Background
CT perfusion can be useful in the work-up of patients with acute stroke. The value of the additional diagnostic information gained should, however, be weighed against treatment delays and the added contrast and radiation exposure that are incurred by obtaining a CTP study. AHA stroke guidelines recommend against the routine use of CTP in the early time-window (up to 6 h) because most early-window trials did not use CTP for patient selection. Treatment decisions regarding tPA can typically be based on non-contrast CT alone and treatment decisions regarding early-window endovascular therapy can be based on NCCT and CTA. In the early time-window, it is therefore reasonable to only obtain a CTP when the NCCT ± CTA provide insufficient diagnostic information. In contrast, AHA guidelines do recommend CTP or MR imaging for triage beyond 6 h based on the results of two recent trials that showed benefit of endovascular therapy in the 6–24-h-time window in patients selected with CT perfusion or MRI.1–3 Due to cost and workflow considerations, most hospitals will opt to primarily use CT perfusion (CTP) and not MRI for triage. Because CT perfusion protocols are not standardized, these hospitals are now faced with the question of how to acquire, process, and interpret CT perfusion imaging. These aspects are not covered in the guidelines. This brief review aims to provide practical guidance on how to implement CT perfusion for acute stroke.
CTP scanning protocol
CTP is an approximately 1-min dynamic acquisition of a contrast bolus passing through the brain. This acquisition is summarized on CT perfusion maps that show different characteristics such as the timing of the bolus arrival and passage (Tmax and MTT), cerebral blood flow (CBF), and cerebral blood volume (CBV).4 The key factors in the CTP scanning protocol to consider are scan order, total scan duration, contrast injection, radiation dose, brain coverage, and frame rate (time between frames in the scan). These factors influence the reliability of the perfusion maps and ultimately the reliability of lesion volume estimates.
CTP scan order
It is preferred to schedule the CTP after the CTA with a short time-delay between sequences to allow the contrast from the CTA to reach a steady concentration before the CTP is acquired; a delay of at least 60 s usually accomplishes this. From a technical standpoint, obtaining the CTP last is advantageous because it avoids venous contamination of the CTA from CTP contrast. From a practical standpoint, obtaining CTP last allows physicians to only obtain the CTP when it is indicated and thus avoids unnecessary contrast and radiation exposure. Specifically, CTP could be obtained selectively to (1) assess mismatch status in patients with a large vessel occlusion on CTA who may be candidates for endovascular therapy in the delayed time-window, (2) rule-in or rule-out ischemia when the clinical diagnosis is uncertain, or (3) clarify if there is a large vessel occlusion when the CTA results are equivocal.
Scan duration
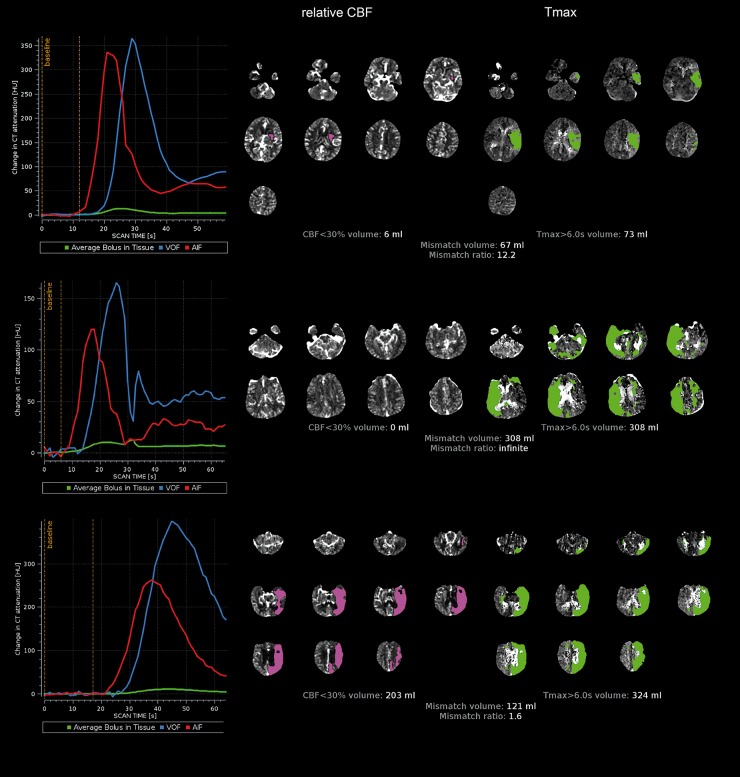
When the CTP scan is too short, the bolus passage is not captured in full, resulting in inaccurate perfusion maps and lesion volume estimates (Figure 1). When the scan is too long, radiation dose is wasted by capturing the tail end of the bolus passage which provides no useful information. The optimal scan duration captures a short pre-contrast baseline (5–10 s) and the up- and downslope of the contrast bolus through a main cerebral vein (Figure 1). This duration varies between patients, but empiric data have shown that this is achieved in >90% of patients with a scan duration of 60 s.5 Accordingly, we recommend a scan duration of 60 to 70 s.
