitive and physiological processes in the central nervous system including pain, mood, appetite, stress, and memory.17,31,32 In addition, the ECS regulates physiological processes outside of the brain, including those in the heart, gastrointestinal tract, reproductive system, and bone, to name a few.33–37 Physiological and pharmacological studies have demonstrated the ECS is widely distributed throughout the gastrointestinal tract, and is involved in regulation of food intake, emesis, gastric secretion, gastric and intestinal motility, visceral sensation, and intestinal inflammation.38 The cannabinoids found in cannabis (phytocannabinoids) act as exogenous ligands for several of the receptors of the ECS.29 Several synthetic cannabinoids structurally analogous to the endocannabinoids have also been developed, and these share a similar biological action.
Figure 1.
A schematic representation of the sites of action of cannabis (depicted by the cannabis leaf) in the brain gut axis. Cannabis acts predominantly on CB1 in the brain and the enteric nervous system of the gastrointestinal tract. CB2 receptors, located on immune cells, are also sites of action of cannabis, and these cells will be increased in states of inflammation. CB1 receptors may also regulate gut barrier function at the level of the epithelium. Visceral pain is regulated by CB1 receptors on spinal primary afferents and in the spinal cord. Nausea and vomiting is regulated by CB1 and possibly CB2 receptors in the brainstem (vomiting) and the insular cortex (nausea). Adapted from Maccarrone and colleagues.39
The two primary endocannabinoid receptors that have been isolated are the G-protein coupled cannabinoid receptors CB1 and CB2.40 CB1 receptors are located primarily on neural tissue including central and peripheral neurons as well as the enteric nervous system (Figure 1).41 CB2 receptors are also expressed in the central nervous system, although most are located in immune tissues, including neutrophils, macrophages, epithelial cells, and subsets of T and B cells.41,42 In general, CB1 signaling mediates neuromodulatory function, while CB2 signaling mediates immunomodulatory activity.
CB1 receptors are important in the control of gastrointestinal motility and gastric intestinal secretion.37,43 CB1 receptor agonists were shown to reduce gastrointestinal propulsion and transit in animal models.44,45 Although the action on secretory function has been less studied, one study did demonstrate that gastric acid secretion was reduced with CB1 activation.46 This suggests a therapeutic potential for the treatment of diarrhea in patients with IBD.
CB1 receptor activation has also been demonstrated to increase appetite, and to promote food intake and energy conservation.47
Several in vitro studies have demonstrated the importance of both the CB1 and CB2 in modulating intestinal inflammation. CB1 and CB2 receptor agonists reduced experimentally induced intestinal inflammation in several murine studies, whereas antagonists were shown to exacerbate inflammation.48–50 The ECS was physiologically involved in protecting against excessive colonic inflammation with activation of these receptors, dampening smooth muscle irritation and controlling cellular pathways involved in inflammatory responses.50 Activation of the CB1 and CB2 receptors was also demonstrated to reduce visceral sensitivity and pain associated with colonic distention in animal models.51–53
In addition to CB1 and CB2, other molecular targets for the cannabinoids have been identified, including the G-protein coupled receptors 18, 55, and 119 (GRP55 and GRP119); the peroxisome proliferator-activated receptor (PPAR); the serotonin-1A receptor (5-HT1A); and the transient receptor potential vanilloid 1 (TRPV1.)38,54–56 The administration of cannabis, through its interaction with the various receptors of the ECS, modulates the gastrointestinal system by increasing appetite and reducing nausea, gastric secretions, intestinal contractility, peristalsis, visceral sensation as well as intestinal inflammation (Figure 1).38
Cannabis use in patients with IBD
Patients with IBD often turn to complementary medications, including various forms of cannabis, to combat symptoms related to their disease. Patients have reported using cannabis to relieve symptoms of abdominal pain, nausea, diarrhea, anorexia, as well as to improve mood and quality of life.57–59 In an anonymous questionnaire-based study, IBD patients reported that cannabis improved abdominal pain (83.9%), abdominal cramping (76.8%), joint pain (48.2%), and, to a lesser extent, diarrhea (28.6%).57
Large epidemiological studies to determine the proportion of people with IBD that use cannabis have been difficult to perform due to the legalities associated with cannabis use. Several patient-reported studies indicate that between 6.8 and 17.6% of IBD patients actively use cannabis.27,57–60
A Spanish study demonstrated that 10% of IBD patients used cannabis; however, only one-third of these patients informed their physician about active use.27 A larger internet based survey of 1666 IBD patients across the United States reported that only 12.8% had discussed use with their physician.61
A Canadian study performed in 2011 evaluated 291 patients with IBD and found that 11.6% with UC and 16% with CD were active users,58 with 51% of patients with UC and 48% of patients with CD reporting cannabis use during their lifetime. In this latter study, patients with a history of abdominal surgery, chronic analgesic use, and complementary medicine use were more likely to use cannabis for symptom relief. A large population-based study explored the patterns of cannabis use in patients with IBD.60 They demonstrated that patients with IBD were more likely to have used cannabis as compared with non-IBD controls.60 Patients with IBD also had an earlier age of onset of cannabis use (15.7 years versus 19.6 years). There does appear to be a high rate of cannabis use among younger patients with IBD, with a study in a pediatric IBD clinic (18–21 years of age) reporting that 70% of 53 patients surveyed were current or past marijuana users.62
Modes of administration
There are a number of potential ways of consuming cannabis, including inhalation, oral ingestion, or through topical application. Inhalation, which includes smoking or inhaling vapors formed by local heating (‘vaping’) rapidly elevate THC levels in the blood stream, with an effect starting in 15–30 min and typically lasting 2–3 h.63 Oral preparations include ingestible oils, liquid extracts, or incorporating cannabis products with food. Consuming oral preparations results in a lower bioavailability and delayed onset of action, typically starting after around an hour and lasting for 6–8 h.64 Various topical preparations are available and are generally used for localized pain management.65 A number of factors influence cannabis absorption, including recent meals, and components of these meals, inhalation techniques, and the temperature of the vaporizer, which lead to significant variability in bioavailability.66
Cannabis as a therapeutic option for IBD
Crohn’s disease
A small observational study performed in Israel in 2011 was the first published report of cannabis as a therapeutic option in CD.67 Of the 30 patients reviewed, 21 demonstrated significant improvement in subjective disease activity, as defined by a ⩾ 4-point reduction in Harvey Bradshaw Index (HBI) following cannabis treatment. Cannabis use was associated with reduced need for other medications as well as surgery. Safety data was not reported in this study. The same research group performed the first randomized controlled trial of medical cannabis in CD,68 with 21 patients randomized to cannabis (twice daily cigarettes containing 115 mg of THC) or placebo. Clinical remission, as defined by a Crohn’s Disease Activity Index (CDAI) <150, was achieved in 45% in the cannabis group and 10% in the placebo group; however, this did not meet statistical significance. Of those treated with cannabis, 90% demonstrated a clinical response (decrease in CDAI of >100) and 25% were also able to stop corticosteroid therapy. Cannabis use was associated with significant improvements in quality of life, pain scores, and appetite, but did not demonstrate improvement in objective markers such as C reactive protein (CRP). There were no differences in adverse effects between groups.
