Abstract
Equid alphaherpesvirus 1 (EHV-1) infections can have a major impact on the horse industry and equine welfare by causing abortion or respiratory or neurologic disease. A single nucleotide polymorphism (A2254→G2254) in open reading frame (ORF) 30, encoding the catalytic subunit of the DNA polymerase, has been shown to be a strong predictive marker for neuropathogenicity. Given that a previously established real-time PCR (rtPCR) protocol yielded unsatisfactory results concerning determination of the EHV-1 genotype, we developed and evaluated a new conventional PCR protocol enabling identification of the genotype by sequencing and restriction enzyme analysis (REA). Thirty samples from horses with signs typical for EHV-1 infection were tested by rtPCR and our new conventional PCR. The results showed that compared to rtPCR, the conventional PCR protocol combined with sequencing and REA was more reliable concerning unambiguous determination of the EHV-1 genotype. Results of our new assay confirmed previous findings, according to which the non-neuropathogenic genotype A2254 is predominantly found in animals with fever, respiratory signs, and abortions or perinatal mortality, whereas the neuropathogenic genotype G2254 is primarily detected in animals suffering from neurologic disease. In some samples, results pointed towards coinfection with both genotypes. Further studies are required in order to elucidate the significance of infections with genotype A2254 and G2254 in neurologic and non-neurologic cases, respectively.
Keywords: equid alphaherpesvirus 1, neuropathogenic, PCR, restriction enzyme analysis, sequencing
Introduction
Equid alphaherpesvirus 1 (EHV-1; family Herpesviridae, genus Varicellovirus) causes respiratory disease, abortion, neonatal death or weakness, and neurologic disorders, also known as equine herpesvirus myeloencephalopathy (EHM), in horses worldwide.9,12 The virus is closely related to EHV-4, which causes infections predominantly of the upper respiratory tract.10 Disease following EHV-1 infection can occur as an isolated case or as outbreaks affecting several horses in a stable.1,9 Although mild rhinopneumonitis has only a minor health impact, abortion and EHM are outcomes of EHV-1 infection that impair equine welfare and cause high economic losses in stud farms and in the entire horse industry.3,9,10,18 Reports of EHM following EHV-1 infection have increased in Europe and North America.2,4,5,13-15,21,25 EHM has therefore been mentioned as a potentially emerging disease in horses by the U.S. Department of Agriculture, Animal and Plant Health Inspection Service (USDA APHIS. Equine herpesvirus myeloencephalopathy: a potentially emerging disease, 2007, https://www.aphis.usda.gov/animal_health/emergingissues/downloads/ehv1final.pdf).
Although herpesviruses have co-evolved with their hosts6 and, in general, have high genetic stability,24 several genetic subgroups of EHV-1 circulate in the field.12 Upon investigation of EHV-1 genomes by PCR and sequencing, a single nucleotide polymorphism (SNP) was found in open reading frame (ORF) 30, encoding the catalytic subunit of the viral DNA polymerase, which is strongly predictive for the (in)ability of a virus strain to cause neurologic disease.12 More precisely, the point mutation A→G at position 2254 of ORF30 leads to a single amino acid substitution from asparagine (N752) to aspartic acid (D752) and is supposed to be a major factor contributing to neuropathogenicity. Accordingly, EHV-1 strains with the genotype A2254 are linked to non-neuropathogenic (NNP) infections, whereas strains with the mutated genotype G2254 are potentially neuropathogenic (NP).12,23 However, genotype G2254 alone is not decisive for the development of neurologic signs. As in many other herpesviral infections, the clinical outcome depends on additional factors, including age,11 physical condition, immune status of the infected animal, infective dose, and on whether it is a primary infection, reinfection, or reactivation from the latent state.10 The mechanism by which a point mutation in the viral DNA polymerase predisposes to neurologic disease is not yet fully understood. Horses infected with neuropathogenic strains of EHV-1 (NP = G2254/D752) were shown to develop higher levels of viremia than horses infected with non-neuropathogenic strains (NNP = A2254/N752).9,23 Furthermore, viremia in horses infected with neuropathogenic EHV-1 lasted longer than in animals infected with non-neuropathogenic EHV-1.23 The amino acid variation in the DNA polymerase seems to have an effect on viral replication in peripheral blood leukocytes, which are crucial for the development of cell-associated viremia and subsequent vascular damage in the central nervous system (CNS).9,23 Henceforth, we refer to the non-neuropathogenic genotype A2254 as NNP EHV-1 and to the neuropathogenic genotype G2254 as NP EHV-1.
In our diagnostic unit, we have applied a real-time PCR (rtPCR) protocol targeting the polymorphic site of ORF30 with probes specific for NNP or NP EHV-1 to detect and distinguish the 2 genotypes.17 However, results were repeatedly ambiguous, with similar signals for both genotypes, not allowing a clear determination of the infecting genotype. A protocol published in 2012 used different primers but the same probes, and therefore was precluded as a possible alternative.21 In order to find a more reliable method, a previously published protocol of a bead-based nested PCR combined with sequencing and restriction enzyme analysis (REA) was successfully validated with clinical specimens known to be positive for EHV-1 or EHV-4.1 However, nested PCR harbors the risk for contamination, and in addition is more work- and cost-intensive. Therefore, we adapted the published nested PCR and established and validated a new protocol, which allowed reliable determination of the EHV-1 genotype by sequencing or REA after performing a conventional single-run PCR using only one pair of primers.
