Abstract
Rationale: Glycolytic shift is implicated in the pathogenesis of pulmonary arterial hypertension (PAH). It remains unknown how glycolysis is increased and how increased glycolysis contributes to pulmonary vascular remodeling in PAH.
Objectives: To determine whether increased glycolysis is caused by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3) and how PFKFB3-driven glycolysis induces vascular remodeling in PAH.
Methods: PFKFB3 levels were measured in pulmonary arteries of patients and animals with PAH. Lactate levels were assessed in lungs of animals with PAH and in pulmonary artery smooth muscle cells (PASMCs). Genetic and pharmacologic approaches were used to investigate the role of PFKFB3 in PAH.
Measurements and Main Results: Lactate production was elevated in lungs of PAH rodents and in platelet-derived growth factor–treated PASMCs. PFKFB3 protein was higher in pulmonary arteries of patients and rodents with PAH, in PASMCs of patients with PAH, and in platelet-derived growth factor–treated PASMCs. PFKFB3 inhibition by genetic disruption and chemical inhibitor attenuated phosphorylation/activation of extracellular signal–regulated kinase (ERK1/2) and calpain-2, and vascular remodeling in PAH rodent models, and reduced platelet-derived growth factor–induced phosphorylation/activation of ERK1/2 and calpain-2, collagen synthesis and proliferation of PASMCs. ERK1/2 inhibition attenuated phosphorylation/activation of calpain-2, and vascular remodeling in Sugen/hypoxia PAH rats, and reduced lactate-induced phosphorylation/activation of calpain-2, collagen synthesis, and proliferation of PASMCs. Calpain-2 inhibition reduced lactate-induced collagen synthesis and proliferation of PASMCs.
Conclusions: Upregulated PFKFB3 mediates collagen synthesis and proliferation of PASMCs, contributing to vascular remodeling in PAH. The mechanism is through the elevation of glycolysis and lactate that results in the activation of calpain by ERK1/2–dependent phosphorylation of calpain-2.
Keywords: glycolysis, platelet-derived growth factor, calpain, extracellular signal–regulated kinase, vascular smooth muscle
At a Glance Commentary
Scientific Knowledge on the Subject
Glycolytic shift is implicated in the pathogenesis of human and experimental pulmonary arterial hypertension. It remains unknown how glycolysis is increased and how increased glycolysis causes pulmonary vascular remodeling in pulmonary arterial hypertension.
What This Study Adds to the Field
We demonstrated, for the first time to our knowledge, that increased glycolysis driven by upregulated 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3) induces extracellular signal–regulated kinase (ERK)1/2 phosphorylation and calpain activation leading to collagen synthesis and proliferation of pulmonary artery smooth muscle cells in pulmonary arterial hypertension. The data from multidisciplinary approaches provide rationale for manipulating PFKFB3 in the treatment of pulmonary arterial hypertension.
A key feature of pulmonary arterial hypertension (PAH) is pulmonary vascular remodeling that is associated with accumulation of extracellular matrix, including collagen, and vascular smooth muscle cell (SMC) proliferation and hypertrophy, leading to thickening of pulmonary arterioles (1, 2). It has been shown that glycolytic shift is implicated in the pathogenesis of PAH (3–5). This metabolic shift, termed “the Warburg effect,” favors ATP production via glycolysis and lactate formation. Mitochondrial metabolism is abnormal in PAH (6–8). Increased 18F-labeled deoxyglucose imaging has been identified in the lungs of patients with idiopathic pulmonary arterial hypertension (IPAH), although the precise origin of the signal was unclear (8, 9). In glycolytic flux, one of the rate-limiting checkpoints is mediated by 6-phosphofructo-1 kinase (PFK-1) catalysis of fructose-6-phosphate (F6P) to fructose-1,6-bisphosphate (F1,6P2). PFK-1 is activated by its allosteric activator, fructose-2,6-bisphosphate (F2,6P2). F2,6P2 is synthesized by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB), isoform 3 (PFKFB3) in vascular cells and leukocytes and by other isoforms in hepatocytes (PFKFB1), cardiomyocytes (PFKFB2), and testis (PFKFB4) (10, 11). Enhanced expression of PFKFB3 results in an elevation of Fru-2,6-P2 leading to increased PFK-1 activity and consequently glycolytic flux (12, 13). Recent studies demonstrated that PFKFB3 plays a critical role in processes, such as proliferation, cell cycle progression, and apoptosis (14, 15). We hypothesize that glucose metabolic shift to glycolysis occurs proactively because of increased PFKFB3 function in proliferation and collagen synthesis of pulmonary arterial smooth muscle cells (PASMCs) in PAH.
Calpain belongs to the family of calcium-dependent, cytosolic, cysteine endopeptidases (16, 17). Two major isoforms, calpain-1 and -2, are composed of a distinct large subunit and a common small subunit (18, 19). Regulation of calpain occurs by such mechanisms as calcium binding; autolysis; phospholipid binding; post-translational modification; or release from the specific inhibitor, calpastatin (17). We reported that platelet-derived growth factor (PDGF) activates calpain in PASMCs, and inhibition of calpain prevents vascular remodeling in PAH (20, 21). Moreover, PAH mediators induce extracellular signal–regulated kinase (ERK1/2) activation that causes activation of calpain-2 in PASMCs from patients and animals with PAH (20). It is still not clear how ERK1/2 is activated in PASMCs in PAH.
In the present study, we demonstrate that enhanced expression of PFKFB3 and increased lactate induce ERK1/2 phosphorylation and calpain activation in PAH. We found that inhibition of PFKFB3 attenuated PDGF-induced calpain activation, collagen synthesis, and proliferation in PASMCs. Inhibition of ERK1/2 prevented calpain activation, collagen synthesis, and proliferation induced by lactate. More importantly, our data show that SMC-specific knockout of PFKFB3, a PFKFB3 inhibitor, and an ERK1/2 inhibitor attenuate PAH and vascular remodeling in PAH animal models. These observations indicate that PFKFB3 in PASMCs may be a novel target for PAH treatment.
Methods
Extended description of the materials and method is provided in the online supplement.
Human Tissues
Lung sections and PASMCs of unused donor and IPAH were provided by the pulmonary hypertension breakthrough initiative. The clinical information of these patients is shown in Table E1 in the online supplement. These patient cells are primary PASMCs from peripheral type-III pulmonary arteries (<1 mm in diameter). The cells were cultured and passaged in the same way as the Lonza PASMCs.
Rodent PAH Models
Mouse line of inducible SMC-specific knockout of PFKFB3 (PFKFB3flox/flox; SMMHC-CreER) were created. Hypoxic PAH mouse model, monocrotaline (MCT), and Sugen/hypoxia PAH rat model were used. All animal studies were performed using unbiased approaches as described previously (22, 23).
Echocardiography, Assessment of Pulmonary Hypertension, and Histologic Analysis
Transthoracic echocardiography was performed with a Visual Sonics Vevo 2100 ultrasound machine. Pulmonary artery acceleration time (PAAT), cardiac output (CO), and tricuspid annular plane systolic excursion were measured. Right ventricular systolic pressure (RVSP) was measured by inserting a 25-gauge needle connected to a pressure transducer into the right ventricle through the diaphragm. The Fulton index [right ventricle/(left ventricle + septum)] was calculated as a parameter of right ventricular hypertrophy. The lung sections were stained with hematoxylin and eosin and analyzed for pulmonary arterial wall thickness.
Cell Experiments
Primary human PASMCs were purchased from Lonza, Inc. (#CC-2581) and cultured in smooth muscle growth medium-2 (SmGM-2; Lonza). Lactate level, calpain activity, cell proliferation, apoptosis, and phosphorylation of ERK1/2 and calpain-2 were analyzed.
Immunofluorescence Confocal Microscopy and Western Blot Analysis
Lung tissue slides from PAH animal models and from normal patients and patients with PAH were double stained for PFKFB3/α-actin, p-ERK1/2/α-actin, p-calpain-2/α-actin, spectrin breakdown product (SBDP)/α-actin, collagen-I/α-actin, and Ki67/α-actin and were examined using a Zeiss LSM 510 confocal microscope. Western blot was performed to determine the protein levels of PFKFB3, calpain-1, calpain-2, collagen-I, calpastatin, p-ERK1/2, and total ERK1/2.
Study Approval
Animal experiments were performed under protocols approved by the Augusta University Institutional Animal Care and Use Committee. Waiver of informed consent of human lung samples was approved by the Augusta University Institutional Review Board.