A larger anonymous questionnaire-based study looking at cannabis use in 313 consecutive IBD patients looked at pattern of use and subjective, beneficial, and adverse effects associated with self-administration of cannabis.57 Cannabis was used by 17.6% of patients, with reported improvements in abdominal pain, cramping, joint pain, and diarrhea. The use of cannabis for more than 6 months in patients with CD was a strong predictor for requiring surgery, with an odds ratio of around 5. Cannabis use may be providing symptomatic benefit in these patients without modulating disease activity, thereby leading to progressive disease and increased complications including surgery. The results of this study, and other questionnaire-based studies, should be interpreted with caution due to a number of inherent biases. Participation was voluntary and anonymous, which may lead to selection bias in choosing patients that did use cannabis and wanted to promote cannabis. There may be a significant reporting bias if patients over or underestimate perceived benefit and side effects. Additionally, prior clinical trials and surveys did not include colonoscopy to assess mucosal healing. Colonoscopy is necessary to differentiate whether symptoms improved due to reduced inflammatory disease activity.
A more recent study investigated oral CBD in 19 patients with medically refractory CD.69 These patients were refractory to standard therapy (steroids, thiopurines, or TNF antagonists), and were randomized to receive 10 mg of oral CBD or placebo twice daily for 8 weeks. This was a negative study, with no improvement in disease activity as measured by the CDAI as well as several laboratory parameters between groups. There were, however, no significant differences in adverse effects in the treatment group as compared with placebo. This pilot study introduced the use of an oral formulation of cannabis as a therapeutic option in IBD. Further studies looking at different dosing and larger numbers of patients are required to assess efficacy in CD.
Ulcerative colitis
There have been even fewer clinical studies looking at cannabis as a treatment option in UC as compared with CD. The first randomized controlled trial was published in 2018 and investigated the safety and efficacy of a CBD rich botanical extract in patients with UC.70 Patients (n = 60) with mild-to-moderate ulcerative colitis, on a stable dose of mesalamine therapy, were randomized to receive a once daily oral capsule containing 50 mg CBD-rich botanical extract or placebo for 10 weeks. The CBD extract was not well tolerated, with 90% of patients reporting treatment-related adverse effects as compared with 48% receiving placebo. Adverse effects did lead to higher rates of discontinuation in the treatment group, the main side effect being dizziness, with resolution following treatment cessation. There was no difference in the primary endpoint of clinical remission (Mayo score of ⩽2 with no subscore >1) between groups (28% for CBD-extract and 26% for placebo.) There was, however, a trend toward improved quality of life scores and improvement in patients’ global impression of change based on per-protocol analysis.
An abstract presentation at European Crohn’s and Colitis Organization Congress (ECCO) 2018 reported on effects of smoking cannabis cigarettes as compared with placebo in 28 patients with moderate-to-severe UC.71 Those randomized to cannabis demonstrated improved clinical disease activity scores, with no significant improvement in endoscopic disease activity scores. Biomarkers, including fecal calprotectin and CRP, however, did not change significantly.
IBD-combined
A prospective observational study assessed the impact of inhaled cannabis on quality of life and disease activity.72 Patients (n = 13) with long standing IBD, (11 CD and 2 UC) were prescribed cigarettes (50 g dry processed plant/month) and followed up for 3 months. Patients reported improvements in health perception (p = 0.001), social functioning (p = 0.0002), ability work (p = 0.0005), physical pain (p = 0.004), and depression (p = 0.007). There were also significant improvements in disease activity in the patients with CD, measured by the HBI as well as a significant improvement in weight.
An unpublished meta-analysis of 100 patients from nonrandomized studies and randomized controlled trials up until July 2018, looking at safety and efficacy of cannabis/cannabinoids in IBD was presented at the ECCO Congress 2019.73 The authors concluded that cannabinoids were not effective in inducing remission of disease (RR = 1.29, 95% CI 0.68–2.47). They did report, however, significant differences in disease activity scores in the intervention group compared with the control group. There was improvement in symptom and quality of life scores associated with cannabinoids. The data suggest a limited efficacy of cannabis in inducing remission of disease activity. The current studies, however, are small, with various formulations, doses, and mechanisms of delivery. The main observational and randomized clinical trials are summarized in Table 1.
Table 1.