Materials and methods
Animals and sample material
Samples from 30 horses examined by the EHV-1/4 multiplex rtPCR7 at the Institute of Virology, University of Zurich between 2012 and 2018 were used for the establishment of a novel protocol for detection and differentiation of NNP and NP EHV-1. For validation of the new assay, the samples were also tested by a published rtPCR that differentiates the EHV-1 genotypes via specific probes.17 The samples were obtained from aborted fetuses, premature foals, and adult horses with clinical signs of disease indicative of herpesviral infection. DNA was extracted from cerebrospinal fluid (1), placenta (2), lung and liver (2), EDTA blood (4), and nasal swabs (21; Table 1). Clinical data, especially whether the animals suffered from neurologic disorders, were obtained from the referring veterinarians.
Table 1.
Results of testing 30 equine samples by EHV-1/4 multiplex rtPCR.7
| Case | Sample | EHV-1/4 rtPCR |
|---|---|---|
| 1 | Cerebrospinal fluid | EHV-1 (33) |
| 2 | Organs (lung and liver) | EHV-1 (14) |
| 3 | EDTA blood | EHV-1 (34) |
| 4 | Nasal swab | EHV-1 (15) |
| 5 | EDTA blood | EHV-1 (33) |
| 6 | Nasal swab | EHV-1 (20) |
| 7 | Nasal swab | EHV-4 (35) |
| 8 | Nasal swab | EHV-4 (30) |
| 9 | Placenta | EHV-1 (26) |
| 10 | Nasal swab | EHV-1 (23) |
| 11 | Nasal swab | EHV-1 (32) |
| 12 | Nasal swab | EHV-1 (31) |
| 13 | Nasal swab | EHV-1 (36) |
| 14 | Nasal swab | EHV-1 (37) |
| 15 | Nasal swab | EHV-1 (15) |
| 16 | EDTA blood | EHV-1 (36) |
| 17 | Nasal swab | EHV-1 (34) |
| 18 | Nasal swab | EHV-1 (32) |
| 19 | Organs (lung and liver) | EHV-1 (27) |
| 20 | Nasal swab | EHV-1 (24) |
| 21 | Nasal swab | EHV-1 (35) |
| 22 | Nasal swab | EHV-1 (21) |
| 23 | Placenta | EHV-1 (24) |
| 24 | Nasal swab | EHV-1 (31) |
| 25 | Nasal swab | EHV-1 (28), EHV-4 (23) |
| 26 | EDTA blood | EHV-1 (35) |
| 27 | Nasal swab | EHV-1 (37) |
| 28 | Nasal swab | EHV-1 (29) |
| 29 | Nasal swab | EHV-1 (35) |
| 30 | Nasal swab | EHV-1 (30) |
EHV-1 = equid alphaherpesvirus 1. Numbers in parentheses are the Ct values for the EHV strain.
Sample preparation and DNA extraction
For leukocyte isolation, EDTA blood samples were mixed with 4 volumes of lysis buffer (0.15 M NH4Cl, 10 mM CHKO3, 0.1 mM EDTA disodium salt [pH 7.2]) and centrifuged at 4°C at 868 × g for 10 min. The supernatant was discarded, and the lysis step was repeated. After resuspension of the leukocyte pellet in 40 mL of phosphate-buffered saline (PBS), the samples were centrifuged for another 10 min. The supernatant was discarded, and the leukocytes resuspended in the remaining liquid. The suspension was transferred to a 1.5-mL tube and centrifuged at 16,060 × g for 1 min. The remaining PBS supernatant was removed, leaving the leukocyte pellet in the tube. DNA was extracted from the leukocytes, cerebrospinal fluid, nasal swabs, placental samples, and organic tissue (QIAamp DNA mini kit; Qiagen, Hilden, Germany) according to the manufacturer’s instructions. After performing the EHV-1/4 multiplex rtPCR, the extracted DNA was stored at −20°C until further use.
EHV-1/4 multiplex rtPCR
For detection of EHV-1 and EHV-4, a multiplex rtPCR targeting the gB gene of both herpesviruses was performed (Table 2).7 The PCR mix had a volume of 25 µL and contained 12.5 µL of TaqMan universal PCR master mix (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA), 400 nM of each primer, 200 nM of the EHV-4 TaqMan probe, 100 nM of the EHV-1 TaqMan probe, and diethylpyrocarbonate (DEPC)-treated water to which 5 µL of the extracted DNA were added. Samples were run in duplicate, undiluted and in 1:10 dilution in order to exclude PCR inhibition, and amplified in a combined thermocycler/fluorometer (7900HT; Applied Biosystems) with the standard thermal cycling protocol of 2 min at 50°C and 10 min at 95°C, followed by 40 cycles of 15 s at 95°C and 60 s at 60°C.
