Abstract
Background:
Metabolic syndrome (MetS) has been associated with colorectal adenomas and cancer. However, MetS definitions have changed over time, leading to a heterogeneity of patients included in previous studies and a substantial inextensibility of observations across time or eastern and western populations. Our aim was to evaluate the association of ‘harmonized’ criteria-defined MetS and its individual components with colorectal neoplasia and cancer in a western population.
Methods:
In this multicenter, cross-sectional study, we prospectively evaluated consecutive outpatients who underwent open-access colonoscopy over a 3-month period. MetS was diagnosed according to the 2009 ‘harmonized’ criteria.
Results:
Out of 5707 patients enrolled, we found 213 cancers (3.7%), 1614 polyps (28.3%), 240 nonpolypoid lesions (4.2%), 95 laterally spreading tumors (1.6%). Polyps presented histological low-grade dysplasia in 72.9% of samples, while in 9.8%, high-grade dysplasia or in situ carcinoma was present; dysplasia rates for nonpolypoid lesions were 66.2% (low-grade) and 2.9% (high-grade/in situ carcinoma), while for laterally spreading tumors, 29.6% and 37%, respectively. Overall, MetS prevalence was 41.6%. MetS correlated with both adenomas [odds ratio (OR): 1.76, 95% confidence interval (CI) 1.54–2.00] and cancer (OR: 1.92, 95% CI 1.42–2.58). MetS was the only risk factor for such colonic lesions in subjects younger than 50 years. For all colonic neoplasia, we found MetS and not its individual components to be significantly associated.
Conclusions:
MetS is risk factor for cancer and adenoma in Whites, especially when younger than 50 years. MetS patients might be considered as a high-risk population also in colorectal cancer screening programs.
Keywords: colon cancer, colorectal adenoma, CRC, metabolic syndrome, MetS
Introduction
Colorectal cancer (CRC) is an important health issue, since one million new cases are diagnosed worldwide each year, with half a million related deaths.1,2 In most cases it is sporadic in nature, with age being the only major risk factor reported.3 Metabolic syndrome (MetS), that is, the combination of cardiovascular risk factors, such as obesity, hypertension, diabetes and dyslipidemia,4–6 has been identified as a potential risk factor for cancer,7 including sporadic CRC.8–10 The prevalence of MetS ranges between 34.8% and 41.9% in the US and 18% and 46% in Europe, depending on age, geographic location or demographic features of the population studied,11,12 thus raising relevant public health implications. In fact, although screening programs based on age and fecal occult blood have proven effective in reducing CRC mortality,13 identification of other potential risk factors, such as MetS, may further improve cost effectiveness of these screening strategies by favoring a more detailed risk-based stratification of the target populations. Although the concept of metabolic syndrome has been widely accepted for a long time,14,15 there was no largely recognized international definition until 1998 and onward,16–19 up to the ‘harmonized’ classification in 200920 that deeply revised the concept of MetS. This has led to the identification of heterogeneous target populations based on the adopted definition. Two recent meta-analyses, although showing an association between MetS and colon cancer, have calculated I2 values indicative of a significant inhomogeneity among studies,21,22 thus weakening their conclusions.
The primary objective of this study, therefore, was to prospectively evaluate the association between MetS, defined according to the 2009 criteria, and colonic neoplastic lesions in consecutive patients undergoing colonoscopy; secondary objectives were to evaluate the role of individual components of MetS in indicating a specific risk for neoplastic disease and, finally, if MetS is a risk factor independent of age.
Methods
Study participants and design
This study has been conducted in 50 open-access endoscopy units, evenly distributed throughout Italy. Every unit prospectively enrolled consecutive outpatients undergoing colonoscopy in a 3-month period.
The indications for performing colonoscopy were abdominal symptoms (impaired bowel habitus, pain, hematochezia, anemia, weight loss, abdominal mass on physical examination), follow up [previous polypectomy, previous colonic surgery, inflammatory bowel disease (IBD)], colon cancer screening [with or without previous positive fecal occult blood (FOBT), voluntary colonoscopy if over 50 years of age, positive family history for CRC]. Patients were excluded in case of failure of cecum intubation, poor bowel cleaning, defined as persistence of solid or semisolid debris that could not be effectively cleaned,23 age below 30 years, impossibility to obtain anthropometric measurements, polyposis syndrome, advanced neoplastic disease, impossibility to obtain written informed consent. Before implementation, this study was approved by the ethical committee of each participating center and informed consent was obtained from all participants.
For each subject, blood pressure, height, weight, and waist circumference were measured. Interviewers completed a medication history for each participant, including use of insulin, oral hypoglycemic agents, antihypertensive drugs, and hypolipidemic agents. Medical history assessment included also occupation, use of low-dose acetylsalicylic acid, and hormone replacement therapy in women. Self-reported lack of physical activity was also recorded.24
Laboratory results for fasting plasma glucose, high-density lipoprotein cholesterol (HDL-C), total cholesterol and triglyceride levels were obtained. Since biochemistry data could not be centralized, blood tests had to be performed in sites complying with the updated European guidelines on accreditation of medical laboratories.
Quality control
Eligible endoscopy units were required to have performed at least 1000 colonoscopies in the previous year and to have already participated in at least one previous multicenter study.25 This ensured that all endoscopists adhered to previously validated quality criteria regarding colonoscopic examination and identification of superficial lesions, in order to avoid heterogeneous diagnoses and noncomparable results. In fact, all endoscopists had already been trained to recognize and classify colonic superficial lesions according to the Paris classification.26 A 1-day start-up preparatory meeting of at least one investigator from each participating unit was held 3 months prior to the initiation of the study, in order to discuss the protocol and instruct all participants to achieve homogeneous blood pressure and anthropometric measurements.
Blood pressure was obtained sphygmomanometrically, according to standard protocols and techniques.27 Height was measured without shoes to the nearest centimeter; weight was measured without shoes or heavy outer clothing. Circumferences were measured to the nearest centimeter using a stretch-resistant tape that provides constant tension over light clothing. Waist circumference was measured halfway between the lower ribs and the iliac crest at the end of a normal expiration, when the lungs are at their functional residual capacity, while hip circumference was measured at the largest circumference around the buttocks with the tape parallel to the floor, as per World Health Organization recommendations.28
Outpatients were consecutively enrolled; endoscopists were blinded to the metabolic status of the examinees.
Definitions
Metabolic syndrome
According to the ‘harmonized’ criteria set forth by a joint scientific multisocietal committee in 2009,20 MetS is defined as the coexistence of three or more of the following risk factors: waist circumference equal or greater than 102 cm in men and equal or greater than 88 cm in women, serum triglyceride concentration of 150 mg/dl or greater; HDL-C concentration of less than 40 mg/dl for men or less than 50 mg/dl for women; blood pressure of 130/85 mmHg or greater; fasting plasma glucose concentration of 100 mg/dl or greater. Individuals who were using antidiabetics, antihypertensive or hypolipidemic drugs were treated as meeting the criteria for the affected variable.
