Abstract
Rationale: Lung function and growth are adversely associated with nitrogen dioxide (NO2) exposure. Lower levels of circulating club cell secretory protein (CC16) in childhood are also associated with subsequent decreased lung function. NO2 exposure may induce epithelial damage in lungs and alter club cell proliferation and morphology.
Objectives: To determine if increased ambient NO2 levels at participants’ home addresses in early life were associated with decreased levels of CC16 from age 6 to 32 years.
Methods: Participants were enrolled at birth in the Tucson Children’s Respiratory Study and had circulating CC16 measured at least once between age 6 and 32. Linear mixed models were used to determine the association between estimated ambient NO2 exposure at participants’ home address at birth or age 6 with CC16 levels from age 6 to 32.
Measurements and Main Results: NO2 exposures at birth or age 6 were available for 777 children with one or more CC16 measurement. We found a negative association between NO2 exposure and CC16 levels, with a 4.7% (95% confidence interval, −8.6 to −0.7) decrease in CC16 levels from age 6 to 32 per interquartile range increase in NO2 exposure (6.0 ppb) at the participants’ birth address. We observed modification by race (p interaction = 0.04), with stronger associations among participants with at least one black parent (−29.6% [95% confidence interval, −42.9% to −13.2%] per interquartile range). NO2 at participant’s age 6 address was not significantly associated with CC16 levels (−1.9%; 95% confidence interval, −6.3 to 2.6).
Conclusions: Higher exposure to NO2 at birth is associated with persistently low levels of CC16 from 6 to 32 years.
Keywords: air pollution, club cell, biomarker
At a Glance Commentary
Scientific Knowledge on the Subject
Lower levels of circulating club cell secretory protein in childhood are associated with reduced lung function in adulthood. Increased exposure to nitrogen dioxide is also associated with reduced lung function growth in children. Evidence from in vitro and in vivo studies suggests that nitrogen dioxide exposure may induce epithelial damage in the lungs and alter club cell growth, yet no previous publication has assessed the effects of nitrogen dioxide exposure in children on subsequent circulating club cell secretory protein levels.
What This Study Adds to the Field
We used data from a longitudinal birth cohort study to determine the associations between nitrogen dioxide exposures in early life and circulating club cell secretory protein from age 6 to 32 years. We determined that higher exposures to nitrogen dioxide at the birth address are associated with persistently lower levels of circulating club cell secretory protein from childhood (age 6) into adult life (age 32). There was no association between nitrogen dioxide exposures at age 6 with circulating club cell secretory protein levels.
Nitrogen dioxide (NO2) is a highly reactive oxidizing gas that arises from the reaction of nitric oxide and oxygen in the atmosphere (1). Both NO2 and nitric oxide are by-products of fossil fuel combustion and are among the most common pollutants measured in both outdoor and indoor air (2). Primary outdoor sources include diesel vehicles and power plants, whereas common indoor sources are the pilot light for gas cook stoves and hot water heaters. NO2 may be the best proxy pollutant to assess spatial exposure to the urban air pollution mixture (3), and nonwhites in the United States are significantly more likely to live in areas with increased NO2 exposure (4).
Deficits in children’s lung function and growth are associated with exposure to NO2 in both cross-sectional and prospective studies (5–7). A 20-year-long study found that long-term reductions in levels of NO2 were associated with significant improvements in children’s lung function growth (8). Multiple studies document that inhaled NO2, because of its reactive nature, can induce epithelial injury in the lungs (1, 9, 10). Of particular concern is evidence that during periods of lung development, epithelial cells may be uniquely susceptible to NO2-induced death (1). Furthermore, NO2 exposure is associated with altered club (formerly Clara) cell proliferation and morphology in animal studies (9).
These club cells release a secretory protein, referred to as CC16, which is a homodimeric pneumoprotein with antiinflammatory properties that may protect the lungs from oxidative stress (11–13). Because CC16 measured in serum has been correlated with levels in BAL fluid and club cell density, serum CC16 is proposed as a novel biomarker for early respiratory impairment from both acute and/or chronic lung injury (11, 14). Decreased levels of circulating CC16 are prospectively associated with lung cancer mortality, chronic obstructive pulmonary disease development, and lung function deficits in children and adults (15–17).
We postulated that early life exposure to NO2 may be associated with lung damage, which is reflected in lower CC16 levels. To test this hypothesis, we used data from the Tucson Children’s Respiratory Study (CRS), a longitudinal birth cohort study that enrolled newborns to examine risk factors for acute lower respiratory tract illnesses in early childhood and chronic lung disorders later in life (18, 19). We determined whether ambient NO2 levels at participants’ birth and age 6 addresses were associated with levels of CC16 from age 6 to 32 years. If early CC16 levels can be shown to reflect lung injury from NO2 exposure, early life interventions that target children with low CC16 levels could be developed to prevent potential long-term health effects in high-risk children. Some of these results have been previously reported in the form of an abstract (20).
Methods
Study Population
The CRS is a longitudinal nonselected birth cohort study. All healthy infants born to mothers using the services of the largest health maintenance organization between 1980 and 1984 were eligible to participate and were contacted by a study nurse. Of the 1,596 eligible families contacted, 1,246 infants (78%) were enrolled. A detailed description of the cohort is published (18, 19). For the purpose of the current analysis, demographic characteristics of the child and parents, and home address, were obtained from a questionnaire administered at enrollment (within 2 wk after birth). Blood samples were obtained at age 6 (mean = 6.1; SD = 0.8), 11 (mean = 10.8; SD = 0.6), 16 (mean = 16.6; SD = 0.6), 22 (mean = 22; SD = 0.9), 26 (mean = 26.4; SD = 0.8), and 32 years of age (mean = 31.8; SD = 1.0). The study was approved by the University of Arizona Human Subjects Committee. Informed consent was obtained from a parent of a participating child and/or from the participants themselves starting at age 16 years.