Table 2.
Primers and probes used to detect EHV-1 and EHV-4 DNA.7 For positions of EHV-1 primers and probes, the nucleotide sequence of GenBank accession D00401 served as reference.
| Primer or probe | Sequence (5’–3’) | Position in gB gene |
|---|---|---|
| EHV-1 | ||
| MGB F1 | CATGTCAACGCACTCCCA | 1247-1264 |
| MGB R1 | GGGTCGGGCGTTTCTGT | 1293-1309 |
| MGB probe | 6FAM-CCCTACGCTGCTCC-MGB-NFQ | 1277-1290 |
| EHV-4 | ||
| MGB F1 | GGGCTATTGGATTACAGCGAGAT | 2302-2324 |
| MGB R1 | TAGAATCGGAGGGCGTGAAG | 2340-2360 |
| MGB probe | VIC-CAGCGCCGTAACCAG-MGB-NFQ | 2326-2339 |
EHV-1 = equid alphaherpesvirus 1; F = forward primer; MGB = minor groove binder; NFQ = non-fluorescent quencher; R = reverse primer.
NNP/NP EHV-1 rtPCR
The rtPCR for differentiation of NNP and NP EHV-1 strains was performed; primers and probes (Table 3) target the polymorphic site of the ORF30 region of EHV-1.17 The NNP probe binds to the EHV-1 genome with genotype A2254; the NP probe binds to the EHV-1 genome with the mutated genotype G2254. The PCR mix had a final volume of 25 µL and contained 12.5 µL of TaqMan universal PCR master mix (Applied Biosystems), 400 nM of each primer, 80 nM of the TaqMan probes, a top-up of DEPC-treated water, and 5 µL of the extracted DNA. The samples were run under the same conditions as described above.
Table 3.
Primers and probes used to detect NNP and NP EHV-1 DNA.17 The DNA polymerase gene of EHV-1 (ORF30; GenBank accession NC_001491) served as reference for positions of primers and probes.
| EHV-1 primer or probe | Sequence (5’–3’) | Position in ORF30 |
|---|---|---|
| 29F | ATCTGGCCGGGCTTCAAC | 2228-2245 |
| 82R | GGTCACCCACCTCGAACGT | 2263-2281 |
| NNP probe | VIC-ATCCGTCAACTACTCG-MGB | 2247-2262 |
| NP probe | 6FAM-ATCCGTCGACTACTCG-MGB | 2247-2262 |
EHV-1 = equid alphaherpesvirus 1; F = forward primer; NNP = non-neuropathogenic; NP = neuropathogenic; ORF = open reading frame; R = reverse primer.
NNP/NP EHV-1 PCR with sequencing and REA
The protocol for detection and distinction of NNP and NP EHV-1 was modified from a bead-based, sequence-capture nested PCR1 (Supplementary Fig. 1) into a conventional PCR using only the inner pair of primers for amplification of a 256-bp fragment of the ORF30 region of the EHV-1 genome (Table 4; Supplementary Fig. 2). To compensate for the lower sensitivity by using a single reaction, the cycle number was increased from 35 in the original protocol to 45 (Supplementary Fig. 3).1 In detail, a 25-µL reaction mixture was set up containing 2.5 µL of PCR buffer (10×; Qiagen), 200 µM of each deoxynucleotide triphosphate, 1 µM of each primer, 4 units of HotStarTaq DNA polymerase (5 U/µL; Qiagen), and 5 µL of DNA, topped up with DEPC-treated water. Thermal cycling was performed (FlexCycler2; Analytik Jena, Jena, Germany). The cycling protocol consisted of an initial denaturation at 95°C for 15 min, 45 cycles of 30 s at 94°C, 30 s at 61°C, and 60 s at 72°C, followed by a final elongation at 72°C for 10 min. After the amplification, the samples were cooled to 4°C. PCR products were subjected to electrophoresis at 100 V for ~1 h in 1.5% agarose gel. Bands of the expected size (256 bp) were excised, and DNA was extracted (QIAquick gel extraction kit; Qiagen) according to the manufacturer’s instructions. For sequencing, the concentration of the extracted PCR product was measured (ND-1000 spectrophotometer; NanoDrop Technologies, Wilmington, DE). Fifty nanograms of DNA were mixed with 3 µL of primer ORF30-F-7 (10 µM) and topped up with DEPC-treated water to a final volume of 12 µL. Sequencing was performed (Microsynth, Balgach, Switzerland), electropherograms were visualized and compared (4Peaks; Nucleobytes, Netherlands), and sequences were analyzed with NCBI BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi). For REA, 0.2–0.4 µg of gel-extracted DNA was mixed with 2 µL of buffer O (Thermo Fisher Scientific) and 0.6 µL of SalI restriction endonuclease (Thermo Fisher Scientific). DEPC-treated water was added to a final volume of 30 µL. After incubation at 37°C for 6 h, DNA fragments were separated on 1.5% agarose gel at 100 V for ~1 h. The presence of a single band at ~256 bp indicated uncleaved PCR product from genotype A2254 (NNP). Given that the point mutation A2254→G2254 creates a cleavage site for SalI, the presence of 2 bands of 161 bp and 95 bp, respectively, indicated a cleaved PCR product of EHV-1 genotype G2254 (NP).