Statistical Analysis
Results are shown as means ± SE for n experiments. One-way ANOVA and two-tailed Student’s t test analysis was used to determine the significance of differences between groups. A value of P less than 0.05 was considered significant.
Results
Higher Levels of PFKFB3 Are Expressed in PASMCs of Patients with IPAH and PAH Rodents
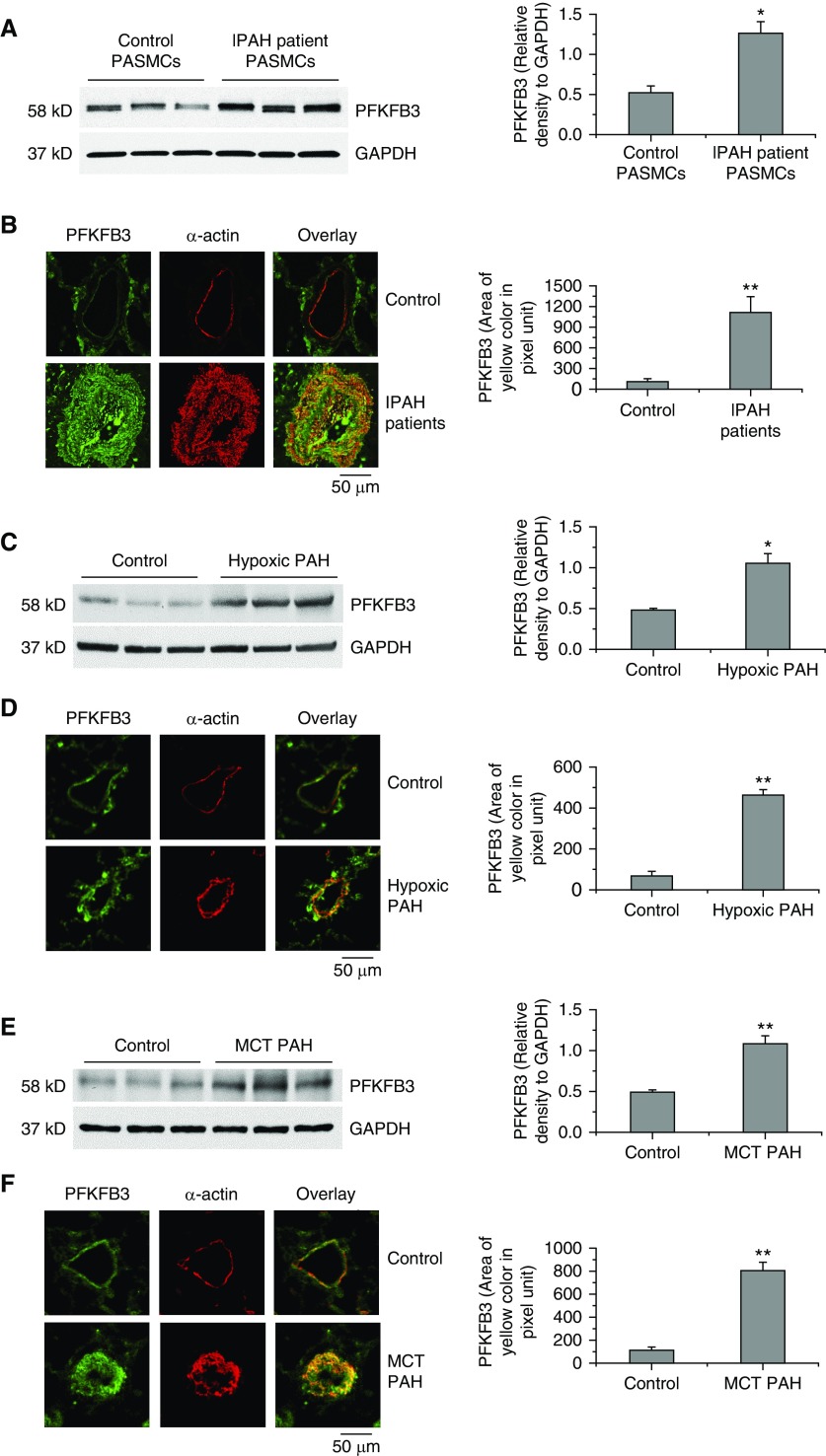
To determine the role of PFKFB3 in PAH, we determined protein levels of PFKFB3 in PASMCs of patients with IPAH and hypoxic PAH mice and MCT PAH rats. PFKFB3 protein levels were much higher in PASMC homogenates of PAH patients (Figure 1A) and in lung homogenates of hypoxic PAH mice and MCT rats (Figures 1C and 1E). Moreover, PASMCs of patients with IPAH and of hypoxic PAH mice and MCT rats contained higher levels of PFKFB3 (Figures 1B, 1D, and 1F).
Figure 1.
Higher levels of PFKFB3 are expressed in pulmonary artery smooth muscle cells (PASMCs) of patients with idiopathic pulmonary arterial hypertension (IPAH) and hypoxic pulmonary arterial hypertension (PAH) mice and monocrotaline (MCT) PAH rats. (A, C, and E) Protein levels of PFKFB3 in PASMCs of control subjects and patients with IPAH and the lung homogenates of control and hypoxic PAH mice and control and MCT PAH rats were measured by Western blot. The blots are representative immunoblots of PFKFB3 from six experiments. (B, D, and F) Lung slides of human, mouse, and rat were double stained for PFKFB3 (green) and α-actin (red). The images are representative of lung tissues from control subjects and patients with PAH (B), from control mice and hypoxic mice (D), and from control rats and MCT PAH rats (F). Results are expressed as mean ± SE; n = 6. *P < 0.05 and **P < 0.01 versus control (normal). PFKFB3 = 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3.
SMC-Specific Knockout of PFKFB3 Attenuates Hypoxia-induced PAH
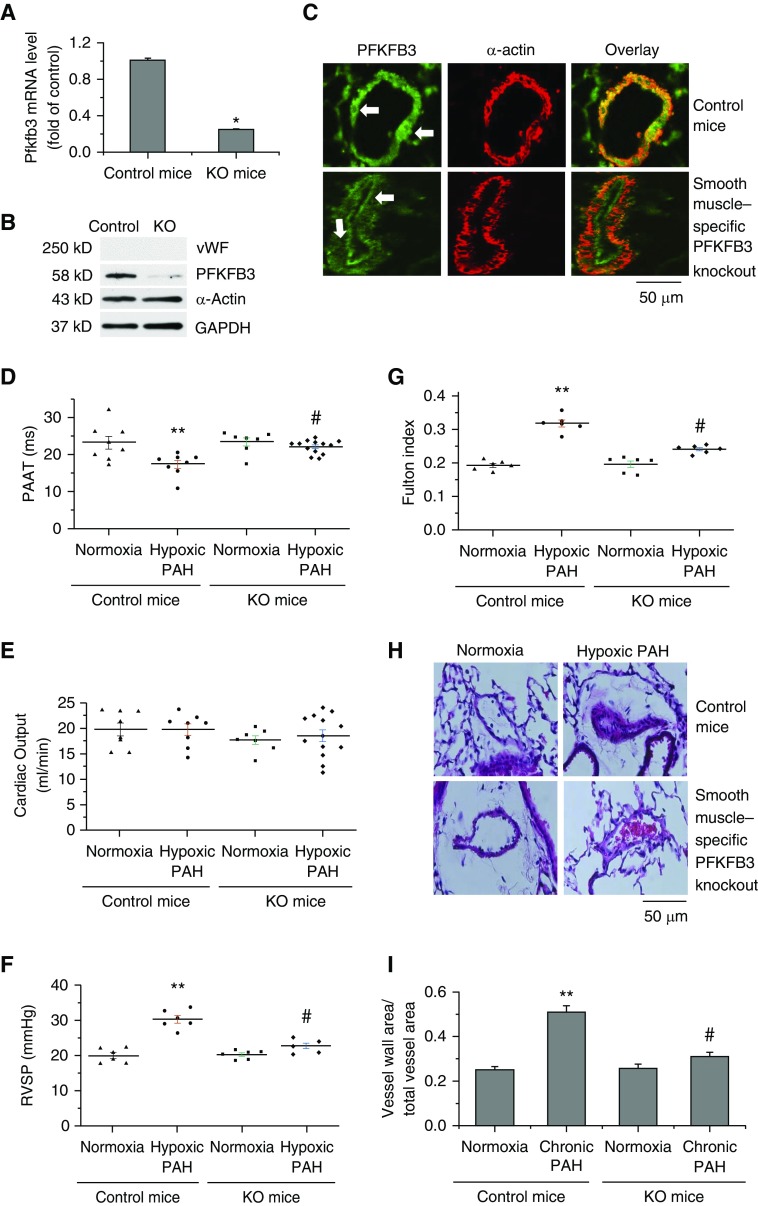
PFKFB3 in PASMCs was inhibited in a mouse line of SMC-specific knockout of PFKFB3. The lungs from PFKFB3flox/flox; SMMHC-CreER mice, compared with control mice, exhibit lower PFKFB3 mRNA and protein levels in PASMCs (Figures 2A–2C), indicating that PFKFB3 was successfully knocked out specifically in PASMCs. Echocardiography showed that PAAT was shorter in hypoxic PAH mice than normoxic mice and SMC-specific knockout of PFKFB3 attenuated decreases in PAAT induced by hypoxia (Figure 2D). There was no significant difference in CO between the two groups of mice under normoxia or hypoxia (Figure 2E). RVSP and Fulton index were higher in hypoxic mice than normoxic mice. However, RVSP and Fulton index were comparable between PFKFB3flox/flox; SMMHC-CreER mice exposed to normoxia and those exposed to hypoxia (Figures 2F and 2G). Moreover, hypoxia-induced increases in pulmonary artery wall thickness, collagen-I protein, and SMC proliferation and decreases in SMC apoptosis in pulmonary arterioles was attenuated in PFKFB3flox/flox; SMMHC-CreER mice (Figures 2H and 2I; see Figures E1 and E2). Together, these results show that knockout of PFKFB3 specifically in PASMCs attenuates hypoxia-induced PAH and vascular remodeling.
