Abstract
Neuronal preconditioning in vitro or in vivo with a stressful but non-lethal stimulus leads to new protein expression that mediates a profound neuroprotection against glutamate excitotoxicity and experimental stroke. The proteins that mediate neuroprotection are relatively unknown and under discovery. Here we find that the expression of the AAA + ATPase Thorase is induced by preconditioning stimulation both in vitro and in vivo. Thorase provides neuroprotection in an ATP-dependent manner against oxygen–glucose deprivation (OGD) neurotoxicity or glutamate N-Methyl-D-aspartate (NMDA) receptor-mediated excitotoxicity in vitro. Knock-down of Thorase prevents the establishment of preconditioning induced neuroprotection against OGD or NMDA neurotoxicity. Transgenic overexpression of Thorase provides neuroprotection in vivo against middle cerebral artery occlusion (MCAO)-induced stroke in mice, while genetic deletion of Thorase results in increased injury in vivo following stroke. These results define Thorase as a neuroprotective protein and understanding Thorase signaling could offer a new therapeutic strategy for the treatment of neurologic disorders.
Keywords: AAA + ATPase, ATAD1, neuroprotection, preconditioning, stroke
Introduction
The nervous system possesses the remarkable ability to respond to sublethal stress, setting in motion transcriptional programs that enable it to survive subsequent lethal insults.1,2 There are diverse signaling networks that are involved in the neuroprotective response with CREB, MEF2, NFκB and HIF-1α transcription factors playing prominent roles.3–9 However, the downstream targets for these transcriptional programs, which are ultimately responsible for the neuroprotective response, are poorly characterized. Previously, we used a functional cloning strategy to identify genes that were induced by oxygen–glucose deprivation (OGD) preconditioning in cortical neuronal cultures.10 Through this screen, we identified 31 neuroprotective genes. One of the genes designated 697-27 was a previously uncharacterized transcript. We have further characterized clone 697-27, which we named Thorase after Thor, the Norse God of Thunder and Lightening. It encodes for a protein of 361 amino acids with a predicted molecular weight of 37 kDa.11 Thorase, also named ATAD1 is an AAA + ATPase that maintains mitochondrial function and regulates AMPA receptor-dependent synaptic plasticity and behavior through control of the surface expression of AMPA receptors.11–13
Since Thorase was identified in a functional screen for protective proteins, its role as a neuroprotective protein was further explored in models of glutamate excitotoxicity and stroke. Here we show that Thorase is induced by preconditioning stimuli and that it is neuroprotective in models of stroke through its AAA + ATPase activity.
Materials and methods
All procedures on mice were approved by the Johns Hopkins University Institutional Animal Care and Use Committee and performed in accordance with National Institutes of Health guidelines and the ARRIVE guidelines (http://www.nc3rs.org.uk/arrive-guidelines).
Primary cortical cultures and transduction of cultured neurons with recombinant lentivirus
Primary cortical neuron cultures were prepared from gestational day 14–15 fetal mouse as described.14 In mature cultures, neurons represent about 80% of the total number of cells. Neurons were infected at DIV7 with shRNA expressing lentivirus or DIV10 for WT-Thorase or Mut-Thorase expressing lentivirus.
Lentivirus generation
Wild type-Thorase or Mutant-Thorase full length coding fragment was cloned into cFUGW vector (UBC-promoter) between BamH I and Age I sites. The shRNA vector was also based on cFUGW with tandem U6-shRNA (short-hairpin) cassettes at Pac 1 site. The shRNA against DsRed sequence was used as control. The oligonucleotides for DsRed shRNA were Up = 5′ GAG TTC CAG TAC GGC TCC AAG AAG CTT GTT GGA GCC GTA CTG GAA CTC TTT TTT ACG CGT; Down = 5′ GTC ACA CGC GTA AAA AAG AGT TCC AGT ACG GCT CCA ACA AGC TTC TTG GAG CCG TAC TGG AAC TC; Oligonucleotides for Thorase shRNA2 were Up = TGA AGC AAA TTG GTG TGA AAT TCA AGA GAT TTC ACA CCA ATT TGC TTC TTT TTT C; Down = TCG AGA AAA AAG AAG CAA ATT GGT GTG AAA TCT CTT GAA TTT CAC ACC AAT TTG CTT CA; Thorase shRNA3: Up = TGT GGT ATG GAG AAT CTC AGT TCA AGA GAC TGA GAT TCT CCA TAC CAC TTT TTT C; Down = TCG AGA AAA AAG TGG TAT GGA GAA TCT CAG TCT CTT GAA CTG AGA TTC TCC ATA CCA CA. Lentiviral vectors were generated by the transient transfection of 293T cells by using the FuGENE HD Transfection Reagent by Roche Applied Science as described previously.11 In brief, cells were cotransfected with the vector plasmid (cFUGW-GFP, cFUGW-WT-Thorase, cFUGW-Mut-Thorase, cFUGW-DsRed shRNA, cFUGW-Thorase shRNA2, or cFUGW-Thorase shRNA3), the transcomplementation plasmids (pLP1 and pLP2), and the plasmid encoding the vesicular stomatitis virus envelope glycoprotein (VSVG). Sodium butyrate was added to the media 4–6 h after transfection. The medium was replaced 12 h after transfection and collected 36 h and 60 h later. Supernatants were centrifuged at 800 × g for 10 min (min), then treated with DNase and passed through a filter with 0.45-µm pores. Viral particles were then concentrated by ultracentrifugation (90 min, 25,000 r/min, rotor SW28) and resuspended in 0.1 M PBS.
Western blotting
Brain tissue or cell lysates were size-separated through SDS/PAGE and processed for analysis by using a Supersignal Chemiluminescence detection kit (Pierce, Rockford, IL) as described by the manufacturer. Anti-Thorase monoclonal antibody was purchased from UC Davis/NIH NeuroMab Facility. Anti-β-actin and anti-β-tubulin antibodies were purchased from Sigma Applied Science.