CC16 Measurements
Circulating CC16 was measured in stored samples from each follow-up period. Serum samples were used for all time points, with the exception of 78 plasma samples from Year 6. In sensitivity analyses we excluded the plasma samples. Measurement techniques and the adjustments for plasma samples have been described in detail previously (16). All samples were cryopreserved at −80°C and circulating CC16 was measured using a commercially available ELISA kit (kit range: 1.57–50.0 ng/ml; BioVendor). All samples had detectable values.
NO2 Exposure Assessment
We estimated NO2 exposure at each enrollment (1980–1984) and age 6 (1986–1990) address using a land-use regression (LUR) model developed from NO2 measurements obtained in eastern Pima County (where Tucson is located) between 1987 and 1991. NO2 concentrations were measured in residential yards using passive Palmes diffusion tubes (21). Participating homes were from the Pima County Workers Study, who were recruited through county government departments with a wide range of occupations, resulting in a study cohort with varied socioeconomic status, demographic indicators, and geographic distribution. In 1987, the year closest to CRS enrollment, 166 homes were sampled for one to two 1-week periods, resulting in 311 observations.
We developed and evaluated our NO2 LUR by adapting protocols for the European Study of Cohorts for Air Pollution Effects (ESCAPE), which has been conducted in 36 study areas in Europe (22). We created 89 geographic predictor variables based on ESCAPE protocols (22). Additional variables important to the Tucson environment were included (e.g., distance to airports, active mining operations, and agricultural land). Following the ESCAPE method, simple regressions were performed with all predictors and the one with the highest R2 and slope in the predefined direction (e.g., area of nearby natural space is negatively related to air pollution) became the “seed” model. Other predictors were added one-by-one and kept if: 1) the adjusted R2 increased by more than 1%, 2) the slope met the predefined direction, and 3) the slope direction of other predictors did not change. After all predictors were evaluated, variables in the model were removed one-by-one if their P value was greater than 0.1, beginning with the variable with the largest P value. Model performance was evaluated using a leave-one-out cross-validation method. Details on the source data and calculations for each variable are provided in online supplement (see Table E1 in the online supplement).
Using ArcGIS 10.1 (ESRI), we geocoded the enrollment and age 6 address for each participant. These coordinates were used as receptor points in the LUR to estimate ambient NO2 exposure in ppb at each address as:
where Pop5km is the population density within 5 km (persons/m2); Elev is the elevation (m); and Distair and Distrail are the distance to the nearest airport and rail line, respectively (m). To predict exposures corresponding to each participant’s birth or age 6 address, these variables were obtained for each address corresponding to the calendar year of enrollment (1980–1984) or age 6 (1984–1992) from the sources listed in Table E1, as in previous ESCAPE studies. Estimates were scaled based on differences between 1987 and the exposure year in NO2 measurements from the regional monitoring network (7).
Statistical Analysis
All analyses were performed with R (V3.3.4; R Foundation for Statistical Computing). NO2 exposures were scaled by dividing by the interquartile range to aid interpretability of the coefficients (i.e., a 1-unit increase is equal to the interquartile range). Because the distribution of CC16 values was right skewed, and concentrations are theoretically log-normally distributed (23), values were natural log-transformed. We used Pearson chi-square test to assess if the demographic characteristics of those who were included in the analyses were different from participants who were excluded. Simple linear regression was used to determine if there were associations between NO2 exposures and participant characteristics.
To estimate longitudinal associations between NO2 exposure and CC16 levels throughout life, we used linear mixed models and included participants with complete covariate and exposure data, and with CC16 measurements for at least one time point. Results are presented as percent change in CC16 per interquartile range increase in NO2. Percent change was calculated from the β coefficients according to methods for log-transformed outcome variables (24). To select covariates for inclusion, we identified covariates a priori that are important in the air pollution–lung function literature, and examined associations between NO2 exposure and those covariates (maternal education, maternal age, race/ethnicity, delivery method, marital status, maternal smoking, child sex, home heating, socioeconomic status, birth order, and parity) and covariates previously associated with CC16 in our cohort (25) (maternal age, sex, parental education, maternal smoking at age 6, CC16 genotype, race/ethnicity). We excluded variables that were strongly associated with the exposure but not with the outcome to avoid inducing instrumental variable bias (26). The remaining variables were included if they resulted in a change-in-estimate of the NO2-CC16 relationship by greater than 10% or if they enhanced the SE of the estimate.
Final model covariates included fixed effects for child race/ethnicity, child sex, maternal smoking at enrollment, follow-up visit (6–32 yr), and random effects by subject. Unadjusted models omitted the fixed effects (with the exception of follow-up visit) but adjusted for random effects by subject. We examined associations between NO2 exposure at birth and at age 6 separately, and CC16 levels at age 6, 11, 16, 22, 26, and 32 years. We assessed modification by several variables including race/ethnicity (non-Hispanic white, any Hispanic parent, any black parent, other), maternal education (high school or less vs. greater than high school), maternal smoking, child sex, and indices of neighborhood socioeconomic status and housing conditions derived from the U.S. census (27), with an alpha cutoff of 0.05 on the interaction term, using a likelihood ratio test. To assess if later CC16 measurements were associated with active smoking, we conducted a sensitivity analysis with active smoking between age 18 and 32 years.