Table 4.
Primers used to detect NNP and NP EHV-1 DNA.1 The DNA polymerase gene of EHV-1 (ORF30; GenBank accession NC_001491) served as reference for positions of the primers.
| Primer | Sequence (5’–3’) | Position in ORF30 |
|---|---|---|
| ORF30-F-7 | GGGAGCAAAGGTTCTAGACC | 2094-2113 |
| ORF30-R-3 | AGCCAGTCGCGCAGCAAGATG | 2328-2348 |
EHV-1 = equid alphaherpesvirus 1; F = forward primer; NNP = non-neuropathogenic; NP = neuropathogenic; ORF = open reading frame; R = reverse primer.
Results
EHV-1/4 multiplex rtPCR
By EHV-1/4 multiplex rtPCR, 27 of the 30 samples tested positive for EHV-1; samples 7 and 8 tested positive for EHV–4. One sample (25) gave detectable signals for both viruses, whereby the cycle threshold (Ct) value for EHV-4 was lower than that for EHV-1 (Table 1).
NNP/NP EHV-1 rtPCR
Seven samples were found positive for NNP EHV-1, and 4 samples tested positive for NP EHV-1 (Table 5). The EHV-1 Ct values were in the same range as in the EHV-1/4 multiplex rtPCR (Tables 1, 5), suggesting a comparable analytical sensitivity of the 2 rtPCR assays. Interestingly, 14 samples, all of which had been identified as positive for EHV-1 by the EHV-1/4 rtPCR, gave detectable signals for both EHV-1 genotypes NNP and NP. In 13 samples, the Ct values for NNP were lower than those for NP. Only one sample (16) had a lower Ct value for NP compared to NNP. Five horses tested negative for EHV-1 by NNP/NP rtPCR. Two of these samples had tested positive for EHV-1 with rather high Ct values of 33 (1) and 35 (21) by means of the EHV-1/4 multiplex rtPCR (Tables 1, 5). The negative results in the NNP/NP EHV-1 rtPCR may be attributed to a low viral load or poor sample quality. Two samples (7 and 8) that tested positive for EHV-4 by the EHV-1/4 rtPCR were found to be negative for EHV-1 in the EHV-1 NNP/NP rtPCR, thereby corroborating the diagnostic specificity of the NNP/NP EHV-1 real-time assay. Sample 25, which yielded detectable signals for both EHV-1 (Ct 28) and EHV-4 (Ct 23) in the EHV-1/4 multiplex rtPCR, tested negative in the NNP/NP EHV-1 rtPCR, indicating a single infection with EHV-4 and putative cross-reaction with EHV-1 in the EHV-1/4 multiplex rtPCR (Tables 1, 5).
Table 5.
Results of the NNP/NP EHV-1 rtPCR17 and the newly established conventional PCR followed by sequencing and restriction enzyme analysis. The main clinical findings of each horse are listed as reported by the referring veterinarians.
| Case | NNP/NP EHV-1 rtPCR | NNP/NP EHV-1 PCR |
Clinical signs | |
|---|---|---|---|---|
| Sequencing* | REA† | |||
| 1 | Negative | Negative | ND | EHM |
| 2 | NNP (13), NP (17) | NNP | NNP | Abortion |
| 3 | NP (33) | NP | NP | EHM |
| 4 | NP (17) | NP | NP | EHM |
| 5 | NNP (36), NP (38) | NNP | NNP | EHM |
| 6 | NNP (23), NP (26) | NNP | NNP | EHM |
| 7 | Negative | Weakly pos. | ND | Fever |
| 8 | Negative | Negative | ND | Fever |
| 9 | NNP (28), NP (31) | NNP | NNP | Abortion |
| 10 | NNP (25), NP (27) | NNP | NNP | Fever |
| 11 | NNP (34), NP (36) | NNP/NP | NNP/NP | Fever |
| 12 | NNP (33), NP (35) | NNP/NP | NNP/NP | Fever |
| 13 | NNP (38) | NP | NNP/NP | Fever |
| 14 | NNP (38) | Negative | ND | Fever |
| 15 | NNP (17), NP (24) | NNP | NNP | Weak foal |
| 16 | NNP (39), NP (34) | NP | NNP/NP | Fever, respiratory disease |
| 17 | NNP (38) | NP | NP | Fever, respiratory disease |
| 18 | NNP (33), NP (39) | NNP | NNP | Fever, respiratory disease |
| 19 | NNP (18), NP (24) | NNP | NNP | Weak foal |
| 20 | NNP (26), NP (32) | NNP | NNP | Fever, respiratory disease |
| 21 | Negative | Negative | ND | EHM |
| 22 | NP (21) | NP | NP | EHM |
| 23 | NNP (26) | NNP | NNP | Weak foal |
| 24 | NP (35) | NP | NP | EHM |
| 25 | Negative | Weakly pos. | ND | Fever, respiratory disease |
| 26 | NNP (39) | NNP | NNP | Fever |
| 27 | NNP (38) | NNP | NNP | Fever |
| 28 | NNP (28), NP (30) | NNP | NNP | Fever |
| 29 | NNP (39) | Negative | ND | Fever |
| 30 | NNP (28), NP (31) | NNP | NNP | EHM |
EHM = equine herpesvirus myeloencephalopathy; EHV-1 = equid alphaherpesvirus 1; ND = not done; NNP = non-neuropathogenic; NP = neuropathogenic. Numbers in parentheses are the Ct values for the EHV strain.