Summary of observational studies and clinical trials of cannabis in inflammatory bowel disease.
| Study | Year | Country | Study design | IBD | Number | Product | Safety | Findings |
|---|---|---|---|---|---|---|---|---|
| Naftali 67 | 2011 | Israel | Retrospective Observational | CD | 30 | Oral or Inhaled Cannabis | Not reported | Improvement in disease activity (⩾4 point reduction in HBI score). A reduction in need for other medications |
| Lahat 72 | 2012 | Israel | Prospective Observational | CD and UC | 13 | 50 g dry processed cigarettes per month | Not reported | Improvement in quality of life scores and disease activity indices (HBI) |
| Naftali 68 | 2013 | Israel | Prospective Placebo Controlled Trial | CD | 21 | Cigarettes containing 115 mg THC twice daily | No difference in adverse effects between groups | No difference in clinical remission. (CDAI score <150) Benefits in clinical response (decrease in CDAI of >100) and steroid use. Improvement in symptoms (sleep and appetite) |
| Naftali 69 | 2017 | Israel | Prospective Placebo Controlled Trial | CD | 19 | Oral CBD 10 mg twice daily | No difference in adverse effects between groups | No beneficial effects in IBD. (Decrease in CDAI >70) Safe and well tolerated |
| Irving 70 | 2018 | United Kingdom | Double Blind placebo controlled, Parallel-group | UC | 60 | Oral capsule containing 50 mg CBD rich botanical extract taken twice daily | Higher mild- moderate adverse effects in treatment group (90% versus 48% in placebo) | Not effective in inducing remission. (Mayo score of ⩽2 with no sub score >1) Improved quality of life and global impression of change scores. |
CBD, cannabidiol; CD, Crohn’s disease; CDAI, Crohn’s Disease Activity Index; HBI, Harvey-Bradshaw Index; IBD, inflammatory bowel diseases; THC, Δ9-tetrahydrocannabinol; UC, ulcerative colitis.
Safety and adverse effects
The long-term safety profile of cannabis in patients with IBD has not been established. The current studies, which include use of oral and inhaled formulations, describe common side effects, including headache, sleepiness, nausea, and dizziness.67–70 The study of the CBD-rich botanical extract reported increased rates of adverse effects in patients randomized to the therapeutic product as compared with placebo; however, the majority of these were mild-to-moderate in severity.70 Adverse effects did lead to higher rates of discontinuation in the treatment group. The main adverse effect reported was dizziness, with resolution following treatment cessation. There were no significant differences in adverse effects in the other randomized trials. Cannabis use has been associated with a number of adverse short- and long-term physical and psychological health effects in studies of the general population.74 The adverse effects are exacerbated in young people.
Short term
The acute effects of cannabis are usually transient, lasting a few minutes to hours after consumption.75 Cannabis has a number of psychotropic effects, the main reason for its recreational use. This varies between individuals, but can include euphoria, a heightened awareness of music and color, and increased appetite. However, it can have a number of negative central effects, including impaired working and episodic memory loss/lapse.76,77 Memory impairment is usually dose dependent, with the onset of effect occurring more rapidly if inhaled rather than if consumed orally. Cannabis has also been shown increase impulsivity, reduce inhibition, and impair decision-making.78
Cannabis and its active component, THC, is known to produce transient, dose-dependent psychotic symptoms, including hallucinations and delusions, in human studies.79 Heavy use, particularly with high potency forms, and early age of onset of use, increase the risk of psychotic symptoms and development of psychotic illness.79–81 Other short-term adverse effects that have been described include panic, anxiety, tachycardia, and dry mouth.28
There has been a recent increase in the consumption of foods containing cannabis (edibles) in both the recreational and medicinal setting.82 The most significant difference between ingestion and inhalation of cannabis is the delayed onset of action associated with ingestion.64 This may lead to greater than intended consumption of the drug before it has taken effect, resulting in serious adverse effects, overdose, and even death.82–84 To promote safe use and prevent adverse effects, it is important to educate patients on how edibles affect the body.
Long term
Prolonged cannabis has been associated with a number of long-term neurocognitive side effects. One of the main long-term consequences is the risk of addiction. Among individuals who experiment with cannabis, 9% became dependent, with higher rates of dependence if initially consumed as a teenager.85,86 As with other addictive substances, attempting to stop may lead to withdrawal symptoms, which makes cessation difficult.87 Cannabis has also been labeled a gateway drug, with increased susceptibility to other drug addictions.88
Although long-term memory impairments have been reported, confounding factors such as baseline cognitive function, concurrent use of other cognitively impairing substances, mental health comorbidities, and age of use make a causal relationship difficult to establish.75 Functional neuroimaging studies have demonstrated subtle differences in brain activity between cannabis users and controls; however, the clinical implications of these differences are difficult to determine.89 Use during adolescence, a period of brain development, is associated with reduced functional connectivity of the brain.90,91 This is reflected in a significant decline in IQ scores in adolescents that frequently use cannabis, with a greater decline in more persistent users.92
Cannabis use has been associated with a number of mental health issues. Epidemiological research suggests chronic use increases the risk of psychoses and may exacerbate the clinical course of patients with schizophrenia.80,81,93 There has also been an association with anxiety and depression.94–96
Prolonged cannabis use is associated with a number of physiological effects. Cannabis has also been demonstrated to have detrimental effects on male and female fertility, and may also impact fetal neurodevelopment.97–99 There has also been an association with increased cardiovascular risks, including ischemic strokes and myocardial infarctions, particularly with larger doses of cannabis.100 Smoking cannabis has also been associated with chronic bronchitis and increased rates of respiratory infection.101
The most commonly reported gastrointestinal side effect is the development of cannabis hyperemesis syndrome.102,103 This syndrome, seen in chronic cannabis users, is characterized by recurrent episodes of severe nausea, intractable vomiting, and abdominal pain, with a diagnosis based on the Rome IV criteria.104,105 The pathophysiology of the syndrome remains unknown; however, there may be an underlying genetic predisposition.106 Symptomatic relief may be achieved by taking a hot shower, with resolution of the problem with cannabis cessation.107
As with any medical therapy, the use of cannabis is associated with risks and adverse effects. Physicians should be aware of these potential risks. The limited studies in IBD have not reported any serious side effects but the follow-up duration was limited. Further studies are required to determine the long-term safety profile of cannabis in the management of IBD.