Colonic lesions and histology
Masses or severely excavated and ulcerated lesions were classified as advanced neoplasia/cancer. Colonic superficial neoplastic lesions (SNLs) were classified according to the Paris classification26 and subgrouped as polypoid (pedunculated or sessile, 0-Ip and 0-Is, respectively) and nonpolypoid lesions (NPLs) if measuring less than 2.5 mm in height. LSTs (laterally spreading tumors) were defined as lesions measuring less than 2.5 mm in height and more than 10mm in width, as in previous studies.25,29,30 For each type of lesion, size and number were recorded; in case of multiple lesions, measurements were performed on the largest. Location of colorectal neoplasia was defined according to anatomic site. Cecum, ascending colon, hepatic flexure, transverse colon, and splenic flexure were defined as proximal colon; descending and sigmoid colon were defined as distal colon, whereas the rectum was considered separately. Subjects who had lesions in both the proximal and the distal colon were defined as having lesions on both sides. A positive colonoscopy was defined as presence of one or more of the aforementioned lesion types.
All detected lesions, where possible, were resected endoscopically. Biopsies were obtained only in case of suspected advanced cancers or nonresectable lesions that, when confirmed as such, were referred for surgery or palliation. Therefore, histological analysis of superficial lesions was based on resected lesions; in case of multiple resections, the most advanced histological pattern was considered for analysis. All specimens were reviewed by an experienced and dedicated pathologist at each center, who was blinded to the patients’ metabolic status.
Histological findings were classified as hyperplastic lesions, low-grade intraepithelial neoplasia (LGIN: serrated adenoma, tubular adenoma, tubulovillous adenoma, villous adenoma), high-grade intraepithelial neoplasia (HGIN: SNLs revealing high-grade dysplasia or intraepithelial neoplasia or carcinoma in situ31), while lesions with invasive features were defined as submucosal carcinoma, as per current literature.25 Lesions presenting either LGIN or HGIN were subsequently grouped together under the term adenoma, for statistical analysis.
Statistical analysis
Descriptive analysis included calculations of rates and proportions for categorical data, as well as means and standard deviations (SDs) for continuous data. Differences between means of continuous variables were analyzed using the Student’s t test, while the χ2 test was used for categorical variables. All p values were two tailed, and p values below 0.05 were considered statistically significant.
A standard logistic regression model was used to estimate the association between positive colonoscopy, CRC, SNLs, MetS and demographic, anthropometric, lifestyle and biochemical data by univariate analysis. Significantly associated variables were considered for multivariable analysis, with the additional inclusion of age, sex, absence of physical activity and body mass index (BMI) > 30, unless otherwise specified in individual tables or figures. Results are presented as odds ratio (OR) with a 95% confidence interval (95% CI). Analyses were performed using the SPSS version 17.0 statistical package (SPSS, Inc., Chicago, IL).
Results
In the present study, 5707 subjects out of 5824 have been enrolled; 117 were excluded mainly due to missing data. Demographic, anthropometric and laboratory data are shown in Table 1. Indications for colonoscopy were presence of symptoms in 48%, follow up 38%, colon cancer screening 17%. No previous colonoscopy was reported in 62% of cases.
Table 1.
Baseline characteristics of study participants.
| Population | Positive colonoscopy (n = 2010) |
Negative colonoscopy
(n = 3697) |
||
|---|---|---|---|---|
| Sex | ||||
| Male | 53.3% | 59.7% | 49.9% | p < 0.001 |
| Female | 46.7% | 40.3% | 50.1% | (Between sexes) |
| Age, years (mean ± SD) | 61 ± 13.1 | 64.3 ± 10.76 | 59 ± 13.87 | p < 0.001 |
| No physical activity | 78.9% | 81.5% | 77.4% | p < 0.001 |
| Metabolic variables | ||||
| Waist circumference (cm; mean ± SD) | 94.2 ± 13.4 | 96.79 ± 13.1 | 92.8 ± 13.4 | p < 0.001 |
| Men | 97.3 ± 12.1 | 99.05 ± 11.7 | 96.17 ± 11.7 | p < 0.001 |
| Women | 90.7 ± 13.9 | 93.47 ± 14.21 | 89.48 ± 13.57 | p < 0.001 |
| Blood pressure | ||||
| Diastolic blood pressure | 77.56 ± 3.4 | 78.81 ± 9.25 | 76.96 ± 9.39 | p < 0.001 |
| Systolic blood pressure | 128 ± 16.9 | 130.73 ± 16.82 | 126.79 ± | p < 0.001 |
| Fasting glucose (⩾100 mg/dl) | 34.60% | 42.4% | 30.3% | p < 0.001 |
| Triglycerides (⩾150 mg/dl) | 35.80% | 42.5% | 32.3% | p < 0.001 |
| HDL cholesterol | 42.90% | 46% | 41.2% | p < 0.001 |
| BMI (kg/m2; mean ± SD) | 26.35 ± 4.3 | 27.02 ± 4.21 | 25.98 ± 4.36 | p < 0.001 |
| <25 | 40.8% | 33.4% | 44.8% | |
| 25–30 | 41% | 45.3% | 38.7% | |
| ⩾30 | 18.2% | 21.4% | 16.5% | |
| ASA within group (%) | 5.9% | 5.9% | 5.8% | p = n.s. |
| Statins within group (%) | 14.1% | 17.9% | 12% | p < 0.001 |
| Metabolic syndrome in each group | 41.60% | 53.9% | 35% | p < 0.001 |
| 0 component | 11.2% | 7.7% | 13.1% | |
| 1 component | 23.4% | 19.2% | 25.8% | |
| 2 components | 23.7% | 19.3% | 26.2% | |
| 3 components | 20.8% | 26.6% | 17.7% | |
| 4 components | 14.3% | 18.3% | 12.1% | |
| 5 components | 6.6% | 9.1% | 5.2% | |
ASA, acetylsalicylic acid; BMI, body mass index; HDL, high-density lipoprotein; n.s., nonsignificant; SD, standard deviation.
Colonoscopy was positive for SNL/CRC in 35.2% (2010/5707) of the procedures, for a total of 2162 lesions observed. Of these, 90.1% were SNL and 9.8% were cancers. Four cases of familiar adenomatous polyposis were diagnosed at this stage and therefore excluded. The majority of neoplasia were sessile polyps (62%); 20.8% were pedunculated, 15.4% were flat elevated and 1.7% were flat depressed lesions. Detailed histological grading of endoscopic findings is shown in Table 2.
