Abstract
Conjunctivitis is an uncommon finding in commercial swine herds, and the etiology of the disease is rarely studied. We investigated cases of conjunctivitis in 3 wean-to-finish swine farms. Eye swabs and tissues were obtained from clinically affected pigs (8–22 wk of age), from unaffected pigs in contact with affected pen-mates, and from age-matched pigs from an unaffected herd. Real-time PCR (rtPCR) testing for Mycoplasma hyorhinis demonstrated consistent detection and high bacterial load in samples from affected herds (clinically affected animals and non-clinical pen-mates). Ct values in affected pigs were 18.9–25.3; values were 36.4–38.6 in unaffected pigs from unaffected herds. Additionally, M. hyorhinis was identified within inflamed palpebral conjunctivae by in situ hybridization. The association of rtPCR and in situ detection of M. hyorhinis, along with the lack of detection of other potential pathogens and noninfectious causes, suggests the involvement of M. hyorhinis in the etiology and pathogenesis of the reported swine conjunctivitis.
Keywords: conjunctivitis, in situ hybridization, Mycoplasma hyorhinis, pigs
Mycoplasma hyorhinis is a commensal bacterium that primarily colonizes the upper respiratory tract of swine. This bacterium has also been reported as the causative agent of polyserositis, polyarthritis, and otitis in pigs. A variety of infectious agents have been associated with conjunctivitis in pigs, including classical swine fever virus (CSFV; family Flaviviridae, genus Pestivirus, species Pestivirus C), African swine fever virus (ASFV; family Asfarviridae, genus Asfivirus), pseudorabies virus (PRV; family Herpesviridae, genus Varicellovirus, species Suid alphaherpesvirus 1), Chlamydia spp., porcine cytomegalovirus (PCMV; family Herpesviridae, genus Roseolovirus, species Suid betaherpesvirus 2), influenza A virus (IAV; family Orthomyxoviridae, genus Alphainfluenzavirus), and porcine reproductive and respiratory syndrome virus (PRRSV; family Arteriviridae, genus Betaarterivirus). Mycoplasma-like organisms have been shown to be involved in outbreaks of conjunctivitis in swine herds.6,11 Even though Mycoplasma spp. are known to be causative agents of conjunctivitis in other animal species, such as cats, birds, sheep, and cattle,2–5,8 the significance of mycoplasmas in the etiology of conjunctivitis in swine is unclear. We report herein investigation of an outbreak of conjunctivitis in commercial swine herds, demonstrating M. hyorhinis as the potential causative agent.
Conjunctivitis was clinically identified in 3 unrelated wean-to-finish swine farms in Minnesota, Ohio, and Oklahoma. Pigs from all farms were housed in standard nursery and/or finishing facilities. The onset of clinical signs was observed in pigs 8–10 wk old; clinical signs persisted until pigs reached 22 wk old. At the early stage of conjunctivitis, clinical signs consisted of conjunctival redness, palpebral edema, and serous ocular discharge (8–10 wk old; Fig. 1A). At the late stage, inflammatory signs of hyperemia and edema persisted; ocular discharges changed from serous to predominantly mucopurulent (22 wk old). The prevalence of the clinical signs was 30–60% within each herd. Anorexia and failure-to-thrive in affected pigs were the major concerns of producers and veterinarians. Noninfectious causes of conjunctivitis, such as lesions caused by foreign bodies, allergic reactions, or insect bites, which could be implicated in the clinical presentation, were not reported by the veterinary practitioners.
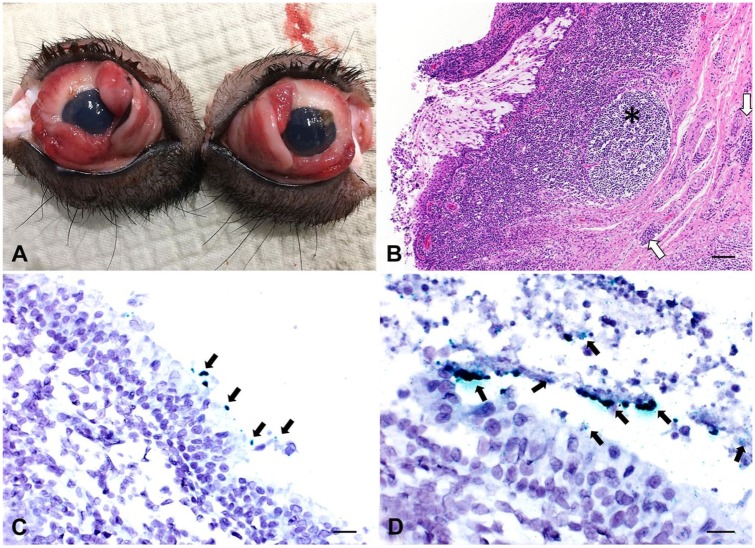
Figure 1.