Cannabis in pregnancy
There is very limited data exploring the effects of cannabis during pregnancy and lactation.108 Cannabinoids are small lipophilic molecules and have been demonstrated to cross the placenta in several animal models.108,109 They have also been detected in breast milk; however, concentrations are low, at only 0.8% of the maternal exposure.110,111 In comparison, alcohol passes freely into breast milk at approximately the same concentration as in maternal blood.112
There is conflicting evidence about the impact of cannabis during pregnancy, with a number of observational studies in humans confounded by inaccurate reporting in use and confounding factors associated with use that may impact pregnancy outcomes.113 Several meta-analyses suggest that cannabis use may be associated with adverse outcomes, including low birth weigh, stillbirth, and preterm delivery.114–116 There is emerging evidence that cannabis may impact neurological development, impairing brain maturation, predisposing the neonate to neurodevelopmental disorders and long-term effects on cognitive function.108,109,114 Until more evidence about the short- and long-term effects of cannabis in pregnancy is available, women should be advised not to use it during pregnancy and while breastfeeding.
Guidelines and clinical recommendations
Due to the current lack of evidence for the efficacy of cannabis in IBD, clinical guidelines and recommendations to assist physicians remain limited. Two recent Cochrane database reviews that reviewed the evidence of cannabis in CD and UC, respectively, were unable to make any firm conclusions on the safety or efficacy of cannabis in IBD.117
The Canadian Association of Gastroenterology released a position statement in 2018 on the use of cannabis for several gastroenterologic and hepatologic disorders.118 They stated that cannabis does not appear to alter the course of the disease in IBD (for better or worse) based on the current evidence available. They have also recommended that medical cannabis should not replace current approved medical therapies for patients with IBD.
A clinical, scientific, and regulatory review commissioned by the American Crohn’s and Colitis Foundation recently concluded that, although cannabis may help control symptoms and improve quality of life in patients with IBD, it has not been demonstrated to modify disease behavior.13 This article outlined the current legal, regulatory, and logistic hurdles in investigating medical cannabis for IBD in the United States.
Conclusion and future directions
There is emerging evidence that cannabis may play a role in the management of patients with IBD. Many patients are already using cannabis to help manage symptoms associated with the disease, and physicians cannot ignore this when taking histories and managing their patients.
The current studies that have explored the use of cannabis in IBD have demonstrated improvement in a number of gastrointestinal symptoms as well as patient quality of life. There has been no clear evidence, however, that cannabis modulates inflammation or improves disease activity. The majority of the current literature is retrospective observational data. The few randomized trials that have been performed are small and insufficiently powered to detect any clinically significant differences between cannabis and placebo. Large prospective randomized controlled studies with standardized preparations of cannabis and long-term follow up are required to assess the effectiveness of cannabis in IBD. There have been many regulatory obstacles in research involving medical cannabis in a number of countries around the world, including the United States. A recent review, released by the Crohn’s and Colitis Foundation (United States) has called for government and regulatory policy change to facilitate further research into cannabinoid-based therapies.13
Patients often do not report cannabis use to their treating physician. As with any drug, cannabis is associated with potential risks and long-term adverse effects. To better counsel patients, it is important for physicians managing IBD to be aware of how patients are using cannabis to manage their symptoms, the current evidence for its use, as well as potential adverse effects.
In summary, cannabis may improve clinical symptoms but has not demonstrated any improvement in disease activity in IBD. The risks and benefits of use should be considered for each individual patient. Cannabis should not replace current effective therapies in IBD, but can be used as a complementary adjunct in certain patients. IBD patients who are taking cannabis should be advised against taking it if their medical and family history dictates otherwise (e.g. pregnancy, age, family history of mental illness, etc.). Further large clinical trials looking at different formulations, doses, modes of administration, and long-term safety and efficacy are required to evaluate the effectiveness of cannabis in IBD.
Acknowledgments
KAS holds the Crohn’s Colitis Canada Chair in IBD Research at the University of Calgary.
GGK holds the Canadian Institute of Health Research Embedded Clinician Research Chair.
Footnotes
Author contributions: SP conducted the literature review and wrote the draft manuscript. SP and CHS planned the manuscript outline. All authors edited the manuscript and approved the final manuscript for submission.
Funding: The author(s) received no financial support for the research, authorship, and publication of this article.
Conflict of interest statement: SP has no conflicts.
GGK has received honoraria for speaking or consultancy from Abbvie, Janssen, Pfizer, and Takeda. He has received research support from Janssen, Abbvie, GlaxoSmith Kline, Merck, and Shire. He shares ownership of a patent: TREATMENT OF INFLAMMATORY DISORDERS, AUTOIMMUNE DISEASE, AND PBC. UTI Limited Partnership, assignee. Patent 62/555,397. 7 September 2017.
KAS is a consultant to Arena Pharmaceuticals, LaSanta Botanicals Ltd and Takeda Pharmaceuticals.
CHS has received honoraria for speaking or consultancy for Janssen, Takeda, Ferring, Abbvie, Pfizer, Shire and has received research support from Janssen.
ORCID iD: Cynthia H. Seow  https://orcid.org/0000-0002-1551-9054
https://orcid.org/0000-0002-1551-9054
Contributor Information
Sherman Picardo, Inflammatory Bowel Disease Unit, Department of Gastroenterology, Cumming School of Medicine, University of Calgary, AB, Canada.
Gilaad G. Kaplan, Inflammatory Bowel Disease Unit, Department of Gastroenterology, Cumming School of Medicine, University of Calgary, AB, Canada Department of Community Health Sciences, University of Calgary, AB, Canada.