For sequencing, an adenine base (A) in the polymorphic site (position 2254 of the ORF30) was interpreted as infection with NNP and a guanine base (G) as infection with NP EHV-1.
Samples displaying 1 band of uncleaved product at 256 bp after REA and gel electrophoresis tested NNP EHV-1 positive; samples displaying 2 bands of cleaved product (161 bp and 95 bp) were positive for NP EHV–1. Samples with indeterminate results in sequencing and REA were identified as NNP/NP.
NNP/NP EHV-1 PCR followed by sequencing and REA
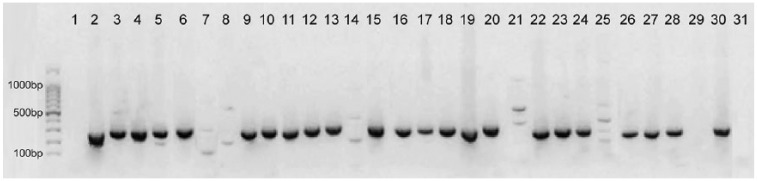
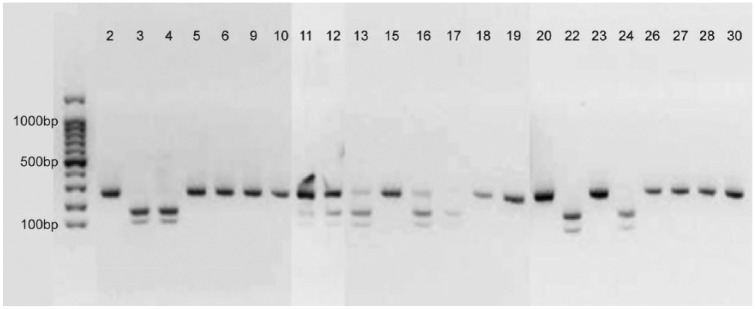
After conventional PCR and gel electrophoresis, 23 of 30 samples showed a band at the expected size of 256 bp (Fig. 1). Sequence analysis revealed that 14 samples were positive for genotype A2254, suggesting infection with NNP EHV-1 (Table 5). These results were confirmed by REA; all of the 14 samples displayed a single band of uncleaved PCR product (Fig. 2). In 7 samples, sequencing analysis revealed a G at position 2254, indicating infection with the EHV-1 neuropathotype. REA confirmed infection with genotype G2254 in 5 of these 7 samples, displaying 2 bands of cleaved product, whereas the restriction pattern of the other 2 samples (13 and 16) showed characteristics of both NNP and NP (3 bands). Upon REA, 3 bands were also detected in samples 11 and 12 (Fig. 2), and in these cases sequence analysis showed nucleotide ambiguities (A/G), thereby making it impossible to clearly determine the nucleotide at position 2254 (N2254; Table 5). Seven samples were not subjected to sequencing and REA, after giving only very faint (7 and 25) or no bands (1, 8, 14, 21, 29) in the gel electrophoresis (Fig. 1).
Figure 1.
Results of agarose gel electrophoresis of the 30 samples after NNP/NP equid alphaherpesvirus 1 PCR. The expected product has a size of 256 bp. In the far left lane of the gel, a 100-bp DNA ladder (lowest lane = 100 bp, strongest lane = 500 bp) was electrophoresed for determination of PCR product sizes. In lane 31, a negative control (DEPC-treated water) was loaded.
Figure 2.
Results of agarose gel electrophoresis after restriction enzyme analysis with SalI of samples determined positive for equid alphaherpesvirus 1 (EHV-1) by NNP/NP EHV-1 PCR. In the far left lane of the gel, a 100-bp DNA ladder was electrophoresed for determination of PCR product sizes.