Figure 2.
Smooth muscle–specific knockout of PFKFB3 attenuates chronic hypoxia–induced pulmonary arterial hypertension (PAH). Five days after a regimen of tamoxifen administration, male control mice and male PFKFB3flox/flox; SMMHC-CreER mice were exposed to room air (normoxia) or 10% oxygen (hypoxic PAH) for 4 weeks. (A and B) Pulmonary arteries (<1 mm in diameter) were isolated from lungs of PFKFB3flox/flox; SMMHC-CreER mice and control mice under a dissect microscope and were denuded of surrounding tissues and endothelium. Total RNA and protein were extracted for QRT-PCR and Western blot analysis of PFKFB3 mRNA and protein levels. (C) Lung slides from PFKFB3flox/flox; SMMHC-CreER mice and control mice were double stained for α-actin (red) and PFKFB3 (green, white arrows). (D and E) Echocardiography showing pulmonary artery acceleration time and cardiac output. (F) Changes in right ventricular systolic pressure. (G) Changes in Fulton index [right ventricle/(left ventricle + septum)]. (H) Representative images of lung sections of control mice and PFKFB3flox/flox; SMMHC-CreER mice exposed to normoxia or hypoxia. (I) Changes in ratio of vascular wall area to total vessel area in the lung sections of control mice and PFKFB3flox/flox; SMMHC-CreER mice, exposed to normoxia or hypoxia. Results are expressed as mean ± SE; n = 6 experiments. *P < 0.05 versus control mice, **P < 0.05 versus normoxia, and #P < 0.05 versus hypoxic pulmonary arterial hypertension group of control mice. KO = knockout; PAAT = pulmonary artery acceleration time; PFKFB3 = 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3; RVSP = right ventricular systolic pressure; vWF = von Willebrand factor.
PFKFB3 Inhibitor 3PO Attenuates Progression of MCT-induced PAH in Rats
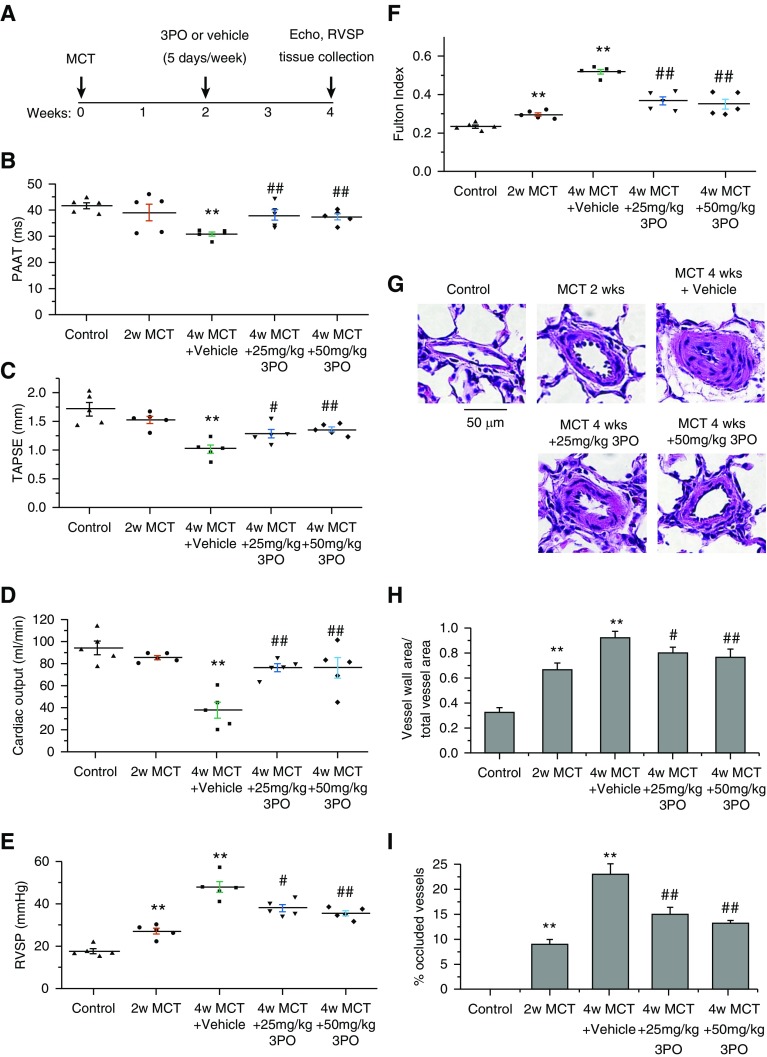
PFKFB3 inhibitor 3PO was given 2 weeks after MCT injection. In echocardiography, 3PO in two doses attenuated decreases in PAAT, tricuspid annular plane systolic excursion, and CO in MCT PAH rats (Figures 3B–3D), suggesting inhibition of PFKFB3 reduces pulmonary hypertension and improves right ventricular function in MCT-induced PAH. The increases in RVSP, Fulton index, pulmonary artery wall thickness, numbers of occluded vessels, collagen-I, and SMC proliferation and decreases in SMC apoptosis in pulmonary arterioles were attenuated in 3PO-treated MCT PAH rats (Figures 3E–3I, E3, and E4).
Figure 3.
The PFKFB3 inhibitor 3PO suppresses monocrotaline (MCT)-induced pulmonary arterial hypertension in rats. Male Sprague-Dawley rats 8 weeks of age were injected subcutaneously without or with MCT (60 mg/kg). After 2 weeks, control rats and MCT-injected rats (MCT 2 wk) were subjected to echocardiography and determination of pulmonary hypertension and pulmonary vascular remodeling. At the same time (the beginning of the third week), another group of MCT-injected rats started to receive the PFKFB3 inhibitor 3PO (intraperitoneally; MCT 4 wk + 25 mg/kg and MCT 4 wk + 50 mg/kg) or saline (MCT 4 wk + vehicle) once a day and 5 days per week. Echocardiography, pulmonary hypertension, and pulmonary vascular remodeling were assessed 2 weeks later (4 wk after MCT injection). (A) Protocol for the time course of the experiment. (B–D) Echocardiography showing pulmonary artery acceleration time, tricuspid annular plane systolic excursion, and cardiac output. (E) Changes in right ventricular systolic pressure. (F) Changes in Fulton index. (G) Representative images of lung sections of control rats and MCT pulmonary arterial hypertension rats treated with or without 3PO. (H) Changes in ratio of vascular wall area to total vessel area in the lung sections. (I) Percentage of occluded vessels. Results are expressed as mean ± SE; n = 5. **P < 0.01 versus control; #P < 0.05 and ##P < 0.01 versus 4 weeks MCT + vehicle group. PAAT = pulmonary artery acceleration time; PFKFB3 = 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3; RVSP = right ventricular systolic pressure; TAPSE = tricuspid annular plane systolic excursion.