Keith A. Sharkey, Hotchkiss Brain Institute and Snyder Institute for Chronic Diseases, Department of Physiology and Pharmacology, Cumming School of Medicine, University of Calgary, AB, Canada
Cynthia H. Seow, Inflammatory Bowel Disease Unit, Department of Gastroenterology, Cumming School of Medicine, University of Calgary, AB, Canada; Department of Community Health Sciences, University of Calgary, 3280 Hospital Drive NW, TRW building, Room 6D18, Calgary, AB T2N 4Z6, Canada.
References
- 1. Mowat C, Cole A, Windsor A, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011; 60: 571–607. [DOI] [PubMed] [Google Scholar]
- 2. Devlen J, Beusterien K, Yen L, et al. The burden of inflammatory bowel disease. Inflamm Bowel Dis 2014; 20: 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology 2017; 152: 313–321.e2. [DOI] [PubMed] [Google Scholar]
- 4. Coward S, Clement F, Benchimol EI, et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology 2019; 156: 1345–1353.e4. [DOI] [PubMed] [Google Scholar]
- 5. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017; 390: 2769–2778. [DOI] [PubMed] [Google Scholar]
- 6. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012; 142: 46–54.e42. [DOI] [PubMed] [Google Scholar]
- 7. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol 2015; 12: 720–727. [DOI] [PubMed] [Google Scholar]
- 8. Patton GC. Cannabis use and mental health in young people: cohort study. BMJ 2002; 325: 1195–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sokol H, Seksik P, Cosnes J. Complications and surgery in the inflammatory bowel diseases biological era. Curr Opin Gastroenterol 2014; 30: 378–384. [DOI] [PubMed] [Google Scholar]
- 10. Frolkis AD, Dykeman J, Negrón ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology 2013; 145: 996–1006. [DOI] [PubMed] [Google Scholar]
- 11. Allez M, Karmiris K, Louis E, et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohn’s Colitis 2010; 4: 355–366. [DOI] [PubMed] [Google Scholar]
- 12. Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther 2011; 33: 987–995. [DOI] [PubMed] [Google Scholar]
- 13. Herkenham M, Lynn AB, Little MD, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA 1990; 87: 1932–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Friedman D, Sirven JI. Historical perspective on the medical use of cannabis for epilepsy: ancient times to the 1980s. Epilepsy Behav 2017; 70: 298–301. [DOI] [PubMed] [Google Scholar]
- 15. Zuardi AW. History of cannabis as a medicine: a review. Rev Bras Psiquiatr 2006; 28: 153–157. [DOI] [PubMed] [Google Scholar]
- 16. Peschel W, Politi M. 1 H NMR and HPLC/DAD for cannabis sativa L. chemotype distinction, extract profiling and specification. Talanta 2015; 140: 150–165. [DOI] [PubMed] [Google Scholar]
- 17. Battista N, Di Tommaso M, Bari M, et al. The endocannabinoid system: an overview. Front Behav Neurosci 2012; 6: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schuelert N, McDougall JJ. Cannabinoid-mediated antinociception is enhanced in rat osteoarthritic knees. Arthritis Rheum 2008; 58: 145–153. [DOI] [PubMed] [Google Scholar]
- 19. Sánchez Robles EM, Bagües Arias A, Martín Fontelles MI. Cannabinoids and muscular pain. Effectiveness of the local administration in rat. Eur J Pain 2012; 16: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 20. Starowicz K, Malek N, Przewlocka B. Cannabinoid receptors and pain. Wiley Interdiscip Rev Membr Transp Signal 2013; 2: 121–132. [Google Scholar]
- 21. Ibrahim MM, Rude ML, Stagg NJ, et al. CB2 cannabinoid receptor mediation of antinociception. Pain 2006; 122: 36–42. [DOI] [PubMed] [Google Scholar]
- 22. Guabiraba R, Russo RC, Coelho AM, et al. Blockade of cannabinoid receptors reduces inflammation, leukocyte accumulation and neovascularization in a model of sponge-induced inflammatory angiogenesis. Inflamm Res 2013; 62: 811–821. [DOI] [PubMed] [Google Scholar]
- 23. Agudelo M, Newton C, Widen R, et al. Cannabinoid receptor 2 (CB2) mediates immunoglobulin class switching from IgM to IgE in cultures of murine-purified B lymphocytes. J Neuroimmune Pharmacol 2008; 3: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Basu S, Ray A, Dittel BN. Cannabinoid receptor 2 is critical for the homing and retention of marginal zone B lineage cells and for efficient T-independent immune responses. J Immunol 2011; 187: 5720–5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Formukong EA, Evans AT, Evans FJ. Analgesic and antiinflammatory activity of constituents of cannabis sativa L. Inflammation 1988; 12: 361–371. [DOI] [PubMed] [Google Scholar]
- 26. Naftali T, Mechulam R, Lev LB, et al. Cannabis for inflammatory bowel disease. Dig Dis 2014; 32: 468–474. [DOI] [PubMed] [Google Scholar]
- 27. García-Planella E, Marín L, Domènech E, et al. Use of complementary and alternative medicine and drug abuse in patients with inflammatory bowel disease. Med Clin (Barc) 2007; 128: 45–48. [DOI] [PubMed] [Google Scholar]
- 28. Gerich ME, Isfort RW, Brimhall B, et al. Medical marijuana for digestive disorders: high time to prescribe? Am J Gastroenterol 2015; 110: 208–214. [DOI] [PubMed] [Google Scholar]
- 29. Andre CM, Hausman JF, Guerriero G. Cannabis sativa: the plant of the thousand and one molecules. Front Plant Sci 2016; 7: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mechoulam R, Hanuš LO, Pertwee R, et al. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat Rev Neurosci 2014; 15: 757–764. [DOI] [PubMed] [Google Scholar]