Thorase-TAP protein purification and ATPase activity assay
Thorase-TAP proteins were purified using InterPlay TAP purification kit (Agilent Technologies, USA) following the protocol from the manufacturer. Briefly, brain tissue was powderized under dry ice and solubilized with lysis buffer provided in the kit with protease inhibitors. The solubilized lysates were centrifuged (15,000 × g for 15 min) and the supernatant was mixed with streptavidin resin followed by incubation at 4℃ for 2 h. The resins were washed four times with the streptavidin buffer and then bound proteins eluted with the streptavidin elution buffer. The eluted samples were then mixed with calmodulin resin in calmodulin binding buffer at 4℃ for 2 h followed by four washes with calmodulin binding buffer. The bound proteins were eluted with calmodulin elution buffer. The eluted samples were dialyzed using ATPase buffer (50 mM Tris pH 7.5, 150 mM NaCl, 5% Glycerol, and 4 mM MgCl2) overnight. The purity of the purified proteins was evaluated by 10% SDS-PAGE followed by silver staining. The ATPase activity of purified Thorase-TAP proteins was determined by using the ADP colorimetric assay kit (Sigma, MAK033-1KT) following manufacturer's instructions. The purified proteins (0.05 µg/µl) were resuspended in ATPase buffer and then incubated with different concentration of ATP solution (10 µM–80 µM) for 30 min at 37℃. ATPase buffer containing same amount of ATP was used as controls for ATP degradation. The samples were then mixed with ADP Reaction Mix Buffer for 30 min at room temperature in the dark to evaluate ADP formation. The absorbance at 570 nm (A570) for each sample was measured to determine ADP concentrations. A standard ADP curve was used to determine the rate of ATP hydrolysis to ADP. The ATPase activity (nmole/min/mg protein) curve was plotted using GraphPad prism to determine the binding constant (Km) of the purified TAP-Thorase.
Cytotoxicity
Primary cultured cortical neurons (14 DIV) were exposed to OGD as described.15 Briefly, OGD was performed by complete change of media with deoxygenated, glucose-free Earle's balanced salt solution, bubbled with 10% H2/85% N2/5% CO2. Cultures were kept in an anaerobic chamber (Biospherix Ltd., USA) for 15 min or 90 min at 37℃. OGD was terminated by replacement of the Earle's balanced salt solution with oxygenated growth media. Primary cultured cortical neurons (14 DIV) were exposed to NMDA as described previously.16 Cells were washed two times with control salt solution (CSS) containing (in mM): 120 NaCl, 5.4 KCl, 1.8 CaCl2, 25 Tris-Cl, 15 glucose, pH 7.4, exposed to 50 µM NMDA/10 µM glycine, 200 µM NMDA/10 µM glycine, 400 µM NMDA/10 µM glycine or 500 µM NMDA/10 µM glycine in CSS for 5 min at 37℃. Exposure was terminated by replacement with fresh media. Neurotoxicity was assessed 24 h after the last experimental exposure by computer-assisted cell counting after staining with 1 µg/ml Hoechst 33,342 (Molecular Probes) and 5 µM propidium iodide (Sigma, St. Louis, MO). The numbers of total and dead cells were counted with the Axiovision 4.7 software (Zeiss, Thornwood, NY). The percentages of neuron death in was quantified and expressed as the mean ± SEM from three independent experiments performed in triplicate. Data were analyzed by ANOVA with the with Tukey–Kramer's post hoc test for independent means.
Generation of Thorase-TAP transgenic mice
The Tet off transgenic construct pPrP.TetP-Thorase-TAP was generated by cloning the Thorase-TAP DNA fragment into the XhoI site of pPrP.TetP vector. The integrity of the cloned construct was verified by sequencing. The transgenic construct pPrP.TetP-Thorase-TAP was linearized by NotI digestion before the microinjection into the embryos of the B6C3F2 strain. The one- or two-cell embryos were transferred into B6D2F1 pseudopregnant female mice (Transgenic Animal Core of National Cancer Institute). Pups were screened by tail genotyping with PCR (GoTaq Green Master Mix, Promega) using two pair primers (for tet region, forward: CGG GTC GAG TAG GCG TGT AC; reverse: TCT AGA TGA TCC CCG GGT ACC GAG; PCR product = 173 bp; for Thorase-TAP region, forward primer: CTG AAG CAT TCT GCC TTC CTA GTG GTA C; reverse: ATA ACC CCT CCC CCA GCC TAG ACC A; PCR product = 1380 bp) to select positive founders. Eight high copy founders were mated with CamKIIα-tTA mice to drive the expression of Thorase-TAP in the forebrains. The primers for genotyping of CamKIIα-tTA were same as previously described17 (forward: TGA AAG TGG GTC CGC GTA C and reverse: TAC TCG TCA ATT CCA AGG GC). The PCR product is 391 bp. According to the expression of Thorase-TAP protein in the brain by Western blot, four lines were selected and mated with C57/BL6 mice for two-three generations to establish the transgenic lines.
Middle cerebral artery occlusion
To avoid the possibility of sex-specific sensitivity to cell death, 10 male Thorase cKO (Thoraseflox/flox, cre+) mice and 9 male WT (Thoraseflox/flox, cre−) littermates as well as 13 male Thorase-TAP mice (tet+, tTA+) and 13 male WT littermates (tet+, tTA−) were subjected to the MCAO as described previously.18 The mice (age: 8–12 weeks) were anesthetized with 1.5–2% isoflurane and maintained at 37 ℃ throughout the surgical procedure using a heating pad and rectal probe (Harvard Apparatus, USA). A monofilament coated with silicone was inserted via a small nick in the right external carotid artery and slowly pushed through the right internal carotid artery till it reached to the base of the middle cerebral artery to block the blood flow into the middle cerebral artery brain territory. After 45 min of occlusion in Thorase KO mice or 60 min in Thorase-TAP transgenic mice, the monofilament was retracted to allow reperfusion. For MCAO ischemic preconditioning (IPC), 15 min of occlusion was performed in WT mice. Blood flow to the brain during the entire occlusion phase and reperfusion was monitored by laser-Doppler flowmetry with a probe placed directly on the exposed skull over the lateral parietal cortex (MCA territory); 24 h later, animal brains were removed, sectioned into five 2-mm thick coronal sections using a mouse brain matrix. Fresh brain sections were stained in 2% 2,3,5 -triphenyltetrazolium chloride (TTC) solution and fixed in 10% formalin overnight. TTC-stained brain section was imaged using a high-resolution scanner (HP Corporation, USA). Border between infarct and non-infarct tissue was outlined using Image J software (NIH, USA). The infarction volume was calculated by summing the infarct volumes of sections and expressed in percentage by using the formula: (contralateral volume-ipsilateral undamaged volume) × 100/contralateral volume to eliminate effects of edema as described previously.19 The investigator performing the surgery and analyzing infarct volume was blinded to the genotype or the MCAO procedure of the mice.
Assessment of neurobehavioral activity
The spontaneous motor activities of mice were recorded for 5 min in an animal cage at 24 h, three days and seven days after MCAO as described previously.18 Briefly, neurological deficits were evaluated by an observer blinded to the treatment and genotype of the animals with a scale of 0–4 (0 no neurological deficit, 4 severe neurological deficit) according to the criteria: 0 = mice appeared normal, explored the cage environment and moved around in the cage freely; 1 = mice hesitantly moved in cage and did not approach all sides of the cage, 2 = mice showed postural and movement abnormalities and had difficulty approaching all walls of the cage, 3 = mice with postural abnormalities tried to move in the cage but did not approach one wall of the cage, 4 = mice were unable to move in the cage and stayed at the center.