Table 2.
Histological patterns of superficial neoplastic lesions (n = 1949).
| Polyp |
NPL |
LST |
||||
|---|---|---|---|---|---|---|
| n | % (within category) | n | % (within category) | n | % (within category) | |
| Hyperplastic | 255 | 15.7% | 70 | 29.1% | 5 | 3.7% |
| LGIN | 1177 | 72.9% | 159 | 66.2% | 45 | 29.6% |
| HGIN/Cis | 159 | 9.8% | 7 | 2.9% | 36 | 37.0% |
| Submucosal cancer | 23 | 1.4% | 4 | 1.6% | 9 | 29.6% |
| Total | 1614 (82.8%) | 240 (12.3%) | 95 (4.8%) | |||
Cis, Carcinoma in situ; HGIN, high-grade intraepithelial neoplasia; LGIN, low-grade intraepithelial neoplasia; LST, laterally spreading tumor; NPL, nonpolypoid lesion; Submucosal cancer, neoplasia extending beyond the lamina propria.
MetS was found in 41.6% of subjects enrolled; it was significantly more prevalent among females (females 43.4% versus males 40.1%; p < 0.05). In both sexes, MetS positively correlated with older age (mean age of MetS versus non-MetS, males 65.3 ± 10.5 versus 58 ± 13.7 years; p < 0.001, females 65.8 ± 10.7 versus 56.1 ± 13.6 years; p < 0.001). Regarding geographic distribution, MetS was found more frequently in individuals from southern Italy (south versus central or northern Italy: OR 1.48, 95% CI: 1.11–2.19; p < 0.001). Northern and central populations were similarly affected.
Male sex (OR 1.48, 95% CI: 1.33–1.66), age > 50 years (OR 3.57, 95% CI: 3.01–4.24), absence of self-reported regular physical exercise (OR 1.29, 95% CI: 1.12–1.48), obesity (OR 1.38, 95% CI: 1.20–1.58) and MetS (OR 2.17, 95% CI: 1.94–2.43) were significantly associated to a positive colonoscopy at univariate analysis. At multivariable analysis, MetS (OR 1.84, 95% CI: 1.63–2.08; p < 0.001), age > 50 years (OR 3.00, 95% CI: 2.51–3.59; p < 0.001) and male sex (OR 1.50, 95% CI: 1.34–1.69; p < 0.001) remained statistically significant when corrected for age, sex, absence of physical exercise and obesity. Notably, obesity did not show any association with colonoscopy outcomes. Overall, MetS significantly associated by multivariable analysis with both adenoma and cancer (respectively, OR 1.76, 95% CI: 1.54–2.00 and OR 1.92, 95% CI: 1.42–2.58; p < 0.001), as did age > 50 years (respectively, OR 3.16, 95% CI: 2.56–3.90 and OR 2.83, 95% CI: 1.59–5.04; p < 0.001); this remained true when colonic and rectal lesions were considered separately. Male sex significantly associated with colorectal adenoma (OR 1.47, 95% CI: 1.30–1.67; p < 0.001) but not cancer, while the opposite was true for subjects reporting no regular physical exercise (OR 1.98, 95% CI: 1.25–3.14; p < 0.01; see Table 3). No differences in terms of anatomical distribution of lesions were observed between sexes; we observed a tendency for a more proximal localization in MetS subjects, although this did not achieve statistical significance. MetS was significantly associated to the presence of both polypoid and NPLs (p < 0.05), but not to their size and number; moreover, no association was found with LSTs.
Table 3.
Multivariable-adjusted estimates of odds ratios for superficial neoplastic lesions and colorectal cancer.
| Variables | Adenoma OR (95% CI) |
p | Cancer OR (95% CI) |
p |
|---|---|---|---|---|
| Age > 50 years | 3.16 (2.56–3.90) | <0.001 | 2.83 (1.59–5.04) | <0.001 |
| Male sex | 1.47 (1.30–1.67) | <0.001 | 1.23 (0.93–1.63) | n.s. |
| No physical activity | 0.99 (0.84–1.16) | n.s. | 1.98 (1.25–3.14) | <0.01 |
| Metabolic syndrome | 1.76 (1.54–2.00) | <0.001 | 1.92 (1.42–2.58) | <0.001 |
| Obesity | 1.05 (0.90–1.23) | n.s. | 0.92 (0.65–1.3) | n.s. |
CI, confidence interval; n.s., nonsignificant; OR, odds ratio.
In order to take into account the possibility that colonoscopic neoplasias were more prevalent in symptomatic patients, we performed a multivariable analysis correcting for all indications for colonoscopy (detailed in the Materials and Methods section), as well as sex, age, absence of physical activity and obesity; MetS was again confirmed as independently associated with adenomas (OR 1.78, 95% CI: 1.58–2.00, p < 0.05) and CRC (OR 1.86; 95% CI: 1.40–2.49, p < 0.05). In a subgroup analysis on patients undergoing screening colonoscopies (n = 967), we found 464 lesions (367 adenomas and 24 cancers). In this subgroup, at multivariable analysis, MetS was again associated to adenomas (OR 2.23, 95% CI: 1.70–2.92, p < 0.05). Association with cancer was not statistically significant (OR 1.19, 95% CI: 0.53–2.70, p > 0.05), possibly due to the low number of occurrences (n = 28).
None of the components of MetS was individually associated with a positive colonoscopy in multivariable analysis (Table 4). However, a statistically significant association was observed when at least three components were simultaneously present, as per MetS definition, in both adenomas and CRC (p < 0.05; Figure 1). MetS as a risk factor for neoplastic lesions was independent of BMI; its role was maintained in lean (BMI < 25, OR 2.27, 95% CI: 1.84–2.79), overweight (BMI ⩾ 25 but <30, OR 1.74, 95% CI: 1.47–2.05), and obese subjects (BMI ⩾ 30, OR 2.55, 95% CI: 1.90–3.41, p < 0.05).
Table 4.
Univariate and multivariable analysis of metabolic factors associated with neoplastic findings at colonoscopy.
| Variable | Univariate OR (95% CI) |
p | Multivariable adjusted*
OR (95% CI) |
p |
|---|---|---|---|---|
| Elevated blood pressure | 2.04 (1.80–2.32) | <.0001 | 1.10 (0.94–1.29) | 0.2192 |
| Fasting plasma glucose | 1.69 (1.51–1.89) | <.0001 | 1.00 (0.86–1.15) | 0.982 |
| Waist circumference | 1.46 (1.31–1.63) | <.0001 | 1.06 (0.92–1.23) | 0.3993 |
| Triglycerides | 1.55 (1.38–1.73) | <.0001 | 0.92 (0.79–1.08) | 0.3151 |
| HDL cholesterol | 1.22 (1.09–1.36) | 0.0007 | 0.84 (0.72–1.08) | 0.2573 |
Adjustment was made for each component of MetS, as well as age, sex and absence of physical activity.