PDGF Increases Protein Levels of PFKFB3 and Lactate Production in PASMCs, and Higher Lactate Levels in Lungs of PAH Rodents
Growth factors, such as PDGF (20, 21, 24) and glycolysis (25), are important elements in PAH pathogenesis. As shown in Figure E5A, incubation of PASMCs with PDGF-BB (5–30 ng/ml) for 24 hours caused increases in PFKFB3 protein levels. Moreover, the lactate concentration in the culture medium was significantly higher in PASMCs treated with PDGF (Figure E5B), indicating that PDGF induces an increase in glycolysis. We have previously reported that PDGF levels are elevated in lungs of PAH models (21). As shown in Figures E5C and E5D, lactate levels were remarkably higher in lungs of hypoxic PAH mice and MCT PAH rats, indicating that glycolysis is increased in PAH. In addition, another PAH mediator endothelin-1 (ET-1) also induced PFKFB3 expression in PASMCs (see Figures E6A and E6B).
Inhibition of PFKFB3 Attenuates PDGF-induced Collagen Synthesis and PASMC Proliferation and Decreases in PASMC Apoptosis
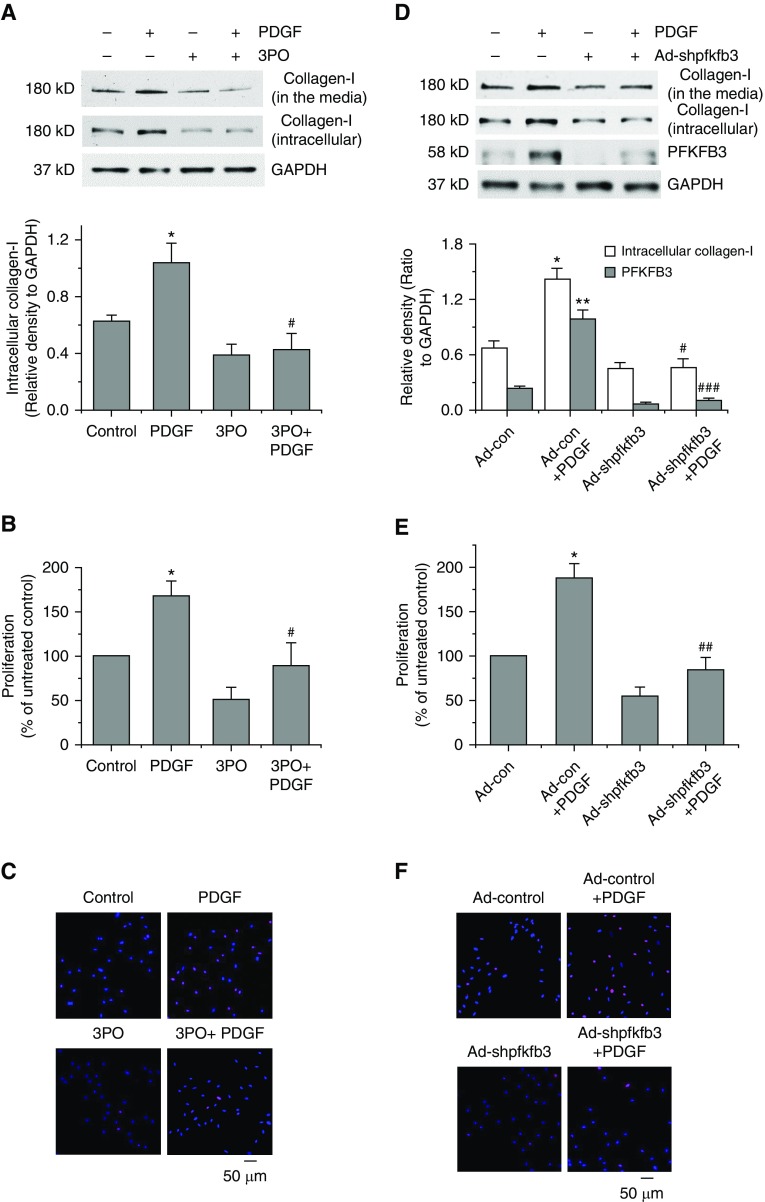
As shown in Figures 4A–4C and Figure E7A, incubation of PASMCs with PDGF for 24 hours caused increases in collagen-I protein levels and PASMC proliferation and decreases in PASMC apoptosis. PFKFB3 inhibitor 3PO significantly attenuated PDGF-induced increases in collagen-I protein levels and proliferation and decreases in apoptosis. Furthermore, transduction of PASMCs with PFKFB3 shRNA adenovirus markedly reduced PDGF-induced increases in collagen-I protein levels and PASMC proliferation (Figures 4D–4F), indicating that PFKFB3 plays an important role in PDGF-induced collagen synthesis and cell proliferation.
Figure 4.
Inhibition of PFKFB3 attenuates platelet-derived growth factor (PDGF)-induced collagen synthesis and proliferation of pulmonary artery smooth muscle cells (PASMCs). (A–C) PASMCs were incubated with PDGF-BB (10 ng/ml) in the presence and absence of PFKFB3 inhibitor 3PO (10 μM) for 24 hours, after which collagen-I protein levels in cell lysates and culture medium (A) and cell proliferation using BrdU assay (B) and Ki67 staining (C) were measured. (D–F) PASMCs were transduced with the PFKFB3 shRNA, adenovirus (10 pfu), and empty sham adenovirus (10 pfu). After 48 hours, PASMCs were incubated with PDGF-BB (10 ng/ml) for 24 hours, then protein levels of collagen-I and PFKFB3 (D) and cell proliferation using BrdU assay (E) and Ki67 staining (F) were measured. Results are expressed as mean ± SE; n = 4. *P < 0.05 and **P < 0.01 versus control; #P < 0.05, ##P < 0.01, and ###P < 0.001 versus PDGF. PFKFB3 = 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3.
Moreover, PFKFB3 inhibitor 3PO also attenuated ET-1–induced increases in collagen-I protein level and cell proliferation (Figures E6C and E6D).
Inhibition of PFKFB3 Attenuates Calpain Activation in Pulmonary Arteries in PAH and in PDGF-treated PASMCs
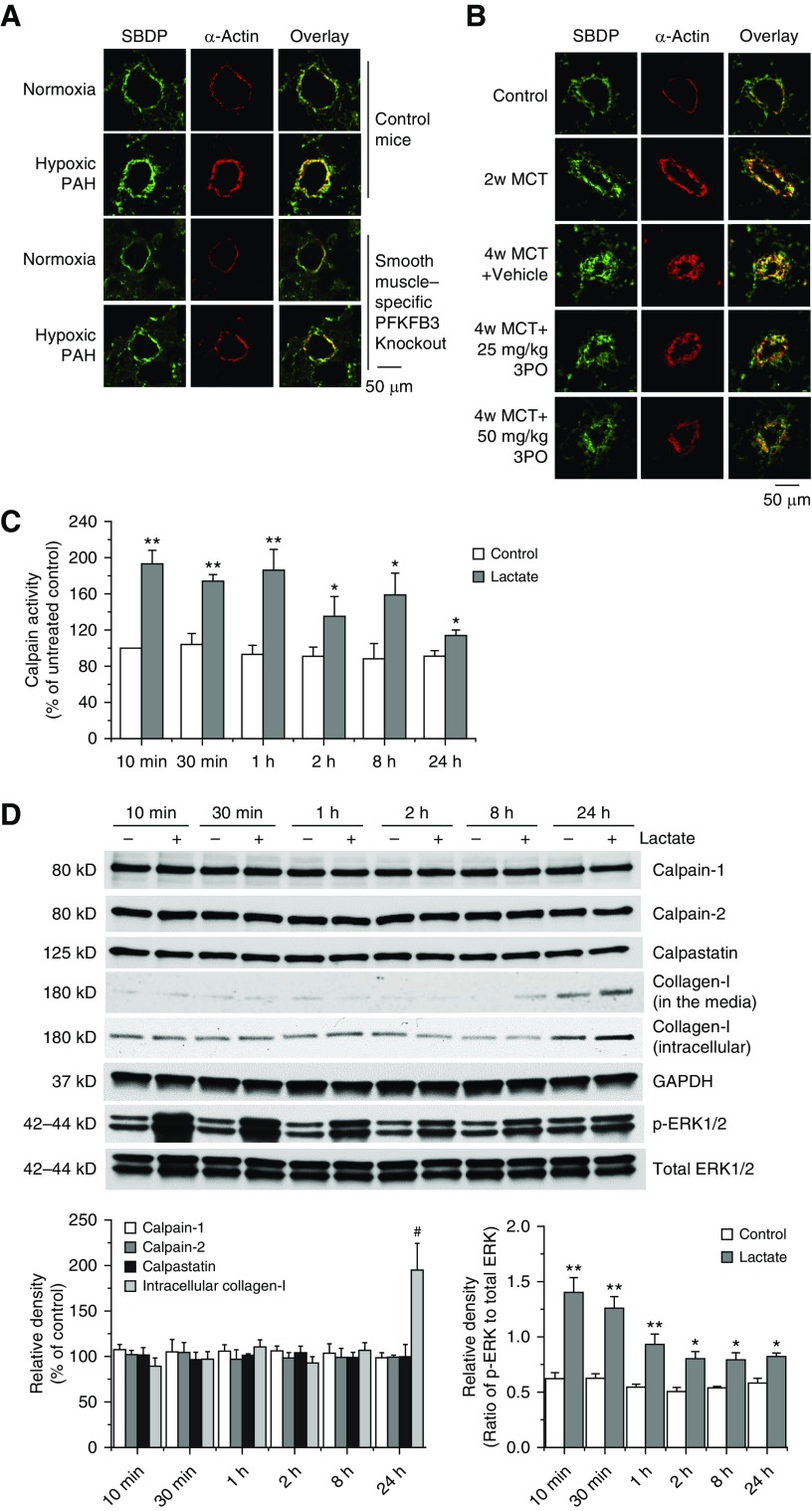
We have previously reported that calpain is activated in pulmonary arteries and causes increased collagen synthesis and PASMC proliferation in PAH (20, 21). To study whether increased glycolysis in PAH causes calpain activation, SBDP was measured in lungs of PAH rodents as described previously (21, 26, 27). As shown in Figures 5A and 5B, SBDP levels were significantly elevated in PASMCs in hypoxic mice and MCT PAH rats. SMC-specific PFKFB3 knockout and 3PO attenuated increases in SBDP levels in PASMCs in hypoxic mice and MCT PAH rats, suggesting that PFKFB3 causes calpain activation in hypoxia- and MCT-induced PAH. Similarly, PFKFB3 inhibitor 3PO prevented PDGF-induced increase in calpain activity in PASMCs (Figure E8A). Furthermore, overexpression of calpain-2 in PFKFB3-deficient PASMCs restores collagen synthesis and cell proliferation in PDGF-treated PASMCs (Figure E8B). These results indicate that calpain-2 is the downstream mediator of PFKFB3 signaling.