For the assessment of the forelimb functional recovery, the cylinder test was performed in a transparent glass cylinder (9 cm in diameter and 15 cm in height) as described previously.18 The cylinder was placed in the center of a chamber containing two video cameras on opposite sides for recording mouse forelimb movements. The use of mouse forelimb was recorded for 10 min and analyzed by an observer blinded to the treatment and genotype of the animals following the criteria: (1) Ipsilateral (right) forelimb use (number of touches to the cylinder wall) independent of the left limb (2) Contralateral (left) forelimb use independent of the right limb (3) Simultaneous use of both limbs. The percent use of the contralateral (left) limb was quantified by subtracting ipsilateral forelimb touches from the total number of touches (ipsilateral + contralateral + simultaneous) made by the mouse during the period of observation. A higher percent use of the contralateral (left) limb indicated a less of reliance on the ipsilateral forelimb and a better forelimb functional recovery.
Statistical analysis
Quantitative data are presented as the mean ± standard error of the mean (SEM). Statistical significance was either assessed via an unpaired two-tailed Student's t test or an ANOVA test with Tukey–Kramer post hoc analysis. For neurological behavioral, data were first subjected to a Kolmogorov–Smirnov normality and an equal variance test. The significant differences were determined at p < 0.05 for group main effects. The points with significant differences were identified by post hoc analysis using the Holm–Sidak method for multiple comparisons.
Results
Preconditioning enhances Thorase expression
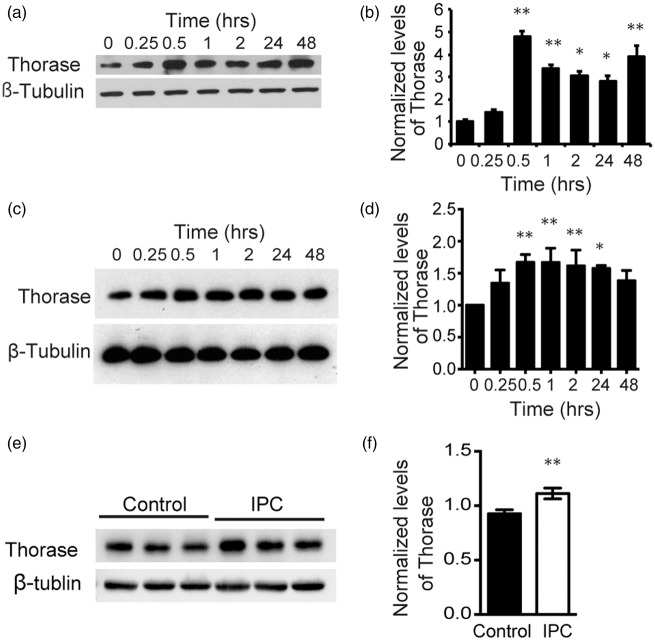
To determine whether preconditioning induces an elevation in Thorase expression, primary cortical neurons were exposed to OGD for 15 min or treated with 50 μM NMDA for 5 min (Figure 1). OGD preconditioning increases the expression levels of Thorase by approximately 5 fold within 30 min. This induction of expression is stable for up to 48 h (Figure 1(a) and (b)). NMDA preconditioning substantially increases the expression of Thorase by greater than 2 fold for 24 h (Figure 1(c) and (d)). In addition, we found that Thorase was inducible in vivo after 15 min ischemic preconditioning (IPC) in wild type mice (Figure 1(e) and (f)). Thus, preconditioning stimulation is sufficient to induce increased expression of Thorase for a sustained period of time.
Figure 1.
Thorase is a preconditioning induced gene. (a) Immunoblot of Thorase expression over time after OGD preconditioning (15 min OGD) in primary cultured cortical neurons. (b) Quantification of the data in (a) normalized to β-tubulin. Data are the mean ± standard error of the mean (SEM) from three independent experiments, **p < 0.01, *p < 0.05 by ANOVA with Tukey–Kramer post hoc test compared with no OGD preconditioning treatment “0”. (c) Immunoblot of Thorase expression over time after NMDA preconditioning (50 µM, 5 min) in primary cortical neurons. (d) Quantification of the data in (c) normalized to β-tubulin. Data are the mean ± SEM from three independent experiments, **p < 0.01, *p < 0.05 by ANOVA with Tukey–Kramer post hoc test compared with no NMDA preconditioning treatment “0”. (e) Immunoblot of Thorase expression in the cortex 24 h after 15 min IPC. Contralateral cortex was used as a control. (f) Quantification of the data in (e) normalized to β-tubulin. **p < 0.01 (n = 6) by Student's t test.
Overexpression of Thorase elicits neuroprotection
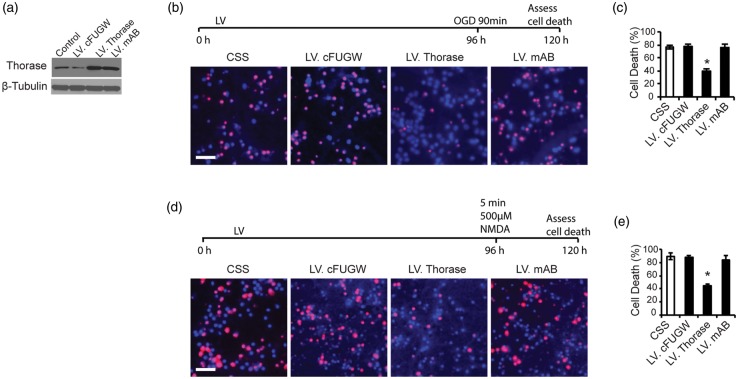
To ascertain whether Thorase is neuroprotective, we utilized a lentivirus that overexpresses wild type Thorase, and to determine whether the ATPase function of Thorase was responsible for its potential neuroprotective actions, we used a lentivirus that expresses the Walker A and Walker B Thorase mutant11 (Figure 2). Both lentiviral constructs efficiently transduce cortical neurons and lead to substantial overexpression of Thorase as previously described11 (Figure 2(a)). Overexpression of Thorase provides approximately 50% protection against OGD-induced cell death (Figure 2(b) and (c)) and NMDA-induced cell death (Figure 2(d) and (e)), whereas the Walker A/B mutant Thorase (mAB) fails to protect (Figure 2(b) to (e)). These results taken together indicate that Thorase overexpression elicits neuroprotection via its ATPase activity.
Figure 2.