Figure 1.
(Top panel): Good quality scan. The arterial input function (AIF) and venous output function (VOF) are of similar shape, the AIF precedes the VOF by a few seconds, there is a short pre-contrast baseline (5–10 s), and the up- and downslope of the VOF are fully captured. The CBF and Tmax maps demonstrate anatomically plausible, unilateral lesions and no artifacts. (Middle panel): A scan with motion. The AIF and VOF are both jagged indicating motion. The CBF map demonstrates no lesion below the 30% threshold. The Tmax map contains bilateral lesions indicative of artifacts. While this patient likely has “at risk” tissue, the calculated mismatch volume is an overestimate of the true mismatch volume. (Bottom panel): The AIF and VOF curves indicate a late-arriving and wide contrast bolus. The contrast passage is not fully captured during the 60 s scan, as indicated by a “truncated” VOF (scan ends during downslope of VOF). This can lead to spurious results in the Tmax and CBF volume estimates which should therefore be interpreted with caution.
Contrast injection
Timing of the contrast bolus injection, relative to CTP scan start time, determines the duration of the baseline. Obtaining at least a few baseline frames is critical to calculate reliable perfusion maps. Longer baselines are not informative. Empiric data have shown that a short (5–10 s) baseline is obtained in most patients when the first frame of the CTP is acquired 4 s after start of the contrast injection. To ensure consistency and avoid overly lengthy bolus passages, it is important to administer the bolus with a power injector. In line with consensus guidelines and the protocols used in recent studies, we recommend to administer 40 mL contrast at 4–6 mL/s followed by 40 mL saline at 4–6 mL/s.6,7 The tissue enhancement achieved with this injection protocol is generally considered the minimum to yield acceptable quality images. Use of less contrast reduces the amount of enhancement and thus increases image noise which can lead to inaccurate lesion volume estimates.
Radiation dose
A major limitation of CT as compared to MR perfusion is its low contrast to noise ratio. The maximum signal increase in brain tissue is typically 3–20 Hounsfield Units, which is low given that the background noise typically has a standard deviation of at least 5 Hounsfield Units. To optimize contrast-to-noise, CTP scans should be acquired at 70–80 kV and timing parameters should be optimized, so that most of the administered radiation is used to capture the contrast passage and is not wasted on an excessively long baseline or tail (see the ‘Contrast injection’ section). An increase in radiation dose will increase signal-to-noise proportionally, but will also increase the risk of radiation injury. The radiation dose should therefore be based on the as low as reasonably achievable (ALARA) principle. While the optimal radiation dose may differ some depending on the type of scanner and post-processing software used, in our experience a radiation dose (CT Dose Index; CTDIvol) of 300 mGy typically is the lowest dose that will yield good-quality perfusion maps and accurate volume estimates.