Comparison of the rtPCR and the new conventional PCR for NNP/NP EHV-1
Of the 14 samples that were found EHV-1 NNP (A2254) positive by conventional PCR followed by sequencing and digestion with SalI restriction endonuclease, only 3 showed an unambiguous result in the NNP/NP rtPCR (i.e., a Ct value only for one genotype; Table 5). The other 11 samples yielded detectable signals for both NNP and NP with Ct values differing by 2–7. However, in all 11 cases, the Ct value for NNP was lower than that for NP. Seven samples were sequenced as EHV-1 NP (G2254) after conventional PCR. Four of these were consistent also in REA following conventional PCR as well as in the NNP/NP rtPCR (Table 5). Results of the other 3 samples were contradictory upon comparison of sequencing, REA, and NNP/NP rtPCR: samples 13 and 16 were NP (G2254) positive according to conventional PCR and sequencing but showed a mixed restriction pattern for both NNP and NP. In the NNP/NP rtPCR, sample 13 was NNP positive, whereas sample 16 had signals for both NNP and NP. In sample 17, results from sequencing and REA correlated, indicating infection with NP EHV-1, but were contradictory to the NNP/NP rtPCR results, which suggested infection with NNP EHV-1 (Table 5, Fig. 2). The 2 samples (11 and 12) that had an undetermined sequencing result (N2254) could also not be assigned reliably to a genotype either by REA or by NNP/NP rtPCR (Ct values differed by only 2). Two samples (14 and 29) tested negative in the conventional PCR but gave weak signals in the NNP/NP rtPCR (Ct values 38 and 39, respectively; Table 5). Five samples did not give any results with either assay. Of these samples, 3 tested positive for EHV-4 in the EHV-1/4 multiplex rtPCR (Tables 1, 5). The other 2 samples, however, tested EHV-1 positive with Ct values of 33 (1) and 35 (21; Table 1).
Comparison of NNP/NP EHV-1 genotype status and clinical signs
The clinical signs of the animals analyzed in our study were typical for EHV-1 or EHV-4 infection (i.e., fever, respiratory signs, abortion or perinatal death, and neurologic disorders such as ataxia, reduced tail tone, recumbency, as well as bladder and rectal paralysis; Table 5). Of the 30 horses investigated, 9 had exhibited neurologic signs, which were ascribed to EHV-1 infection by EHV-1/4 multiplex rtPCR. Of these 9 horses, 4 tested positive for the NP genotype (G2254) of EHV-1 using our protocol. The remaining 5 horses were identified as EHV-1 negative (2 cases) or as EHV-1 NNP (A2254) positive (3 cases; Fig. 3). Sixteen animals did not show neurologic signs, but rather exhibited fever and respiratory disease. In 6 of these animals, the infecting genotype was characterized as NNP EHV-1 and, in 1 case, as NP EHV-1 by sequencing and REA. In another 4 horses, the results from sequencing and REA did not allow a final statement concerning the infecting EHV-1 pathotype. Horses 14 and 29 tested EHV-1 negative using the adapted protocol. Horses 7 and 8, which according to the EHV-1/4 multiplex rtPCR were infected with EHV-4, and a third animal (25), which was positive for both EHV-1 and EHV-4, all tested EHV-1 negative by conventional NNP/NP EHV-1 PCR. In these 3 animals, neurologic signs were not observed, which can be expected, given that EHV-4 has not been associated with neurologic disease to date. The 5 aborted fetuses or premature foals were identified consistently as positive for NNP EHV-1 (A2254) according to sequencing and REA (Table 5).
Figure 3.
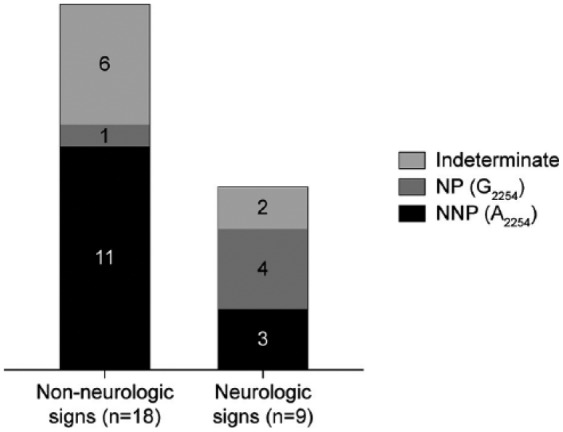
Identified equid alphaherpesvirus 1 (EHV-1) genotypes in 27 EHV-1–positive horses without neurologic signs and in horses with clinical signs of equine herpesvirus myeloencephalopathy.
Discussion
We concluded that the specificity of our single-run PCR for EHV-1 was satisfactory, given that EHV-4–positive samples would be recognized as such upon sequencing. Four samples that were EHV-1 positive in the EHV-1/4 rtPCR tested negative in the conventional PCR. The analytical sensitivity of conventional PCR is often considered lower compared to real-time assays. However, in our study, viral genomes were successfully detected by the conventional PCR in samples that had Ct values up to 37 in the EHV-1/4 rtPCR, demonstrating a high degree of sensitivity of our test. This was further corroborated by the fact that the 4 samples that tested “false negative” in the conventional NNP/NP EHV-1 PCR also tested negative or very weakly positive (Ct 38 and Ct 39) in the NNP/NP EHV-1 rtPCR. Additional mutations in the ORF30 region hampering DNA amplification could not be ruled out. In any event, the differentiation test for NNP/NP strains is not intended for primary detection of an EHV-1 infection but as a follow-up test after EHV-1 infection has been determined by other means (e.g., EHV-1/4 multiplex rtPCR).
