Abstract
Detection of equine acute kidney injury (AKI) is hindered by limited markers of early renal damage in horses. N-acetyl-β-D-glucosaminidase (NAG), a lysosomal enzyme in renal tubular epithelium released into urine during tubular insult, has shown promise for early identification of AKI in humans and other species. We validated an assay for NAG in equine urine and measured urinary NAG in 7 azotemic and 7 non-azotemic client-owned adult horses. The enzymatic NAG assay was validated using within- and between-run coefficients of variation (CVs), recovery following standard addition, and linearity of dilution. Intra- and inter-run CVs (21% and 3.2%, respectively), average recovery following standard addition (99–109%), and linearity under serial dilution (R2 = 0.997) were satisfactory. Urine NAG index was significantly correlated with urinary fractional excretion of sodium (FENa; ρ = 0.76, p < 0.001) and plasma creatinine (ρ = 0.74, p = 0.001). Median urine NAG indices were higher in azotemic horses (p = 0.006), in horses with increased urinary FENa (p = 0.006), and in horses with increased urine gamma-glutamyl transferase index (p = 0.032). Urine NAG can be measured in horses and shows positive correlation with 2 current renal biomarkers. Additional work is needed to establish normal equine reference intervals and characterize the increase of urine NAG index in horses in relation to tubular injury.
Keywords: acute kidney injury, horses, N-acetyl-beta-D-glucosaminidase, renal, urine
Introduction
Kidney injury in horses can result from a variety of etiologies, including hypotension, endotoxemia, pigmenturia, infectious agents, nephrotoxic medications, and exogenous toxins, and may progress to clinically significant renal dysfunction.8 Estimation of glomerular filtration rate, proposed as the closest representation of renal function, is typically complex and time-consuming and is not practical in most clinical situations.31 Currently available methods to assess kidney damage and effects on renal function in equine practice include measurement of plasma creatinine and urine specific gravity (USG), examination of urine sediment, and calculation of urine fractional excretion of electrolytes and urine gamma-glutamyl transferase (GGT) index.31 Although these tests are generally widely available, there are limitations to their abilities to detect acute kidney injury (AKI) and estimate severity of damage, especially following administration of fluid therapy or alpha-2 adrenergic agonists.28,31,36
N-acetyl-β-D glucosaminidase (NAG) is a lysosomal enzyme found in proximal renal tubular epithelial cells. Its large molecular size prevents filtration from systemic sources at the glomerulus, but low amounts of urine NAG are detectable in normal subjects because of physiologic exocytosis into the tubular lumen.26 Urine NAG is increased following tubular damage or dysfunction from a variety of causes, including drug toxicity, exposure to heavy metals, and diabetic nephropathy.41–43 By comparing NAG enzyme activity to urine creatinine concentration, which is expressed as an index, the effects of alterations in urine concentration on enzyme measurements can be minimized, allowing use of single urine sample measurements.13,40
In rats, dogs, sheep, and human neonates, administration of known nephrotoxic drugs, including gentamicin and ketoprofen, has been shown to increase urine NAG indices, often prior to or in the absence of significant changes in serum creatinine.2,7,18,27,41,44 Correlation between urine NAG index and severity of renal lesions was demonstrated in experimental canine models of aminoglycoside nephrotoxicity.2,44 Urine NAG indices in healthy humans do not seem to be affected by exercise, hydration status, or urine flow rate.13,20,35 No influence of circadian rhythm was found in healthy cats.39 Therefore, urine NAG demonstrates considerable promise in facilitating diagnosis of tubular injury prior to detectable changes in more traditional markers. Additionally, urine NAG appears to be superior to serum creatinine or urine production for predicting mortality or need for dialysis in humans with AKI.17
The ability to identify equine kidney injury in an early stage is critical for instituting appropriate treatment to minimize the potential for permanent compromise of renal function. From investigation in other species, urine NAG shows the potential to improve early detection of renal insult and could be useful for monitoring patients being treated with aminoglycoside antibiotics, nonsteroidal anti-inflammatory drugs (NSAIDs), or other potentially nephrotoxic medications. Urine NAG could also provide a more sensitive way to evaluate tubular effects of drugs during research into novel therapeutics or new dosing strategies. We attempted to validate an assay for measurement of NAG in equine urine and to compare urine NAG index with other markers of renal injury utilized in equine practice. We hypothesized that urine NAG index would be significantly correlated with plasma creatinine, urinary fractional excretion of sodium (FENa), and urine GGT index.
Materials and methods
Animal use and study protocol were approved by the Colorado State University Institutional Animal Care and Use Committee (protocol 16-6663AA); consent was obtained from all clients prior to horse enrollment. This was a prospective observational study.
Assay validation
Validation of a commercial photometric assay (N-acetyl-β-D-glucosaminidase; Roche Diagnostics, Mannheim, Germany), based upon cleavage of sodium 3-cresolsulfonphthaleinyl-N-acetyl-β-D-glucosaminide to measure the activity of NAG, was performed using equine urine samples. The assay was loaded onto a standard biochemical analyzer (Cobas 6000 series; Roche Diagnostics, Indianapolis, IN) according to the manufacturer’s instructions, and assay validation experiments to characterize performance of equine urine NAG measurement were guided by American Society for Veterinary Clinical Pathology published recommendations (Principles of quality assurance and standards for veterinary clinical pathology, 2006 rev. Available from: https://www.asvcp.org/page/QALS_Guidelines). The urine creatinine assay has been validated previously within our laboratory.