CI, confidence interval; HDL, high-density lipoprotein; MetS, metabolic syndrome; OR, odds ratio.
Figure 1.
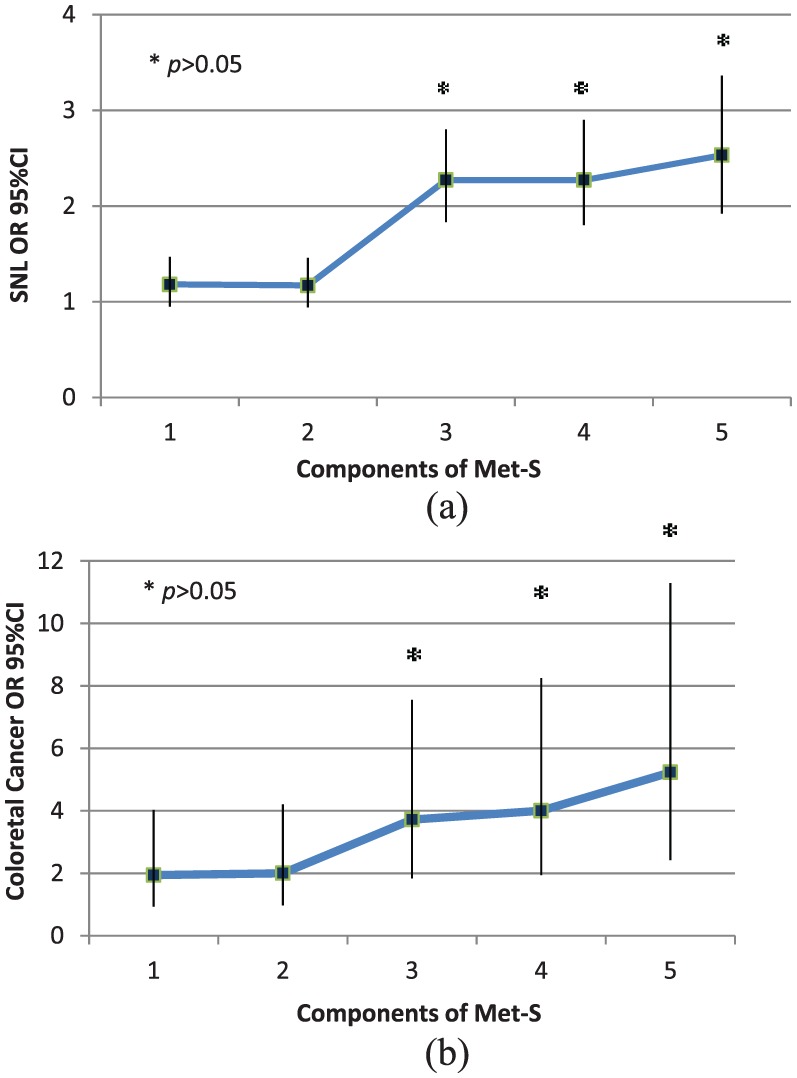
Occurrence of superficial neoplastic lesions or colorectal cancer according to the number of components of MetS present.
Occurrence of superficial neoplastic lesions (a) or colorectal cancer (b) according to the number of components of MetS present. For both SNL and CRC, statistical significance was achieved when at least three components were simultaneously present.
CI, confidence interval; CRC, colorectal cancer; MetS, metabolic syndrome; OR, odds ratio; SNL, superficial neoplastic lesions.
Finally, MetS was an important predictor of a positive outcome at colonoscopy in subjects younger than 50 years, which represented 19.9% of the studied population (555 male, 581 female, mean age 41.6 ± 6.38 years). Although the prevalence of colonic lesions within this age group was expectedly lower than in the elderly (15.8% versus 40.1%, respectively), in subjects with MetS, it was almost three times higher (2.7% versus 0.8%; p < 0.05). In addition, when analyzed in a multivariable model corrected for IBD, family history, sex, physical activity, and obesity, MetS was the only factor associated with the presence of colonic lesions (OR 2.59, CI 95%: 1.78–3.79, p < 0.05).
Discussion
Previous studies from Asian populations have shown that MetS is a risk factor for colonic adenomas.32–34 In one study35 conducted on a Korean population, MetS was associated to adenomas in the rectosigmoid colon; however, in this study, rectosigmoidoscopy only was performed, and modified Adult Treatment Panel criteria (using BMI instead of waist circumference) were used to diagnose MetS. Morita and colleagues33 confirmed the association of MetS with adenomas in the proximal colon for lesions greater than 5 mm. However, this study was restricted to male patients and a positive correlation was found only when the lowest cut-off for waist circumference was applied, thereby increasing the sensitivity of MetS diagnostic criteria. The importance of MetS was also highlighted in a recent 5-year follow-up study by Chiu and colleagues, showing that MetS is a significant risk factor for advanced adenoma in negative baseline colonoscopy and low-risk patients.36
Although the association between MetS and colorectal cancer has been previously investigated,37–39 available studies are widely heterogeneous in terms of the criteria used to diagnose the syndrome; in fact, some studies included features not previously adopted by any internationally formalized definition.37,40–43 Some authors have used BMI, an indicator of obesity rather than of a multifactorial metabolic disturbance, as a discriminatory component for MetS.39,43–45 Studies were also different in terms of design (cohort or association) and endpoints (mortality or incidence);45–47 variable adjustments for confounding factors and ethnicity may also account for disparity. Moreover, although it is known that colon and rectal cancer are different in terms of prevalence, natural history and prognosis, distinctions between them,38,40 or between male and female sex38,41 have variably been described, thus, making the results difficult to pool and interpret. Finally, evolving definitions of MetS have previously required mandatory criteria such as insulin resistance16 and waist circumference;18 as such, those individual criteria were, by nature, associated with endoscopic neoplasia because of selection bias.
For these reasons, in this cross-sectional, multicenter, observational study, we have applied the latest ‘harmonized’ criteria for the diagnosis of MetS20 in a well-defined population of 5707 White patients undergoing colonoscopy. This includes also patients with metabolic disturbances preceding prediabetes or diabetes, hypertension or obesity, thus, strengthening the role of MetS as a cluster of factors and not a mere association of unrelated phenotypes. Our data demonstrate that patients affected by MetS have a significantly increased risk of colonic and rectal adenomas and cancer at endoscopy.