Thorase elicits neuroprotection. (a) Immunoblot of Thorase expression in primary cultured cortical neurons after transfection with lentivirus expressing EGFP (LV.cFUGW), wild type Thorase (LV.Thorase) or mutant Thorase (LV. mAB) with both mutated Walker A and Walker B domains. Control cultures were not transfected. Immunoblot is representative of three independent experiments. (b) Cell viability assessed by PI/Hoechst staining in primary cortical cultures transfected with LV.cFUGW, LV.Thorase or LV. mAB following exposure to OGD for 90 min. Scale bar, 25 μM. (c) Percentages of neuron death were quantified and expressed as the mean ± SEM from three independent experiments performed in triplicate (n = 9). *p < 0.05 by ANOVA with Tukey–Kramer post hoc test compared to LV.cFUGW viral control. (d) Cell viability assessed in transfected primary cortical cultures after exposure to 500 µM NMDA (5 min) by PI/Hoechst staining. Scale bar, 25 μM. (e) Percentages of neuron death were quantified and expressed as the mean ± SEM from three independent experiments performed in triplicate (n = 9). *p < 0.05 by ANOVA with Tukey–Kramer post hoc test compared to LV.cFUGW viral control.
Thorase deficiency blocks preconditioning induced neuronal survival
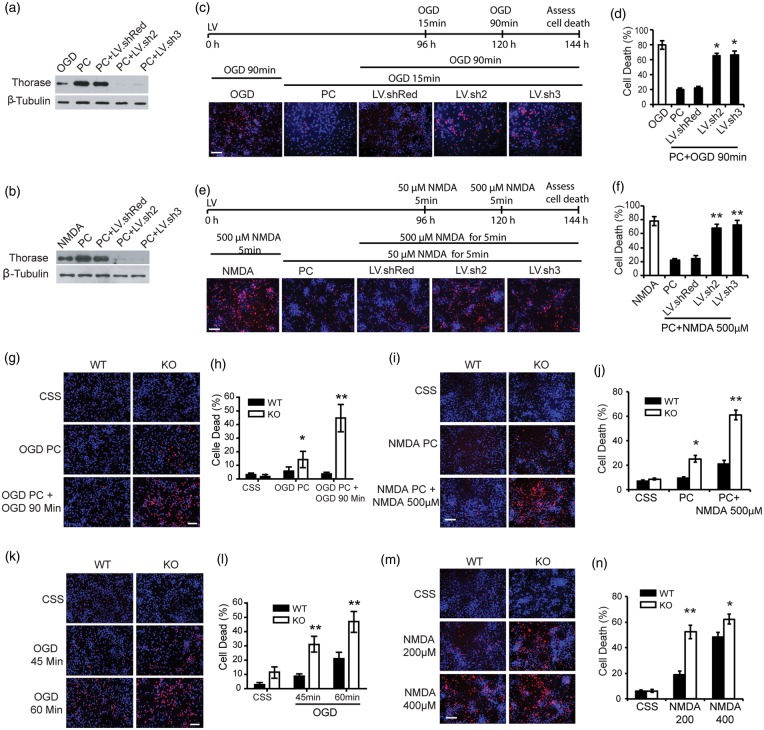
Two lentiviral shRNA hairpin constructs were generated to knockdown the expression of Thorase (Figure 3). These shRNAs to Thorase effectively prevent the upregulation of Thorase following OGD preconditioning (Figure 3(a)) and NMDA preconditioning (Figure 3(b)). Moreover, both shRNA constructs reduce constitutively expressed Thorase (Figure 3(a) and (b)). A shRNA construct to DsRed that engages the RISC complex has no effect on Thorase levels (Figure 3(a) and (b)). Both Thorase shRNA constructs substantially reduce the neuroprotective response due to preconditioning, whereas the DsRed shRNA construct has no effect on the preconditioning response for both OGD preconditioning (Figure 3(c) and (d)) or NMDA-induced preconditioning (Figure 3(e) and (f)). To confirm that Thorase is required for preconditioning, Thorase knockout cortical cultures were subjected to OGD-induced preconditioning (Figure 3(g) and (h)) and NMDA-induced preconditioning (Figure 3(i) and (j)). Knockout of Thorase disrupts OGD-induced preconditioning protection (Figure 3(g) and (h)) and NMDA-induced preconditioning (Figure 3(i) and (j)). Although the Thorase knockout cultures fail to undergo preconditioning, we observed a decrease in cell survival compared to wild-type cultures. Therefore, to determine whether basal levels of Thorase may be involved in neuronal cell survival, Thorase knockout cultures were subjected to exposures of 45 and 60 min of OGD or 200 μM and 400 μM of NMDA. Cell viability was compared to wild type cortical cultures. Thorase knockout cortical cultures have statistically enhanced cell death in response to 45 and 60 min of OGD (Figure 3(k) and (l)) or 200 μM and 400 μM NMDA (Figure 3(m) and (n)). Taken together, these data indicate that the relative levels of Thorase expression are important in determining the strength of neurotoxic stimulation required to kill cortical neuronal cultures and lowering Thorase levels is sufficient to decrease neuronal survival to normally sub-lethal insults.
Figure 3.
Knockdown or knockout of Thorase blocks preconditioning and increases sensitivity to neuronal injury. (a and b) Immunoblot of Thorase expression in primary cortical cultures transfected with lentivirus expressing DsRed shRNA (LV.shRed), Thorase-shRNA2 (LV.sh2) or Thorase-shRNA3 (LV.sh3) and exposure to 15 min OGD preconditioning (PC) (a) or 50 µM NMDA/10 µM glycine (PC) (b). No viral infected neurons were used as control. (c) Cell viability assessed by PI/Hoechst staining in primary cortical cultures transfected with DsRed shRNA (LV.shRed), Thorase-shRNA2 (LV.sh2) or Thorase-shRNA3 (LV.sh3) and exposed to 15 min OGD followed 24 h later to 90 min OGD. Scale bar, 25 μM. (d) Percentages of neuron death in (c) were quantified and expressed as the mean ± SEM from three independent experiments performed in triplicate (n = 9). *p < 0.05 by ANOVA with Tukey–Kramer post hoc test compared to LV.shRed viral control. (e) Cell viability assessed by PI/Hoechst staining in primary cortical cultures transfected with DsRed shRNA (LV.shRed), Thorase-shRNA2 (LV.sh2) or Thorase-shRNA3 (LV.sh3) and exposed to 50 μM NMDA and 24 h later to 500 µM NMDA. Scale bar, 25 μM. (f) Percentages of neuron death in (e) were quantified and expressed as mean ± SEM from three independent experiments performed in triplicate (n = 9). **p < 0.01 by ANOVA with Tukey–Kramer post hoc test compared to LV.shRed viral control. (g) Cell viability assessed by PI/Hoechst staining in primary cultured Thorase KO and WT neurons exposed to 15 min OGD followed by 90-min OGD 24 h later. Scale bar, 25 μM. (h) Percentages of neuron death in (g) were quantified and expressed as mean ± SEM from three independent experiments performed in triplicate (n = 9). *p < 0.05, **p < 0.01 by ANOVA with Tukey–Kramer post hoc test compared to WT control. (i) Cell viability assessed by PI/Hoechst staining in primary cultured Thorase KO and WT neurons treated with 50 µM NMDA followed 24 h later by 500 μM NMDA. Scale bar, 25 μM. (j) Percentages of neuron death in (i) were quantified and expressed as the mean ± SEM from three independent experiments performed in triplicate (n = 9). *p < 0.05, **p < 0.01 by ANOVA with Tukey–Kramer post hoc test compared to WT control. (k) Cell viability in primary cultured Thorase KO and WT neurons exposed to 45 min or 60 min OGD. Scale bar, 25 μM. (l) Quantification of cell viability in (k). Experiments were performed three independent experiments performed in triplicate (n = 9). The data represent the mean ± SEM. *p < 0.05, **p < 0.01 by ANOVA with Tukey–Kramer post hoc test compared to WT control. (m) Cell viability in primary cultured Thorase KO and WT neurons treated with 200 μM or 400 μM NMDA. Scale bar, 25 μM. (n) Quantification of cell viability in (m). Experiments were performed three independent experiments performed in triplicate (n = 9). The data represent the mean ± SEM. *p < 0.05, **p < 0.01 by ANOVA with Tukey–Kramer post hoc test compared to WT control.