Equine test material
A 12-y-old Thoroughbred gelding that had been presented for nuclear scintigraphy was selected for urine collection based on an unremarkable physical exam, lack of hematologic or biochemical abnormalities, and no history of recent illness or medication administration in the previous 4 wk. Urine was obtained by free-catch during mid-stream of a spontaneous voiding. Urine specific gravity was measured using a refractometer, and qualitative urinalysis was performed with a urine dipstick (Chemstrip 10; Roche Diagnostics). The urine sample was centrifuged at 1,500 × g for 10 min, and urine creatinine (Creatinine Jaffé Gen.2; Roche Diagnostics), sodium (Sodium ion–selective electrode; Roche Diagnostics), protein (Total protein urine/CSF Gen.3; Roche Diagnostics), and GGT (GGT-2; Roche Diagnostics) concentrations were measured in the supernatant using a standard biochemistry analyzer (Cobas 6000 series; Roche Diagnostics). Unremarkable dipstick analysis of urine and normal USG (1.020–1.050 in clinically euhydrated animal), FENa (<1%), urine protein-to-creatinine ratio (UPC; <1.0), and urine GGT index (<2.8 U/mmol creatinine) were verified to confirm suitability of the sample for assay validation.3,31,34 The remainder of the supernatant was refrigerated (4°C) until assay validation, which began within 24 h of collection. The urine sample was brought to room temperature prior to analysis.
Acceptable performance
Based on published recommendations of total allowable error for NAG activity (58%) and urine NAG index (56%) in human medicine, and clinical judgement, total allowable error for NAG activity and urine NAG index in equine samples was set at 50% (Minchinela J, et al. Desirable specifications for total error, imprecision, and bias, derived from intra- and inter-individual biological variation. 2014. Available from: https://www.westgard.com/biodatabase-2014-update.htm; EFLM biological variation database, https://biologicalvariation.eu/).
Assay precision (coefficients of variation)
An aliquot of the equine test sample was measured 5 times within a single run each day for 3 consecutive days. For each day, an aliquot was removed, used for testing, and then discarded. The rest of the sample was stored at 4°C. Within-day (intra-run) coefficients of variation (CVs) were calculated for each day. Because stability of native equine urine samples over an extended period of time was uncertain, between-day (inter-run) CV was determined using commercial quality control material for NAG (N-acetyl-β-D-glucosaminidase control; Roche Diagnostics), obtained from the manufacturer, and creatinine (Liquichek urine chemistry control, Level 1 and 2; Bio-Rad, Hercules, CA) measured on independent runs across 17 d.
Assay bias (recovery after standard addition)
Standard NAG (29.7 milliunits [mU]/mL) was added to aliquots of the equine test sample in 2 amounts, 0.1 mL (2.97 mU; added to 1.5 mL of urine) and 0.2 mL (5.94 mU; added to 1 mL of urine). The urine NAG activity and creatinine concentration were measured 5 times for each of the 2 spiked samples. To determine percentage recovery, the average net amount (activity) of urine NAG recovered was compared to the expected activity based on amount added.
Linearity under dilution
A 10-mL aliquot was prepared by adding 5 mL of NAG standard to 5 mL of the original urine sample. Serial dilution with deionized water (50:50 v/v) was performed 4 times for a total of 5 samples, and the urine NAG activity in each resulting sample was measured 3 times. The final dilution curve was constructed using the mean of the 3 measurements for each dilution. Percent error at the lower measured value was also calculated.
Correlation to existing indicators of renal injury
Animals
Two groups of client-owned adult horses (4–20 y old) were used for our study. Group N consisted of 7 non-azotemic horses with normal plasma creatinine (≤159 μmol/L [1.8 mg/dL]), no clinical suspicion of renal disease, and no reported treatment with NSAIDs or aminoglycoside antibiotics within 4 wk of sample collection that were euthanized for unrelated reasons (n = 6); the horse from which urine was collected for assay validation was also included in this group. Group A consisted of 7 hospitalized adult equine patients in which azotemia was identified prior to presentation or after admission. There were no restrictions on breed, sex, previous treatments, or medical history. Pregnant mares were excluded from both groups.
Samples
For animals presented for euthanasia, blood was collected in a heparinized tube prior to administration of any drugs, and urine was collected via a urinary catheter within several minutes of euthanasia with an overdose of intravenous (IV) pentobarbital (Euthanasia Solution; VetOne, Boise, ID). Blood was obtained from hospitalized patients via direct venipuncture or through a jugular catheter. Urine samples were collected via midstream free-catch except for one occasion in which a urinary catheter was passed as part of diagnostic evaluation. An attempt was made to obtain urine prior to initiating treatment with IV fluids, but appropriate therapy was not delayed for the purposes of our study. Three samples were obtained from one azotemic patient on separate days over the course of hospitalization, for a total of 9 samples in group A. Urine was centrifuged as described previously, and the supernatant was refrigerated at 4°C until analysis within 48 h (group N samples, sample A1, A3, A5, A6) or frozen at −80°C for up to 2 mo prior to analysis (remaining samples from group A). Postmortem renal histologic examination was performed in horses N1–N6 by the same board-certified veterinary pathologist and pathology resident.