To our knowledge, no consistent data from western countries on the relationship between MetS and colonic lesions are available. Large previous studies on western populations using older or not universally accepted definitions of MetS, many retrospective in nature, have variably reported a positive association between MetS and colonic adenoma or cancer,39,41,43,44,46,48,49 noting important differences between sexes. This is the first large prospective cross-sectional study that establishes an association between MetS, as defined by the current ‘harmonized’ criteria, and adenoma/CRC in a western population. In contrast to previous studies, we have shown that MetS is an independent significant risk factor for neoplastic lesions in the colon and rectum, both in men and women, in a large White population.
Our data demonstrate that patients affected by MetS have a significantly increased risk of colonic and rectal adenomas and cancer at endoscopy. None of the components of MetS when considered individually or in pairs, were associated with an increased risk of neoplasia. On the contrary, simultaneous presence of at least three out of five criteria abruptly increased this risk in a statistically significant fashion, regardless of age. This observation further strengthens the notion that MetS is more than the sum of its parts, potentially indicating an independent underlying pathophysiological drive for epithelial transformation at any age. A previous large retrospective case-control European study,45 utilizing multiple MetS definitions, including the ‘harmonized’ criteria, found a significant relative risk for colon cancer in affected patients irrespective of the definition used. However, the authors found that this risk was adequately accounted for by the presence of an abnormal glucose metabolism and abdominal obesity, rather than the syndrome itself. Furthermore, cases included only final CRC diagnoses, but not adenomatous lesions or specific endoscopic outcomes.
The potential implication of these findings is that a set of particular dysmetabolic alterations giving rise to MetS might be an important factor that modulates the transition from a hyperproliferative to an adenomatous epithelium.50 Environmental risk factors and dysmetabolic conditions (obesity, increased visceral adipose tissue, diabetes or insulin resistance, dyslipidemia) have been largely investigated in relation to CRC.8,51,52 Some have reported positive associations between CRC and obesity with an incremental risk of 1.24 for men and 1.09 for women per 5 kg/m2 increase.51 Others have shown that fat tissue distribution (waist circumference, hip circumference, waist-to-hip ratio), more than BMI, correlates with the risk of colorectal cancer or adenoma,32,53 and that a higher visceral adipose tissue volume is associated to an increased risk for adenoma at baseline and follow-up colonoscopy.54,55 Both adult-onset diabetes mellitus and glucose intolerance have been associated with an increased risk of colon cancer56,57 or adenoma.58,59 Elevated arterial blood pressure,60 hypertriglyceridemia and hypercholesterolemia61,62 have also been associated with CRC. The mechanisms underlying these associations are not completely understood, yet insulin resistance has been proposed as a key mechanism in promoting cancer through adipokine imbalance, reactive oxygen species and inflammatory cytokine release, such as tumor necrosis factor-alpha and interleukin-6, that act as ancillary pathways in this context.8,50,63–66
In our study, the association between MetS and colonic neoplasia was independent from obesity, as defined by BMI class. In fact, the correlation between this complex metabolic disturbance and colorectal adenoma or cancer was similar in either lean, overweight, or obese patients. Moreover, after accounting for MetS status, BMI-defined obesity showed no residual risk for colonic neoplasia, probably because MetS is a much more specific state of metabolic imbalance, transcending simple overweight/obesity. It has been noted that while BMI is generally an accurate index of obesity in the general population, it is less well correlated to the metabolic disturbances implied by such a status. In fact, it is precisely this drawback of BMI that led to the adoption of waist circumference as a marker of metabolic disturbance and a relevant component of MetS. It has been hypothesized that the partial lack of correlation between these anthropometric indices is due to the fact that waist circumference, a more specific marker of abdominal obesity, is a better predictor of the metabolically active visceral adipose tissue, as opposed to the less active peripheral fat.19,50,54 Previously, colon cancer risk has been reported as more strongly associated with abdominal obesity, persisting even after adjusting for BMI, while the relation between BMI and colon cancer was notably attenuated after adjustment for waist circumference.67 Thus, MetS should be considered more as including a subset of obese individuals, in whom body mass alone may not be a major factor associated with an increased cancer risk. This is of particular relevance to colonic neoplasia in which the role of other risk factors beyond age and genetics remains to be elucidated.
One additional interesting observation of our study is that patients younger than 50 years were also at risk for neoplasia at colonoscopy, if affected by MetS. This is in agreement with a Korean study on a prescreening population, showing that colorectal neoplasias were significantly more frequent in subjects 45–49 years old, if affected by MetS.68 These data might suggest that MetS, as defined by the ‘harmonized’ criteria, could be a useful addition to the current screening protocols, in addition to FOBT positivity and age beyond 50 years.
The main limitation of this study is the inclusion of consecutive subjects at colonoscopy, with a significant proportion of symptomatic patients. This design was deemed necessary due to local differences in colorectal cancer screening protocols across Italy, which are implemented on a regional basis. Yet, the per-protocol and post-hoc analyses confirm the role of MetS as a potential risk factor for colorectal neoplasia independently of symptoms.
In conclusion, MetS is associated with an increased risk for colorectal adenoma and carcinoma at colonoscopy, seemingly owing to its underlying dysmetabolic status, regardless of sex and age. This could be particularly useful when targeting younger populations for screening. To this day, aside from age and FOBT positivity, no other dependable clinical marker is used for risk stratification and prevention. Further studies are needed to establish the potential prospective value of MetS as a marker of increased risk for colonic neoplasia, as well as its real-world impact on CRC screening programs and prevention.
Acknowledgments
AM and MN: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; final approval of the article.
MAB, LB, LC, EG, and GR: study concept and design; acquisition of data; critical revision of the manuscript for important intellectual content; final approval of the article.
FT: study concept and design; statistical analysis; final approval of the article.
KE: acquisition of data; analysis and interpretation of data; drafting of the manuscript; final approval of the article.
NEOCOSM study group participants: study concept and design; acquisition of data. Please note that all participants in the NEOCOSM study should have the same credit for co-authorship for bibliometric and indexing purposes.