Figure 5.
Smooth muscle cell–specific knockout of PFKFB3 and the PFKFB3 inhibitor 3PO attenuates calpain activation in pulmonary arteries in hypoxia- and monocrotaline (MCT)-induced pulmonary arterial hypertension, and lactate increases calpain activity, ERK1/2 phosphorylation, and collagen levels in pulmonary artery smooth muscle cells (PASMCs). (A) Five days after a regimen of tamoxifen administration, control mice and PFKFB3flox/flox; SMMHC-CreER mice were exposed to room air (normoxia) or 10% oxygen (chronic hypoxia) for 4 weeks. Lung slides were double stained for α-actin (red) and spectrin breakdown product (SBDP) (green). n = 6. (B) The PFKFB3 inhibitor 3PO was given to rats 2 weeks after MCT injection. Lung slides were double stained for α-actin (red) and SBDP (green). n = 5. (C and D) PASMCs were incubated with sodium lactate (10 mM) for 10 minutes to 24 hours, then calpain activity (C) and protein levels of calpain-1, calpain-2, calpastatin, collagen-I, p-ERK1/2, and total ERK1/2 (D) were measured. Results are expressed as mean ± SE; n = 4. *P < 0.05 and **P < 0.01 versus control; #P < 0.05 versus 10 minutes. ERK1/2 = extracellular signal–regulated kinase 1/2; PAH = pulmonary arterial hypertension; PFKFB3 = 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3.
Lactate Increases Calpain Activity and ERK1/2 Phosphorylation in PASMCs
As shown in Figure 5C, incubation of PASMCs with sodium lactate (10 mM) for 10 minutes to 24 hours induced a rapid increase in calpain activity. The calpain activation was at peak at 10 minutes to 1 hour and lasted for at least 24 hours. The calpain activation was not accompanied with changes in the protein levels of calpain-1, calpain-2, and calpastatin (Figure 5D), suggesting that lactate stimulates calpain activity via post-translational mechanisms.
We have previously reported that ERK1/2 phosphorylates Ser-50 of calpain-2, which is crucial in PDGF-induced calpain activation (20). Interestingly, lactate induced ERK1/2 phosphorylation in the same temporal manner as calpain activation (Figure 5D), suggesting that ERK1/2 may mediate lactate-induced calpain activation.
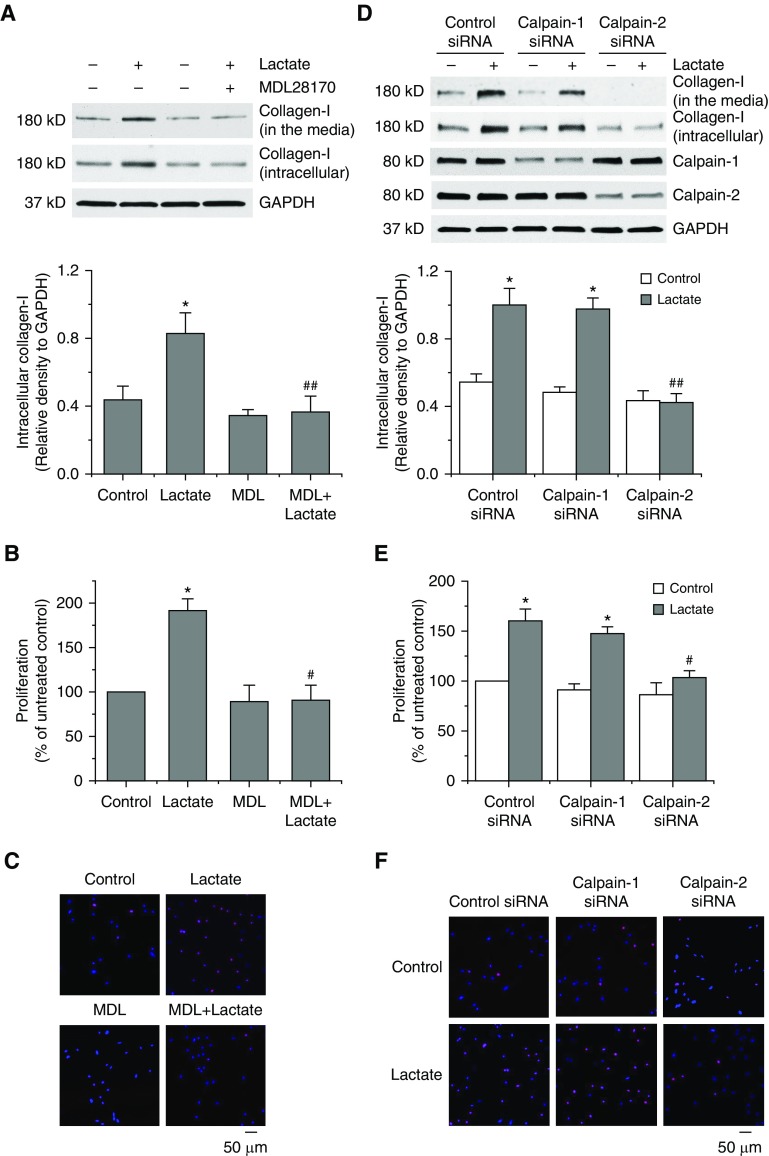
Inhibition of Calpain-2 Attenuates Lactate-induced Collagen Synthesis and PASMC Proliferation
Incubation of PASMCs with sodium lactate (10 mM) caused increases in collagen-I protein levels (Figure 5D). Moreover, calpain inhibitor MDL28170 prevented lactate-induced increases in collagen protein levels and proliferation and decreases in apoptosis (Figures 6A–6C and E7B). Furthermore, knocking down calpain-2, but not calpain-1, inhibited lactate-induced increases in collagen protein levels and proliferation of PASMCs (Figures 6D–6F). These data show that it is calpain-2, rather than calpain-1, that is responsible for lactate-induced collagen synthesis and PASMC proliferation.
Figure 6.
Inhibition of calpain-2 abolishes the lactate-induced collagen synthesis and proliferation of pulmonary artery smooth muscle cells (PASMCs). (A–C) PASMCs were incubated with sodium lactate (10 mM) in the presence and absence of calpain inhibitor MDL28170 (20 μM) for 24 hours, after which collagen-I protein levels in cell lysates and culture medium (A) and cell proliferation using BrdU assay (B) and Ki67 staining (C) were measured. (D–F) PASMCs were transfected with siRNAs against calpain-1, calpain-2, or control siRNA, respectively. After 72 hours, the cells were incubated with sodium lactate (10 mM) for 24 hours, then protein levels of collagen-I, calpain-1, and calpain-2 (D) and cell proliferation using BrdU assay (E) and Ki67 staining (F) were measured. Results are expressed as mean ± SE; n = 4. *P < 0.05 versus control; #P < 0.05 and ##P < 0.01 versus lactate. MDL = MDL28170.