Results
Of the original 1,246 children enrolled, 1,137 had enrollment and 806 children had age 6 addresses, for which NO2 exposure could be estimated and were included in the analyses (Table 1). NO2 values were estimated from the LUR models as described and reflect estimates at the child’s address during each time period. NO2 levels were slightly higher during the enrollment period than during the first follow-up period (geometric means birth vs. age 6; P = 0.05). At enrollment, children classified as “Other” race/ethnicity had significantly lower exposures at their home address than children of any other race/ethnicity category (see Table E2). There were no other significant differences in NO2 exposure at enrollment by participant characteristics. However, at age 6, children who had a Hispanic or black parent had higher NO2 exposure levels, as did children with younger mothers or those whose mothers had lower educational attainment.
Table 1.
Distribution of Ambient NO2 Concentrations (ppb) at Reported Residence at Enrollment and First Follow-up (Age 6)
| n | GM | GSD | Median | IQR | Range | |
|---|---|---|---|---|---|---|
| Enrollment (1980–1984) | 1,137 | 10.96 | 1.41 | 11.00 | 6.00 | 4.18–27.30 |
| First follow-up (1986–1990) | 806 | 10.23 | 1.48 | 10.74 | 6.69 | 2.73–20.39 |
Definition of abbreviations: GM = geometric mean; GSD = geometric standard deviation; IQR = interquartile range; NO2 = nitrogen dioxide.
Characteristics and CC16 levels for the study population included in the analysis are presented by follow-up period in Table 2. As previously reported, CC16 levels in this cohort increased with age (25). There were no large differences in demographic characteristics of the participants by follow-up period (Table 2). However, CRS participants included in this analysis were more likely to be non-Hispanic white or Hispanic and have mothers with more education and that were less likely to smoke than those who were not included in the analysis (see Table E3).
Table 2.
Characteristics of Participants Included in Analyses in Each Follow-up Period
| Enrollment | 6 yr | 11 yr | 16 yr | 22 yr | 26 yr | 32 yr | |
|---|---|---|---|---|---|---|---|
| n | 1,137* | 479† | 550† | 432† | 416† | 358† | 234† |
| CC16, ng/ml, GM (GSD) |
7.9 (1.5) | 7.5 (1.5) | 8.4 (1.5) | 11.5 (1.5) | 9.5 (1.6) | 10.5 (1.5) | |
| NO2 at enrollment, ppb, median (IQR) | 11.0 (6.0) | 10.8 (6.3) | 10.9 (6.2) | 10.7 (6.2) | 10.8 (6.2) | 10.7 (6.0) | 10.8 (6.2) |
| NO2 at age 6, ppb, median (IQR) | 10.5 (6.5) | 10.8 (6.5) | 10.7 (6.4) | 10.5 (6.4) | 10.3 (6.4) | 10.4 (6.9) | |
| Child sex, % (n) | |||||||
| M | 48.3 (550) | 49.9 (239) | 48.7 (268) | 48.8 (211) | 48.5 (204) | 47.2 (169) | 43.6 (102) |
| F | 51.6 (587) | 50.1 (240) | 51.3 (282) | 51.2 (221) | 51.5 (217) | 52.8 (189) | 56.4 (132) |
| Child ethnicity race, % (n) |
|||||||
| Non-Hispanic white | 59.4 (675) | 62.2 (298) | 58.6 (322) | 60.2 (260) | 61.3 (255) | 62.0 (222) | 60.3 (141) |
| Any Hispanic | 25.5 (290) | 27.8 (133) | 31.5 (173) | 29.6 (128) | 29.6 (123) | 28.5 (102) | 30.3 (71) |
| Any black | 4.1 (47) | 3.6 (17) | 3.3 (18) | 3.2 (14) | 2.2 (9) | 2.5 (9) | 2.6 (6) |
| Other | 11.0 (125) | 6.4 (31) | 6.7 (37) | 6.9 (30) | 7.0 (29) | 7.0 (25) | 6.8 (16) |
| Maternal age at enrollment, % (n) |
|||||||
| <20 | 5.1 (58) | 4.4 (21) | 4.5 (25) | 3.2 (14) | 3.1 (13) | 3.3 (12) | 2.1 (5) |
| 20–25 | 32.1 (365) | 29.4 (141) | 29.6 (163) | 29.9 (129) | 29.3 (122) | 33.5 (120) | 33.8 (79) |
| 26–30 | 38.2 (434) | 40.1 (192) | 40.2 (221) | 43.1 (186) | 42.3 (176) | 38.5 (138) | 40.2 (94) |
| >30 | 24.6 (280) | 26.1 (125) | 25.6 (141) | 23.8 (103) | 25.2 (105) | 24.6 (88) | 23.9 (56) |
| Maternal education at enrollment, % (n) |
|||||||
| ≤12 yr | 31.3 (355) | 28.2 (135) | 28.2 (155) | 24.6 (106) | 25.0 (104) | 25.1 (90) | 25.8 (60) |
| >12 yr | 68.7 (780) | 71.8 (344) | 71.8 (394) | 75.4 (325) | 75.0 (312) | 74.9 (268) | 74.3 (173) |
| Maternal smoking at enrollment, % (n) |
|||||||
| Yes | 17.2 (195) | 15.1 (72) | 14.2 (78) | 13.7 (59) | 13.9 (58) | 15.1 (54) | 13.7 (32) |
| No | 82.8 (941) | 84.9 (406) | 85.8 (471) | 86.3 (373) | 86.1 (358) | 84.9 (304) | 86.3 (202) |
| Current smoking, % (n) |
|||||||
| Yes | — | — | — | 10.6 (43) | 28.9 (120) | 26.0 (92) | 16.7 (39) |
| No | — | — | — | 89.4 (362) | 71.1 (296) | 74.0 (262) | 83.3 (195) |
Definition of abbreviations: CC16 = club cell secretory protein; GM = geometric mean; GSD = geometric standard deviation; IQR = interquartile range; NO2 = nitrogen dioxide.