Overexpression of Thorase protects against neuronal injury following stroke
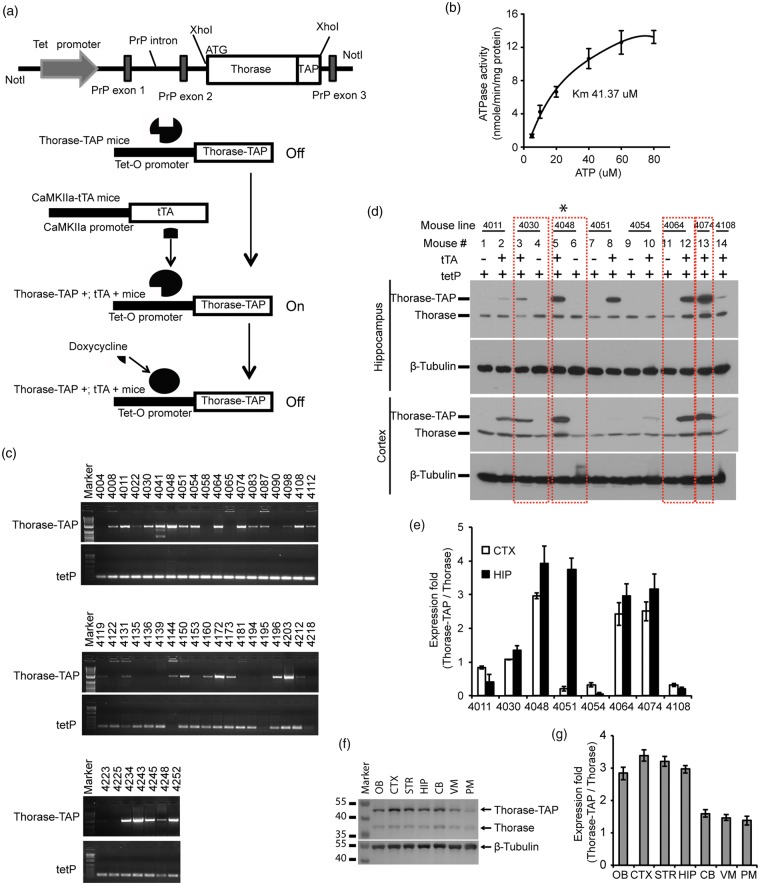
To investigate whether Thorase is neuroprotective in vivo, we generated a conditional transgenic mouse model in which the expression of a C-terminal tandem affinity purification (TAP) tag of Thorase was generated by fusing a streptavidin binding peptide and a calmodulin binding peptide to the C-terminus of mouse Thorase under the control of a tetracycline responsive regulator (Figure 4(a)). TAP-tagged Thorase retains AAA + ATPase activity (Figure 4(b)). Twenty-eight founders expressing TetP-Thorase were identified via PCR screening for the tetracycline promoter (173 bp) and Thorase-TAP sequences (1380 bp) (Figure 4(c)). Eight of the male founder mice were crossed with the CamKIIα-tTA transgenic mice20 and mice expressing both CamKIIα-tTA and Thorase-TAP were identified by PCR (Supplementary Figure 1(d)).
Figure 4.
Generation and characterization of conditional Thorase transgenic mice. (a) Schematic of the TetP-PrP2-Thorase-TAP transgenic construct. (b) ATPase activity analysis of Thorase-TAP. (c) Representative PCR genotyping of Thorase-TAP (1380 bp) and tetP (173 bp) using tail genomic DNA from founder mice. (d) Western blot of the expression of Thorase-TAP and endogenous Thorase in cortical and hippocampal regions of eight Thorase-TAP transgenic lines (4011, 4030, 4048, 4054, 4064, 4074 and 4108) in the presence or absence of tTA expression. β-Tubulin was used as protein loading control. Quantification of Thorase-TAP expression compared to endogenous Thorase in the CTX: cortex and HIP: hippocampus from the different lines of Thorase-TAP mice as shown in panel (d). (f) Representative Western blot of Thorase distribution in the brain subregions of Thorase-TAP mice (2048 line). OB: olfactory bulb; CTX: cortex; HIP: hippocampus; STR: striatum; CB: cerebellum; PM: pons and medulla. (f) Representative Western blot of Thorase distribution in the brain subregions of Thorase-TAP mice (2048 line). OB: olfactory bulb; CTX: cortex; HIP: hippocampus; STR: striatum; CB: cerebellum; PM: pons and medulla. (g) Quantification of Thorase-TAP expression compared to endogenous Thorase in different brain subregions of Thorase-TAP mice as shown in panel (f).
Expression of Thorase was detected in the cortex and hippocampus compared to littermate controls containing TetP-Thorase gene alone. Line 4030 overexpresses Thorase ∼1 fold in the cortex and ∼1.5 fold in the hippocampus (Figure 4(d) and (e)). Line 4048 overexpresses Thorase ∼3 fold in the cortex and ∼3.5 fold in the hippocampus (Figure 4(d) and (e)). Lines 4064 and 4074 overexpress Thorase ∼2.5 fold in the cortex and ∼ 3 fold in the hippocampus (Figure 4(d) and (e)). Line 4048 that overexpressed Thorase at levels similar to those induced by OGD preconditioning was selected for detailed characterization. Western blot analysis indicates that Thorase is overexpressed three fold throughout the forebrain, olfactory bulb, ventral midbrain and cerebellum with very little overexpression in the pons and medulla (Figure 4(f) and (g)).
