Abstract
Glioblastoma remains difficult to treat with chemotherapy and patients with tumors expressing high levels of (O6-methylguanine DNA methyltransferase (MGMT) usually respond poorly to standard temozolomide therapy. We have previously shown that the selective AURKA inhibitor alisertib potently inhibits growth of glioblastoma cells. Here we demonstrate that alisertib potentiates the antiproliferative effects of carboplatin and irinotecan in glioblastoma cells using colony formation assays. Alisertib’s potentiation of these drugs was often synergistic, including against glioblastoma tumor stem-like cells, as demonstrated by Chou-Talalay and Bliss analyses. Upon examining MGMT levels of cell lines by western blotting, we found that high MGMT expression correlated with more pronounced potentiation of carboplatin’s growth inhibitory effects by alisertib, while low MGMT expression correlated with stronger potentiation of irinotecan by alisertib. This pattern was also observed when these drug combinations were tested for their ability to induce apoptosis via annexin V binding assays. MGMT knockdown increased apoptosis caused by combined alisertib and irinotecan, while exogenous MGMT overexpression increased apoptosis from alisertib and carboplatin combination treatment. These results suggest that tumor MGMT expression levels may be predictive of patient response to these drug combinations, and importantly that the combination of alisertib and carboplatin may be selectively effective in glioblastoma patients with high tumor MGMT who are resistant to standard therapy. Since clinical experience with alisertib, carboplatin and irinotecan as single agents already exists, these findings may provide rationale for the design of clinical trials for their use in combination treatment regimens.
Keywords: AURKA, alisertib, carboplatin, irinotecan, glioblastoma, cytotoxicity, growth inhibition, synergy, sequence dependence
INTRODUCTION
Aurora A kinase (AURKA) is a serine-threonine kinase critical to cell cycle progression. It regulates several pro-proliferative pathways and multiple phases of mitosis (1). The small-molecule, selective AURKA inhibitor alisertib (MLN8237) inhibits tumor cell proliferation and causes formation of abnormal mitotic spindles, followed by apoptosis, differentiation or senescence (1). AURKA is overexpressed in gliomas (1), including glioblastoma, which is associated with short patient survival and represents a significant therapeutic challenge. Alisertib has been demonstrated to inhibit the growth of glioblastoma tumor stem-like cells in vitro and in vivo (2), suggesting that it may be a potentially effective agent against glioblastoma.
Because discovery of novel therapies for glioblastoma is critical, and effective chemotherapeutic approaches for refractory diseases may require a combination of agents, we have tested for possible synergistic antiglioma effects between alisertib and other treatments. We previously reported that alisertib potentiated the cytotoxicity of the first line glioblastoma adjuvant therapies temozolomide (TMZ) and ionizing radiation (1, 2), as well as the novel taxane TPI 287 (3). Both carboplatin and irinotecan are currently used against a wide variety of neoplasms and are occasionally used to treat intracranial tumors in children and adults, including glioblastoma (4, 5). We therefore used colony formation and annexin V binding assays to test for possible synergistic antiglioma effects between alisertib and carboplatin or irinotecan in glioblastoma cells.
MATERIALS AND METHODS
Cell lines.
U87, U251, T98 and LN18 cells were purchased from the American Type Culture Collection. GB30 neurosphere cells were established as previously described (6). STR profiling of GB30 and U1242 cells was performed at the University of Arizona Genetics Core for authentication.
Standard colony formation assays.
All monolayer glioma cell lines were cultured in DMEM with 10% fetal calf serum and 1% penicillin/streptomycin in 5% CO2 at 37°C. Synergy was determined by colony formation assays (CFAs) in which 600 cells were seeded per 60 mm dish and the following day treated with increasing concentrations of alisertib, carboplatin, irinotecan, or alisertib combined with either carboplatin or irinotecan. Drug vehicle (sterile water) was added to untreated controls. IC50 values for each drug were determined for each cell line (Table S1). Doses were calculated as percentages of the IC50, and performed in triplicate. Treated dishes were incubated for 72 hr, after which they were washed with Dulbecco’s phosphate buffered saline (DPBS), and fresh media added. Approximately 3-4 days later, dishes were washed with DPBS, fixed with methanol, stained with Giemsa, rinsed in deionized water and air dried. Colonies of ≥ 20 cells were counted using a dissecting microscope. Percent survival was calculated as the average number of colonies in 3 dishes for a given drug concentration divided by the average number of colonies in 3 untreated control dishes.
Glioblastoma neurosphere cell colony formation in soft agar.