Mycoplasma hyorhinis–associated conjunctivitis is swine. A. The peak lesions occurred in 12-wk-old pigs. In this group, there was hyperemia and mild serous discharge with blepharedema. B. The epithelium of the ocular conjunctiva is eroded and ulcerated, with exposure of the lamina propria. The conjunctival fibrous layer is markedly expanded by a lymphoplasmacytic inflammatory infiltrate, with lymphofollicular hyperplasia (asterisk) and perivascular cuffing (arrows). H&E. Bar = 100 µm. C. Green M. hyorhinis–positive signals (arrows) detected in the epithelium of palpebral conjunctiva at the early clinical stage of conjunctivitis. In situ hybridization (ISH). Bar = 50 µm. D. Green M. hyorhinis–positive signals (arrows) detected within ulcerative regions of the palpebral conjunctiva at late clinical stage of conjunctivitis. ISH. Bar = 20 µm.
Eye swabs were collected from pigs at early and late stages of the clinical disease, as well as from contact pen-mates with no apparent clinical signs. Eye swab samples were also collected from pigs without a history of conjunctivitis (age-matched pigs) from a non-related herd. Tissue samples (eye globes along with their conjunctiva) from autopsied pigs were submitted to the Veterinary Diagnostic Laboratory at the University of Minnesota (UMN-VDL; Saint Paul, MN) for histologic evaluation and in situ hybridization (ISH) tests. In total, we obtained 12 tissue samples (3 from Minnesota, 3 from Ohio, 3 from Oklahoma, and 3 from an unaffected farm in South Dakota) and 36 eye swabs (11 from affected pigs at early stage of the disease, 11 from affected pigs at late stage of the disease, 10 from contact pen-mates with no apparent clinical signs, and 4 from pigs housed in an unaffected herd) (Table 1).
Table 1.
Real-time PCR results for Mycoplasma hyorhinis from conjunctival swabs collected during an outbreak of conjunctivitis in commercial swine herds.
| Pig clinical condition | No. of samples by state |
Ct value (mean ± SD) | |||
|---|---|---|---|---|---|
| MN | OH | OK | SD | ||
| Affected | |||||
| Early stage (n = 11) | 6 | 3 | 2 | 0 | 21.3 ± 2.4 |
| Late stage (n = 11) | 5 | 3 | 3 | 0 | 23.5 ± 1.8 |
| Unaffected | |||||
| Contact with affected pen mates (n = 10) | 4 | 6 | 0 | 0 | 29.7 ± 3.1 |
| Non-contact (unaffected herd) (n = 6) | 2 | 0 | 0 | 4 | 37.5 ± 1.1 |
OH = Ohio; OK = Oklahoma; MN = Minnesota; SD = South Dakota.