Laboratory analysis
Routine plasma biochemistry analysis was performed promptly after blood collection to determine plasma creatinine and sodium concentrations. Urine supernatant was brought to room temperature before creatinine, sodium, GGT, and NAG were measured using an automated chemistry analyzer. On each day of analysis, a NAG control and 2 creatinine controls (low and high creatinine concentrations) were analyzed and results deemed acceptable prior to measurement of urine NAG activity and creatinine in study samples. Serial plasma creatinine measurements were performed for hospitalized patients as part of clinical monitoring, with frequency determined by the attending clinician.
Data analysis
Urine NAG indices were calculated as follows:
Urinary FENa and urine GGT indices were calculated as described previously.1,22
Statistical analysis
Normality of distribution for age, plasma creatinine, FENa, urine GGT index, and urine NAG index for groups N and A were assessed using histogram plots. All distributions appeared non-normal; therefore, nonparametric statistical methods were utilized. Spearman rank correlation was used to assess the correlation between urine NAG index and each of the following indicators of renal tubular injury: plasma creatinine, urine FENa, and urine GGT index. Animals were divided into groups based on whether creatinine was increased (>159 μmol/L) or normal, whether FENa was increased (>1%) or normal, and whether urine GGT index was increased (>2.8 U/mmol creatinine) or normal. The median urine NAG indices were compared between each set of dichotomous groups using one-sided Wilcoxon rank sum tests. Median age and urine pH for groups N and A were compared using 2-sided Wilcoxon rank sum tests. Statistical evaluations were carried out with the actual urine NAG results and then repeated using the determined lower limit of linearity for any measured urine NAG activity that was less than the lowest activity evaluated in linear dilution. Given that 3 group A samples were collected from 1 horse on separate days of hospitalization, statistical analyses were performed on the full set of samples and on 3 subsets of independent samples (each including a different sample [A2a, A2b, or A2c]). In the case of discrepancies regarding statistical significance with different subsets (only applicable for Spearman correlation between urine NAG and GGT indices), the decision was made to err on the side of conservative interpretation (i.e., no statistical significance). Significance was set at p ≤ 0.05, and analysis was performed using commercial software (R v.3.4.2, https://www.r-project.org/; RStudio v.1.1.383, https://www.rstudio.com/).
Results
Assay validation
Dipstick analysis of urine used for assay validation was unremarkable; USG, FENa, UPC, and urine GGT index were considered normal based on criteria described above. The intra-run CV for urine NAG activity for each of the 3 d was 14–25%, average 21%; the inter-run CV was 3.2%. Intra- and inter-run CVs for urine NAG index were similar (20% and 3.2%, respectively), and intra- and inter-run CVs for urine creatinine were lower (1.0% and 2.9%, respectively). Average net percent recovery of NAG activity added to aliquots of equine urine supernatant was 109% for the lower concentration (2.16 U/L) and 99% for the higher concentration (5.22 U/L). When average net percent recovery of urine NAG index was calculated based on NAG activity and expected urine creatinine concentration following addition of NAG standard, the results were higher but within allowable limits (112% for lower concentration of NAG, 105% for higher concentration of NAG). Urine NAG activity showed linearity over the full range of solutions measured during serial dilution (final mean concentrations of 0.93–10.1 U/L), with high coefficient of determination (R2 = 0.997). Percent error for the lowest concentration was 47.9%.
Correlation to existing indicators of renal injury
The median age of horses in group N (15 y, range: 8–20 y) was not significantly different (p = 0.177) from the median age of group A horses (10 y, range: 4–16 y). A summary of horse signalment, reasons for euthanasia, and primary disease processes is available in Supplementary Table 1. Horse N1 was sedated with detomidine (Dormosedan; Zoetis, Kalamazoo, MI) and butorphanol (Torbugesic; Zoetis) intravenously for endoscopy several hours prior to euthanasia and urine collection. Horses N2–N7 did not receive any medications or IV fluid therapy within the 24 h prior to urine collection except for IV xylazine (XylaMed; VetOne) or detomidine immediately before euthanasia in some cases. Most of the samples for group A were collected via free-catch during micturition after IV fluid therapy had been initiated; sample A2a was obtained via urinary bladder catheterization following sedation with IV xylazine and within hours of administration of 35 L of IV fluids prior to referral. Samples A2b and A2c were collected from horse A2 on subsequent days of hospitalization (following ~475 L and ~530 L of IV fluid therapy, respectively) because azotemia persisted. Sample A4 was collected after <0.5 L of IV fluids were administered in the hospital. Other group A samples were obtained after the horses had received ~7.5–55 L of IV fluids. Horse A1 had been sedated with IV xylazine and detomidine 1–2 h prior to urine collection. Urine was obtained from horse A6 via catheter immediately after euthanasia was performed following ~1 h of general anesthesia. Renal histopathology was performed postmortem on horses in group N that were euthanized (N1–N6) to provide additional information on kidney health. Clinically significant lesions were not observed. Renal histopathology was not available for N7 or for any of the horses in group A.