Four EHV-1–positive samples gave indeterminate results upon sequencing and REA, yielding characteristic features for both genotypes. For samples 11 and 12, electropherograms showed high signals for both nucleotides A and G in position 2254, resulting in N2254. In the REA, the band of uncleaved product (256 bp) was of stronger intensity than the 2 bands of cleaved product (161 bp and 95 bp). The electropherogram of samples 13 and 16 showed clearly higher signals for nucleotide G than for A at position 2254 (data not shown), leading to a sequencing result of G2254. Accordingly, the intensity of cleaved products after REA with SalI was more pronounced than the intensity of the uncleaved product. We concluded that, although the results for these samples are ambiguous, sequencing and REA results were in agreement when looking at the electropherograms in detail, suggesting coinfection with NNP and NP EHV–1. Indeed, dual infections with the NNP genotype A2254 and the NP genotype G2254 have been reported previously and may be the result of infection from 2 sources or mutation of the persisting virus within the host. In a previous study of 13 horses infected with NP EHV-1, 11 also harbored NNP EHV-1.4 After having had contact with a case of EHM, 10 of 27 EHV-1–positive horses were reported to be dually infected with NP and NNP EHV-1.17 In 2 other reports, dual infections were detected in 10 of 18 and 3 of 204 horses, respectively.19,21 Notably, most of the dually infected horses in the described studies did not develop clinical signs. In all of these studies, rtPCR assays were performed for allelic discrimination of NNP and NP EHV-1. In our study, 14 samples yielded signals for both genotypes in the NNP/NP EHV-1 rtPCR. However, we could not conclude whether these signals were the result of coinfections with genotypes A2254 and G2254 or the result of cross-reactions between the two. Given that the probes for NNP and NP EHV-1 differ only by a single nucleotide, cross-reactivity cannot be completely ruled out. Using the NNP/NP rtPCR protocol, 11 samples gave a clear result, yielding detectable signals for only 1 genotype. This stands in contrast with our PCR protocol, which allowed sound determination of the infecting EHV-1 genotype in 19 samples; only 4 samples gave indeterminate results. The clearer differentiation by the conventional PCR followed by sequencing and REA compared to rtPCR may be the result of recognizing only or mainly the predominant strain in a sample, or the result of less cross-reactivity. Subjecting the samples with unclear results to untargeted next-generation sequencing may shed some light on this question. However, given that the predominant strain is likely to be more important for the clinical outcome in the infected animal, usage of the conventional PCR is favored in any case. Additionally, our conventional PCR combined with sequencing gives more insight into genome variations and possible SNPs other than A/G2254. In our study, alignment of the 23 sequences obtained showed only nucleotide variations at position 2254.
Comparison of the EHV-1 genotypes determined by our new PCR protocol with clinical signs showed that the majority of the 18 EHV-1–positive horses lacking neurologic signs, aborted fetuses and premature foals included, were infected with NNP EHV-1 and that only 1 horse tested positive for NP EHV-1. In the animals that had neurologic signs compatible with EHM, 4 were infected with NP EHV-1, whereas 3 tested positive for NNP EHV–1. Although sample sizes were too small to perform statistical analysis, our results confirm previous findings that EHV-1 genotype A2254 is isolated mainly from cases of respiratory disease and abortion, whereas genotype G2254 is predominantly found in animals suffering from EHM.12,14,15,25 The fact that infections with NNP EHV-1 also occurred in association with EHM, and NP strains in association with abortions, indicates that the A2254/G2254 SNP is not the only viral factor contributing to the neuropathogenic potential of EHV-1.8,12,13,16,20,22,25 We cannot exclude other agents as main causality for the neurologic signs. NNP EHV-1 was previously reported in 13%,22 14%,12 and 24%13 of EHM cases. In contrast, the NP genotype G2254 was detected in 2%,13 6%,12 10%,22 11%,8 and 87%25 of non-neurologic cases. This clearly demonstrates that the genotyping of field isolates needs to be interpreted carefully, keeping in mind that the assays used to determine the infecting EHV-1 pathotype are not 100% specific and that coinfections may occur.12,13,17 Nevertheless, closer characterization of EHV-1 strains is recommended, given that the risk for neurologic disease was shown to be 162 times higher in horses infected with genotype G2254 than in those infected with genotype A2254.13 Although our adaptation of a conventional PCR in combination with sequencing and/or REA represents a rapid, robust, and reliable way to discriminate NP and NNP EHV-1 strains, further studies are required to determine additional neurovirulence markers of EHV-1.
Acknowledgments
We thank Prof. Mathias Ackermann for his helpful input.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This work was supported by the University of Zurich.
ORCID iD: Julia Lechmann  https://orcid.org/0000-0002-7389-846X
https://orcid.org/0000-0002-7389-846X
Supplementary material: Supplementary material for this article is available online.