Included participants had at least one CC16 measurement between age 6 and 32 years and their NO2 exposure at enrollment or age 6 could be determined from their address. Participants were asked about their own smoking status beginning at age 16.
Number of participants with ≥1 CC16 measurement between age 6 and 32 years and NO2 exposure was determined at enrollment address.
Number of participants with a CC16 measurement obtained during that follow-up period and their NO2 exposure at enrollment or age 6 could be determined from their address.
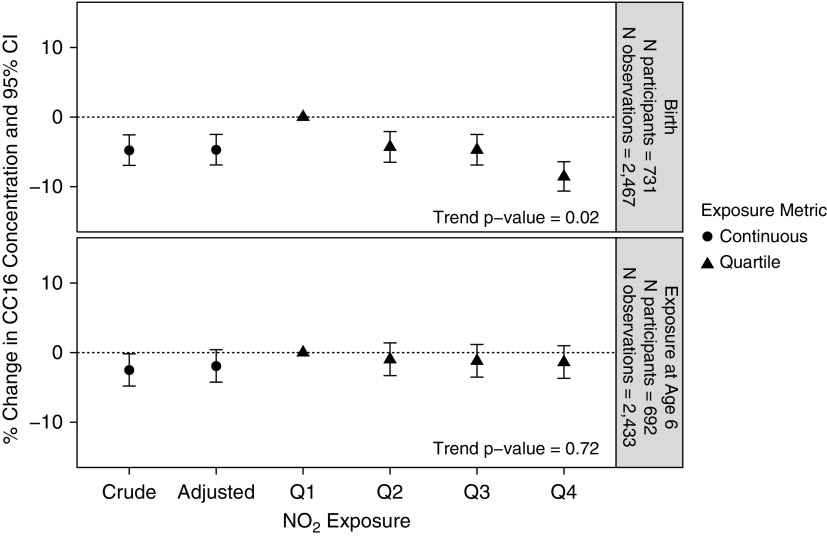
An interquartile increase in NO2 exposure at birth was associated with a decrease in CC16 levels from age 6 to 32 in both unadjusted and adjusted models (unadjusted, −4.8%; 95% confidence interval [CI], −8.6 to −0.7) (adjusted, −4.7%; 95% CI, −8.6 to −0.7) (Figure 1). Children in the highest quartile of exposure at birth had 8.6% lower (95% CI, −15.0 to −1.65) average levels of CC16 compared with children in the lowest quartile of exposure at birth. These trends were consistent in models stratified by follow-up period (see Figure E1). There was no significant association between children’s NO2 exposures at age 6 and their CC16 levels from age 6 to 32 in either unadjusted or adjusted models (Figure 1). Results were consistent when models included only children with reported exposures for both time periods. When models were stratified by follow-up period, there was an increase in CC16 levels at age 6 with concurrent NO2 exposures at age 6 (see Figure E1), and a decrease in CC16 levels at age 22–32. These trends were not significant.
Figure 1.
Percent change in club cell secretory protein levels from ages 6 to 32 years in association with an interquartile range increase in nitrogen dioxide exposure at birth and age 6 addresses, respectively. Associations are also shown per quartile. All models include random effects for subject and fixed effects for visit. Adjusted models include fixed effects for child’s race/ethnicity, child’s sex, and maternal smoking at enrollment. Participant counts include those with complete covariate, exposure, and outcome data. Percent change was calculated from the β coefficients according to methods for log-transformed outcome variables (24). CC16 = club cell secretory protein; CI = confidence interval; NO2 = nitrogen dioxide.
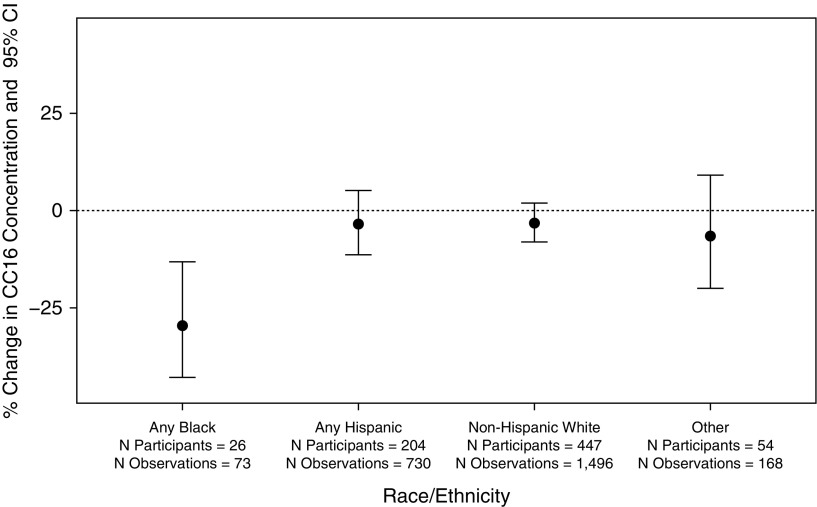
We observed a significant interaction between race/ethnicity and NO2 exposure at birth on CC16 levels (p for interaction = 0.04). The strongest NO2/CC16 association was among black individuals, though this estimate was quite imprecise (−29.6%; 95% CI, −42.9% to −13.2%) (Figure 2). Our cohort had a relatively small black population (n participants = 26; n observations = 73), and thus these exploratory results should be interpreted with caution. Given that their exposures at birth were not significantly higher (see Table E2), this may indicate an increase in susceptibility to NO2 exposure. Although we hypothesized that race may be a proxy for socioeconomic status in our cohort, we did not observe modification by maternal age, maternal education, or smoking. To ensure that this was not an effect of living in a neighborhood with lower socioeconomic status or poorer housing conditions, we also assessed effect modification by indices we previously derived from U.S. Census variables (27). There was no effect modification by these variables either. In our cohort, participants with any black parent were not more likely than other participants to have younger mothers, mothers who smoked, mothers with lower education, or live in neighborhoods with lower socioeconomic status or poorer housing. In a sensitivity analysis without black children, each interquartile increase in NO2 exposure at birth was associated with a decrease in CC16 levels from age 6 to 32 years (adjusted, −3.5%; 95% CI, −7.5 to 0.7). Although not a significant association, these results are still suggestive of a negative association between NO2 exposure and CC16 levels among children of other racial or ethnic heritage.