The diagnostic investigation for potential pathogens was conducted at the UMN-VDL. Tissue samples were evaluated by histopathology, and eye swabs and tissue samples were screened for viral, bacterial, and fungal agents. Immunohistochemistry (IHC) and/or PCR were performed for CSFV, ASFV, PRV, Chlamydia spp., M. hyopneumoniae, M. hyorhinis, PCMV, IAV, and PRRSV. A real-time PCR (rtPCR) was used to determine the presence and concentration of M. hyorhinis DNA in eye swabs. The rtPCR assay was developed and validated at the UMN-VDL. A hydrolysis probe (5’-/6FAM/CCGGATATAGTTATTTATCGCATGA/MGBNFQ/-3’), and forward (5’-CGTACCTAACCTACCTTTAAGACTGGG-3’) and reverse (5’-GTGAAGCTGTGAAGCTCCTTTCT-3’) primer sequences, were developed targeting a specific region (position 131-182) of the 16S rRNA gene of the M. hyorhinis genome (NR_113686.1). An ISH assay was developed to assess the expression of M. hyorhinis 16S rRNA within eye tissues. Briefly, unstained paraffin sections of tissue were deparaffinized in xylene and rehydrated through a series of alcohol washes. The rehydrated sections were tested using the RNAscope technique12 (Advanced Cell Diagnostics, Newark, CA). Hydrogen peroxide was added over the tissue sections at room temperature for 10 min. Slides were then boiled in citrate buffer for 15 min, and incubated with protease at 40°C for 30 min. A M. hyorhinis probe was hybridized in tissue sections with the hybridization buffer at 40°C for 2 h. Then, according to the manufacturer’s instructions, amplifiers were added for 15 or 30 min at 40°C in order to amplify the hybridization signal. Green colorimetric staining (alkaline phosphatase) detected the M. hyorhinis hybridization signal, and counterstaining was performed using hematoxylin. Microscopic assessment was conducted by 2 independent blinded pathologists.
At the early stage of the clinical disease (8–10 wk old), erosive lymphoplasmacytic conjunctivitis associated with lymphofollicular hyperplasia was observed predominantly in the palpebral conjunctiva with minimal or no extension to the bulbar conjunctiva (Fig. 1B). Tissue samples collected at the late stage of the clinical course revealed ulceration associated with lymphoplasmacytic and neutrophilic infiltration distributed throughout the palpebral and bulbar conjunctiva, and lymphofollicular hyperplasia.
CSFV, ASFV, PRRSV, IAV, PRV, and M. hyopneumoniae infections were ruled out by their absence on PCR assays. Samples were negative for Chlamydia spp. by culture and capture ELISA (QuickVue; Quidel, San Diego, CA). PCMV was inconsistently detected by PCR in affected and unaffected pigs. Inclusion bodies suggestive of rhinitis caused by PCMV were not observed during histologic examination. Staphylococcus chromogenes and S. aureus were also isolated from eye swabs of affected and unaffected animals. Tissue samples from unaffected pigs did not show evidence of histopathologic change.
Eye swabs and conjunctival tissues from clinically affected animals and unaffected contact pen-mates were consistently positive for M. hyorhinis by rtPCR (Table 1). ISH-positive signals for M. hyorhinis were observed associated with histologic lesions of the conjunctival epithelium and connective tissue, and where the substantia propria was exposed as a result of ulceration (Figs. 1C, D). Bacterial isolation of M. hyorhinis was attempted from samples of all herds. However, overgrowth of S. aureus limited isolation of M. hyorhinis, despite indication of significant bacterial concentration, as evidenced by low cycle threshold (Ct) values of rtPCR in broth. ISH-positive signals were not observed in tissue samples from unaffected pigs (data not shown).
Conjunctivitis is not usually considered a disease with significant economic impact in intensive pig production. Sporadic cases are usually related to the presence of irritating materials in the environment (dust, gases, and chemicals), and/or presence of foreign bodies in the eye region. Chlamydia spp. have been common infectious agents associated with conjunctivitis outbreaks in pig herds; however, there are limited reports in the literature that describe diagnostic investigation of conjunctivitis in pigs, and incidence of pathogens causing conjunctivitis may be higher. M. hyorhinis has been isolated from the conjunctivae of pigs with and without clinical disease6; based on serologic evaluation, the author suggested the involvement of a distinct variant species of M. hyorhinis. Mycoplasma-like particles have been described by ultrastructural evaluation without association with other pathogens11; unfortunately, these investigators did not perform experimental infection of M. hyorhinis isolates to verify the reproducibility of clinical signs.
Several animal species are susceptible to conjunctivitis caused by Mycoplasma spp. M. gallisepticum has been associated with outbreaks of conjunctivitis in birds.3 M. felis has been isolated from cats exhibiting clinical conjunctivitis. Cats inoculated with M. felis isolates showed moderate-to-severe conjunctival hyperemia.7 Conjunctivitis and keratoconjunctivitis in small ruminants associated with M. conjunctivae have been consistently reported in the literature.4,10 Cattle can also suffer from conjunctivitis caused by M. bovoculi and M. bovis.1,8,9 Finally, M. genitalium was reported to be associated with a case of chronic conjunctivitis in a human patient affected by mild urethritis caused by the same agent.