- 31. Pertwee RG, Ross RA. Cannabinoid receptors and their ligands. Prostaglandins, Leukot Essent Fat Acids 2002; 66: 101–121. [DOI] [PubMed] [Google Scholar]
- 32. Rodríguez de, Fonseca F, Del Arco I, Bermudez-Silva FJ, et al. The endocannabinoid system: physiology and pharmacology. Alcohol Alcohol 2005; 40: 2–14. [DOI] [PubMed] [Google Scholar]
- 33. Brents LK. Marijuana, the endocannabinoid system and the female reproductive system. Yale J Biol Med 2016; 89: 175–91. [PMC free article] [PubMed] [Google Scholar]
- 34. du Plessis SS, Agarwal A, Syriac A. Marijuana, phytocannabinoids, the endocannabinoid system, and male fertility. J Assist Reprod Genet 2015; 32: 1575–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alfulaij N, Meiners F, Michalek J, et al. Cannabinoids, the heart of the matter. J Am Heart Assoc 2018; 7: pii: e009099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bab I, Ofek O, Tam J, et al. Endocannabinoids and the regulation of bone metabolism. J Neuroendocrinol 2008; 20: 69–74. [DOI] [PubMed] [Google Scholar]
- 37. Pertwee RG. Cannabinoids and the gastrointestinal tract. Gut 2001; 48: 859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Izzo AA, Sharkey KA. Cannabinoids and the gut: new developments and emerging concepts. Pharmacol Ther 2010; 126: 21–38. [DOI] [PubMed] [Google Scholar]
- 39. Maccarrone M, Bab I, Bíró T, et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol Sci 2015; 36: 277–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Duncan M, Davison JS, Sharkey KA. Review article: endocannabinoids and their receptors in the enteric nervous system. Aliment Pharmacol Ther 2005; 22: 667–683. [DOI] [PubMed] [Google Scholar]
- 41. Massa F, Storr M, Lutz B. The endocannabinoid system in the physiology and pathophysiology of the gastrointestinal tract. J Mol Med 2005; 83: 944–954. [DOI] [PubMed] [Google Scholar]
- 42. Wright K, Rooney N, Feeney M, et al. Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing. Gastroenterology 2005; 129: 437–453. [DOI] [PubMed] [Google Scholar]
- 43. Coutts AA, Izzo AA. The gastrointestinal pharmacology of cannabinoids: an update. Curr Opin Pharmacol 2004; 4: 572–579. [DOI] [PubMed] [Google Scholar]
- 44. Coutts AA, Irving AJ, Mackie K, et al. Localisation of cannabinoid CB1 receptor immunoreactivity in the guinea pig and rat myenteric plexus. J Comp Neurol 2002; 448: 410–422. [DOI] [PubMed] [Google Scholar]
- 45. Mancinelli R, Fabrizi A, Del Monaco S, et al. Inhibition of peristaltic activity by cannabinoids in the isolated distal colon of mouse. Life Sci 2001; 69: 101–111. [DOI] [PubMed] [Google Scholar]
- 46. Coruzzi G, Adami M, Coppelli G, et al. Inhibitory effect of the cannabinoid receptor agonist WIN 55,212–2 on pentagastrin-induced gastric acid secretion in the anaesthetized rat. Naunyn Schmiedebergs Arch Pharmacol 1999; 360: 715–718. [DOI] [PubMed] [Google Scholar]
- 47. Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci 2005; 8: 585–589. [DOI] [PubMed] [Google Scholar]
- 48. Kimball ES, Schneider CR, Wallace NH, et al. Agonists of cannabinoid receptor 1 and 2 inhibit experimental colitis induced by oil of mustard and by dextran sulfate sodium. Am J Physiol Liver Physiol 2006; 291: G364–G371. [DOI] [PubMed] [Google Scholar]
- 49. Storr MA, Keenan CM, Zhang H, et al. Activation of the cannabinoid 2 receptor (CB2) protects against experimental colitis. Inflamm Bowel Dis 2009; 15: 1678–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Massa F, Marsicano G, Hermann H, et al. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest 2004; 113: 1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sanson M, Bueno L, Fioramonti J. Involvement of cannabinoid receptors in inflammatory hypersensitivity to colonic distension in rats. Neurogastroenterol Motil 2006; 18: 949–956. [DOI] [PubMed] [Google Scholar]
- 52. Fioramonti J, Bueno L. Role of cannabinoid receptors in the control of gastrointestinal motility and perception. Expert Rev Gastroenterol Hepatol 2008; 2: 385–397. [DOI] [PubMed] [Google Scholar]
- 53. Kikuchi A, Ohashi K, Sugie Y, et al. Pharmacological evaluation of a novel cannabinoid 2 (CB2) ligand, PF-03550096, in vitro and in vivo by using a rat model of visceral hypersensitivity. J Pharmacol Sci 2008; 106: 219–224. [DOI] [PubMed] [Google Scholar]
- 54. Guerrero-Alba R, Barragán-Iglesias P, González-Hernández A, et al. Some prospective alternatives for treating pain: the endocannabinoid system and its putative receptors GPR18 and GPR55. Front Pharmacol 2018; 9: 1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. O’Sullivan SE, Kendall DA. Cannabinoid activation of peroxisome proliferator-activated receptors: Potential for modulation of inflammatory disease. Immunobiology 2010; 215: 611–616. [DOI] [PubMed] [Google Scholar]
- 56. Resstel LBM, Tavares RF, Lisboa SFS, et al. 5-HT 1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br J Pharmacol 2009; 156: 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Storr M, Devlin S, Kaplan GG, et al. Cannabis use provides symptom relief in patients with inflammatory bowel disease but is associated with worse disease prognosis in patients with Crohn’s disease. Inflamm Bowel Dis 2014; 20: 472–480. [DOI] [PubMed] [Google Scholar]
- 58. Lal S, Prasad N, Ryan M, et al. Cannabis use amongst patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol 2011; 23: 891–896. [DOI] [PubMed] [Google Scholar]