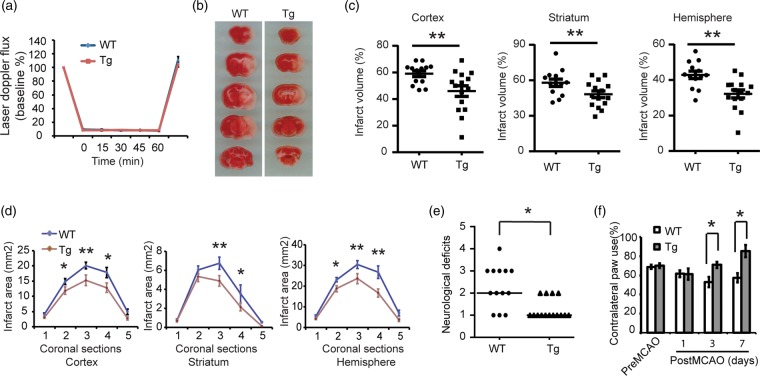
To determine whether Thorase overexpression is neuroprotective in vivo, wild-type littermates and Thorase-transgenic mice were subjected to transient occlusion of the middle cerebral artery. Over the 60-min period of occlusion, cortical perfusion monitored by laser-Doppler flowmetry was reduced equivalently in wild type mice (8.41 ± 0.24% of baseline; mean ± SEM.) and Thorase transgenic mice (8.13 ± 0.10% of baseline, mean ± SEM.). The reduction was stable throughout the occlusion period and recovered to preischemic levels immediately upon removal of the filament in both groups (Figure 5(a)). Despite the similar intensity of the ischemic insult, infarct volume was reduced by 22.1% in the cortex, 16.8% in the striatum and 25.1% in the entire hemisphere of the Thorase transgenic mice compared to their wild type counterparts (Figure 5(b) and (c)). Moreover, the reduction in infarct size was not skewed to a particular level (Figure 5(d)). Likewise, Thorase transgenic mice showed less neurological deficits 24 h after stroke (Figure 5(e)) and higher percent use of the contralateral (left) limb paw touch compared to their WT littermates, especially on third and seventh day after stroke (Figure 5(f)), suggesting a fast forelimb functional recovery and improved neurological function in Thorase transgenic mice. There were no baseline neurobehavioral differences in Thorase transgenic and wild type mice (Figure 5(e) and (f)). Thus, Thorase overexpression protects against stroke-induced neuronal injury.
Figure 5.
Overexpression of Thorase protects against neuronal injury following stroke. (a) Laser-Doppler flux measured over the lateral parietal cortex in the core of the ischemic region in WT (n = 13) and Thorase transgenic mice (Tg) (n = 13) mice. Values are means ± SEM, expressed as a percentage of the preischemic baseline values. (b) Representative images of TTC staining of brain slices from Thorase Tg and WT littermate controls subjected to 60 min of MCAO. (c) Quantification of infarct volumes in the cortex, striatum and whole hemisphere after 60 min of MCAO in WT and Thorase-Tg mice. Data are expressed as a percentage of the entire ischemic hemisphere and are means ± SEM. *p < 0.05 from WT by Student's t-test. (d) Quantification of infarct area among the five coronal levels (level 1 is most anterior) after 60 min of MCAO in WT and Thorase-Tg mice. Data represent the mean ± SEM, *p ≤ <0.05 and **p < 0.01 from WT by ANOVA with Tukey–Kramer's post hoc test. (e) Spontaneous neurobehavioral activity following MCAO was assessed on a scale of 0-4 (0 no neurological deficit, 4 severe neurological deficit). Data represent the median/range, *p < 0.05 determined by using a one-tailed Wilcoxon Mann–Whitney U-test for non-parametric rank-sum analysis. (f) Forelimb functional recovery assessed by the percent use of the contralateral (left) limb paw touch scored by an observer blind to the treatment and genotype. Data represent mean ± SEM, *p < 0.05 by ANOVA with Tukey–Kramer's post hoc test.
Thorase is required for neuronal survival against neuronal injury following stroke
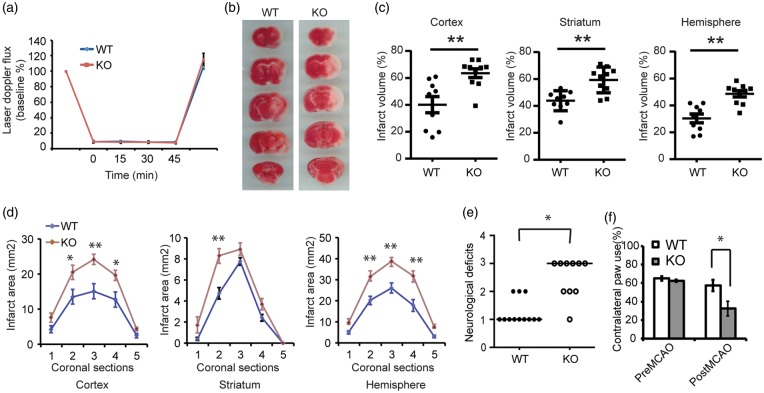
To determine whether Thorase is required for neuronal survival in vivo, we utilized a conditional knockout (cKO) mouse of Thorase, in which Thorase was deleted from the forebrain of mice via crossing Thoraseflx/flx mice with CamKIIα-iCre as previously described.11 Wild type littermates and Thorase cKO mice were subjected to transient occlusion of the middle cerebral artery. Initial studies indicated that the Thorase cKO mice experience increased sensitivity to transient occlusion of the middle cerebral artery in that the Thorase cKO mice had substantially reduced survival following a 60-min period of occlusion. Thus, animals were subjected to 45 min of occlusion. Over the 45-min period of occlusion, cortical perfusion monitored by laser-Doppler flowmetry was reduced equivalently in wild type mice (8.78 ± 0.22% of baseline, mean ± SEM) and Thorase cKO mice (8.25 ± 0.27% of baseline, mean ± SEM). The reduction was stable throughout the occlusion period and recovered to preischemic levels immediately upon removal of the filament in both groups (Figure 6(a)). Despite the similar intensity of the ischemic insult, infarct volume was increased by 58.4% in the cortex, 35.2% in the striatum and 60.2% in the entire hemisphere of the Thorase cKO mice compared to their wild type counterparts (Figure 6(b) and (c)). Moreover, the increase in infarct size was not skewed to a particular level (Figure 6(d)). Thorase cKO mice showed enhanced neurological deficits after stroke (Figure 6(e) and (f)). Although 45 min of occlusion was performed, most of the Thorase cKO mice died within five days after MCAO surgery. There were no baseline neurobehavioral differences in Thorase cKO and wild type mice (Figure 6(e) and (f)). Taken together, the data support the notion that Thorase is required for neuronal survival in stroke-induced neuronal injury.