Glioblastoma GB30 neurosphere cells (6) of less than 20 passages were cultured in neurosphere medium: DMEM/F12 (Cellgro), with 1% N2 supplement (Invitrogen), 1% penicillin/streptomycin (Sigma), and 20 ng/mL bFGF and EGF (R&D Systems) under standard conditions. Cells were seeded at a density of 3×103 cells/well in 0.4% low melting point agarose (Invitrogen, MA) in six-well plates (Corning, Corning, NY), and topped with 3 mL neurosphere media. Wells were treated with alisertib and/or carboplatin, or alisertib and/or irinotecan. Sterile water was added to control wells. Media and drugs were changed every 3 days. After 10 days the plates were stained with 0.05% crystal violet. Colonies of ≥ 20 cells were counted as above.
Drug treatment sequence dependence.
Sequence dependence CFAs contained 5 groups: one for each individual drug, one with the drugs used concomitantly, and two sequence groups. For sequence groups, dishes were incubated with one drug for 72 hr, after which they were washed with DPBS, and the second drug added. After 72 hr, dishes were again washed and fresh media added. Approximately 24 hr later, they were washed, fixed and stained, and counted as described above. Each group had associated triplicate control dishes.
siRNA knockdown.
Cells at approximately 60% confluence in 6-well plates were transfected with 25 nM ON-TARGETplus SMARTpool siRNA targeting human MGMT (Dharmacon, L-008856-01-0005) or ON-TARGETplus control nontargeting pool siRNA (Dharmacon, D-001810-10-05) in OptiMEM with DharmaFECT 1 for 24 hr. Following transfection, cells were treated with drugs and annexin-binding assays were performed. MGMT knockdown was confirmed by western blot.
Stable transfection.
Low MGMT expressing cells U251, U87 and GB30 were transfected with human MGMT (NM 002412) Myc-DDK-tagged ORF clone plasmid (Origene RC229131) or empty pCMV6-Entry Myc-DDK-tagged vector (Origene PS100001) using OptiMEM and Lipofectamine 3000 (Invitrogen, Waltham, MA). Cells were seeded in 6-well plates at 1×106 cells/well and transfected with 2.5μg human MGMT- pCMV6 or empty vector. Plasmid-containing cells were grown in the presence of G418 and colonies highly expressing MGMT (as determined by western blotting) were selected and propagated to generate stable MGMT-expressing lines.
Glioblastoma neurosphere cell proliferation assays.
GB30 cells stably transfected with MGMT or empty vector as described above were seeded at 1 × 104 cells/mL in 6 well plates and triplicate wells were treated with alisertib, carboplatin, irinotecan, alisertib + carboplatin or alisertib + irinotecan for 3 days. Cells were counted using a Countess II FL cell counter.
Annexin V binding.
Drug-treated cells were stained with Alexa Fluor 594 annexin V conjugate (Thermo Fisher) and analyzed with a Countess II FL cell counter equipped with a Texas Red light cube (Thermo Fisher) per the manufacturer’s instructions.
Western Blotting.
Cell lysates were prepared as previously described (1), with the addition of 1 mM sodium orthovanadate and 5 mM sodium fluoride to the lysis buffer. Protein (10 μg) was run on 12% Bis-Tris gels (Thermo Fisher) and blotted onto PVDF membranes. Anti-human MGMT (Invitrogen, 35-7000, 1:500), anti-human cleaved PARP (Cell Signaling Technologies 9541, 1:1000), anti-human cleaved caspase-3 (Cell Signaling Technologies 9661, 1:1000), and anti-human β-actin (Sigma, A2228, 1:10,000) primary antibodies were incubated for 90 min at 25°C. HRP-conjugated anti-mouse and anti-rabbit secondary antibodies (Cell Signaling Technologies 7076, 1:2000 and 7074, 1:2000, respectively) were incubated for 30 min at 25°C.
Calculating Synergy.
Each dosing experiment was completed three times, and the effect (E) of a drug or combination at a given concentration was the mean of these replicates divided by the mean of the untreated control replicates. For each drug concentration, two models were considered to identify potential synergy: An effect based model (Bliss) and a dose-effect based model (Loewe). The Bliss independence model combination index (7, 8) was calculated as the expected combination effect (assuming that statistically the drugs act independently) divided by the observed combination effect (CI = (Ea + Eb - Ea*Eb)/Eab). The Loewe independence model (9) was assessed using the Chou-Talalay combination index (7, 10) (CI=a/A+b/B; where a and b are the doses in combination for a given effect, and A and B are the single doses required for this effect). For both models, CI>1 indicates antagonism, and CI<1 indicates synergy.
Statistical analysis for annexin V binding and cell proliferation assays.
Data are presented as mean ± S.D. Means of groups of 3 replicates were compared by a two-tailed Student’s t test. Difference between groups were considered significant when p < 0.05.
RESULTS
Alisertib and carboplatin demonstrate variable synergy in glioblastoma cells.