- 59. Ravikoff Allegretti J, Courtwright A, Lucci M, et al. Marijuana use patterns among patients with inflammatory bowel disease. Inflamm Bowel Dis 2013; 19: 2809–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Weiss A, Friedenberg F. Patterns of cannabis use in patients with inflammatory bowel disease: a population based analysis. Drug Alcohol Depend 2015; 156: 84–89. [DOI] [PubMed] [Google Scholar]
- 61. Kerlin AM, Long M, Kappelman M, et al. Profiles of patients who use marijuana for inflammatory bowel disease. Dig Dis Sci 2018; 63: 1600–1604. [DOI] [PubMed] [Google Scholar]
- 62. Phatak UP, Rojas-Velasquez D, Porto A, et al. Prevalence and patterns of marijuana use in young adults with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2017; 64: 261–264. [DOI] [PubMed] [Google Scholar]
- 63. Varlet V, Concha-Lozano N, Berthet A, et al. Drug vaping applied to cannabis: is “Cannavaping” a therapeutic alternative to marijuana? Sci Rep 2016; 6: 25599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schauer GL, King BA, Bunnell RE, et al. Toking, vaping, and eating for health or fun. Am J Prev Med 2016; 50: 1–8. [DOI] [PubMed] [Google Scholar]
- 65. Jorge LL, Feres CC, Teles VE. Topical preparations for pain relief: efficacy and patient adherence. J Pain Res 2010; 4: 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers 2007; 4: 1770–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Naftali T, Lev LB, Yablecovitch D, et al. Treatment of Crohn’s disease with cannabis: an observational study. Isr Med Assoc J 2011; 13: 455–458. [PubMed] [Google Scholar]
- 68. Naftali T, Bar-Lev Schleider L, Dotan I, et al. Cannabis induces a clinical response in patients with Crohn’s disease: a prospective placebo-controlled study. Clin Gastroenterol Hepatol 2013; 11: 1276–1280.e1. [DOI] [PubMed] [Google Scholar]
- 69. Naftali T, Mechulam R, Marii A, et al. Low-dose cannabidiol is safe but not effective in the treatment for Crohn’s disease, a randomized controlled trial. Dig Dis Sci 2017; 62: 1615–1620. [DOI] [PubMed] [Google Scholar]
- 70. Irving PM, Iqbal T, Nwokolo C, et al. A Randomized, double-blind, placebo-controlled, parallel-group, pilot study of cannabidiol-rich botanical extract in the symptomatic treatment of ulcerative colitis. Inflamm Bowel Dis 2018; 24: 714–724. [DOI] [PubMed] [Google Scholar]
- 71. Naftali T, Bar Lev Schlieder L, Sklerovsky Benjaminov F, et al. P398 cannabis induces clinical and endoscopic improvement in moderately active ulcerative colitis (UC). J Crohn’s Colitis 2018; 12: S306–S306. [Google Scholar]
- 72. Lahat A, Lang A, Ben-Horin S. Impact of cannabis treatment on the quality of life, weight and clinical disease activity in inflammatory bowel disease patients: a pilot prospective study. Digestion 2012; 85: 1–8. [DOI] [PubMed] [Google Scholar]
- 73. Doeve B, van Schaik F, van de Meeberg M, et al. P448 cannabis and cannabinoids for the treatment of inflammatory bowel disease: a systematic review and meta-analysis. J Crohn’s Colitis 2019; 13: S335–S336. [Google Scholar]
- 74. Ford TC, Hayley AC, Downey LA, et al. Cannabis: an overview of its adverse acute and chronic effects and its implications. Curr Drug Abuse Rev 2018; 10: 6–18. [DOI] [PubMed] [Google Scholar]
- 75. Curran HV, Freeman TP, Mokrysz C, et al. Keep off the grass? Cannabis, cognition and addiction. Nat Rev Neurosci 2016; 17: 293–306. [DOI] [PubMed] [Google Scholar]
- 76. Curran V, Brignell C, Fletcher S, et al. Cognitive and subjective dose-response effects of acute oral Δ 9 -tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology (Berl) 2002; 164: 61–70. [DOI] [PubMed] [Google Scholar]
- 77. Crane NA, Schuster RM, Fusar-Poli P, et al. Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychol Rev 2013; 23: 117–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Crean RD, Crane NA, Mason BJ. An evidence-based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med 2011; 5: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Murray RM, Englund A, Abi-Dargham A, et al. Cannabis-associated psychosis: neural substrate and clinical impact. Neuropharmacology 2017; 124: 89–104. [DOI] [PubMed] [Google Scholar]
- 80. Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet 2007; 370: 319–328. [DOI] [PubMed] [Google Scholar]
- 81. van Os J. Cannabis use and psychosis: a longitudinal population-based study. Am J Epidemiol 2002; 156: 319–327. [DOI] [PubMed] [Google Scholar]
- 82. Barrus DG, Capogrossi KL, Cates SC, et al. Tasty THC: promises and challenges of cannabis edibles. Methods Rep RTI Press. Epub ahead of print 15 November 2016. DOI: 10.3768/rtipress.2016.op.0035.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chen YC, Klig JE. Cannabis-related emergencies in children and teens. Curr Opin Pediatr 2019; 31: 291–296. [DOI] [PubMed] [Google Scholar]
- 84. Cao D, Srisuma S, Bronstein AC, et al. Characterization of edible marijuana product exposures reported to United States poison centers. Clin Toxicol 2016; 54: 840–846. [DOI] [PubMed] [Google Scholar]
- 85. Lopez-Quintero C, Cobos JP, de los Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the national epidemiologic survey on alcohol and related conditions (NESARC). Drug Alcohol Depend 2011; 115: 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet 2009; 374: 1383–1391. [DOI] [PubMed] [Google Scholar]
- 87. Gorelick DA, Levin KH, Copersino ML, et al. Diagnostic criteria for cannabis withdrawal syndrome. Drug Alcohol Depend 2012; 123: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Agrawal A, Neale M, Prescott CA, et al. A twin study of early cannabis use and subsequent use and abuse/dependence of other illicit drugs. Psychol Med 2004; 34: 1227–1237. [DOI] [PubMed] [Google Scholar]