USG data showed adequate concentrating ability in group N; USG results for group A were confounded by prior IV fluids or alpha-2 adrenergic agonist administration (Supplementary Table 2). Fractional excretion of sodium was normal in all group N samples and mildly to markedly increased in 7 of 9 samples from group A. Urine GGT index was within the reference interval (RI) for group N samples except for sample N1; 5 of 9 samples in group A showed increased urine GGT index. Urine dipstick analysis (data not shown) did not reveal abnormal findings, such as hematuria, pigmenturia, or glucosuria, in group N; qualitative evidence of protein in some samples was not unexpected given urine alkalinity and, based on UPC of <1 in all samples, was considered to represent false positives associated with the reagent pad.3 Samples A1, A2a, and A4 demonstrated hematuria or pigmenturia on the dipstick reagent pad. Hematuria was confirmed on urine sediment examination for sample A2a and suspected in sample A1 (urine sediment exam not performed) in the absence of clinical or laboratory signs of hemolysis or rhabdomyolysis. Pigmenturia in sample A4 was attributed to myoglobinuria because: no erythrocytes were observed on urine sediment examination; there was no evidence of intravascular hemolysis; plasma biochemistry showed markedly elevated creatine kinase and aspartate aminotransferase. Urine sediment was not examined in other group A samples or in any of the group N samples.
There was a significant positive correlation between urine NAG index and FENa (ρ = 0.76, p < 0.001) and between urine NAG index and plasma creatinine (ρ = 0.74, p = 0.001; Fig. 1). Correlation between urine NAG index and urine GGT index did not reach significance as defined for our study (ρ = 0.45, p = 0.079). Median urine NAG index was significantly higher in horses that were azotemic (group A; median = 0.47 U/mmol creatinine, range: 0.08–0.77 U/mmol creatinine) compared to horses that were non-azotemic (group N; median = 0.03 U/mmol creatinine, range: 0.01–0.38 U/mmol creatinine; p = 0.006; Fig. 2). Median urine NAG index was also elevated in horses that had increased FENa (p = 0.006) and in horses that had increased urine GGT index (p = 0.032) relative to horses in which these parameters were within normal limits (Fig. 2). Additionally, urine NAG indices were recalculated using a urine NAG activity of 0.93 U/L, the lowest activity evaluated in linear dilution, for all samples in which the measured activity was <0.9 U/L. When statistical analyses were repeated using the modified urine NAG indices, conclusions regarding significance were unchanged compared to those reported above, aside from a small increase in p value (0.057) for Wilcoxon rank sum comparison of horses with increased urine GGT index versus horses with normal urine GGT index, which would suggest no significant difference in median urine NAG index.
Figure 1.
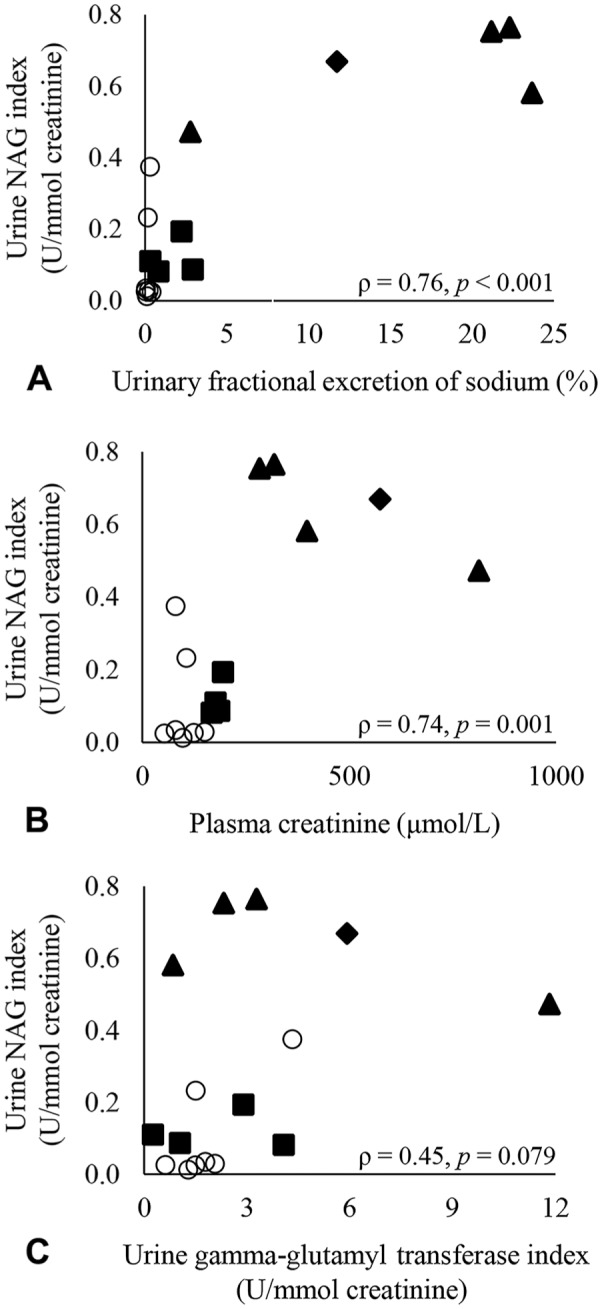
Correlation of urine N-acetyl-β-D-glucosaminidase (NAG) index with A. urinary fractional excretion of sodium, B. plasma creatinine, and C. urine gamma-glutamyl transferase index. Each sample (group N: n = 7 samples; group A: n = 9 samples) is represented by a data point. Group N (non-azotemic) is represented by open circles. Group A (azotemic) is shown in solid markers and is subdivided based on suspected origin of azotemia (suspect renal azotemia = solid triangle; suspect prerenal azotemia = solid square; unknown origin azotemia = solid diamond).
Figure 2.