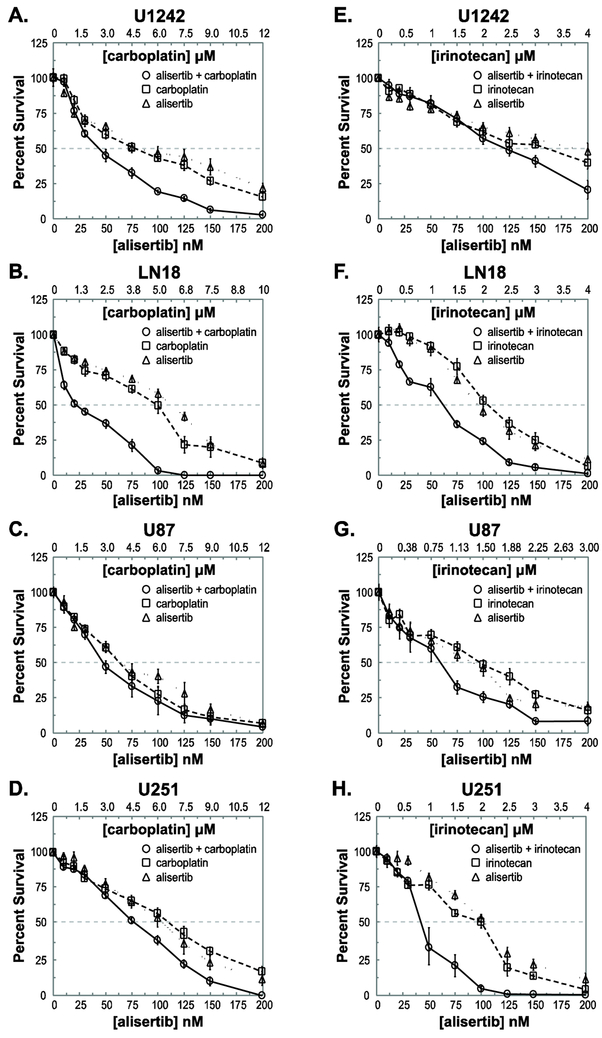
Synergy between alisertib and carboplatin was cell line dependent. The combination of alisertib and carboplatin exhibited synergy over a wide concentration range in U1242, LN18 and T98 cells (Fig. 1A-B; Fig. S1A; Table S2A). The strongest synergy between alisertib and carboplatin was observed in LN18 cells. Concentrations ranging from 0.2x to 2.0x IC50 (20-200 nM alisertib and 0.6-6 μM carboplatin) consistently demonstrated strong synergy by both Bliss and Chou-Talalay analyses (Fig. 1B, Table S2A). In U1242 cells, synergy was observed between 1.0x and 2.0x IC50 drug concentrations or between 100-200 nM alisertib and 6-12 μM carboplatin (Fig. 1A; Table S2A). In T98 cells, synergy was observed between 0.3x and 2.0x IC50 drug concentrations (30-200 nM alisertib and 1.8-12 μM carboplatin) (Fig. S1A; Table S2A). In contrast, no synergy on average was seen between alisertib and carboplatin in U87 cells, while minimal synergy was observed in U251 cells and GB30 tumor stem-like neurosphere cells (Fig. 1C-D and S1B; Table S2A).
Figure 1: Cytotoxicity of alisertib and carboplatin in glioblastoma cells.
Cells were seeded, treated with alisertib, carboplatin or alisertib + carboplatin for 72 hr, and cultured an additional 3–4 days. GB30 cells were seeded in soft agar and exposed to drugs for 10 days. Drug concentrations are expressed as multiples of approximate IC50 values for colony formation. A-D. CFAs of U1242, LN18, U87 and U251 cells treated with alisertib, carboplatin or alisertib and carboplatin. E-H. CFAs of U1242, LN18, U87 and U251 cells treated with alisertib, irinotecan or alisertib and irinotecan. All experiments were performed twice. Average percent survivals of both experiments are shown.
Alisertib and irinotecan are synergistic in glioma cell lines in which alisertib and carboplatin are not.
Unlike with alisertib and carboplatin, alisertib and irinotecan were potently synergistic over a wide range of concentrations in U251 cells, with synergy observed between 0.5x and 2.0x IC50 drug concentrations, or 50-200 nM alisertib and 1-4 μM irinotecan (Fig. 1H). This synergy was confirmed by Chou-Talalay analysis (Table S2B). Alisertib and irinotecan also demonstrated synergy in U87 cells, albeit over a narrower range of concentrations than in U251 cells (Fig. 1G). Yet, evidence for synergy between alisertib and irinotecan in U87 cells was much more compelling than between alisertib and carboplatin in the same cell line (Fig. 1C) and was supported by Chou-Talalay analysis (Table S2). Likewise, in GB30 cells stronger synergy between alisertib and irinotecan was observed between 0.75x and 2.0x IC50 concentrations (24-64 nM alisertib and 0.375-1.0μM irinotecan) (Fig. S1D) than was seen with alisertib and carboplatin in these cells (Fig. S1B). This was confirmed by Chou-Talalay analysis (Table S2). In further contrast to what was observed with alisertib and carboplatin, little synergy was observed between alisertib and irinotecan in U1242 cells and only mild to moderate synergy was seen in T98 cells (Fig. 1E and S1C; Table S2B). Although synergy between alisertib and irinotecan was observed in LN18 cells (Fig. 1F), it was not nearly of the same magnitude nor was it over as broad a drug concentration range as that seen between alisertib and carboplatin (Fig 1B; Table S2).