References
- 1. Allen GP. Antemortem detection of latent infection with neuropathogenic strains of equine herpesvirus-1 in horses. Am J Vet Res 2006;67:1401–1405. [DOI] [PubMed] [Google Scholar]
- 2. Allen GP. Development of a real-time polymerase chain reaction assay for rapid diagnosis of neuropathogenic strains of equine herpesvirus–1. J Vet Diagn Invest 2007;19:69–72. [DOI] [PubMed] [Google Scholar]
- 3. Allen GP. Risk factors for development of neurologic disease after experimental exposure to equine herpesvirus-1 in horses. Am J Vet Res 2008;69:1595–1600. [DOI] [PubMed] [Google Scholar]
- 4. Allen GP. et al. Prevalence of latent, neuropathogenic equine herpesvirus-1 in the Thoroughbred broodmare population of central Kentucky. Equine Vet J 2008;40:105–110. [DOI] [PubMed] [Google Scholar]
- 5. Barbić L. et al. Two outbreaks of neuropathogenic equine herpesvirus type 1 with breed-dependent clinical signs. Vet Rec 2012;170:227. [DOI] [PubMed] [Google Scholar]
- 6. Davison AJ. Evolution of the herpesviruses. Vet Microbiol 2002;86,69–88. [DOI] [PubMed] [Google Scholar]
- 7. Diallo IS. et al. Multiplex real-time PCR for the detection and differentiation of equid herpesvirus 1 (EHV-1) and equid herpesvirus 4 (EHV-4). Vet Microbiol 2007;123:93–103. [DOI] [PubMed] [Google Scholar]
- 8. Fritsche AK, Borchers K. Detection of neuropathogenic strains of equid herpesvirus 1 (EHV-1) associated with abortions in Germany. Vet Microbiol 2011;147:176–180. [DOI] [PubMed] [Google Scholar]
- 9. Goodman LB. et al. A point mutation in a herpesvirus polymerase determines neuropathogenicity. PLoS Pathog 2007;3:e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ma G. et al. Equine herpesviruses type 1 (EHV-1) and 4 (EHV-4)–masters of co-evolution and a constant threat to equids and beyond. Vet Microbiol 2013;167:123–134. [DOI] [PubMed] [Google Scholar]
- 11. Maxwell LK. et al. Efficacy of the early administration of valacyclovir hydrochloride for the treatment of neuropathogenic equine herpesvirus type-I infection in horses. Am J Vet Res 2017;78:1126–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nugent J. et al. Analysis of equid herpesvirus 1 strain variation reveals a point mutation of the DNA polymerase strongly associated with neuropathogenic versus nonneuropathogenic disease outbreaks. J Virol 2006;80:4047–4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perkins GA. et al. Investigation of the prevalence of neurologic equine herpes virus type 1 (EHV-1) in a 23-year retrospective analysis (1984–2007). Vet Microbiol 2009;139:375–378. [DOI] [PubMed] [Google Scholar]
- 14. Pronost S. et al. Neuropathogenic and non-neuropathogenic variants of equine herpesvirus 1 in France. Vet Microbiol 2010;145:329–333. [DOI] [PubMed] [Google Scholar]
- 15. Pronost S. et al. Outbreak of equine herpesvirus myeloencephalopathy in France. A clinical and molecular investigation. Transbound Emerg Dis 2012;59:256–263. [DOI] [PubMed] [Google Scholar]
- 16. Pronost S. et al. Relationship between equine herpesvirus-1 myeloencephalopathy and viral genotype. Equine Vet J 2010;42:672–674. [DOI] [PubMed] [Google Scholar]
- 17. Pusterla N. et al. Characterization of viral loads, strain and state of equine herpesvirus-1 using real-time PCR in horses following natural exposure at a racetrack in California. Vet J 2009;179:230–239. [DOI] [PubMed] [Google Scholar]
- 18. Pusterla N. et al. Equine herpesvirus-1 myeloencephalopathy. A review of recent developments. Vet J 2009;180:279–289. [DOI] [PubMed] [Google Scholar]
- 19. Pusterla N. et al. Prevalence of latent alpha-herpesviruses in Thoroughbred racing horses. Vet J 2012;193:579–582. [DOI] [PubMed] [Google Scholar]
- 20. Smith KL. et al. The increased prevalence of neuropathogenic strains of EHV-1 in equine abortions. Vet Microbiol 2010;141:5–11. [DOI] [PubMed] [Google Scholar]
- 21. Smith KL. et al. New real-time PCR assay using allelic discrimination for detection and differentiation of equine herpesvirus-1 strains with A2254 and G2254 polymorphisms. J Clin Microbiol 2012;50:1981–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tewari D. et al. Equine herpesvirus 1 (EHV-1) nucleotide polymorphism determination using formalin fixed tissues in EHV-1 induced abortions and myelopathies with real-time PCR and pyrosequencing. J Virol Methods 2013;193:371–373. [DOI] [PubMed] [Google Scholar]
- 23. van de Walle GR. et al. A single-nucleotide polymorphism in a herpesvirus DNA polymerase is sufficient to cause lethal neurological disease. J Infect Dis 2009;200:20–25. [DOI] [PubMed] [Google Scholar]
- 24. Vaz PK. et al. Evidence of widespread natural recombination among field isolates of equine herpesvirus 4 but not among field isolates of equine herpesvirus 1. J Gen Virol 2016;97:747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walter J. et al. Clinical observations and management of a severe equine herpesvirus type 1 outbreak with abortion and encephalomyelitis. Acta Vet Scand 2013;55:19. [DOI] [PMC free article] [PubMed] [Google Scholar]