Figure 2.
Percent change in club cell secretory protein levels from ages 6 to 32 years in association with an interquartile range increase in nitrogen dioxide (NO2) exposure at birth, by race/ethnicity. All models include random effects for subject, fixed effects for visit, child’s sex and maternal smoking at enrollment, and an interaction term for child’s race/ethnicity and NO2 exposure at birth. Participant counts include those with complete covariate, exposure, and outcome data. Percent change was calculated from the β coefficients according to methods for log-transformed outcome variables (24). CC16 = club cell secretory protein; CI = confidence interval.
Discussion
To the best of our knowledge, this is the first study to prospectively document an association between early life NO2 exposures and CC16 levels in humans. We demonstrated that ambient NO2 exposures at birth address were associated with decreased circulating CC16 levels from age 6 to 32 years. However, there was no association between ambient NO2 exposures at age 6 home address with CC16 levels. There was effect modification by race/ethnicity. Participants with at least one non-Hispanic black parent had significantly larger reductions in CC16 levels per interquartile range of NO2 exposure compared with participants from other racial/ethnic backgrounds. CC16 may be a sensitive biomarker of early life damage to the respiratory tract following exposure to oxidizing air pollutants, such as NO2.
Our study confirms findings from the only other study we identified that assessed the relationship between NO2 exposure and CC16 levels in humans. Workers chronically exposed to nitrogen oxides at a nitric acid production factory were significantly more likely to have decreased levels of CC16, compared with unexposed workers (10). In our study, we demonstrated that increased NO2 exposure levels at birth were associated with decreased CC16 levels through age 32 years. Of the participants included in our analyses with measured CC16 at enrollment and age 6, a total of 56% had moved residences between birth and age 6. However, exposures at age 6 were not significantly associated with CC16 levels from age 6 to 32 years. This supports conclusions from animal studies that there may be critical periods of lung development where epithelial cells may be uniquely susceptible to NO2-induced death (1). Thus, children with increased NO2 exposures during this critical window may be predisposed to having fewer club cells and reduced potential to produce CC16.
One of the complexities of CC16 as a biomarker of lung damage is the finding from in vitro and in vivo studies that show that acute or recent exposures to NO2 may temporarily increase bronchial epithelial permeability, thereby allowing more CC16 into circulation, resulting in temporarily elevated levels (1, 9, 10). Although not significant, increased NO2 exposures at age 6 were associated with increased CC16 levels at age 6 in our study (see Figure E1). However, similar to exposures at birth, increased NO2 exposures at age 6 years were associated with decreased levels of CC16 in adulthood (age 22–32 yr). These findings indicate that the critical window of exposure may include more of early childhood.
Although there have not been any studies examining associations between ambient NO2 exposure and CC16 levels in humans during critical periods of lung growth, a few studies in adults document that daily exposures to ambient particulate matter (PM) are associated with increased CC16 concentrations (28, 29). These associations are stronger with PM attributed to combustion sources (30), which are also more likely to be correlated with NO2 in most ambient environments. Timonen and colleagues (29) and Jacquemin and colleagues (30) hypothesize that increased CC16 levels associated with ambient PM exposures on the same day may indicate that acute PM exposure is also associated with increased epithelial permeability. In one study, concurrent increase in CC16 levels with PM exposures are associated with temporary deficits in lung function (28), indicating that the immediate decrease in lung function following exposure to PM may be attributed to loss of pulmonary epithelium integrity (28). They demonstrated in a mediation model that increased levels of CC16 accounted for 4–36% of the lung function deficits associated with PM exposure. Thus, it is plausible that the positive association we observed in our cohort for concurrent NO2 exposure and CC16 levels at age 6 may be explained by a similar mechanism (28), because NO2 is often correlated with PM in ambient environments.
Previously, we reported that diminished CC16 levels (age 6–32 yr) in our cohort were associated with socioeconomic factors (i.e., Hispanic ethnicity, younger maternal age, maternal smoking, lower parental education attainment) (25). Although children with lower socioeconomic status are likely to have higher NO2 exposures, in our study increased NO2 exposure at birth was only associated with maternal smoking and none of the other factors. Interestingly, increased NO2 exposure at age 6 was associated with all of these factors, yet there was not a significant association between NO2 exposures at age 6 and longitudinal CC16 levels. In our cohort, socioeconomic status is not likely the explanation for the association of NO2 exposure with CC16 levels (25). Future work is needed to determine if there are other factors that could explain these associations, such as additional environmental exposures, nutritional status, or access to health care.