- 89. Batalla A, Bhattacharyya S, Yücel M, et al. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS One 2013; 8: e55821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zalesky A, Solowij N, Yucel M, et al. Effect of long-term cannabis use on axonal fibre connectivity. Brain 2012; 135: 2245–2255. [DOI] [PubMed] [Google Scholar]
- 91. Filbey F, Yezhuvath U. Functional connectivity in inhibitory control networks and severity of cannabis use disorder. Am J Drug Alcohol Abuse 2013; 39: 382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA 2012; 109: E2657–E2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Caspi A, Moffitt TE, Cannon M, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-o-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry 2005; 57: 1117–1127. [DOI] [PubMed] [Google Scholar]
- 94. Patton GC, Coffey C, Carlin JB, et al. Cannabis use and mental health in young people: cohort study. BMJ 2002; 325: 1195–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Buckner JD, Zvolensky MJ, Smits JAJ, et al. Anxiety sensitivity and marijuana use: an analysis from ecological momentary assessment. Depress Anxiety 2011; 28: 420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bovasso GB. Cannabis abuse as a risk factor for depressive symptoms. Am J Psychiatry 2001; 158: 2033–2037. [DOI] [PubMed] [Google Scholar]
- 97. Hsiao P, Clavijo RI. Adverse effects of cannabis on male reproduction. Eur Urol Focus 2018; 4: 324–328. [DOI] [PubMed] [Google Scholar]
- 98. Merlob P, Stahl B, Klinger G. For debate: does cannabis use by the pregnant mother affect the fetus and newborn? Pediatr Endocrinol Rev 2017; 15: 4–7. [DOI] [PubMed] [Google Scholar]
- 99. Richardson KA, Hester AK, McLemore GL. Prenatal cannabis exposure – The ‘first hit’ to the endocannabinoid system. Neurotoxicol Teratol 2016; 58: 5–14. [DOI] [PubMed] [Google Scholar]
- 100. Jouanjus E, Raymond V, Lapeyre-Mestre M, et al. What is the current knowledge about the cardiovascular risk for users of cannabis-based products? A systematic review. Curr Atheroscler Rep 2017; 19: 26. [DOI] [PubMed] [Google Scholar]
- 101. Owen KP, Sutter ME, Albertson TE. Marijuana: respiratory tract effects. Clin Rev Allergy Immunol 2014; 46: 65–81. [DOI] [PubMed] [Google Scholar]
- 102. Simonetto DA, Oxentenko AS, Herman ML, et al. Cannabinoid hyperemesis: a case series of 98 patients. Mayo Clin Proc 2012; 87: 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Heise L. Cannabinoid hyperemesis syndrome. Adv Emerg Nurs J 2015; 37: 95–101. [DOI] [PubMed] [Google Scholar]
- 104. Wild K, Wilson H. Cannabinoid hyperemesis. Emerg Med J 2012; 29: 67–69. [DOI] [PubMed] [Google Scholar]
- 105. Aziz I, Palsson OS, Whitehead WE, et al. Epidemiology, clinical characteristics, and associations for Rome IV functional nausea and vomiting disorders in adults. Clin Gastroenterol Hepatol 2019; 17: 878–886. [DOI] [PubMed] [Google Scholar]
- 106. Venkatesan T, Levinthal DJ, Li BUK, et al. Role of chronic cannabis use: cyclic vomiting syndrome vs cannabinoid hyperemesis syndrome. Neurogastroenterol Motil 2019; 31: e13606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Allen JH. Cannabinoid hyperemesis: cyclical hyperemesis in association with chronic cannabis abuse. Gut 2004; 53: 1566–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Grant KS, Petroff R, Isoherranen N, et al. Cannabis use during pregnancy: pharmacokinetics and effects on child development. Pharmacol Ther 2018; 182: 133–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mark K, Terplan M. Cannabis and pregnancy: maternal child health implications during a period of drug policy liberalization. Prev Med (Baltim) 2017; 104: 46–49. [DOI] [PubMed] [Google Scholar]
- 110. Bertrand KA, Hanan NJ, Honerkamp-Smith G, et al. Marijuana use by breastfeeding mothers and cannabinoid concentrations in breast milk. Pediatrics 2018; 142: e20181076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Djulus J, Moretti M, Koren G. Marijuana use and breastfeeding. Can Fam Physician 2005; 51: 349–50. [PMC free article] [PubMed] [Google Scholar]
- 112. Haastrup MB, Pottegård A, Damkier P. Alcohol and breastfeeding. Basic Clin Pharmacol Toxicol 2014; 114: 168–173. [DOI] [PubMed] [Google Scholar]
- 113. Cook JL, Blake JM. Cannabis: implications for pregnancy, fetal development, and longer-term health outcomes. J Obstet Gynaecol Canada 2018; 40: 1204–1207. [DOI] [PubMed] [Google Scholar]
- 114. Metz TD, Stickrath EH. Marijuana use in pregnancy and lactation: a review of the evidence. Am J Obstet Gynecol 2015; 213: 761–778. [DOI] [PubMed] [Google Scholar]
- 115. Gunn JKL, Rosales CB, Center KE, et al. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open 2016; 6: e009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Conner SN, Bedell V, Lipsey K, et al. Maternal marijuana use and adverse neonatal outcomes. Obstet Gynecol 2016; 128: 713–723. [DOI] [PubMed] [Google Scholar]
- 117. Kafil TS, Nguyen TM, MacDonald JK, et al. Cannabis for the treatment of ulcerative colitis. Cochrane database Syst Rev 2018; 11: CD012954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Andrews CN, Devlin SM, Le Foll B, et al. Canadian association of gastroenterology position statement: use of cannabis in gastroenterological and hepatic disorders. J Can Assoc Gastroenterol 2019; 2: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]