To the Editor:
Cystic fibrosis (CF) is a genetic disease caused by dysfunctional CF transmembrane conductance regulator (CFTR) anion secretion that leads to airway dehydration and poor pathogen clearance. These defects result in bacterial infection in the lower airways, persistent inflammation, tissue damage, and a progressive decline in lung function. Gram-negative Pseudomonas aeruginosa chronically infects patients with CF, and the Burkholderia cepacia complex is associated with severe but less common CF lung infections (1, 2). The antimicrobial compound SPLUNC1 (short palate, lung, and nasal epithelial clone; gene name BPIFA1) is found in airway secretions and targets gram-negative bacteria (3), but its relevance to CF pathogenesis remains unclear. CF airways are characterized by a mildly acidic airway surface liquid (ASL) pH, about 0.5 units lower than normal ASL pH of ∼7.1, as a result of diminished bicarbonate transport through mutant CFTR (4, 5). Some antimicrobial peptides are pH sensitive, and it has been hypothesized that the acidic CF ASL leads to loss of antimicrobial function (6). Here, we hypothesized that SPLUNC1 is inactivated by acidic CF ASL leading to reduced antimicrobial activity in CF airways. Some of the results of these studies have been previously reported in the form of an abstract (7).
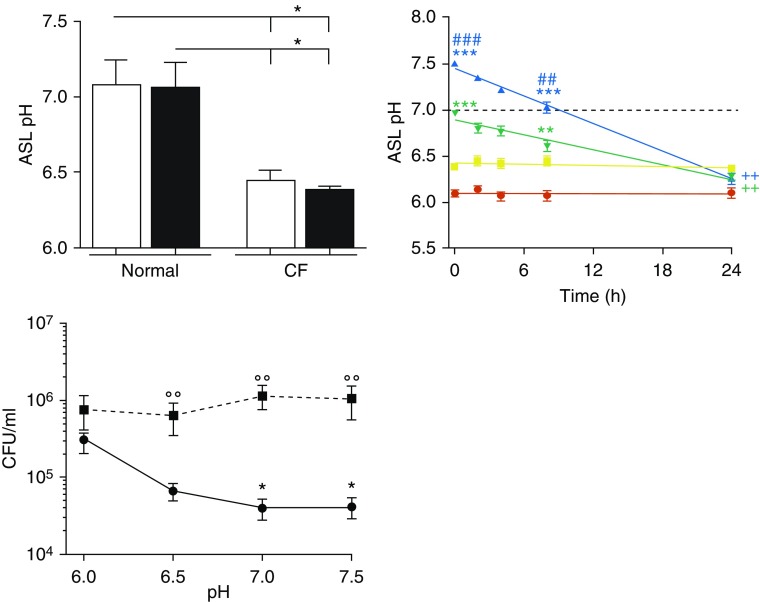
Although not all studies report that CF ASL has reduced pH (8), we and others have found CF ASL to be acidic (4, 5, 9), including CF human bronchial epithelial cells (HBECs) ASL and CF patient nasal secretions (4). Normal and CF human bronchial epithelial cells, obtained from main stem bronchi, were infected or not with 103 cfu/ml B. cenocepacia strain J2315 and were measured for ASL pH, using fluorescent dyes as described (4). Normal ASL pH was 7.2 and was unaffected by a 2-hour J2315 infection. CF ASL pH was significantly lower (pH 6.4) and also unaffected by J2315 (Figure 1, left). To determine whether CF ASL pH could be normalized, and whether this could restore antimicrobial activity, 100 μl Ringer’s solution buffered from pH 6.0 to 7.5 with 100 mM MES (2-[N-morpholino]ethanesulfonic acid) or HEPES was added mucosally to CF HBECs. ASL pH was measured. These buffers maintained ASL pH at their target values for ∼8 hours. However, despite the extensive buffering, by 24 hours, CF ASL pH was ≤6.5 (Figure 1, middle). Next, both 8 and 24 hours after the addition of pH-buffered Ringer’s solution, CF ASL was collected, and this lavage was incubated with 103 cfu/ml J2315 for 2 hours. At 8 hours, when the pH buffering was still effective, bacterial burden was significantly reduced, especially for the pH 7.0- and 7.5-buffered ASL. However, at 24 hours, when the buffered ASLs became acidified, bacterial burden was significantly elevated (Figure 1, right). Because pH can be hard to measure, studying the functionality of pH-sensitive proteins is an orthogonal approach to address this. Low pH reduces antimicrobial peptide/protein activity (6). Hence, acidic CF ASL may negatively affect SPLUNC1 function. Here, we demonstrated that SPLUNC1’s antimicrobial activity was pH sensitive, and increasing ASL pH restored its antimicrobial function for up to 8 hours, but when pH decreased (i.e., by 24 h), antimicrobial function similarly declined.
Figure 1.