In our analysis, children with any non-Hispanic black parent had significantly larger deficits in CC16 levels per interquartile increase in NO2 exposures at birth compared with children from any other racial/ethnic background (see Figure E2). Because only 3.6% of our study population had any black parent, which is representative of Tucson, Arizona, further analyses in cohorts with a larger proportion of black children are needed to confirm this increased potential susceptibility.
However, there is a strong suggestion from the literature that black children may be more susceptible to NO2 exposures. Multiple studies demonstrated significantly stronger associations between NO2 exposures and hospital admissions or emergency room visits for asthma among black children compared with other children (31–34). This effect modification remained significant after controlling for insurance status and socioeconomic variables in a cohort of low-income children. Other studies demonstrated that racial disparities in childhood asthma are not just a function of income disparities (35–37). In a laboratory-controlled study, adult black males demonstrated significantly larger lung function deficits following exposure to ozone, another oxidizing air pollutant (38). Multiple hypotheses have been proposed, including differences in access to health care (39), oxidant defense genes (40, 41), or levels of micronutrients (42). For example, increased vitamin A levels are associated with increases in CC16 levels (43), and increased levels of vitamin E protect the lungs from the effects of NO2 (1). Non-Hispanic black children in the United States have lower levels of vitamins A and E after controlling for income and socioeconomic factors, compared with children from other racial/ethnic backgrounds (44). Further work is needed to better understand why black individuals are more susceptible to oxidizing air pollutant (i.e., NO2, ozone) exposures and the role that CC16 may have in this effect.
One of the key limitations of our study is that we only had exposure estimates for NO2 and not for other air pollutants. Because air pollutants are often highly correlated, it is plausible that the effects we observed may be attributed to other air pollutants, such as ozone or PM. However, we were not able to assess for or control for those other exposures. Although there is biologic evidence of the effect of NO2 on CC16 levels, club cells, and epithelial integrity, future studies should investigate exposures to mixtures of ambient air pollutants to determine their independent and joint effects on longitudinal CC16 levels.
In summary, in our nonselected birth cohort with 32 years of follow-up, we found that increased NO2 exposure at birth but not at age 6 was associated with a decrease in CC16 levels through adult life (age 6–32 yr). Although the numbers were small, participants with at least one non-Hispanic black parent had significantly larger deficits in association with NO2 exposure at their birth. Thus, CC16 may be a sensitive biomarker of early life lung damage from the oxidizing air pollutant NO2 during critical windows of lung development, particularly among black children. Confirmation of these findings in other cohorts with a larger proportion of black participants could support the utility of CC16 as a means of identifying those children most susceptible to the effects of air pollution.
Supplementary Material
Acknowledgments
Acknowledgment
The authors gratefully acknowledge the contributions of Lynn Taussig, who started the Tucson Children’s Respiratory Study; and Michael Lebowitz and Mary Kay O’Rourke, who started the Pima County Workers Study. They thank Catherine J. Holberg and Bruce Saul for data management, and the study nurses, Marilyn Lindell, Lydia de la Ossa, and Nicole Pargas, for data collection and participant follow-up.
Footnotes
Supported by NIH (ES006694, AI135108, HL56177, HL103970, and ES028743), the Arizona Technology and Research Initiative Fund, and U.S. Environmental Protection Agency Cooperative Agreement No. CR811806. The publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the U.S. Environmental Protection Agency.
Author Contributions: P.I.B., S.G., A.L.W., and F.D.M. contributed to study conception and design and obtained funding. P.I.B. and N.L. contributed to acquisition of exposure data. M.H. led the analysis of CC16 in the samples by her laboratory. S.G., D.A.S., and J.Z. provided and assisted with calculation of covariates. P.I.B. and M.F. conducted the epidemiologic analyses. D.B. provided statistical guidance. P.I.B., N.L., and M.F. drafted the initial report. All authors contributed to interpretation of the data, to revision of the report, and approved the version submitted.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201808-1488OC on February 21, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Persinger RL, Poynter ME, Ckless K, Janssen-Heininger YM. Molecular mechanisms of nitrogen dioxide induced epithelial injury in the lung. Mol Cell Biochem. 2002;234–235:71–80. [PubMed] [Google Scholar]
- 2.Seinfeld JH, Pandis SN. Atmospheric chemistry and physics: from air pollution to climate change. Hoboken, NJ: John Wiley & Sons; 2016. [Google Scholar]