NEOCOSM study group participants.
| City | Chief of unit | Coworkers |
|---|---|---|
| Arezzo | SBRAMA Fabio | AGNOLUCCI Angiolo, AGLIETTI Alessandro |
| Aviano | CANNIZZARO Renato | CANNIZZARO Renato, MAIERO Stefania |
| Bassano del Grappa | MASTROPAOLO Gaetano | MASTROPAOLO Gaetano, MONICA Fabio |
| Belluno | GERMANA’ Bastianello | GERMANA’ Bastianello, LECIS Pier Enrico |
| Brescia | CESTARI Renzo | CESTARI Renzo, LANCINI Gian Paolo |
| Campobasso | INGROSSO Marcello | INGROSSO Marcello, MARANGI Stefania |
| Campobasso | CARRATO Alfredo | CARRATO Alfredo |
| Caserta | FORTE Giovanni Battista | GRIMALDI Enzo, ASTRETTO Silvia |
| Castellana Grotte | DI MATTEO Giovanni | CUPPONE Renato, BURATTINI Osvaldo |
| Catania | VIRGILIO Clara | VIRGILIO Clara, MIRAGLIA Stefania |
| Catanzaro | SACCA’ Natale | SACCA’ Natale, RODINO’ Stefano |
| Chieti | NERI Matteo | MILANO Angelo, SERIO Mariaelena, EFTHYMAKIS Konstantinos |
| Civitanova Marche | MOBILI Massimo | BALATSINOU Chrysanthi, MANCINI Stefano |
| Como | SPINZI Giancarlo | MANDELLI Giovanna, TOLDI Anna |
| Cosenza | LEO Pietro | LEO Pietro, LEDONNE Ester |
| Cremona | BUFFOLI Federico | BUFFOLI Federico, IIRITANO Elena |
| Cuneo | MANCA Aldo | ASNAGHI Giuliano |
| Firenze | ANNESE Vito | ANNESE Vito, BONANOMI Andrea Giovanni |
| Forlì | RICCI Enrico | RICCI Enrico, DE PADOVA Angelo |
| Genova | SAVARINO Vincenzo | GEMIGNANI Lorenzo, GIAMBRUNO Elisa |
| Genova | COCCIA Gianni | COCCIA Gianni, ALLEGRETTI Annaglays |
| Macerata | TOMBESI Giorgio | FELICIANGELI Giuseppe |
| Matera | DE MAIO Giovanni | DE MAIO Giovanni, CORAZZA Luciano |
| Merano | PIERAMICO Oreste | PIERAMICO Oreste |
| Messina | FAMILIARI Luigi | FAMILIARI Luigi, PALLIO Socrate |
| Milano | MUTIGNANI Massimiliano | MUTIGNANI Massimiliano, Salerno Raffaele |
| Napoli | DI GIORGIO Pietro | DI GIORGIO Pietro |
| Napoli | NARDONE Gerardo | ROCCO Alba, NARDONE Gerardo |
| Napoli | TEMPESTA Alfonso | MARONE Pietro, D’ANGELO Valentina |
| Napoli | ROMANO Marco | ROMANO Marco, GRAVINA Antonietta Gerarda |
| Novara | DEL PIANO Mario | DEL PIANO Mario, TARI Roberto |
| Osimo | TOMARELLI Luigi Maria | SILVESTRELLI Marco, BOUSERHAL Toufic |
| Polistena | CARDONE Francesco Carmelo | CARDONE Francesco Carmelo |
| Rionero in Vulture | CIUFFI Mario | TREMOLATERRA Fabrizio, CIUFFI Mario |
| Roma | DI GIULIO Emilio | DI GIULIO Emilio, CORLETO Vito |
| Roma | CICALA Michele | DI MATTEO Francesco Maria |
| Roma | COSTAMAGNA Guido | PETRUZZIELLO Licio, CESARO Paola, COSTAMAGNA Guido |
| Roma | PALLONE Francesco | DEL VECCHIO BLANCO Giovanna, COPPOLA Manuela |
| Roma | STROPPA Italo | STROPPA Italo |
| San Giovanni Rotondo | ANDRIULLI Angelo | ANDRIULLI Angelo, BISCAGLIA Giuseppe |
| Salerno | CORDUA Anna Maria | ROMANO Maurizio, BORGHERESI Patrizia |
| San Cataldo | SCARPULLA Giuseppe | SCARPULLA Giuseppe, CAMILLERI Salvatore |
| San Donato Milanese | VECCHI Maurizio | VECCHI Maurizio, POLIANI Luca |
| Siena | FROSINI Giorgio | FROSINI Giorgio, RENTINI Silvia |
| Torre del Greco | CIPOLLETTA Livio | BIANCO Maria Antonia, ROTONDANO Gianluca |
| Trani | GUGLIELMI Francesco William | GUGLIELMI Francesco William, REGANO Nunzia |
| Trieste | BURI Luigi | BURI Luigi, SIMETH Catrin |
| Udine | ZILLI Maurizio | ZILLI Maurizio, CHECCHIN Davide |
| Vasto | SPADACCINI Antonio | SPADACCINI Antonio, SILLA Michele |
| Verona | GABBRIELLI Armando | GABBRIELLI Armando, BERNARDONI Laura |
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: An unrestricted grant for logistical support was provided by Bracco SpA, Italy.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Konstantinos Efthymakis  https://orcid.org/0000-0002-9537-6507
https://orcid.org/0000-0002-9537-6507
Contributor Information
Angelo Milano, Department of Medicine and Aging Sciences and Center of Aging Sciences and Translational Medicine (CeSI-MeT), ‘G.D.’ Annunzio University and Foundation, Chieti, Italy; Digestive Endoscopy and Gastroenterology Unit, ‘SS Annunziata’ University Hospital, Chieti, Italy.
Maria Antonia Bianco, Division of Gastroenterology and Digestive Endoscopy Unit, Hospital ‘A Maresca’, Torre del Greco, Italy.
Luigi Buri, Gastroenterology and Digestive Endoscopy Unit, Cattinara Hospital, Trieste, Italy.
Livio Cipolletta, Division of Gastroenterology and Digestive Endoscopy Unit, Hospital ‘A Maresca’, Torre del Greco, Italy.
Enzo Grossi, Medical Department Bracco SpA, Milan, Italy.
Gianluca Rotondano, Division of Gastroenterology and Digestive Endoscopy Unit, Hospital ‘A Maresca’, Torre del Greco, Italy.
Francesco Tessari, Electronic Data Processing, Idea 99 Srl, Padova, Italy.
Konstantinos Efthymakis, Department of Medicine and Aging Sciences and Center of Aging Sciences and Translational Medicine (CeSI-MeT), ‘G.D.’ Annunzio University and Foundation, Chieti, Italy; Digestive Endoscopy and Gastroenterology Unit, ‘SS Annunziata’ University Hospital, Chieti, Italy.
Matteo Neri, Department of Medicine and Aging Sciences and Center of Aging Sciences and Translational Medicine (CeSI-MeT), ‘G.D.’ Annunzio University and Foundation, Chieti, Italy; Digestive Endoscopy and Gastroenterology Unit, ‘SS Annunziata’ University Hospital, Chieti, Italy.