Inhibition of ERK1/2 Attenuates Lactate-induced Increases in Calpain Activation, Collagen Synthesis, and PASMC Proliferation
Incubation of PASMCs with lactate (10 mM) caused remarkable increases in calpain activity, collagen-I protein levels, and cell proliferation (Figure E9). Moreover, PD98059 (10 μM) and knocking down of ERK1/2 significantly attenuated lactate-induced increases in calpain activity, collagen-I protein levels, and proliferation and decreases in PASMC apoptosis (Figures E7C and E9). These data confirm that ERK1/2 has an important role in lactate-induced calpain activation, collagen synthesis, and PASMC proliferation.
Inhibition of ERK1/2 Blocks Calpain-2 Phosphorylation at Serine 50 in Lactate-treated PASMCs
There are two major phosphorylation sites in calpain-2 (p-Ser50 and p-Ser369), which are altered by PAH mediators including PDGF in PASMCs (20). Incubation of PASMCs with lactate significantly increased ERK1/2 phosphorylation, which was inhibited by PD98059 (Figure E10A). Lactate and PD98059 did not affect the protein levels of calpain-2 (Figure E10A).
Furthermore, lactate caused increases in Ser50 phosphorylation and decreases in p-Ser369 levels in PASMCs (Figure E10B). In addition, PD98059 prevented a lactate-induced increase in phosphorylation of calpain-2 at Ser50, and did not influence decreases in Ser369 phosphorylation induced by lactate (Figure E10B). Collectively, these results indicate that lactate induces calpain activation via ERK1/2-mediated phosphorylation of calpain-2 at Ser50.
SMC-Specific Knockout of PFKFB3 and PFKFB3 Inhibitor 3PO Attenuate ERK1/2 Phosphorylation and Calpain-2 Phosphorylation at Ser50 in PAH Models
The protein levels of p-ERK1/2 and p-Ser50-calpain-2 were significantly elevated in PASMCs in mice with hypoxia and MCT PAH rats and lung homogenates of MCT PAH rats (Figures E11–E13). SMC-specific PFKFB3 knockout attenuated increases in the protein levels of p-ERK1/2 and p-Ser50-calpain-2 in PASMCs in mice with hypoxia and MCT PAH rats, indicating that PFKFB3-mediated glycolysis causes the phosphorylation and activation of ERK1/2 and calpain in PAH.
Inhibition of ERK1/2 Attenuates Progression of Pulmonary Hypertension and Vascular Remodeling in Sugen/Hypoxia PAH Rat Model
In echocardiography, ERK1/2 inhibitor PD98059 attenuated decreases in PAAT and CO in Sugen/hypoxia rats (Figure E14), suggesting ERK1/2 inhibition reduces pulmonary hypertension and improves right ventricular function. The protein levels of p-ERK1/2 were elevated in PASMCs and in lung homogenates of Sugen/hypoxia rats (Figure E15). More importantly, ERK1/2 inhibitor PD98059 significantly reduced increases in ERK1/2 phosphorylation (Figure E15) and in RVSP, Fulton index, pulmonary artery wall thickness, number of occluded vessels, and SMC proliferation and decreases in SMC apoptosis in pulmonary arterioles in Sugen/hypoxia rats (Figures E14 and E16). Furthermore, the protein levels of p-Ser50-calpain-2, SBDP, and collagen-I were much higher in PASMCs and in lung homogenates of Sugen/hypoxia rats than control rats (Figures E17 and E18). PD98059 markedly reduced increases in the protein levels of p-Ser50-calpain-2, SBDP, and collagen-I in PASMCs in Sugen/hypoxia rats (Figures E17 and E18). Together, these findings indicate that the ERK1/2 inhibition attenuates the phosphorylation and activation of calpain-2 and pulmonary hypertension as well as vascular remodeling.
Discussion
A growing body of evidence indicates that a glycolytic shift contributes to increased proliferation and extracellular matrix production of PASMCs, leading to pulmonary vascular remodeling in PAH (3, 28). In the present study we demonstrated, for the first time to our knowledge, that PFKFB3 plays an important role in glycolysis-mediated collagen synthesis and proliferation of PASMCs via increased ERK1/2-stimulated calpain activation.
We found that PASMCs of patients with IPAH and PAH rodents contain higher levels of PFKFB3. It has been shown that PFKFB3 levels are elevated in cancer (29), and that PFKFB3 contributes to hyperproliferation of cancerous cells (29, 30) and participates in glycolysis-associated angiogenesis (15, 31). To study the role of PFKFB3 in vascular remodeling in PAH, a hypoxic mouse model and an MCT-induced rat model were used. Knockout of PFKFB3 specifically in SMCs attenuated hypoxia-induced PAH, PASMC proliferation, and pulmonary vascular remodeling. PFKFB3 inhibitor 3PO inhibited increases in RVSP, PASMC proliferation, collagen deposition, and pulmonary artery wall thickness, and right ventricular hypertrophy in an established PAH model. These data provide the first evidence showing that increased PFKFB3 in PASMCs mediates vascular remodeling in PAH.
Upregulated PDGF signaling is associated with PAH (24, 32), and the PDGF receptor antagonist STI571 reduced and reversed pulmonary hypertension in PAH animal models (24). In response to several stimuli, such as hypoxia, PFKFB3 expression is markedly increased in several cell lines maintaining high glycolytic rates (33). Elevated energy generation by glycolysis contributes to increased proliferation and survival of PASMCs in PAH (34). Our present work here identified a novel link from PAH mediators, such as PDGF and ET-1, through PFKFB3 to the elevated collagen synthesis and proliferation of PASMCs. We found that the PAH mediator PDGF and ET-1 significantly increased PFKFB3 protein level in PASMCs. We recently reported that PDGF- and ET-1–stimulated collagen synthesis and proliferation of PASMCs occurs through calpain activation (20, 21). Here, our data revealed that specific PFKFB3 inhibitor 3PO prevented PDGF-induced increases in calpain activity. In addition, 3PO also abolished increases in collagen synthesis, cell proliferation, and decreases in PASMC apoptosis induced by PDGF and ET-1. Furthermore, knockdown of PFKFB3 by PFKFB3 shRNA adenovirus remarkably suppressed PDGF-induced calpain activity, collagen synthesis, and PASMC proliferation. SMC-specific knockout of PFKFB3 and PFKFB3 inhibitor 3PO attenuates calpain activation in PASMCs in PAH animal models. Collectively, these findings indicate that PFKFB3 governs PDGF- and ET-1–induced signaling pathway via calpain activation in PASMCs.
Elevation in PFKFB3 protein levels in PDGF-treated PASMCs and in the lungs of PAH rodents are associated with increased lactate levels. We provide evidence, for the first time, claiming that PDGF upregulates PFKFB3 expression leading to increased glycolysis and elevation in lactate production in PASMCs in PAH. Increased vascular SMC proliferation and collagen synthesis are characterized by enhanced glycolysis and high lactate levels in vascular remodeling in PAH (35, 36). The underlying mechanism in which lactate induces remodeling of the small pulmonary arteries is not known. Our data show that lactate causes remarkable elevation in calpain activation in PASMCs. Inhibition of calpain prevented lactate-induced collagen synthesis and cell proliferation and decreases in apoptosis of PASMCs. Moreover, our data revealed that calpain-2 rather than calpain-1 is responsible for the increased calpain activation, collagen production, and PASMC proliferation evoked by lactate. These findings are in agreement with our previous report that calpain-2 mediates collagen synthesis and proliferation of PASMCs in PAH (20, 37, 38).
Interestingly, protein levels of calpain-1, calpain-2, and calpastatin were not changed in lactate-challenged PASMCs, indicating that lactate-stimulated calpain activation is caused by post-translational modification of calpain-2. Phosphorylation is one of the most critical post-translational modifications that alter calpain activation (39, 40). Epidermal growth factor initiates serine 50 phosphorylation and activation of calpain-2 by ERK1/2 in the absence of calcium (41). Protein kinase A-mediated phosphorylation of Ser369/370 inhibits epidermal growth factor–induced calpain activation (42). We have recently reported that the PAH mediator PDGF induces changes in the phosphorylation of calpain-2 via ERK1/2 and PP2A leading to calpain activation and increased PASMC proliferation in PAH (20). We also found that higher levels of phospho-Ser50-calpain-2 and lower levels of phospho-Ser369-calpain-2 in PASMCs in patients with PAH and animal models were correlated with higher calpain activities (20).