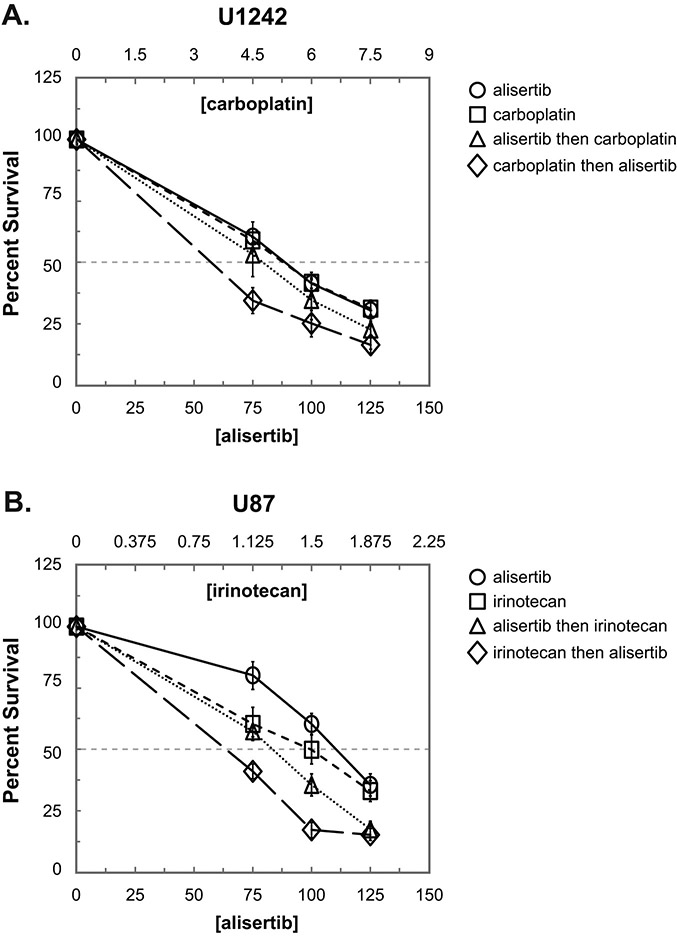
Combination treatment is sequence dependent.
Next, we tested if synergy between each drug combination was sequence dependent. In each case we chose a cell line in which synergy was observed for the particular drug combination. For all drug combinations, concurrent treatment with both agents resulted in the greatest antiproliferative effect (data not shown). In the case of alisertib + carboplatin, treatment with carboplatin followed by alisertib resulted in the greatest inhibition of colony formation in U1242 cells (Fig. 2A). Similarly, irinotecan followed by alisertib resulted in more growth inhibition of U87 cells compared to the reverse order of administration (Fig. 2B).
Figure 2: Effect of drug administration sequence on proliferation inhibition in non-concurrent drug treatment.
Cells were seeded and treated with individual drugs for 72 hr, washed with DPBS, and treated with the second drug for an additional 72 hr. Drug concentrations were chosen as multiples of approximate IC50s for colony formation. A. U1242 cells were treated with alisertib, carboplatin, alisertib followed by carboplatin or carboplatin followed by alisertib. B. U87 cells were treated with alisertib, irinotecan, alisertib followed by irinotecan or irinotecan followed by alisertib. All experiments were performed twice. Average percent survivals of both experiments are shown.
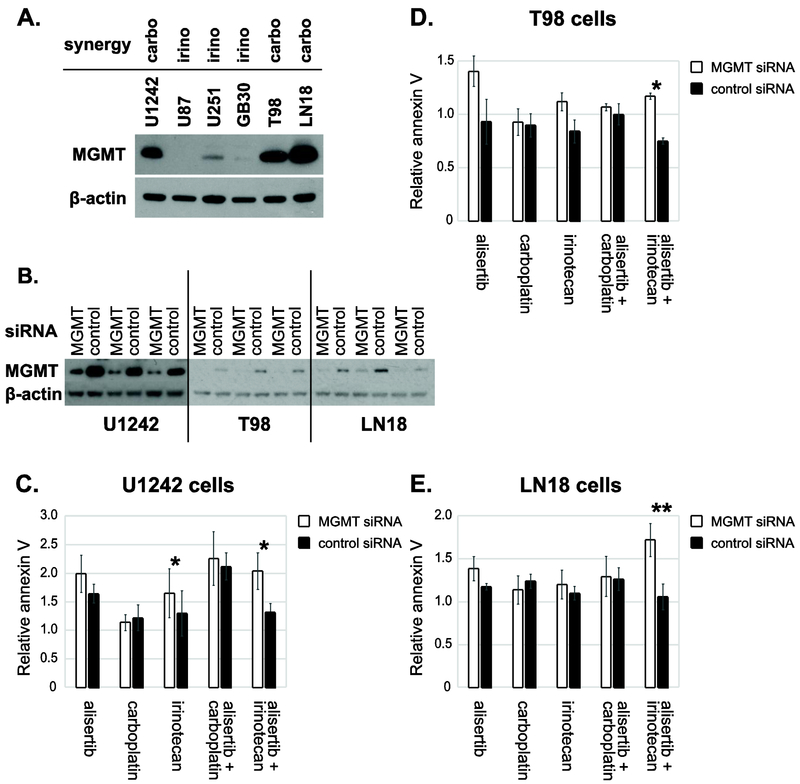
MGMT protein expression predicts potentiation of alisertib by carboplatin and irinotecan.