Figure 6.
Thorase is required for neuronal survival against neuronal injury following stroke. (a) Laser-Doppler flux measured over the lateral parietal cortex in the core of the ischemic region in Thorase KO (n = 10) mice and WT littermates (n = 9). Values are means ± SEM, expressed as a percentage of the preischemic baseline values. (b) Representative images of TTC staining of brain slices from Thorase (KO) and WT littermate controls subjected to 45 min of MCAO. (c) Quantification of infarct volumes in the cortex, striatum and whole hemisphere after 45 min of MCAO in WT and Thorase KO mice. Data were expressed as a percentage of the entire ischemic hemisphere and are means ± SEM. *p < 0.05 from WT littermates by Student's t-test. (d) Quantification of infarct area among the five coronal levels (level 1 is most anterior) after 45 min of MCAO in WT and Thorase KO mice. Data represent the mean ± SEM. *p < 0.05 and **p < 0.01 from WT by ANOVA with Tukey–Kramer's post hoc test. (e) Spontaneous neurobehavioral activity following MCAO was assessed on a scale of 0–4 (0 no neurological deficit, 4 severe neurological deficit). Data represent the median/range. *p < 0.05 determined by using a one-tailed Wilcoxon Mann–Whitney U-test for non-parametric rank-sum analysis. (f) Forelimb functional recovery assessed by the percent use of the contralateral (left) limb paw touch scored by an observer blind to the treatment and genotype. Data represent mean ± SEM, *p < 0.05 by ANOVA with Turkey–Kramer's post hoc test.
Discussion
The major finding of this paper is the discovery that Thorase, an AAA + ATPase, has neuroprotective properties. Thorase was originally identified through a functional cloning strategy aimed at identifying preconditioning-induced neuroprotective genes.10 We have confirmed that Thorase is neuroprotective against OGD toxicity and NMDA excitotoxicity in vitro and ischemic injury due to middle cerebral artery occlusion. The expression of Thorase is enhanced by both OGD and NMDA preconditioning in vitro. Knockdown of Thorase by shRNA prevents the preconditioning response to NMDA and OGD and knockout of Thorase prevents the preconditioning response to OGD suggesting that it participates in the preconditioning phenomenon. Moreover, knockdown of Thorase by shRNA enhances NMDA- and OGD-induced neuronal cell death suggesting that Thorase plays a critical role in the maintenance of neuronal survival. Consistent with this notion is the observation that knockout of Thorase dramatically increases the susceptibility of neurons to OGD and NMDA in vitro and to ischemic injury in vivo. In addition, overexpression of Thorase in vivo protects against neuronal injury following MCAO. Thus, Thorase is a neuroprotective AAA + ATPase that plays an important role in the survival of neurons following preconditioning in the setting of glutamate excitotoxicity and stroke.
Thorase has substantial homology to AAA + ATPases such as NSF and it possesses ATPase activity that is required for its neuroprotective properties. AAA + ATPases play a variety of roles in the cell by serving as molecular motors for diverse cellular processes.21–24 The related ATPases TorsinA, Spastin and Paraplegin ATPases when mutated cause neurodegeneration.25–33 Thus, ATPases play important roles in maintaining normal neuronal function as well as the maintenance of neuronal survival.34–36 Germline knockout of Thorase leads to post-natal lethality at post-natal days 19 to 25.11 The cause of cell death in the germline Thorase knockout mice was not determined but these mice were reported to have generalized seizures at death.11 It is conceivable that the decreased survival of the germline Thorase knockout mice is due to enhanced glutamate excitotoxicity, particularly since Thorase is a major regulator of AMPA receptor function. In a similar manner, the protective function of Thorase in preconditioning and neuronal injury due to NMDA, OGD and middle cerebral artery occlusion is likely to be due to its regulation of AMPA receptor function. Future studies are required to determine the role of Thorase's regulation of AMPA receptor function in neuronal survival.
In summary, Thorase is a neuroprotective AAA + ATPase that plays a prominent role in the preconditioning response and the survival of neurons to glutamate excitotoxicity and ischemic injury. Identification and characterization of Thorase's substrates as well as its role as a neuroprotective AAA + ATPase hold particular promise for better understanding of the neuroprotective response induced by preconditioning and neuronal survival. Enhancing Thorase's activity may be an attractive therapeutic target for treatment of neurologic disorders characterized by neuronal cell death.
Acknowledgements
We thank Dr. Jian Zhang and Dr. Ran An for the technical support of MCAO experiments. We also thank Deborah A Swing and Lino Tessarollo of the Neural Development Section, Mouse Cancer Genetics Program, Center for Cancer Research, National Cancer Institute, Frederick, MD, for generation of transgenic mice.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH grants DA000266 (TMD, VLD) R01 NS067525 (TMD, VLD), R37 NS067525 (TMD, VLD), RO1AG029368 (VLD), American Heart Association 0725470U, 11GRNT7810020, 12SDG8940000 (JZ), Simons Foundation Autism Research Initiative (SFARI) (TMD), National Natural Science Foundation of China 81271415, 31471016, National Key Research and Development Program of China 2016YFA0101001, CAMS Initiative for Innovative Medicine 2016-I2M-1-008 (JZ). TMD is the Leonard and Madlyn Abramson Professor in Neurodegenerative Diseases.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author's contributions
JZ, JY, MK, GU, EP, ZC, KP, HW performed the experimental components of the study, including cell culture, genotyping, Western blot, cytotoxicity assays. OS and SA performed the animal surgery for MCAO. JZ, WH and HW analyzed and interpreted the data from behavioral tests, including statistical analysis. JZ, TMD and VLD designed the study and wrote the manuscript. All authors read and approved the final manuscript.