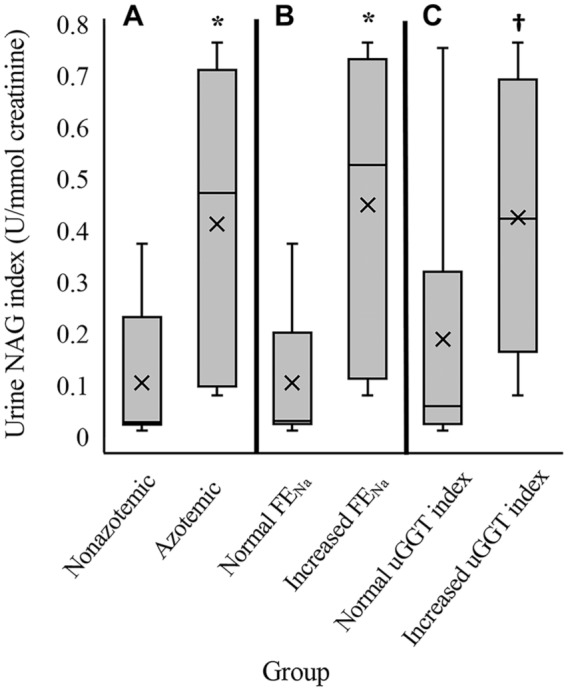
Box-and-whisker plots of urine N-acetyl-β-D-glucosaminidase (NAG) indices for samples divided into groups by A. non-azotemic (n = 7 samples) versus azotemic (n = 9 samples), B. normal urinary fractional excretion of sodium (FENa; n = 8 samples) versus increased FENa (n = 8 samples), and C. normal urine gamma-glutamyl transferase (uGGT) index (n = 10 samples) versus increased uGGT index (n = 6 samples). Horizontal lines = median and quartiles. Whiskers = range of data; × = mean; * = p < 0.01; † = p < 0.05.
Discussion
We showed successful partial validation of the NAG assay for use with equine urine and significant correlation between urine NAG index and both FENa and plasma creatinine, supporting the use of urine NAG as a marker of tubular injury in horses. Moreover, significantly higher urine NAG index in horses with other evidence of renal compromise compared to horses without compromise also suggests that urine NAG index may help identify renal damage, although there was overlap in the ranges of urine NAG index between horses with abnormal and normal renal parameters. Correlation of urine NAG with plasma creatinine in our study is in agreement with findings in children with AKI and adults with diabetic nephropathy.19,32 In dogs with chronic kidney disease, moderate correlation has been demonstrated between urine NAG index and blood urea as well as severity of renal injury (International Renal Interest Society [IRIS] staging of chronic kidney disease).25,33
Previous reference to equine urinary NAG in the literature is limited to a brief discussion of the challenges of detecting NAG activity in alkaline urine from a small number of clinically normal horses (Bayly W. Enzymuria in horses: clinical usefulness. Proc 8th Am Coll Vet Intern Med Forum, Washington, DC; May 1990; 539–541). Information regarding the assay and protocol used for that analysis is not available, and potential differences in methodology might have contributed to the ability to measure equine urine NAG activity described herein (Bayly W, pers. comm. with RL Bayless, April 4, 2018). The median urine NAG index in non-azotemic horses in our study was less than the mean or median urine NAG indices reported for healthy adult cattle, dogs, cats, sheep, and rats using the same assay substrate, although in some cases the published standard deviations or ranges were large.4,7,10,15,29,30,38 This may reflect species-specific variation in the normal urine NAG index and provides support for determination of equine RIs instead of extrapolating from other species.
Histopathology was not available for any horses in group A to confirm renal damage, and previous fluid therapy and/or alpha-2 agonist administration interfered with USG interpretation. On the basis of plasma creatinine response to therapy, tentative classification of azotemia was assigned with the acknowledgement that pre-renal and renal azotemia may be a continuum.8 Plasma creatinine remained elevated in 2 horses (A1, A2) despite prolonged aggressive IV fluid therapy (>5 d). Azotemia in these horses was likely renal in origin, and tubular injury was supported by increased FENa and urine GGT indices. Based on recent onset of clinical abnormalities and lack of owner-reported weight loss, AKI was suspected; plasma creatinine was normal on the only previous bloodwork available (horse A1, 7 y prior). In horses A3–A6, normalization of plasma creatinine within 24 h of IV fluid therapy and normal to mildly increased FENa and urine GGT indices led to suspicion of pre-renal origin of azotemia. Horse A7 was euthanized the day of presentation, so response of azotemia to fluid therapy was unknown; markedly elevated FENa and urine GGT index were consistent with renal tubular damage. Our proposed classification of group A samples shows a tendency for higher urine NAG indices for horses in which azotemia was thought to result from renal damage instead of pre-renal factors; statistical comparison was not performed given the small number of samples from each suspected category of azotemia and lack of confirmation via renal histopathology.