To try to understand the basis of the variable synergy in different cell lines for these drug combinations we compared glioblastoma biomarkers that may potentially affect response to cytotoxic chemotherapy between cell lines. IDH1 and p53 mutational status did not appear to correlate with our observations however there was some suggestion that MGMT expression could (Table S3). To examine whether synergy between alisertib and carboplatin and/or alisertib and irinotecan correlated with MGMT expression, western blotting was used to compare MGMT protein expression levels between cell lines. Cell lines showing more convincing synergy between alisertib and carboplatin compared to alisertib and irinotecan (U1242, T98 and LN18) expressed high levels of MGMT. In contrast, cell lines showing stronger synergy between alisertib and irinotecan (U251, U87 and GB30) expressed much lower MGMT levels (Fig. 3A; Table S2B).
Figure 3: Effects of alisertib and irinotecan versus alisertib and carboplatin are inversely dependent on MGMT levels.
A. Alisertib and MGMT expression in glioma cell lines. Western blotting was performed to determine expression of MGMT in standard cell lines and glioblastoma stem-like cells (GB30). Alisertib synergy with irinotecan (irino) and carboplatin (carbo) is indicated B. MGMT knockdown western blots. C-E. MGMT knockdown results in increased apoptosis of alisertib + irinotecan treated cells. Cells were seeded and transfected with MGMT or control siRNA the following day. After 24 hr, transfection medium was removed, and cells were treated with alisertib (150 nM), carboplatin (6 μM), irinotecan (2 μM), alisertib + carboplatin or alisertib + irinotecan. The following day, cells were stained with an Alexa Fluor 594 annexin V conjugate, and apoptotic cells were quantified with a Countess II FL cell counter. Results are presented as the fold-increase in annexin V-binding relative to untreated control cells. Average fold changes in annexin V binding relative to untreated control cells are presented. *p < 0.05, **p < 0.01, n=3.
To directly test the effects of MGMT expression on synergy between alisertib and irinotecan, we knocked down MGMT in high MGMT expressing cells by siRNA (Fig. 3B) and treated with single agents and drug combinations. Annexin V-binding assays were then performed to quantify apoptosis. MGMT knockdown appeared to mildly potentiate alisertib-induced apoptosis in all 3 high MGMT expressing cell lines tested, but particularly in T98 cells (Fig. 3C–E), however this was not statistically significant (Table S4). Irinotecan-induced apoptosis also appeared to be increased by MGMT knockdown, but this was statistically significant only in U1242 cells. In contrast, MGMT knockdown had little effect on apoptosis in cells treated with carboplatin.
Apoptosis resulting from combined treatment with alisertib and carboplatin was also relatively unchanged by MGMT knockdown. The combination of alisertib and irinotecan however lead to greatly increased apoptosis when MGMT was knocked down (Fig. 3C-E). In all cell lines tested, this drug combination showed the greatest difference in relative apoptosis when compared with control siRNA-transfected cells, p = 0.0202 for U1242, p = 0.0121 for T98 and p = 0.0024 for LN18 (Fig. 3; Table S4).