Alkalinized cystic fibrosis (CF) airway surface liquid (ASL) transiently reduces Burkholderia cenocepacia J2315 growth. Normal and CF ASL pH was measured immediately and 2 hours after phosphate-buffered saline addition without (open bars) and with (solid bars) 103 cfu/ml J2315 infection. All n = 4 donors per group (top left). Next, 100 μl modified Ringer’s solution buffered at pH 6.0 (red), 6.5 (yellow), 7.0 (green), and 7.5 (blue) and labeled with 10 μM pHRodo Red dextran and 10 μM Alexa-Fluor 647 dextran dyes was added apically to CF human bronchial epithelial cells at T = 0 hours and left undisturbed for 24 hours in an environmental chamber. ASL pH was measured over time, using the pHrodo dye. n = 11 donors per group (right). Aliquots of ASL at 8 hours (circles) and 24 hours (squares), initially buffered at the indicated pH, were collected, and 100 μl was incubated with 103 cfu/ml J2315 for 2 hours at 37°C, 5% CO2. Aliquots were serially diluted onto agar plates to determine bacterial counts. n = 5 donors per group (bottom left). *P < 0.05, **P < 0.01, and ***P < 0.001 comparing pH at the respective times with ASL buffered at pH 6. ##P < 0.01 and ###P < 0.001 comparing pH at the respective times with ASL buffered at pH 6.5. ++P < 0.01 comparing t = 24 hours with t = 0 hours of the respective pH-buffered ASL. °°P < 0.01 comparing 8–24 hours at each buffered pH.
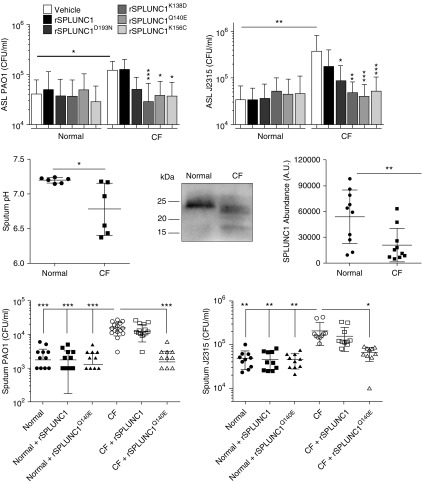
We pretreated CF HBECs in situ with 10 μM wild-type recombinant SPLUNC1 (rSPLUNC1) apically for 1 hour, and then infected them with 103 cfu/ml P. aeruginosa strain PAO1 or J2315 for 2 hours at 37°C, 5% CO2. Aliquots of HBEC ASL lavage were collected, serially diluted, and plated on Luria broth agar plates to determine bacterial burden. Consistent with the previous data, bacterial load remained high. SPLUNC1 possesses an electrostatic surface containing salt bridges that are pH sensitive (4). We used SPLUNC1 mutants with either disrupted salt bridge formation or mutated electrostatic residues near the salt bridges: 10 μM rSPLUNC1K156C, rSPLUNC1Q140E, and rSPLUNC1K138D were able to significantly reduce both PAO1 and J2315 bacterial load in CF ASL compared with nontreated cultures (Figure 2 top). Importantly, bacterial load was reduced, by ∼0.5 to 1 log10, to the levels seen in normal ASL, demonstrating that these SPLUNC1 mutants can maintain antimicrobial activity in CF airways.
Figure 2.
pH-independent SPLUNC1 mutants reduce bacterial burden in cystic fibrosis (CF) airway surface liquid (ASL) and sputum. Normal and CF human bronchial epithelial cells were pretreated with 10 μM rSPLUNC1 and rSPLUNC1 mutants for 1 hour and then infected with 103 cfu/ml PAO1 and J2315 apically for 2 hours, and bacterial burden in the ASL was determined. n = 6 donors per group (top). Normal and CF sputa were collected and measured for pH and for Western blot of SPLUNC1 densitometry of full-length (25 kD) SPLUNC1 (middle). Next, 100 μl normal and CF sputa were coincubated with or without 10 μM rSPLUNC1 or rSPLUNC1Q140E with 103 cfu/ml PAO1 and J2315 for 2 hours at 37°C, 5% CO2. Aliquots were serially diluted and plated on agar to determine bacterial counts (bottom). n = 9–10 donors per group. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the respective vehicle or as indicated with line.
To assess the pH-independent SPLUNC1 mutants’ antimicrobial activities, sputa were collected from normal patients and patients with CF, as described in Webster and colleagues’ supplement Methods (10). Normal sputum pH was ∼7.2, whereas CF sputum pH was significantly reduced to ∼6.8 (Figure 2, middle). In addition, CF sputum had significantly decreased SPLUNC1 levels compared with normal sputum (Figure 2, middle) (10), which may be a result of degradation by proteases in the sputum (3, 10). SPLUNC1 is present in CF HBECs but is nonfunctional (4). We propose that in CF, there is a double effect, whereby SPLUNC1 is inactivated by acidic pH and subsequently degraded as inflammation and proteases become present. We selected one mutant, rSPLUNC1Q140E, for further analysis of antimicrobial effects. Normal and CF sputa were centrifuged at 4,000 × g for 10 minutes. Supernatants were coincubated with or without 10 μM rSPLUNC1 or rSPLUNC1Q140E and with 103 cfu/ml PAO1 or J2315 for 2 hours at 37°C, 5% CO2. Although CF sputum alone or with wild-type rSPLUNC1 had higher bacterial load compared with normal sputum samples, rSPLUNC1Q140E significantly reduced the CF bacterial load, by ∼0.5 to 1 log10, equivalent to normal levels (Figure 2, bottom). Importantly, we measured bacterial growth when there was still rSPLUNC1 in CF sputum, suggesting that this defect was not a result of SPLUNC1 degradation and that pH-insensitive SPLUNC1 mutants maintain antimicrobial activity in CF airway secretions.