The intra-run and inter-run CVs were considerably less than our established total allowable error for both NAG and urinary NAG index. The magnitude of difference between median urinary NAG index in horses with and without evidence of renal compromise (~10-fold) confirmed the clinical relevance of the established total allowable error. Average net percent recovery for urine NAG activity, a measure of assay bias, was satisfactory for both concentrations of NAG. Urine NAG activity was linear for the range of all dilutions that were analyzed during that portion of assay validation (0.93–10.1 U/L). Urine NAG activities from group A samples were within this range except for 2 samples with NAG activity slightly less than the most dilute sample tested (0.7 and 0.8 U/L). Four of the 7 group N samples had urine NAG activity <0.9 U/L. Extrapolation of linearity outside the tested range is not reliable, but given that low values for urine NAG activity are unlikely to be clinically significant, the range of linearity was considered adequate for the purposes of our study. The statistically significant findings observed when urine NAG results below our established lower limit of linearity were considered to be the lower limit of linearity suggests that working within this linear range will likely provide clinically meaningful data. However, there may be benefit to attempting to extend the reportable range of this assay to urine NAG activities measured in non-azotemic horses in our study. Unfortunately, we could not pursue ascertaining linearity at these lower activities.
Effects of patient, sample, or storage factors on urine NAG are not thoroughly understood. In healthy dogs, there is evidence for no significant difference in urine NAG index between young adults and older adults.33 Data on effect of age on urine NAG index in humans are inconsistent.24,40 Group N horses tended to be older than group A horses, although there was no significant difference in median age for both groups. The impact of sex on urine NAG index is also uncertain. A significant effect of sex on urine NAG index has been identified in humans and clinically normal dogs, but no clear difference in urine NAG index between males and females was demonstrated in studies of juvenile rats and healthy cats.4,24,30,37 Potential effects of sex in the overall findings of our study were likely minimized by the similar sex composition of group N and group A.
Instability of NAG in alkaline urine (pH ≥ 8) at 37°C has been reported in other species and shown to be time-dependent.12,21 The potential for NAG to be inactivated in alkaline equine urine (normal urine pH 7.5–8.5) warrants consideration, but urine NAG activity has been successfully measured in cattle and llamas, which also often have alkaluria (normal urine pH 7.5–8.5 and 7–8.5, respectively).5,14,29,31,34 To our knowledge, the effect of urine pH on NAG activity in samples that are frozen soon after collection has not been well-described. For our study, urine pH was recorded for 5 group N samples and 7 group A samples; median urine pH was not significantly different between groups (group N: median 8, range 7.5–9; group A: median 8, range 6–8.5; p = 0.297). Stability of NAG activity has been demonstrated in urine stored at 4°C for 6 d (rats) or −80°C for 1 y (dogs).16,23 In recent literature for humans and animals, it is not uncommon for samples to be frozen at −80°C until urine NAG measurement.9,11,18,19,23,33 Thus, variation in storage times and conditions between samples in our study was not expected to affect urine NAG results. The effect of storage time and conditions should be evaluated prior to routine use of this assay for diagnostic samples.
Small sample sizes pose a limitation for our study, as does non-random sampling, lack of a gold standard, and administration of IV fluids and/or sedation before obtaining urine. Data for expected urine NAG indices in horses with and without renal injury were not available, so sample size was guided by calculations using urine NAG index summary statistics from healthy dogs and dogs in renal failure.39 Although the sample size was expected to be sufficient for detecting a difference in urine NAG index between horses without suspicion of renal injury and horses that were azotemic, it is possible that our study was underpowered for a secondary outcome of detecting a correlation between urine NAG index and urine GGT index, which may have reached significance with more samples. The number of horses in group N was not designed to be sufficiently large to determine a RI for equine urine NAG index. Enrollment from the hospital population via convenience sampling may have introduced sampling bias; even though group N horses lacked evidence of renal tubular injury, all but one horse had comorbidities (typically orthopedic) that led to euthanasia. Use of creatinine as a biomarker for kidney dysfunction has several weaknesses, including limited ability to detect early kidney injury, challenge for differentiating pre-renal, renal, and post-renal causes of azotemia, and influence of endogenous creatinine production.6 Even considering these limitations, measurement of plasma creatinine is common in equine practice and provided an inexpensive, relatively noninvasive, and quantitative basis for categorizing horses into groups for our study. An effort was made to partially account for limited sensitivity of plasma creatinine by excluding horses from group N if renal lesions were detected postmortem; renal histopathology findings did not prompt exclusion of any group N horses. Histopathology of kidney from group A horses would have been helpful for differentiating pre-renal from renal origin of azotemia and, in cases with kidney lesions, estimating chronicity and severity of renal disease. Comparison of NAG activity to urine creatinine concentration, as we performed in our study, has been effective in minimizing the effect of variation in urine flow rate and concentration on measures of NAG in human studies.13,35,40 Thus, although collection of urine samples prior to fluid therapy or use of alpha-2 agonists would have facilitated USG interpretation, these treatments were unlikely to influence the urine NAG index.
Limited availability of NAG standard precluded further investigation into the influence of signalment, urine pH, and storage on urinary NAG or analysis of additional clinical samples. Nonetheless, the ability to measure NAG in equine urine demonstrated herein warrants continued study of this potential biomarker in horses. Given that one of the proposed advantages of NAG is earlier detection of renal damage compared to other currently used biomarkers, monitoring of the urine NAG index in horses prior to development of azotemia would be useful. Other directions for continued examination of urine NAG in equine medicine include determination of urine NAG index RIs in young, middle-aged, and senior horses, monitoring of urine NAG indices in equine patients treated with potentially nephrotoxic medications, and correlation of urine NAG indices with renal histopathology.