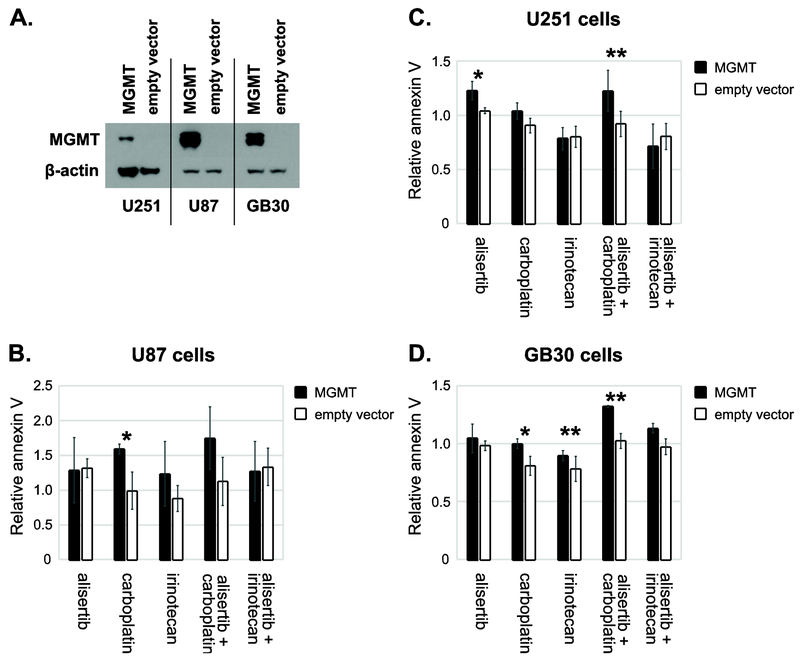
The effects of exogenous overexpression of MGMT in the low MGMT expressing glioblastoma cell lines U87, U251 and GB30 inversely correlated with results seen when MGMT was knocked down in high MGMT expressing lines. Namely, exogenous MGMT enhanced apoptosis caused by alisertib combined with carboplatin in all three of these cell lines (Fig. 4). This was statistically significant for U251 and GB30 glioblastoma tumor stem-like cells (Fig. 4B-D; Table S4). Exogenous MGMT overexpression also appeared to increase apoptosis from carboplatin alone, and in some cell lines, from alisertib and/or irinotecan as single agents, albeit not in a consistent, statistically significant manner (Fig. 4B-D).
Figure 4: Overexpression of exogenous MGMT enhances apoptosis induced by alisertib + carboplatin.
Cell lines were stably transfected with pCMV6 containing human MGMT or empty vector. A. Western blotting was performed to confirm exogenous MGMT expression. Note: a less sensitive ECL substrate was used to obtain these MGMT bands (Pierce ECL Western Blotting Substrate, Thermo 32106) than was used to determine endogenous MGMT expression in Fig 3A (SuperSignal West Femto Maximum Sensitivity Substrate, Thermo 34095). The same ECL substrate was used for β-actin in both cases. B-D. Cells were treated with alisertib (250 nM), carboplatin (10 μM), irinotecan (5 μM), aliserib + carboplatin or alisertib + irinotecan for 24h hr. Cells were stained with Alexa Fluor 594 annexin V conjugate and analyzed with the Countess II FL cell counter. Average fold changes in annexin V binding are presented. *p < 0.05, **p < 0.01; n=3 for U251 and U87, n=2 for GB30.
Western blotting for apoptosis markers in drug treated U251 cells transfected with MGMT or empty vector confirmed annexin V bonding results. Irinotecan and alisertib + irinotecan induced more cleaved caspase-3 and cleaved poly (ADP-ribose) polymerase (PARP) when MGMT was absent, while carboplatin and carboplatin + alisertib induced more apoptosis when MGMT was expressed (Fig S2A).
Consistent with apoptosis assays, CFAs using U251 cells stably transfected with MGMT or empty vector revealed that MGMT enhances the anti-proliferative interaction between alisertib and carboplatin (Fig. S2B). Bliss and Chou-Talalay analysis confirmed synergy at all concentrations tested (Table S4). Although some synergy was observed in cells transfected with empty vector, it was neither as strong nor over as broad of a concentration range (Fig. S2B, Table S5).
Proliferation assays of MGMT-overexpressing GB30 cells showed an inhibitory effect of MGMT on the efficacy of irinotecan, while the efficacy of carboplatin was enhanced by MGMT (Fig. S2D). Alisertib’s anti-proliferative effect was also inhibited by MGMT. The combination of alisertib and carboplatin inhibited proliferation to a greater extent when MGMT was present. This was also the case for alisertib and irinotecan, although to a much lesser extent (Fig S2D).
DISCUSSION
Here we found that alisertib potentiated the cytotoxic effects of both carboplatin and irinotecan, often in a synergistic manner. Surprisingly, the cytotoxic synergy between alisertib and carboplatin versus alisertib and irinotecan were inversely dependent on glioblastoma cell O6-methylguanine DNA methyltransferase (MGMT) levels.
TMZ methylates the O6 position of guanine, which can lead to point mutations, intrastrand DNA cross-linking and cell death (11). MGMT is a DNA repair enzyme capable of removing this abnormal methyl group. In glioblastoma, high MGMT expression is correlated with shorter patient survival and resistance to TMZ (12), and MGMT knockdown has been shown to potentiate TMZ in resistant glioblastoma cells (13). The platinum-based drug carboplatin adds platinum adducts to DNA similarly resulting in crosslinking, but does not alkylate DNA and the relationship between MGMT expression and response to carboplatin is less clear. Although their DNA damaging mechanisms differ, both temozolomide and carboplatin interfere with DNA replication and genomic stability.