- 3.Levy I, Mihele C, Lu G, Narayan J, Brook JR. Evaluating multipollutant exposure and urban air quality: pollutant interrelationships, neighborhood variability, and nitrogen dioxide as a proxy pollutant. Environ Health Perspect. 2014;122:65–72. doi: 10.1289/ehp.1306518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark LP, Millet DB, Marshall JD. National patterns in environmental injustice and inequality: outdoor NO2 air pollution in the United States. PLoS One. 2014;9:e94431. doi: 10.1371/journal.pone.0094431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urman R, McConnell R, Islam T, Avol EL, Lurmann FW, Vora H, et al. Associations of children’s lung function with ambient air pollution: joint effects of regional and near-roadway pollutants. Thorax. 2014;69:540–547. doi: 10.1136/thoraxjnl-2012-203159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mölter A, Agius RM, de Vocht F, Lindley S, Gerrard W, Lowe L, et al. Long-term exposure to PM10 and NO2 in association with lung volume and airway resistance in the MAAS birth cohort. Environ Health Perspect. 2013;121:1232–1238. doi: 10.1289/ehp.1205961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacIntyre EA, Gehring U, Mölter A, Fuertes E, Klümper C, Krämer U, et al. Air pollution and respiratory infections during early childhood: an analysis of 10 European birth cohorts within the ESCAPE Project. Environ Health Perspect. 2014;122:107–113. doi: 10.1289/ehp.1306755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gauderman WJ, Urman R, Avol E, Berhane K, McConnell R, Rappaport E, et al. Association of improved air quality with lung development in children. N Engl J Med. 2015;372:905–913. doi: 10.1056/NEJMoa1414123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barth PJ, Müller B. Effects of nitrogen dioxide exposure on Clara cell proliferation and morphology. Pathol Res Pract. 1999;195:487–493. doi: 10.1016/S0344-0338(99)80052-1. [DOI] [PubMed] [Google Scholar]
- 10.Hałatek T, Gromadzińska J, Wasowicz W, Rydzyński K. Serum clara-cell protein and beta2-microglobulin as early markers of occupational exposure to nitric oxides. Inhal Toxicol. 2005;17:87–97. doi: 10.1080/08958370590899460. [DOI] [PubMed] [Google Scholar]
- 11.Broeckaert F, Bernard A. Clara cell secretory protein (CC16): characteristics and perspectives as lung peripheral biomarker. Clin Exp Allergy. 2000;30:469–475. doi: 10.1046/j.1365-2222.2000.00760.x. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee AB, Zhang Z, Chilton BS. Uteroglobin: a steroid-inducible immunomodulatory protein that founded the Secretoglobin superfamily. Endocr Rev. 2007;28:707–725. doi: 10.1210/er.2007-0018. [DOI] [PubMed] [Google Scholar]
- 13.Lakind JS, Holgate ST, Ownby DR, Mansur AH, Helms PJ, Pyatt D, et al. A critical review of the use of Clara cell secretory protein (CC16) as a biomarker of acute or chronic pulmonary effects. Biomarkers. 2007;12:445–467. doi: 10.1080/13547500701359327. [DOI] [PubMed] [Google Scholar]
- 14.Guerra S, Vasquez MM, Spangenberg A, Halonen M, Martin RJ. Club cell secretory protein in serum and bronchoalveolar lavage of patients with asthma. J Allergy Clin Immunol. 2016;138:932–934. doi: 10.1016/j.jaci.2016.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerra S, Vasquez MM, Spangenberg A, Halonen M, Martinez FD. Serum concentrations of club cell secretory protein (Clara) and cancer mortality in adults: a population-based, prospective cohort study. Lancet Respir Med. 2013;1:779–785. doi: 10.1016/S2213-2600(13)70220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerra S, Halonen M, Vasquez MM, Spangenberg A, Stern DA, Morgan WJ, et al. Relation between circulating CC16 concentrations, lung function, and development of chronic obstructive pulmonary disease across the lifespan: a prospective study. Lancet Respir Med. 2015;3:613–620. doi: 10.1016/S2213-2600(15)00196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhai J, Insel M, Addison KJ, Stern DA, Pederson W, Dy A, et al. Club cell secretory protein deficiency leads to altered lung function. Am J Respir Crit Care Med. 2019;199:302–312. doi: 10.1164/rccm.201807-1345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taussig LM, Wright AL, Morgan WJ, Harrison HR, Ray CG. The Tucson Children’s Respiratory Study: I. Design and implementation of a prospective study of acute and chronic respiratory illness in children. Am J Epidemiol. 1989;129:1219–1231. doi: 10.1093/oxfordjournals.aje.a115242. [DOI] [PubMed] [Google Scholar]
- 19.Wright AL, Taussig LM, Ray CG, Harrison HR, Holberg CJ. The Tucson Children’s Respiratory Study: II. Lower respiratory tract illness in the first year of life. Am J Epidemiol. 1989;129:1232–1246. doi: 10.1093/oxfordjournals.aje.a115243. [DOI] [PubMed] [Google Scholar]
- 20.Beamer PI, Furlong M, Lothrop N, Guerra S, Billheimer D, Stern DA, et al. CC16 levels into adult life are associated with nitrogen dioxide exposure at birth. Am J Respir Crit Care Med. doi: 10.1164/rccm.201808-1488OC. [online ahead of print] 21 Feb 2019; DOI: 10.1164/rccm.201808-1488OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quackenboss JJ, Lebowitz MD, Hayes C. Epidemiological study of respiratory responses to indoor/outdoor air quality. Environ Int. 1989;15:493–502. [Google Scholar]
- 22.Beelen R, Hoek G, Vienneau D, Eeftens M, Dimakopoulou K, Pedeli X, et al. Development of NO 2 and NO x land use regression models for estimating air pollution exposure in 36 study areas in Europe: the ESCAPE project. Atmos Environ. 2013;72:10–23. [Google Scholar]
- 23.Ott WR. A physical explanation of the lognormality of pollutant concentrations. J Air Waste Manage Assoc. 1990;40:1378–1383. doi: 10.1080/10473289.1990.10466789. [DOI] [PubMed] [Google Scholar]
- 24.Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Regression methods in biostatistics: linear, logistic, survival, and repeated measures models. Berlin, Germany: Springer Science & Business Media; 2011. [Google Scholar]