In the present study, we found significant increases in ERK1/2 phosphorylation and calpain activation in lactate-treated PASMCs. The increased calpain activation was correlated with enhanced phosphorylation/activation of ERK1/2. More importantly, ERK1/2 inhibition reduced lactate-induced calpain activation, collagen synthesis, and proliferation and decreases in PASMC apoptosis. Furthermore, we detected elevation in the Ser50 phosphorylation of calpain-2 and reduction in the Ser369 phosphorylation of calpain-2 in PASMCs treated with lactate. Our results also indicate that the ERK1/2 inhibitor PD98059 attenuates lactate-induced phosphorylation/activation of ERK1/2 and phosphorylation of calpain-2 at Ser50 residue. SMC-specific knockout of PFKFB3 and PFKFB3 inhibitor 3PO reduces the increases in ERK1/2 phosphorylation and calpain-2 phosphorylation in PASMCs in PAH rodent lungs. Together, these data show that increased PFKFB3 causes elevation of glycolysis and lactate level, which induces calpain activation via ERK1/2–dependent Ser50 phosphorylation of calpain-2 in PASMCs.
We previously reported that calpain inhibition attenuates pulmonary vascular remodeling in PAH (20, 21). ERK1/2 phosphorylation is increased in PASMCs in a broiler PAH model (43). It is not clear whether and how ERK1/2 participates in PAH. Our data show that ERK1/2 inhibition attenuated pulmonary hypertension and thickening of the SMC layer of pulmonary arterioles in the lungs of rats in Sugen/hypoxia PAH model. Moreover, ERK1/2 inhibition prevented phosphorylation of calpain-2 at Ser50, calpain activation, collagen synthesis, and cell proliferation in PASMCs in Sugen/hypoxia rats. These findings provide solid evidence confirming that ERK1/2 stimulates calpain activation and plays a critical role in vascular remodeling in PAH. Notably, ERK activity is decreased in right ventricular failure (44). Our data showed that the ERK inhibitor PD98059 improved RVSP, PAAT, and CO in Sugen/hypoxia PAH model, suggesting that right ventricular dysfunction in PAH is primarily caused by increased pulmonary arterial pressure.
PFKFB3 is located downstream of hexokinase in glycolytic flux. Inhibition of hexokinase did not reduce but increased PFKFB3 protein level in PASMCs (Figure E19), indicating that PFKFB3 is a separate glycolysis regulator. However, hypoxia-inducible factor (HIF) is an important transcriptional regulator of the hypoxic response and plays a crucial role in glycolytic shift (45) and PAH (46). Hypoxia upregulates PFKFB3 expression through HIF1α (33, 47). We found that HIF1α inhibitor reduced PDGF- and hypoxia-induced PFKFB3 expression (Figure E20). Thus, the role of HIF1α in glycolytic shift and PAH may be achieved through PFKFB3.
Besides PASMCs, pulmonary endothelial cells also participate in the pathogenesis of PAH. Indeed, PFKFB3 levels are also increased in pulmonary artery endothelium in Sugen/hypoxia PAH rats (Figure E21). Notably, endothelial PFKFB3 plays a critical role in angiogenesis (15). Thus, PFKFB3 in lung endothelial cells may also contribute to the development of PAH with their unique mechanisms. Further studies are needed on the role of PFKFB3 in these lung cells in PAH.
In conclusion, we have demonstrated that upregulated PFKFB3 mediates cell proliferation and collagen synthesis in SMCs of pulmonary arteries, contributing to vascular remodeling in PAH. The proposed mechanism (Figure E22) is through the elevation of glycolysis and lactate that results in calpain activation by ERK1/2–dependent phosphorylation of calpain-2 at Ser50. PFKFB3 may be a novel target for PAH treatment.
Supplementary Material
Footnotes
Supported by NIH/NHLBI grant R01 HL134934 (Y.S. and Y.H.), VA Merit Review Award BX002035 (Y.S.), Flight Attendants Medical Research Institute grant 140083_CIA (Y.S.), and American Heart Association Postdoctoral Fellowships 16POST27730023 (L.K.) and 18POST33990193 (A.K.-K.) and Career Development Award 18CDA34110225 (L.K.).
Author Contributions: Conception, design, and experiment: L.K., Y.C., W.H., L.M., A.K.-K., D.K., and Y.S. Analysis and interpretation: L.K., Y.C., W.H., and Y.S. Drafting the manuscript for important intellectual content: L.K., Y.C., A.D.V., S.A.B., Z.D., Y.H., and Y.S.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201812-2290OC on February 28, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Tuder RM. Pathology of pulmonary arterial hypertension. Semin Respir Crit Care Med. 2009;30:376–385. doi: 10.1055/s-0029-1233307. [DOI] [PubMed] [Google Scholar]
- 2.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2008;118:2372–2379. doi: 10.1172/JCI33452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuder RM, Davis LA, Graham BB. Targeting energetic metabolism: a new frontier in the pathogenesis and treatment of pulmonary hypertension. Am J Respir Crit Care Med. 2012;185:260–266. doi: 10.1164/rccm.201108-1536PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thébaud B, Bonnet S, et al. An abnormal mitochondrial-hypoxia inducible factor-1alpha-Kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: similarities to human pulmonary arterial hypertension. Circulation. 2006;113:2630–2641. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 5.Fijalkowska I, Xu W, Comhair SA, Janocha AJ, Mavrakis LA, Krishnamachary B, et al. Hypoxia inducible-factor1alpha regulates the metabolic shift of pulmonary hypertensive endothelial cells. Am J Pathol. 2010;176:1130–1138. doi: 10.2353/ajpath.2010.090832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutendra G, Michelakis ED. The metabolic basis of pulmonary arterial hypertension. Cell Metab. 2014;19:558–573. doi: 10.1016/j.cmet.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Archer SL, Fang YH, Ryan JJ, Piao L. Metabolism and bioenergetics in the right ventricle and pulmonary vasculature in pulmonary hypertension. Pulm Circ. 2013;3:144–152. doi: 10.4103/2045-8932.109960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu W, Koeck T, Lara AR, Neumann D, DiFilippo FP, Koo M, et al. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc Natl Acad Sci USA. 2007;104:1342–1347. doi: 10.1073/pnas.0605080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao L, Ashek A, Wang L, Fang W, Dabral S, Dubois O, et al. Heterogeneity in lung (18)FDG uptake in pulmonary arterial hypertension: potential of dynamic (18)FDG positron emission tomography with kinetic analysis as a bridging biomarker for pulmonary vascular remodeling targeted treatments. Circulation. 2013;128:1214–1224. doi: 10.1161/CIRCULATIONAHA.113.004136. [DOI] [PubMed] [Google Scholar]
- 10.Rider MH, Bertrand L, Vertommen D, Michels PA, Rousseau GG, Hue L. 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis. Biochem J. 2004;381:561–579. doi: 10.1042/BJ20040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ros S, Schulze A. Balancing glycolytic flux: the role of 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatases in cancer metabolism. Cancer Metab. 2013;1:8. doi: 10.1186/2049-3002-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atsumi T, Chesney J, Metz C, Leng L, Donnelly S, Makita Z, et al. High expression of inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (iPFK-2; PFKFB3) in human cancers. Cancer Res. 2002;62:5881–5887. [PubMed] [Google Scholar]
- 13.Huo Y, Guo X, Li H, Wang H, Zhang W, Wang Y, et al. Disruption of inducible 6-phosphofructo-2-kinase ameliorates diet-induced adiposity but exacerbates systemic insulin resistance and adipose tissue inflammatory response. J Biol Chem. 2010;285:3713–3721. doi: 10.1074/jbc.M109.058446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yalcin A, Telang S, Clem B, Chesney J. Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. Exp Mol Pathol. 2009;86:174–179. doi: 10.1016/j.yexmp.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, An X, Guo X, Habtetsion TG, Wang Y, Xu X, et al. Endothelial PFKFB3 plays a critical role in angiogenesis. Arterioscler Thromb Vasc Biol. 2014;34:1231–1239. doi: 10.1161/ATVBAHA.113.303041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovacs L, Su Y. The critical role of calpain in cell proliferation. J Biomol Res Ther. 2014;3:112–119. doi: 10.4172/2167-7956.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki K, Sorimachi H, Yoshizawa T, Kinbara K, Ishiura S. Calpain: novel family members, activation, and physiologic function. Biol Chem Hoppe Seyler. 1995;376:523–529. doi: 10.1515/bchm3.1995.376.9.523. [DOI] [PubMed] [Google Scholar]