Supplemental Material
Supplemental material, DS1_JVDI_10.1177_1040638719867124 for Equine urinary N-acetyl-β-D-glucosaminidase assay validation and correlation with other markers of kidney injury by Rosemary L. Bayless, A Russell Moore, Diana M. Hassel, Brittney J. Byer, Gabriele A. Landolt and Yvette S. Nout-Lomas in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank Drs. Chad Frank and Lauren Harris for histologic examination of postmortem renal tissue, Colorado State University Clinical Pathology and Diagnostic Laboratory for sample processing, and Drs. Ben Ouyang and Ann Hess for statistical consultation.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The study was supported by funding from the Young Investigator Grant Program in the Center for Companion Animal Studies, Colorado State University.
ORCID iD: Rosemary L. Bayless  https://orcid.org/0000-0001-5748-8279
https://orcid.org/0000-0001-5748-8279
Supplementary material: Supplementary material for this article is available online.
References
- 1. Adams R. et al. Evaluation of a technique for measurement of γ-glutamyltranspeptidase in equine urine. Am J Vet Res 1985;46:147–150. [PubMed] [Google Scholar]
- 2. Adelman RD. et al. Comparative nephrotoxicity of gentamicin and netilmicin: functional and morphological correlations with urinary enzyme activities. Curr Probl Clin Biochem 1979; 166–182. [PubMed] [Google Scholar]
- 3. Bohn AA. Laboratory evaluation of the equine renal system. In: Walton RM, ed. Equine Clinical Pathology. Oxford, UK: Wiley, 2014:87–101. [Google Scholar]
- 4. Brunker JD. et al. Indices of urine N-acetyl-β-D-glucosaminidase and γ-glutamyl transpeptidase activities in clinically normal adult dogs. Am J Vet Res 2009;70:297–301. [DOI] [PubMed] [Google Scholar]
- 5. Cebra C. Disorders of the urinary system. In: Cebra C. et al. , eds. Llama and Alpaca Care: Medicine, Surgery, Reproduction, Nutrition, and Herd Health. 1st ed. St. Louis, MO: Elsevier, 2014:464–476. [Google Scholar]
- 6. Finco DR, Duncan JR. Evaluation of blood urea nitrogen and serum creatinine concentrations as indicators of renal dysfunction: a study of 111 cases and a review of related literature. J Am Vet Med Assoc 1976;168:593–601. [PubMed] [Google Scholar]
- 7. Garry F. et al. Enzymuria as an index of renal damage in sheep with induced aminoglycoside nephrotoxicosis. Am J Vet Res 1990;51:428–432. [PubMed] [Google Scholar]
- 8. Geor RJ. Acute renal failure in horses. Vet Clin North Am Equine Pract 2007;23:577–591. [DOI] [PubMed] [Google Scholar]
- 9. Gluhovschi G. et al. Urinary biomarkers in assessing the nephrotoxic potential of gentamicin in solitary kidney patients after 7 days of therapy. Renal Fail 2014;36:534–540. [DOI] [PubMed] [Google Scholar]
- 10. Ida S. et al. Mild to severe lithium-induced nephropathy models and urine N-acetyl-β-D-glucosaminidase in rats. Methods Find Exp Clin Pharmacol 2001;23:445–448. [DOI] [PubMed] [Google Scholar]
- 11. Jansen D. et al. Tubular injury biomarkers to detect gentamicin-induced acute kidney injury in the neonatal intensive care unit. Am J Perinatol 2016;33:180–187. [DOI] [PubMed] [Google Scholar]
- 12. Jung K. et al. Stability of enzymes in urine at 37°C. Clin Chim Acta 1983;131:185–191. [DOI] [PubMed] [Google Scholar]
- 13. Jung K. et al. Different diuresis-dependent excretions of urinary enzymes: N-acetyl-β-D-glucosaminidase, alanine aminopeptidase, alkaline phosphatase, and γ-glutamyltransferase. Clin Chem 1986;32:529–532. [PubMed] [Google Scholar]
- 14. Lackey MN. et al. Urinary indices in llamas fed different diets. Am J Vet Res 1995;56:859–865. [PubMed] [Google Scholar]
- 15. Lapointe C. et al. N-acetyl-β-D-glucosaminidase index as an early biomarker for chronic kidney disease in cats with hyperthyroidism. J Vet Intern Med 2008;22:1103–1110. [DOI] [PubMed] [Google Scholar]
- 16. Lee J-M. et al. Variation of nephrotoxicity biomarkers by urinary storage condition in rats. Toxicol Res 2014;30:305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liangos O. et al. Urinary N-acetyl-β-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol 2007;18:904–912. [DOI] [PubMed] [Google Scholar]
- 18. McWilliam SJ. et al. Mechanism-based urinary biomarkers to identify the potential for aminoglycoside-induced nephrotoxicity in premature neonates: a proof-of-concept study. PLoS One 2012;7:e43809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mishra OP. et al. Predictive ability of urinary biomarkers for outcome in children with acute kidney injury. Pediatr Nephrol 2017;32:521–527. [DOI] [PubMed] [Google Scholar]
- 20. Miyai T, Ogata M. Changes in the concentrations of urinary proteins after physical exercise. Acta Med Okayama 1990;44:263–266. [DOI] [PubMed] [Google Scholar]