References
- 1.Thushara Vijayakumar N, Sangwan A, Sharma B, et al. Cerebral ischemic preconditioning: the road so far. Mol Neurobiol 2016; 53: 2579–2593. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Reis C, Applegate R, 2nd, et al. Ischemic conditioning-induced endogenous brain protection: applications pre-, per- or post-stroke. Exp Neurol 2015; 272: 26–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Culmsee C, Siewe J, Junker V, et al. Reciprocal inhibition of p53 and nuclear factor-kappaB transcriptional activities determines cell survival or death in neurons. J Neurosci 2003; 23: 8586–8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawson TM, Ginty DD. CREB family transcription factors inhibit neuronal suicide. Nat Med 2002; 8: 450–451. [DOI] [PubMed] [Google Scholar]
- 5.Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappa B signalling cascades. Nature 2001; 412: 641–647. [DOI] [PubMed] [Google Scholar]
- 6.Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ 2006; 13: 852–860. [DOI] [PubMed] [Google Scholar]
- 7.Meller R, Minami M, Cameron JA, et al. CREB-mediated Bcl-2 protein expression after ischemic preconditioning. J Cereb Blood Flow Metab 2005; 25: 234–246. [DOI] [PubMed] [Google Scholar]
- 8.Soucek T, Cumming R, Dargusch R, et al. The regulation of glucose metabolism by HIF-1 mediates a neuroprotective response to amyloid beta peptide. Neuron 2003; 39: 43–56. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Tang X, Li M, et al. Regulation of neuroprotective activity of myocyte-enhancer factor 2 by cAMP-protein kinase A signaling pathway in neuronal survival. J Biol Chem 2005; 280: 16705–16713. [DOI] [PubMed] [Google Scholar]
- 10.Dai C, Liang D, Li H, et al. Functional identification of neuroprotective molecules. PLoS One 2010; 5: e15008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Wang Y, Chi Z, et al. The AAA + ATPase thorase regulates AMPA receptor-dependent synaptic plasticity and behavior. Cell 2011; 145: 284–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen YC, Umanah GK, Dephoure N, et al. Msp1/ATAD1 maintains mitochondrial function by facilitating the degradation of mislocalized tail-anchored proteins. EMBO J 2014; 33: 1548–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prendergast J, Umanah GK, Yoo SW, et al. Ganglioside regulation of AMPA receptor trafficking. J Neurosci 2014; 34: 13246–13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samdani AF, Newcamp C, Resink A, et al. Differential susceptibility to neurotoxicity mediated by neurotrophins and neuronal nitric oxide synthase. J Neurosci 1997; 17: 4633–4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Zulueta M, Feldman AB, Klesse LJ, et al. Requirement for nitric oxide activation of p21(ras)/extracellular regulated kinase in neuronal ischemic preconditioning. Proc Natl Acad Sci U S A 2000; 97: 436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Yu SW, Koh DW, et al. Apoptosis-inducing factor substitutes for caspase executioners in NMDA-triggered excitotoxic neuronal death. J Neurosci 2004; 24: 10963–10973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee Y, Karuppagounder SS, Shin JH, et al. Parthanatos mediates AIMP2-activated age-dependent dopaminergic neuronal loss. Nat Neurosci 2013; 16: 1392–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrabi SA, Kang HC, Haince JF, et al. Iduna protects the brain from glutamate excitotoxicity and stroke by interfering with poly(ADP-ribose) polymer-induced cell death. Nat Med 2011; 17: 692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kishimoto K, Li RC, Zhang J, et al. Cytosolic phospholipase A2 alpha amplifies early cyclooxygenase-2 expression, oxidative stress and MAP kinase phosphorylation after cerebral ischemia in mice. J Neuroinflammation 2010; 7: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayford M, Bach ME, Huang YY, et al. Control of memory formation through regulated expression of a CaMKII transgene. Science 1996; 274: 1678–1683. [DOI] [PubMed] [Google Scholar]
- 21.Dalal S, Hanson PI. Membrane traffic: what drives the AAA motor?. Cell 2001; 104: 5–8. [DOI] [PubMed] [Google Scholar]
- 22.Davey MJ, Jeruzalmi D, Kuriyan J, et al. Motors and switches: AAA + machines within the replisome. Nat Rev Mol Cell Bio 2002; 3: 826–835. [DOI] [PubMed] [Google Scholar]
- 23.Erzberger JP, Berger JM. Evolutionary relationships and structural mechanisms of AAA + proteins. Annu Rev Biophys Biomol Struct 2006; 35: 93–114. [DOI] [PubMed] [Google Scholar]
- 24.Martin A, Baker TA, Sauer RT. Rebuilt AAA plus motors reveal operating principles for ATP-fuelled machines. Nature 2005; 437: 1115–1120. [DOI] [PubMed] [Google Scholar]
- 25.Casari G, De Fusco M, Ciarmatori S, et al. Spastic paraplegia and OXPHOS impairment caused by mutations in paraplegin, a nuclear-encoded mitochondrial metalloprotease. Cell 1998; 93: 973–983. [DOI] [PubMed] [Google Scholar]
- 26.Fonknechten N, Mavel D, Byrne P, et al. Spectrum of SPG4 mutations in autosomal dominant spastic paraplegia. Hum Mol Genet 2000; 9: 637–644. [DOI] [PubMed] [Google Scholar]
- 27.Goodchild RE, Kim CE, Dauer WT. Loss of the dystonia-associated protein torsinA selectively disrupts the neuronal nuclear envelope. Neuron 2005; 48: 923–932. [DOI] [PubMed] [Google Scholar]
- 28.Lindsey JC, Lusher ME, McDermott CJ, et al. Mutation analysis of the spastin gene (SPG4) in patients with hereditary spastic paraparesis. J Med Genet 2000; 37: 759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morita M, Ho M, Hosler BA, et al. A novel mutation in the spastin gene in a family with spastic paraplegia. Neurosci Lett 2002; 325: 57–61. [DOI] [PubMed] [Google Scholar]
- 30.Nolden M, Ehses S, Koppen M, et al. The m-AAA protease defective in hereditary spastic paraplegia controls ribosome assembly in mitochondria. Cell 2005; 123: 277–289. [DOI] [PubMed] [Google Scholar]
- 31.Sauter S, Miterski B, Klimpe S, et al. Mutation analysis of the spastin gene (SPG4) in patients in Germany with autosomal dominant hereditary spastic paraplegia. Hum Mutat 2002; 20: 127–132. [DOI] [PubMed] [Google Scholar]
- 32.Sharma N, Baxter MG, Petravicz J, et al. Impaired motor learning in mice expressing torsinA with the DYT1 dystonia mutation. J Neurosci 2005; 25: 5351–5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torres GE, Sweeney AL, Beaulieu JM, et al. Effect of torsinA on membrane proteins reveals a loss of function and a dominant-negative phenotype of the dystonia-associated DeltaE-torsinA mutant. Proc Natl Acad Sci U S A 2004; 101: 15650–15655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heerssen HM, Pazyra MF, Segal RA. Dynein motors transport activated Trks to promote survival of target-dependent neurons. Nat Neurosci 2004; 7: 596–604. [DOI] [PubMed] [Google Scholar]
- 35.Hirokawa N, Takemura R. Molecular motors and mechanisms of directional transport in neurons. Nat Rev Neurosci 2005; 6: 201–214. [DOI] [PubMed] [Google Scholar]
- 36.Midorikawa R, Takei Y, Hirokawa N. KIF4 motor regulates activity-dependent neuronal survival by suppressing PARP-1 enzymatic activity. Cell 2006; 125: 371–383. [DOI] [PubMed] [Google Scholar]