- 19.Sorimachi H, Suzuki K. The structure of calpain. J Biochem. 2001;129:653–664. doi: 10.1093/oxfordjournals.jbchem.a002903. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs L, Han W, Rafikov R, Bagi Z, Offermanns S, Saido TC, et al. Activation of calpain-2 by mediators in pulmonary vascular remodeling of pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2016;54:384–393. doi: 10.1165/rcmb.2015-0151OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma W, Han W, Greer PA, Tuder RM, Toque HA, Wang KKW, et al. Calpain mediates pulmonary vascular remodeling in rodent models of pulmonary hypertension, and its inhibition attenuates pathologic features of disease. J Clin Invest. 2011;121:4548–4566. doi: 10.1172/JCI57734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonnet S, Provencher S, Guignabert C, Perros F, Boucherat O, Schermuly RT, et al. Translating research into improved patient care in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2017;195:583–595. doi: 10.1164/rccm.201607-1515PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Provencher S, Archer SL, Ramirez FD, Hibbert B, Paulin R, Boucherat O, et al. Standards and methodological rigor in pulmonary arterial hypertension preclinical and translational research. Circ Res. 2018;122:1021–1032. doi: 10.1161/CIRCRESAHA.117.312579. [DOI] [PubMed] [Google Scholar]
- 24.Schermuly RT, Dony E, Ghofrani HA, Pullamsetti S, Savai R, Roth M, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115:2811–2821. doi: 10.1172/JCI24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan JJ, Archer SL. Emerging concepts in the molecular basis of pulmonary arterial hypertension: part I. Metabolic plasticity and mitochondrial dynamics in the pulmonary circulation and right ventricle in pulmonary arterial hypertension. Circulation. 2015;131:1691–1702. doi: 10.1161/CIRCULATIONAHA.114.006979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glantz SB, Cianci CD, Iyer R, Pradhan D, Wang KK, Morrow JS. Sequential degradation of alphaII and betaII spectrin by calpain in glutamate or maitotoxin-stimulated cells. Biochemistry. 2007;46:502–513. doi: 10.1021/bi061504y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida K, Inui M, Harada K, Saido TC, Sorimachi Y, Ishihara T, et al. Reperfusion of rat heart after brief ischemia induces proteolysis of calspectin (nonerythroid spectrin or fodrin) by calpain. Circ Res. 1995;77:603–610. doi: 10.1161/01.res.77.3.603. [DOI] [PubMed] [Google Scholar]
- 28.Marsboom G, Wietholt C, Haney CR, Toth PT, Ryan JJ, Morrow E, et al. Lung 18F-fluorodeoxyglucose positron emission tomography for diagnosis and monitoring of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;185:670–679. doi: 10.1164/rccm.201108-1562OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novellasdemunt L, Obach M, Millán-Ariño L, Manzano A, Ventura F, Rosa JL, et al. Progestins activate 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3) in breast cancer cells. Biochem J. 2012;442:345–356. doi: 10.1042/BJ20111418. [DOI] [PubMed] [Google Scholar]
- 30.Clem BF, O’Neal J, Tapolsky G, Clem AL, Imbert-Fernandez Y, Kerr DA, II, et al. Targeting 6-phosphofructo-2-kinase (PFKFB3) as a therapeutic strategy against cancer. Mol Cancer Ther. 2013;12:1461–1470. doi: 10.1158/1535-7163.MCT-13-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154:651–663. doi: 10.1016/j.cell.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 32.Barst RJ. PDGF signaling in pulmonary arterial hypertension. J Clin Invest. 2005;115:2691–2694. doi: 10.1172/JCI26593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Obach M, Navarro-Sabaté A, Caro J, Kong X, Duran J, Gómez M, et al. 6-Phosphofructo-2-kinase (pfkfb3) gene promoter contains hypoxia-inducible factor-1 binding sites necessary for transactivation in response to hypoxia. J Biol Chem. 2004;279:53562–53570. doi: 10.1074/jbc.M406096200. [DOI] [PubMed] [Google Scholar]
- 34.Goncharov DA, Kudryashova TV, Ziai H, Ihida-Stansbury K, DeLisser H, Krymskaya VP, et al. Mammalian target of rapamycin complex 2 (mTORC2) coordinates pulmonary artery smooth muscle cell metabolism, proliferation, and survival in pulmonary arterial hypertension. Circulation. 2014;129:864–874. doi: 10.1161/CIRCULATIONAHA.113.004581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paulin R, Michelakis ED. The metabolic theory of pulmonary arterial hypertension. Circ Res. 2014;115:148–164. doi: 10.1161/CIRCRESAHA.115.301130. [DOI] [PubMed] [Google Scholar]
- 36.Werle M, Kreuzer J, Höfele J, Elsässer A, Ackermann C, Katus HA, et al. Metabolic control analysis of the Warburg-effect in proliferating vascular smooth muscle cells. J Biomed Sci. 2005;12:827–834. doi: 10.1007/s11373-005-9010-5. [DOI] [PubMed] [Google Scholar]
- 37.Cai P, Kovacs L, Dong S, Wu G, Su Y. BMP4 inhibits PDGF-induced proliferation and collagen synthesis via PKA-mediated inhibition of calpain-2 in pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2017;312:L638–L648. doi: 10.1152/ajplung.00260.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abeyrathna P, Kovacs L, Han W, Su Y. Calpain-2 activates Akt via TGF-β1-mTORC2 pathway in pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol. 2016;311:C24–C34. doi: 10.1152/ajpcell.00295.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kovács L, Alexa A, Klement E, Kókai E, Tantos A, Gógl G, et al. Regulation of calpain B from Drosophila melanogaster by phosphorylation. FEBS J. 2009;276:4959–4972. doi: 10.1111/j.1742-4658.2009.07198.x. [DOI] [PubMed] [Google Scholar]
- 40.Zadran S, Jourdi H, Rostamiani K, Qin Q, Bi X, Baudry M. Brain-derived neurotrophic factor and epidermal growth factor activate neuronal m-calpain via mitogen-activated protein kinase-dependent phosphorylation. J Neurosci. 2010;30:1086–1095. doi: 10.1523/JNEUROSCI.5120-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glading A, Bodnar RJ, Reynolds IJ, Shiraha H, Satish L, Potter DA, et al. Epidermal growth factor activates m-calpain (calpain II), at least in part, by extracellular signal-regulated kinase-mediated phosphorylation. Mol Cell Biol. 2004;24:2499–2512. doi: 10.1128/MCB.24.6.2499-2512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiraha H, Glading A, Chou J, Jia Z, Wells A. Activation of m-calpain (calpain II) by epidermal growth factor is limited by protein kinase A phosphorylation of m-calpain. Mol Cell Biol. 2002;22:2716–2727. doi: 10.1128/MCB.22.8.2716-2727.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H, Wang Y, Chen L, Han L, Li L, He H, et al. The role of MIF, cyclinD1 and ERK in the development of pulmonary hypertension in broilers. Avian Pathol. 2017;46:202–208. doi: 10.1080/03079457.2016.1245409. [DOI] [PubMed] [Google Scholar]
- 44.Potus F, Ruffenach G, Dahou A, Thebault C, Breuils-Bonnet S, Tremblay È, et al. Downregulation of microRNA-126 contributes to the failing right ventricle in pulmonary arterial hypertension. Circulation. 2015;132:932–943. doi: 10.1161/CIRCULATIONAHA.115.016382. [DOI] [PubMed] [Google Scholar]
- 45.Regan JN, Lim J, Shi Y, Joeng KS, Arbeit JM, Shohet RV, et al. Up-regulation of glycolytic metabolism is required for HIF1α-driven bone formation. Proc Natl Acad Sci USA. 2014;111:8673–8678. doi: 10.1073/pnas.1324290111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimoda LA, Semenza GL. HIF and the lung: role of hypoxia-inducible factors in pulmonary development and disease. Am J Respir Crit Care Med. 2011;183:152–156. doi: 10.1164/rccm.201009-1393PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minchenko A, Leshchinsky I, Opentanova I, Sang N, Srinivas V, Armstead V, et al. Hypoxia-inducible factor-1-mediated expression of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3) gene: its possible role in the Warburg effect. J Biol Chem. 2002;277:6183–6187. doi: 10.1074/jbc.M110978200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.