In conclusion, we have shown that CF ASL was acidic, which rendered SPLUNC1 inactive and led to increased bacterial growth. Importantly, we demonstrated that SPLUNC1 mutants, particularly rSPLUNC1Q140E, maintained pH-independent antimicrobial activity. These mutants reduced bacterial load in sputum of patients with CF, suggesting that SPLUNC1 mutants may lead to novel therapies for treating CF infections. We have developed SPLUNC1-derived peptides that are stable in the CF lung and can increase CF airway hydration (10). These peptides are currently in phase II clinical trials for CF. Our data suggest that another aspect of SPLUNC1, namely, its ability to reduce gram-negative bacterial infections, is also defective in CF, and that novel strategies to replace this deficit may lead to improved infection control in CF lungs.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the University of North Carolina at Chapel Hill (UNC) CF Center Tissue Core for providing cells, Scott Donaldson and Margret Powell (UNC) for providing CF sputum samples, Heather Wells (UNC) for processing the sputum samples, Colin Bingle (University of Sheffield) for providing the SPLUNC1 construct, Michael Miley and Richard Feng (UNC) for purifying rSPLUNC1 and rSPLUNC1 mutants, and John LiPuma (University of Michigan) for sending the Burkholderia cenocepacia J2315 strain.
Footnotes
This study was funded by NIH R01HL108927, P30DK065988, the Environmental Protection Agency Cooperative Agreement CR 83578501, the UK Cystic Fibrosis Trust (INOVCF, SRC003), and Cystic Fibrosis Foundation R026-CR11.
Originally Published in Press as DOI: 10.1164/rccm.201812-2303LE on April 23, 2019
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Ramsey KA, Ranganathan S, Park J, Skoric B, Adams AM, Simpson SJ, et al. AREST CF. Early respiratory infection is associated with reduced spirometry in children with cystic fibrosis. Am J Respir Crit Care Med. 2014;190:1111–1116. doi: 10.1164/rccm.201407-1277OC. [DOI] [PubMed] [Google Scholar]
- 2.Szczesniak RD, Li D, Su W, Brokamp C, Pestian J, Seid M, et al. Phenotypes of rapid cystic fibrosis lung disease progression during adolescence and young adulthood. Am J Respir Crit Care Med. 2017;196:471–478. doi: 10.1164/rccm.201612-2574OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad S, Tyrrell J, Walton WG, Tripathy A, Redinbo MR, Tarran R. Short palate, lung, and nasal epithelial clone 1 has antimicrobial and antibiofilm activities against the Burkholderia cepacia complex. Antimicrob Agents Chemother. 2016;60:6003–6012. doi: 10.1128/AAC.00975-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garland AL, Walton WG, Coakley RD, Tan CD, Gilmore RC, Hobbs CA, et al. Molecular basis for pH-dependent mucosal dehydration in cystic fibrosis airways. Proc Natl Acad Sci USA. 2013;110:15973–15978. doi: 10.1073/pnas.1311999110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coakley RD, Grubb BR, Paradiso AM, Gatzy JT, Johnson LG, Kreda SM, et al. Abnormal surface liquid pH regulation by cultured cystic fibrosis bronchial epithelium. Proc Natl Acad Sci USA. 2003;100:16083–16088. doi: 10.1073/pnas.2634339100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abou Alaiwa MH, Reznikov LR, Gansemer ND, Sheets KA, Horswill AR, Stoltz DA, et al. pH modulates the activity and synergism of the airway surface liquid antimicrobials β-defensin-3 and LL-37. Proc Natl Acad Sci USA. 2014;111:18703–18708. doi: 10.1073/pnas.1422091112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmad S, Alexis NE, Donaldson SH, Tarran R. SPLUNC1 has decreased antimicrobial activity in cystic fibrosis airway secretions [abstract] Pediatr Pulmonol. 2018;53:S313. [Google Scholar]
- 8.Schultz A, Puvvadi R, Borisov SM, Shaw NC, Klimant I, Berry LJ, et al. Airway surface liquid pH is not acidic in children with cystic fibrosis. Nat Commun. 2017;8:1409. doi: 10.1038/s41467-017-00532-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pezzulo AA, Tang XX, Hoegger MJ, Abou Alaiwa MH, Ramachandran S, Moninger TO, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;487:109–113. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webster MJ, Reidel B, Tan CD, Ghosh A, Alexis NE, Donaldson SH, et al. SPLUNC1 degradation by the cystic fibrosis mucosal environment drives airway surface liquid dehydration. Eur Respir J. 2018;52:1800668. doi: 10.1183/13993003.00668-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.