Brain coverage
In patients with a large vessel occlusion of the anterior circulation, a minimum of 4 cm z-axis coverage is often sufficient to assess the presence of salvageable tissue. However, with such limited coverage, the full extent of the ischemic lesion may not be captured. We therefore recommend obtaining at least 8 cm of z-axis coverage, extending rostrally from above the orbits. Z-axis coverage is primarily determined by the CT detector width, which varies from 4 to 16 cm on most modern scanners. For scanners with relatively narrow detectors, there are a couple of options to expand the z-axis coverage beyond the detector width. One option is to obtain two individual CTP scans at adjacent levels, each requiring its own contrast injection. In this case, the CT table is moved between scans but is steady during each individual scan. An alternative approach to expand coverage is to move the CT table during a single CTP acquisition. In our experience, the steady-table technique provides better quality images because it allows for faster sampling rates and eliminates artifacts due to table motion.
Frame rate
The quality of the perfusion maps can be impacted by low frame rates, and we therefore recommend sampling faster than 2 s when possible. Such rates are not possible in shuttle or spiral-mode due to the time it takes to move the table between scan positions. Slower frame rates—up to 3 s with shuttle mode—are generally assumed to provide adequate quality scans and were routinely used in recent trials.7
CTP processing
Perfusion software packages generally produce two results: (1) Perfusion maps (e.g. Tmax, CBV, CBF) and (2) Segmentations and volumetric measurements of discrete regions (e.g. ischemic core and critical hypoperfusion). Unfortunately, software packages differ in how the perfusion maps are calculated and how the segmentations are performed. For example, whereas one software may generate highly delay-sensitive MTT maps, other packages may generate delay-insensitive maps. These types of differences can result in substantial variability in lesion volumes between packages.8
Because different post-processing methods could yield clinically useful CTP maps and lesion volumes, we do not recommend a specific post-processing method. Instead, we recommend the use of software packages that have been validated, either directly in clinical trials or indirectly through comparison against software that has been used successfully in clinical trials. When evaluating CTP software, it is therefore important to know the specific thresholds that are recommended for that software package, and the data on which these recommendations are based.9
CTP interpretation
The main role of CTP in acute stroke is to determine if there is a substantial amount of tissue that, with timely reperfusion, can be salvaged. The DAWN and DEFUSE 3 trials determined subjects' trial eligibility based on lesion volumes calculated by CTP software. Such a quantitative approach is preferred over a qualitative (‘eyeballing’) approach as it is more reproducible and less subjective.10 It is, however, important to note that the calculated lesion volumes can give a false sense of accuracy. Several factors, outlined below, should therefore be taken into consideration when interpreting the output of CTP software.
Quality of the acquisition and the perfusion maps
The main reasons for image artifacts and inaccurate lesion estimates include head motion, a failed bolus injection, poor scan timing, and low signal-to-noise. Inspection of the arterial input function (AIF) and venous output function (VOF) curves can provide a good indication of scan quality (Figure 1). An AIF curve with multiple spikes is indicative of head motion. Absence of a VOF peak, which is easier to detect than the AIF peak due to the larger caliber of the vessel, typically indicates a failed bolus injection. A truncated VOF indicates that the scan duration is too short. Sufficient contrast-to-noise can be estimated by review of the deep grey structures on the CBF map. The grey matter should be easily discernable from the surrounding white matter, which has lower CBF. Finally, it is important to review the CTP maps to make sure that there are no obvious artifacts and that the z-axis coverage fully captures the ischemic lesion or, if no lesion is present, the vascular territory of interest. Any problems discovered during quality review, should raise suspicion that the calculated lesion volumes may not be accurate.
The CTP in context of the non-contrast CT
CT perfusion images should not be used in isolation to make treatment decisions. It is critical to realize that the CTP images merely reflect the “perfusion-state” of the tissue and fundamentally offer no direct insight into the extent of tissue that has undergone irreversible damage (i.e. the ischemic core).11 This is an important difference between ischemic core identification by CTP and MRI, as a low ADC value on MR reflects microstructural changes with a high probability of irreversible tissue damage.12 While the CTP-derived ischemic core is a fairly close estimate of the true core in most instances, there are some notable exceptions. When the CTP is obtained ultra-early (within one hour of symptom onset), a more restrictive CBF threshold is likely needed to estimate the ischemic core.13 In this time-frame, it is particularly important to review the NCCT, as a completely normal NCCT in the setting of a large ischemic core should raise suspicion of a CTP core overcall. Conversely, the ischemic core can be underestimated when tissue that has already sustained irreversible damage undergoes (partial) reperfusion because of recanalization or improved collateral flow. In this scenario, the non-contrast CT may show signs of infarction (hypodensity) outside of the territory that is identified as the iscemic core on CTP.