References
- 1. Boyle P, Leon ME. Epidemiology of colorectal cancer. Br Med Bull 2002; 64: 1–25. [DOI] [PubMed] [Google Scholar]
- 2. Curado MP, Edwards B, Shin HRet al. Cancer incidence in five continents, Vol. IX. IARC Scientific Publications No. 160, Lyon: IARC, 2007. [Google Scholar]
- 3. Weitz J, Koch M, Debus Jet al. Colorectal cancer. Lancet 2005; 365: 153–165. [DOI] [PubMed] [Google Scholar]
- 4. Grundy SM. Hypertriglyceridemia, insulin resistance, and the metabolic syndrome. Am J Cardiol 1999; 83: 25–29. [DOI] [PubMed] [Google Scholar]
- 5. Alexander CM, Landsman PB, Teutsch SMet al. NCEP-defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes 2003; 52: 1210–1214. [DOI] [PubMed] [Google Scholar]
- 6. Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Ann Rev Med 2005; 56: 45–62. [DOI] [PubMed] [Google Scholar]
- 7. Braun S, Bitton-Worms K, LeRoith D. The link between the metabolic syndrome and cancer. Int J Biol Sci 2011; 7: 1003–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr 2007; 86: s836–s842. [DOI] [PubMed] [Google Scholar]
- 9. Renehan AG, Tyson M, Egger Met al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008; 371: 569–578. [DOI] [PubMed] [Google Scholar]
- 10. Pais R, Silaghi H, Silaghi ACet al. Metabolic syndrome and risk of subsequent colorectal cancer. World J Gastroenterol 2009; 15: 5141–5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol 2008; 28: 629–636. [DOI] [PubMed] [Google Scholar]
- 12. Martínez MA, Puig JG, Mora Met al. Metabolic syndrome: prevalence, associated factors, and C-reactive protein: the MADRIC (MADrid RIesgo Cardiovascular) study. Metabolism 2008; 57: 1232–1240. [DOI] [PubMed] [Google Scholar]
- 13. Shaukat A, Mongin SJ, Geisser MSet al. Long-term mortality after screening for colorectal cancer. N Engl J Med 2013; 369: 1106–1114. [DOI] [PubMed] [Google Scholar]
- 14. Kylin E. Studien ueber das hypertonie-hyperglykemie-hyperurikämiesyndrome. Zentralbl Inn Med 1923; 44: 105–127. [Google Scholar]
- 15. Vague J. La differenciation sexuelle, facteur determinant des formes de l’obesite. Presse Med 1947; 55: 339. [PubMed] [Google Scholar]
- 16. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications, part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15: 539–553. [DOI] [PubMed] [Google Scholar]
- 17. National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 2002; 106: 3143–3421. [PubMed] [Google Scholar]
- 18. Alberti KG, Zimmet P, Shaw Jet al. The metabolic syndrome: a new worldwide definition. Lancet 2005; 366: 1059–1062. [DOI] [PubMed] [Google Scholar]
- 19. Grundy SM, Cleeman JI, Daniels SRet al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement [published corrections appear in Circulation 2005; 112: e297 and Circulation 2005; 112: e298]. Circulation 2005; 112: 2735–2752. [DOI] [PubMed] [Google Scholar]
- 20. Alberti KG, Eckel RH, Grundy SMet al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120: 1640–1645. [DOI] [PubMed] [Google Scholar]
- 21. Esposito K, Chiodini P, Capuano Aet al. Colorectal cancer association with metabolic syndrome and its components: a systematic review with meta-analysis. Endocrine 2013; 44: 634–647. [DOI] [PubMed] [Google Scholar]
- 22. Jinjuvadia R, Lohia P, Jinjuvadia Cet al. The association between metabolic syndrome and colorectal neoplasm: systemic review and meta-analysis. J Clin Gastroenterol 2013; 47: 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rex DK, Petrini JL, Baron THet al. Quality indicators for colonoscopy. Am J Gastroenterol 2006; 101: 873–885. [DOI] [PubMed] [Google Scholar]
- 24. Furberg AS, Thune J. Metabolic abnormalities (hypertension, hyperglycemia and overweight), lifestyle (high energy intake and physical inactivity) and endometrial cancer risk in a Norwegian cohort. Int J Cancer 2003; 104: 669–676. [DOI] [PubMed] [Google Scholar]
- 25. Bianco MA, Cipolletta L, Rotondano Get al. On behalf of the flat lesions Italian network (FLIN). Prevalence of non polypoid colorectal neoplasia: an Italian multicenter observational study. Endoscopy 2010; 42: 279–285. [DOI] [PubMed] [Google Scholar]
- 26. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon. Gastrointest Endosc 2003; 58(Suppl. 6): S3–S43. [DOI] [PubMed] [Google Scholar]
- 27. Pickering TG, Hall JE, Appel LJet al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the subcommittee of professional and public education of the American Heart Association Council on high blood pressure research. Hypertension 2005; 45: 142–161. [DOI] [PubMed] [Google Scholar]
- 28. World Health Organization. Waist circumference and waist-hip ratio report of a WHO expert consultation. Geneva: World Health Organization, 2011. [Google Scholar]
- 29. Tamura S, Nakajo K, Yokoyama Yet al. Evaluation of endoscopic mucosal resection for laterally spreading rectal tumors. Endoscopy 2004; 36: 306–312. [DOI] [PubMed] [Google Scholar]
- 30. Uraoka T, Saito Y, Matsuda Tet al. Endoscopic indications for endoscopic mucosal resection of laterally spreading tumours in the colorectum. Gut 2006; 55: 1592–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut 2002; 51: 130–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim JH, Lim YJ, Kim YHet al. Is metabolic syndrome a risk factor for colorectal adenoma? Cancer Epidemiol Biomarkers Prev 2007; 16: 1543–1546. [DOI] [PubMed] [Google Scholar]
- 33. Morita T, Tabata S, Mineshita Met al. The metabolic syndrome is associated with increased risk of colorectal adenoma development: the self-defense forces health study. Asian Pac J Cancer Prev 2005; 6: 485–489. [PubMed] [Google Scholar]
- 34. Chiu HM, Lin JT, Shun CTet al. Association of metabolic syndrome with proximal and synchronous colorectal neoplasm. Clin Gastroenterol Hepatol 2007; 5: 221–229. [DOI] [PubMed] [Google Scholar]
- 35. Kim MC, Kim CS, Chung THet al. Metabolic syndrome, lifestyle risk factors, and distal colon adenoma: a retrospective cohort study. World J Gastroenterol 2011; 17: 4031–4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chiu HM, Lee YC, Tu CHet al. Effects of metabolic syndrome and findings from baseline colonoscopies on occurrence of colorectal neoplasms. Clin Gastroenterol Hepatol 2015; 13: 1134–1142.e8. [DOI] [PubMed] [Google Scholar]