- 21. Morita A. et al. Stabilities of N-acetyl-β-D-glucosaminidase (NAG) isoenzymes in urine: advantage of NAG isoenzyme B measurement in clinical applications. Clin Chim Acta 1998;278:35–43. [DOI] [PubMed] [Google Scholar]
- 22. Morris DD. et al. Renal clearance and fractional excretion of electrolytes over a 24-hour period in horses. Am J Vet Res 1984;45:2431–2435. [PubMed] [Google Scholar]
- 23. Nabity MB. et al. Urinary biomarkers of renal disease in dogs with X-linked hereditary nephropathy. J Vet Intern Med 2012;26:282–293. [DOI] [PubMed] [Google Scholar]
- 24. Pennemans V. et al. Establishment of reference values for novel urinary biomarkers for renal damage in the healthy population: are age and gender an issue? Clin Chem Lab Med 2013;51:1795–1802. [DOI] [PubMed] [Google Scholar]
- 25. Polzin D. et al. Chronic kidney disease. In: Ettinger S, Feldman E, eds. Textbook of Veterinary Internal Medicine. 6th ed. St. Louis, MO: Elsevier Saunders, 2005:1756–1785. [Google Scholar]
- 26. Price RG. The role of NAG (N-acetyl-β-D-glucosaminidase) in the diagnosis of kidney disease including the monitoring of nephrotoxicity. Clin Nephrol 1992;38:S14–S19. [PubMed] [Google Scholar]
- 27. Raekallio MR. et al. Early detection of ketoprofen-induced acute kidney injury in sheep as determined by evaluation of urinary enzyme activities. Am J Vet Res 2010;71:1246–1252. [DOI] [PubMed] [Google Scholar]
- 28. Roussel AJ. et al. Urinary indices of horses after intravenous administration of crystalloid solutions. J Vet Intern Med 1993;7:241–246. [DOI] [PubMed] [Google Scholar]
- 29. Sato R. et al. Urine N-acetyl-β-D-glucosaminidase activity in healthy cattle. Am J Vet Res 1997;58:1197–1200. [PubMed] [Google Scholar]
- 30. Sato R. et al. Urinary excretion of N-acetyl-β-D-glucosaminidase and its isoenzymes in cats with urinary disease. J Vet Med Sci 2002;64:367–371. [DOI] [PubMed] [Google Scholar]
- 31. Savage CJ. Urinary clinical pathologic findings and glomerular filtration rate in the horse. Vet Clin North Am Equine Pract 2008;24:387–404. [DOI] [PubMed] [Google Scholar]
- 32. Sheira G. et al. Urinary biomarker N-acetyl-β-D-glucosaminidase can predict severity of renal damage in diabetic nephropathy. J Diabetes Metab Disord 2015;14:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smets PMY. et al. Urinary markers in healthy young and aged dogs and dogs with chronic kidney disease. J Vet Intern Med 2010;24:65–72. [DOI] [PubMed] [Google Scholar]
- 34. Stockham SL, Scott MA. Urinary system. In: Fundamentals of Veterinary Clinical Pathology. 2nd ed. Ames, IA: Blackwell, 2008:708–840. [Google Scholar]
- 35. Trachtenberg F. et al. The influence of urinary flow rate in children on excretion of markers used for assessment of renal damage: albumin, γ-glutamyl transpeptidase, N-acetyl-β-D-glucosaminidase, and alpha1-microglobulin. Pediatr Nephrol 2008;23:445–456. [DOI] [PubMed] [Google Scholar]
- 36. Trim CM, Hanson RR. Effects of xylazine on renal function and plasma glucose in ponies. Vet Rec 1986;118:65–67. [DOI] [PubMed] [Google Scholar]
- 37. Tsuji S. et al. Sex differences in the excretion levels of traditional and novel urinary biomarkers of nephrotoxicity in rats. J Toxicol Sci 2017;42:615–627. [DOI] [PubMed] [Google Scholar]
- 38. Uechi M. et al. Evaluation of urinary enzymes in dogs with early renal disorder. J Vet Med Sci 1994;56:555–556. [DOI] [PubMed] [Google Scholar]
- 39. Uechi M. et al. The circadian variation of urinary N-acetyl-β-D-glucosaminidase and γ-glutamyl transpeptidase in clinically healthy cats. J Vet Med Sci 1998;60:1033–1034. [DOI] [PubMed] [Google Scholar]
- 40. Wellwood JM. et al. Urinary N-acetyl-β-D-glucosaminidase activities in patients with renal disease. Br Med J 1975;3:408–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Whiting PH, Brown PAJ. The relationship between enzymuria and kidney enzyme activities in experimental gentamicin nephrotoxicity. Renal Fail 1996;18:899–909. [DOI] [PubMed] [Google Scholar]
- 42. Wu X. et al. The reference dose for subchronic exposure of pigs to cadmium leading to early renal damage by benchmark dose method. Toxicol Sci 2012;128:524–531. [DOI] [PubMed] [Google Scholar]
- 43. Zhang X. et al. Combined detection of urinary micro albumin, α1-microglobulin and N-acetyl-β-D-glucosaminidase in the early diagnosis of diabetic nephropathy. Pak J Med Sci 2017;33:1324–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhou X. et al. Evaluation of the usefulness of novel biomarkers for drug-induced acute kidney injury in beagle dogs. Toxicol Appl Pharmacol 2014;280:30–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS1_JVDI_10.1177_1040638719867124 for Equine urinary N-acetyl-β-D-glucosaminidase assay validation and correlation with other markers of kidney injury by Rosemary L. Bayless, A Russell Moore, Diana M. Hassel, Brittney J. Byer, Gabriele A. Landolt and Yvette S. Nout-Lomas in Journal of Veterinary Diagnostic Investigation