Precision of CTP lesion estimates
With appropriate thresholding, the CTP core identified on CBF (or CBV) can, on average, provide a reasonably close estimate of the ischemic core assessed with DWI/ADC. For each individual case, however, there can be substantial over- or under-call (standard deviation of 20 mL).14 To avoid excluding patients from treatment who may benefit, some software programs intentionally use a relatively stringent CBF threshold, which biases the software towards underestimating the ischemic core. Therefore, knowledge of the precision and bias of a particular CTP software package should be taken into consideration when interpreting its maps and lesion volume estimates.
Conclusion
The expanded role for CT perfusion imaging increases the need for clinicians to understand its capabilities and limitations. To mirror the results of recent clinical trials in routine practice, we recommend the use of validated software and a scanning protocol that meets the criteria specified above. Key limitations of CTP include the inability to detect infarcted tissue that has reperfused, susceptibility to motion, and bolus-related artifacts.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Soren Christensen: Consultancy fees and equity in IschemaView Inc.
References
- 1.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018; 378: 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 378: 11–21. [DOI] [PubMed] [Google Scholar]
- 3.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2018; 49: e46–e110. [DOI] [PubMed] [Google Scholar]
- 4.Konstas AA, Goldmakher GV, Lee TY, et al. Theoretic basis and technical implementations of CT perfusion in acute ischemic stroke, part 1: theoretic basis. AJNR Am J Neuroradiol 2009; 30: 662–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kasasbeh AS, Christensen S, Straka M, et al. Optimal computed tomographic perfusion scan duration for assessment of acute stroke lesion volumes. Stroke 2016; 47: 2966–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heit JJ, Wintermark M. Perfusion computed tomography for the evaluation of acute ischemic stroke: strengths and pitfalls. Stroke 2016; 47: 1153–1158. [DOI] [PubMed] [Google Scholar]
- 7.Wintermark M, Albers GW, Alexandrov AV, et al. Acute stroke imaging research roadmap. Stroke 2008; 39: 1621–1628. [DOI] [PubMed] [Google Scholar]
- 8.Austein F, Riedel C, Kerby T, et al. Comparison of perfusion CT software to predict the final infarct volume after thrombectomy. Stroke 2016; 47: 2311–2317. [DOI] [PubMed] [Google Scholar]
- 9.Kudo K, Sasaki M, Yamada K, et al. Differences in CT perfusion maps generated by different commercial software: quantitative analysis by using identical source data of acute stroke patients. Radiology 2010; 254: 200–209. [DOI] [PubMed] [Google Scholar]
- 10.Campbell BC, Christensen S, Foster SJ, et al. Visual assessment of perfusion-diffusion mismatch is inadequate to select patients for thrombolysis. Cerebrovasc Dis 2010; 29: 592–596. [DOI] [PubMed] [Google Scholar]
- 11.d'Esterre CD, Boesen ME, Ahn SH, et al. Time-dependent computed tomographic perfusion thresholds for patients with acute ischemic stroke. Stroke 2015; 46: 3390–3397. [DOI] [PubMed] [Google Scholar]
- 12.Chemmanam T, Campbell BC, Christensen S, et al. Ischemic diffusion lesion reversal is uncommon and rarely alters perfusion-diffusion mismatch. Neurology 2010; 75: 1040–1047. [DOI] [PubMed] [Google Scholar]
- 13.Najm M, Al-Ajlan FS, Boesen ME, et al. Defining CT perfusion thresholds for infarction in the golden hour and with ultra-early reperfusion. Can J Neurol Sci 2018; 45: 339–342. [DOI] [PubMed] [Google Scholar]
- 14.Cereda CW, Christensen S, Campbell BC, et al. A benchmarking tool to evaluate computer tomography perfusion infarct core predictions against a DWI standard. J Cereb Blood Flow Metab 2016; 36: 1780–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]