In colony formation assays and MGMT knock in experiments, MGMT expression consistently correlated with increased synergy between alisertib and carboplatin. There are conflicting reports on the relationship between MGMT and platinum drugs (14). Although MGMT may be able to repair platinum adducts (12, 14), MGMT has been shown to be downregulated and its degradation accelerated following exposure to platinum drugs (15). Nevertheless, several clinical studies have noted efficacy of platinum drugs in MGMT-expressing gliomas (12, 16).
We observed little change in the alisertib-carboplatin combination’s ability to induce apoptosis when MGMT was knocked down, which suggests another factor in high MGMT expressing cells is determining the combinations’ apparent selectivity for such cells. As noted, platinum drugs can decrease MGMT (15), therefore downregulation of MGMT by carboplatin may be an important component of synergy between these drugs. The greatest degree of synergy between alisertib and carboplatin however was observed in LN18 cells, which had the highest MGMT expression. Regardless of the mechanism of synergy, our data suggest that this combination may be effective against glioblastoma expressing high MGMT levels, which characteristically does not respond well to chemotherapy and has a poorer prognosis.
Irinotecan is a camptothecin analogue whose SN-38 metabolite irreversibly binds topoisomerase I leading to double strand DNA breaks and inhibition of both DNA replication and transcription (17). In contrast to alisertib and carboplatin, combination therapy with alisertib and irinotecan was more synergistic in glioblastoma cell lines expressing low levels of MGMT. When MGMT was exogenously overexpressed in these cells, the combination of alisertib and irinotecan tended to induce less apoptosis when compared with cells transfected with empty vector (Fig. 4). Conversely, when MGMT was knocked down in cells with high MGMT expression, which exhibited less synergy between alisertib and irinotecan in CFAs, the result was a statistically significant increase in induction of apoptosis by this drug combination when compared with cells transfected with control siRNA (Fig. 3). This was true even in LN18 cells, the only cell line to both express high MGMT levels and exhibit synergy between alisertib and irinotecan (Fig. 1F), albeit much less synergy than seen with alisertib and carboplatin (Fig. 1B).
Increased MGMT expression has previously been shown to impede topoisomerase I inhibitor induced cell death (18, 19). Several methods of MGMT-mediated resistance to irinotecan have been proposed. Among these are the enhancement of DNA double strand breaks through an interaction between O6methylguanine and the irinotecan-stabilized DNA-topoisomerase I cleavable complex, or a more direct interaction between MGMT and topoisomerase I (19). Other possibilities include intracellular drug depletion due to off target binding of irinotecan or alisertib to MGMT. Although we did not observe dramatically higher IC50 concentrations for alisertib and irinotecan in MGMT-expressing cell lines, this does not preclude MGMT from being a possible factor in mediating the effectiveness of these drugs. It may not be surprising then that absence of MGMT greatly enhanced the potency of this combination.
Sequence-dependence experiments may hint at a mechanism for synergy in our drug combinations. In cell lines in which carboplatin or irinotecan was synergistic with alisertib, the sequences of carboplatin or irinotecan followed by alisertib treatment were more effective than their reverse. This result suggests that DNA damage by carboplatin and irinotecan sensitized cells to the subsequent effect of AURKA inhibition, perhaps resulting in the latter more readily triggering the mitotic spindle check point and apoptosis. Further studies are needed to explain why glioblastoma cells expressing MGMT showed greater sensitivity to carboplatin and less to irinotecan in synergy assays with alisertib, but this observation may be related to differences in other DNA repair capabilities among cell lines that may in turn be possibly related to high MGMT expression.
Clinically, tumor MGMT expression is frequently determined indirectly by measuring the amount of MGMT promoter methylation. High MGMT expression is equated with MGMT promoter hypomethylation and low MGMT expression with MGMT promoter hypermethylation. This however, is not always the case (20, 21). Even so, some studies have purported that MGMT promoter methylation may be a better predictor of response to adjuvant therapy versus actual MGMT protein levels (22). Evidence of the value of determining MGMT expression by immunohistochemistry however has been recently presented (23). Since our findings are based on MGMT protein levels rather than promoter methylation, this should be taken into consideration in potentially selecting patients for carboplatin, or alisertib + carboplatin or alisertib + irinotecan combination therapies. For such clinical studies, MGMT expression should be determined by both promoter methylation analysis and protein measurement, and the results independently correlated with overall patient responses.
Because MGMT expression is routinely tested in glioblastoma patients in the clinical setting, and alisertib, carboplatin and irinotecan have all been safely used in glioma patients, this study provides rationale for clinical trials of these drug combinations in glioblastoma patients who are refractory to standard therapy. If such drug combinations were shown to be clinically effective, patients could be selected for treatment with alisertib and carboplatin or irinotecan based on tumor MGMT levels.