- 25.Zhai J, Stern DA, Sherrill DL, Spangenberg AL, Wright AL, Morgan WJ, et al. Trajectories and early determinants of circulating CC16 from birth to age 32 years. Am J Respir Crit Care Med. 2018;198:267–270. doi: 10.1164/rccm.201712-2398LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myers JA, Rassen JA, Gagne JJ, Huybrechts KF, Schneeweiss S, Rothman KJ, et al. Effects of adjusting for instrumental variables on bias and precision of effect estimates. Am J Epidemiol. 2011;174:1213–1222. doi: 10.1093/aje/kwr364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beamer PI, Lothrop N, Lu Z, Ascher R, Ernst K, Stern DA, et al. Spatial clusters of child lower respiratory illnesses associated with community-level risk factors. Pediatr Pulmonol. 2016;51:633–642. doi: 10.1002/ppul.23332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C, Cai J, Chen R, Shi J, Yang C, Li H, et al. Personal exposure to fine particulate matter, lung function and serum club cell secretory protein (Clara) Environ Pollut. 2017;225:450–455. doi: 10.1016/j.envpol.2017.02.068. [DOI] [PubMed] [Google Scholar]
- 29.Timonen KL, Hoek G, Heinrich J, Bernard A, Brunekreef B, de Hartog J, et al. Daily variation in fine and ultrafine particulate air pollution and urinary concentrations of lung Clara cell protein CC16. Occup Environ Med. 2004;61:908–914. doi: 10.1136/oem.2004.012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacquemin B, Lanki T, Yli-Tuomi T, Vallius M, Hoek G, Heinrich J, et al. Source category-specific PM2.5 and urinary levels of Clara cell protein CC16: the ULTRA study. Inhal Toxicol. 2009;21:1068–1076. doi: 10.3109/08958370902725292. [DOI] [PubMed] [Google Scholar]
- 31.Alhanti BA, Chang HH, Winquist A, Mulholland JA, Darrow LA, Sarnat SE. Ambient air pollution and emergency department visits for asthma: a multi-city assessment of effect modification by age. J Expo Sci Environ Epidemiol. 2016;26:180–188. doi: 10.1038/jes.2015.57. [DOI] [PubMed] [Google Scholar]
- 32.Grineski SE, Staniswalis JG, Peng Y, Atkinson-Palombo C. Children’s asthma hospitalizations and relative risk due to nitrogen dioxide (NO2): effect modification by race, ethnicity, and insurance status. Environ Res. 2010;110:178–188. doi: 10.1016/j.envres.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strickland MJ, Klein M, Flanders WD, Chang HH, Mulholland JA, Tolbert PE, et al. Modification of the effect of ambient air pollution on pediatric asthma emergency visits: susceptible subpopulations. Epidemiology. 2014;25:843–850. doi: 10.1097/EDE.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wendt JK, Symanski E, Stock TH, Chan W, Du XL. Association of short-term increases in ambient air pollution and timing of initial asthma diagnosis among Medicaid-enrolled children in a metropolitan area. Environ Res. 2014;131:50–58. doi: 10.1016/j.envres.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crocker D, Brown C, Moolenaar R, Moorman J, Bailey C, Mannino D, et al. Racial and ethnic disparities in asthma medication usage and health-care utilization: data from the National Asthma Survey. Chest. 2009;136:1063–1071. doi: 10.1378/chest.09-0013. [DOI] [PubMed] [Google Scholar]
- 36.Law H-Z, Oraka E, Mannino DM. The role of income in reducing racial and ethnic disparities in emergency room and urgent care center visits for asthma: United States, 2001-2009. J Asthma. 2011;48:405–413. doi: 10.3109/02770903.2011.565849. [DOI] [PubMed] [Google Scholar]
- 37.Roy A, Wisnivesky JP. Racial and ethnic differences in the use of environmental control practices among children with asthma. J Asthma. 2010;47:507–512. doi: 10.3109/02770901003734314. [DOI] [PubMed] [Google Scholar]
- 38.Que LG, Stiles JV, Sundy JS, Foster WM. Pulmonary function, bronchial reactivity, and epithelial permeability are response phenotypes to ozone and develop differentially in healthy humans. J Appl Physiol (1985) 2011;111:679–687. doi: 10.1152/japplphysiol.00337.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glad JA, Brink LL, Talbott EO, Lee PC, Xu X, Saul M, et al. The relationship of ambient ozone and PM(2.5) levels and asthma emergency department visits: possible influence of gender and ethnicity. Arch Environ Occup Health. 2012;67:103–108. doi: 10.1080/19338244.2011.598888. [DOI] [PubMed] [Google Scholar]
- 40.Vawda S, Mansour R, Takeda A, Funnell P, Kerry S, Mudway I, et al. Associations between inflammatory and immune response genes and adverse respiratory outcomes following exposure to outdoor air pollution: a HuGE systematic review. Am J Epidemiol. 2014;179:432–442. doi: 10.1093/aje/kwt269. [DOI] [PubMed] [Google Scholar]
- 41.Islam T, McConnell R, Gauderman WJ, Avol E, Peters JM, Gilliland FD. Ozone, oxidant defense genes, and risk of asthma during adolescence. Am J Respir Crit Care Med. 2008;177:388–395. doi: 10.1164/rccm.200706-863OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moreno-Macías H, Dockery DW, Schwartz J, Gold DR, Laird NM, Sienra-Monge JJ, et al. Ozone exposure, vitamin C intake, and genetic susceptibility of asthmatic children in Mexico City: a cohort study. Respir Res. 2013;14:14. doi: 10.1186/1465-9921-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y, Vasquez MM, Zhu L, Lizarraga RE, Krutzsch M, Einspahr J, et al. Effects of retinoids on augmentation of club cell secretory protein. Am J Respir Crit Care Med. 2017;196:928–931. doi: 10.1164/rccm.201608-1611LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kant AK, Graubard BI. Race-ethnic, family income, and education differentials in nutritional and lipid biomarkers in US children and adolescents: NHANES 2003-2006. Am J Clin Nutr. 2012;96:601–612. doi: 10.3945/ajcn.112.035535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.