Real-time PCR coupled with ISH is a valuable tool for outbreak investigations to identify the potential causative agent in association with histologic lesions, especially when there are commensal bacterial organisms involved and lack of antibodies available to perform immunohistochemistry assays. Although we did not fulfill Koch’s postulates in our investigation, certain factors indicate M. hyorhinis as the primary agent in our cases. First, Ct values detected by rtPCR were lower in affected pigs compared with unaffected pen-mates, indicating that although M. hyorhinis was detected in healthy pigs as part of the eye microbiota, in conjunctivitis-affected pigs, higher bacterial loads of M. hyorhinis were present. Additionally, samples from unaffected, non-contact pigs had high Ct values. Although M. hyorhinis was not isolated from conjunctival swabs, M. hyorhinis was detected with the developed probe for the ISH assay when targeting mRNA of the 16S gene. Therefore, positive signals corresponded to metabolically active bacteria present in the tissue. The extensive number of Mycoplasma species that can cause conjunctivitis and keratoconjunctivitis in other animal species also supports M. hyorhinis as the etiology of the swine conjunctivitis in our study. Comprehensive studies involving whole genome comparison among M. hyorhinis strains isolated from clinical cases of conjunctivitis, polyserositis, and asymptomatic carriers are warranted to clarify potential genotypic differences associated with a specific disease phenotype.
Acknowledgments
We thank Lacey Marshall-Lund, Alyssa M. Betlach, and the Immunohistochemistry and Histopathology laboratories at the Veterinary Diagnostic Laboratory at the University of Minnesota for their support.
Footnotes
Declaration of conflicting interests: The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: TPR was supported by the Brazilian government sponsoring agency Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
ORCID iD: Talita P. Resende  https://orcid.org/0000-0002-7705-714X
https://orcid.org/0000-0002-7705-714X
References
- 1. Alberti A. et al. Molecular and antigenic characterization of a Mycoplasma bovis strain causing an outbreak of infectious keratoconjunctivitis. J Vet Diagn Invest 2006;18:41–51. [DOI] [PubMed] [Google Scholar]
- 2. Arnal M. et al. Dynamics of an infectious keratoconjunctivitis outbreak by Mycoplasma conjunctivae on Pyrenean Chamois Rupicapra p. pyrenaica. PLoS One 2013;8:e61887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dhondt AA. et al. Diverse wild bird host range of Mycoplasma gallisepticum in eastern North America. PLoS One 2014;9:e103553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fernández-Aguilar X. et al. Mycoplasma conjunctivae in domestic small ruminants from high mountain habitats in northern Spain. BMC Vet Res 2013;9:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Forsyth MH. et al. Mycoplasma sturni sp. nov., from the conjunctiva of a European starling (Sturnus vulgaris). Int J Syst Bacteriol 1996;46:716–719. [DOI] [PubMed] [Google Scholar]
- 6. Friis NF. A serologic variant of Mycoplasma hyorhinis recovered from the conjunctiva of swine. Acta Vet Scand 1976;17:343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haesebrouck F. et al. Incidence and significance of isolation of Mycoplasma felis from conjunctival swabs of cats. Vet Microbiol 1991;26:95–101. [DOI] [PubMed] [Google Scholar]
- 8. Langford EV, Leach RH. Characterization of a mycoplasma isolated from infectious bovine keratoconjunctivitis: M. bovoculi sp. nov. Can J Microbiol 1973;19:1435–1444. [DOI] [PubMed] [Google Scholar]
- 9. Levisohn S. et al. Diagnosis of a mixed mycoplasma infection associated with a severe outbreak of bovine pinkeye in young calves. J Vet Diagn Invest 2004;16:579–581. [DOI] [PubMed] [Google Scholar]
- 10. Motha MX. et al. Detection of Mycoplasma conjunctivae in sheep affected with conjunctivitis and infectious keratoconjunctivitis. N Z Vet J 2003;51:186–190. [DOI] [PubMed] [Google Scholar]
- 11. Rogers DG. et al. Conjunctivitis associated with a mycoplasma-like organism in swine. J Am Vet Med Assoc 1991;198:450–452. [PubMed] [Google Scholar]
- 12. Wang F. et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn 2012;14:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]