- 37. Colangelo LA, Gapstur SM, Gann PHet al. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer Epidemiol Biomarkers Prev 2002; 11: 385–391. [PubMed] [Google Scholar]
- 38. Stûrmer T, Buring JE, Lee IMet al. Metabolic abnormalities and risk for colorectal cancer in the physicians’ health study. Cancer Epidemiol Biomarkers Prev 2006; 15: 2391–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stocks T, Lukanova A, Johansson Met al. Components of the metabolic syndrome and colorectal cancer risk: a prospective study. Int J Obes 2008; 32: 304–314. [DOI] [PubMed] [Google Scholar]
- 40. Trevisan M, Liu J, Muti Pet al. Markers of insulin resistance and colorectal cancer mortality. Cancer Epidemiol Biomarkers Prev 2001; 10: 937–941. [PubMed] [Google Scholar]
- 41. Bowers K, Albanes D, Limburg Pet al. A prospective study of anthropometric and clinical measurements associated with insulin resistance syndrome and colorectal cancer in male smokers. Am J Epidemiol 2006; 164: 652–664. [DOI] [PubMed] [Google Scholar]
- 42. Russo A, Autelitano M, Bisanti L. Metabolic syndrome and cancer risk. Eur J Cancer 2008; 44: 293–297. [DOI] [PubMed] [Google Scholar]
- 43. Stocks T, Lukanova A, Bjørge Tet al. Metabolic factors and the risk of colorectal cancer in 580,000 men and women in the metabolic syndrome and cancer project (Me-Can). Cancer 2011; 117: 2398–2407. [DOI] [PubMed] [Google Scholar]
- 44. Pelucchi C, Negri E, Talamini Ret al. Metabolic syndrome is associated with colorectal cancer in men. Eur J Cancer 2010; 46: 1866–1872. [DOI] [PubMed] [Google Scholar]
- 45. Aleksandrova K, Boeing H, Jenab Met al. Metabolic syndrome and risks of colon and rectal cancer: the European prospective investigation into cancer and nutrition study. Cancer Prev Res 2011; 4: 1873–1883. [DOI] [PubMed] [Google Scholar]
- 46. Ahmed RL, Schmitz KH, Anderson KEet al. The metabolic syndrome and risk of incident colorectal cancer. Cancer 2006; 107: 28–36. [DOI] [PubMed] [Google Scholar]
- 47. Shen Z, Wang S, Ye Yet al. Clinical study on the correlation between metabolic syndrome and colorectal carcinoma. ANZ J Surg 2010; 80: 331–336. [DOI] [PubMed] [Google Scholar]
- 48. Stürmer T, Buring JE, Lee IMet al. Metabolic abnormalities and risk for colorectal cancer in the physicians’ health study. Cancer Epidemiol Biomarkers Prev 2006; 15: 2391–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ashbeck EL, Jacobs ET, Martínez MEet al. Components of metabolic syndrome and metachronous colorectal neoplasia. Cancer Epidemiol Biomarkers Prev 2009; 18: 1134–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut 2013; 62: 933–947. [DOI] [PubMed] [Google Scholar]
- 51. Renehan AG, Tyson M, Egger Met al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008; 371: 569–578. [DOI] [PubMed] [Google Scholar]
- 52. Pais R, Silaghi H, Silaghi ACet al. Metabolic syndrome and risk of subsequent colorectal cancer. World J Gastroenterol 2009; 15: 5141–5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Moore LL, Bradlee ML, Singer MRet al. BMI and waist circumference as predictors of lifetime colon cancer risk in Framingham study adults. Int J Obes Relat Metab Disord 2004; 28: 559–567. [DOI] [PubMed] [Google Scholar]
- 54. Nam SY, Kim BC, Han KSet al. Abdominal visceral adipose tissue predicts risk of colorectal adenoma in both sexes. Clin Gastroenterol Hepatol 2010; 8: 443–450. [DOI] [PubMed] [Google Scholar]
- 55. Kim B, Kim BC, Nam SYet al. Visceral adipose tissue volume and the occurrence of colorectal adenoma in follow-up colonoscopy for screening and surveillance. Nutr Cancer 2017; 69: 739–745. [DOI] [PubMed] [Google Scholar]
- 56. Le Marchand L, Wilkens LR, Kolonel LNet al. Associations of sedentary lifestyle, obesity, smoking, alcohol use, and diabetes with the risk of colorectal cancer. Cancer Res 1997; 57: 4787–4794. [PubMed] [Google Scholar]
- 57. Deng L, Gui Z, Zhao Let al. Diabetes mellitus and the incidence of colorectal cancer: an updated systematic review and meta-analysis. Dig Dis Sci 2012; 57: 1576–1585. [DOI] [PubMed] [Google Scholar]
- 58. Kono S, Honjo S, Todoroki Iet al. Glucose intolerance and adenomas of the sigmoid colon in Japanese men (Japan). Cancer Causes Control 1998; 9: 441–446. [DOI] [PubMed] [Google Scholar]
- 59. Elwing JE, Gao F, Davidson NOet al. Type 2 diabetes mellitus: the impact on colorectal adenoma risk in women. Am J Gastroenterol 2006; 101: 1866–1867. [DOI] [PubMed] [Google Scholar]
- 60. Stocks T, Van Hemelrijck M, Manjer Jet al. Blood pressure and risk of cancer incidence and mortality in the metabolic syndrome and cancer project. Hypertension 2012; 59: 802–810. [DOI] [PubMed] [Google Scholar]
- 61. Liao KF, Lai HC, Lai SWet al. Association between rectosigmoid adenomas and cardiovascular risk factors: a hospital-based, cross-sectional study. Ann Acad Med Singapore 2009; 38: 630–636. [PubMed] [Google Scholar]
- 62. Tabuchi M, Kitayama J, Nagawa H. Hypertriglyceridemia is positively correlated with the development of colorectal tubular adenoma in Japanese men. World J Gastroenterol 2006; 12: 1261–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005; 365: 1415–1428. [DOI] [PubMed] [Google Scholar]
- 64. Cowey S, Hardy RW. The metabolic syndrome a high-risk state for cancer? Am J Pathol 2006; 169: 1505–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer 2005; 41: 2502–2512. [DOI] [PubMed] [Google Scholar]
- 66. Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer 2009; 9: 361–371. [DOI] [PubMed] [Google Scholar]
- 67. Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr 2007; 86: 556–565. [DOI] [PubMed] [Google Scholar]
- 68. Hong SN, Kim JH, Choe WHet al. Prevalence and risk of colorectal neoplasms in asymptomatic, average-risk screenees 40 to 49 years of age. Gastrointest Endosc 2010; 72: 480–489. [DOI] [PubMed] [Google Scholar]