Supplementary Material
Figure S1: Cytotoxicity of alisertib and carboplatin or irinotecan in additional glioblastoma cells. Cells were seeded, treated with drugs for 72 hr and cultured an additional 3–4 days. Drug concentrations were chosen as multiples of approximate IC50s for colony formation. A-B. CFAs of T98 and GB30 cells treated with alisertib, carboplatin or alisertib and carboplatin. C-D. CFAs of T98 and GB30 cells treated with alisertib, irinotecan or alisertib and irinotecan. All experiments were performed twice. The average percent survival of both experiments is shown.
Figure S2: Overexpression of exogenous MGMT leads to synergy between alisertib and carboplatin in cell lines with low endogenous MGMT. A. U251 cells transfected with MGMT or an empty vector were seeded, and treated for 4 days with alisertib (200 nM), carboplatin (15 μM), irinotecan (4 μM), alisertib + carboplatin or alisertib + irinotecan, and western blotting was performed to determine expression of MGMT and apoptosis markers cleaved PARP and cleaved caspase-3. B-C. CFAs of U251 cells stably transfected with MGMT or empty vector (EV) were performed with alisertib and carboplatin alone and in combination. D. Stably transfected GB30 cells (MGMT or empty vector) were seeded and treated with alisertib (25 nM), carboplatin (3 μM), irinotecan (0.5 μM), alisertib + carboplatin or alisertib + irinotecan for 3 days. Average percent survival of drug-treated cells compared to untreated MGMT or empty vector controls are presented. Mean ± S.D. are shown. *p = 0.0065 and **p = 0.032.
Table S1: Potency of antineoplastic agents in glioblastoma cells in vitro. IC50 values found for each drug and cell line used in CFA experiments are shown.
Table S2: Statistical analyses for potential synergy between A. alisertib and carboplatin, and B. alisertib and irinotecan. Values less than 1.0 indicate synergy in both the Bliss and Chou-Talalay models and are highlighted in bold. Confidence intervals less than 1.0 suggest a higher degree of statistical significance.
Table S3: Glioblastoma cell line mutations.
Table S4: p Values for annexin V binding assays.
Table S5: Statistical analyses for potential synergy between alisertib and carboplatin in U251 cells with exogenous MGMT overexpression versus empty vector.
Acknowledgments
Funding: This study was funded by NIH Grant RO1 NS081125 (NLL).
Footnotes
Conflict of Interest: All authors declare that they have no conflicts of interest.
None of the aforementioned authors have conflicting interest in the publication of this article.
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Lehman NL, O'Donnell JP, Whiteley LJ, Stapp RT, Lehman TD, Roszka KM, Schultz LR, Williams CJ, Mikkelsen T, Brown SL, Ecsedy JA, Poisson LM. Aurora A is differentially expressed in gliomas, is associated with patient survival in glioblastoma and is a potential chemotherapeutic target in gliomas. Cell Cycle. 2012;11(3):489–502. Epub 2012/01/26. doi: 10.4161/cc.11.3.18996. PubMed PMID: 22274399; PMCID: PMC3315093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong X, O'Donnell JP, Salazar CR, Van Brocklyn JR, Barnett KD, Pearl DK, deCarvalho AC, Ecsedy JA, Brown SL, Mikkelsen T, Lehman NL. The selective Aurora-A kinase inhibitor MLN8237 (alisertib) potently inhibits proliferation of glioblastoma neurosphere tumor stem-like cells and potentiates the effects of temozolomide and ionizing radiation. Cancer Chemother Pharmacol. 2014;73(5):983–90. Epub 2014/03/15. doi: 10.1007/s00280-014-2430-z. PubMed PMID: 24627220; PMCID: PMC4975936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zumbar CT, Usubalieva A, King PD, Li X, Mifsud CS, Dalton HM, Sak M, Urio S, Bryant WM, McElroy JP, Farmer G, Lehman NL. The CNS penetrating taxane TPI 287 and the AURKA inhibitor alisertib induce synergistic apoptosis in glioblastoma cells. J Neurooncol. 2018;137(3):481–92. Epub 2018/02/06. doi: 10.1007/s11060-018-2755-2. PubMed PMID: 29396807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang P, Mukthavaram R, Chao Y, Bharati IS, Fogal V, Pastorino S, Cong X, Nomura N, Gallagher M, Abbasi T, Vali S, Pingle SC, Makale M, Kesari S. Novel anti-glioblastoma agents and therapeutic combinations identified from a collection of FDA approved drugs. J Transl Med. 2014;12:13. Epub 2014/01/18. doi: 10.1186/1479-5876-12-13. PubMed PMID: 24433351; PMCID: PMC3898565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poisson M, Pereon Y, Chiras J, Delattre JY. Treatment of recurrent malignant supratentorial gliomas with carboplatin (CBDCA). J Neurooncol. 1991;10(2):139–44. Epub 1991/04/01. PubMed PMID: 1654401. [DOI] [PubMed] [Google Scholar]
- 6.Van Brocklyn JR, Wojton J, Meisen WH, Kellough DA, Ecsedy JA, Kaur B, Lehman NL. Aurora-A inhibition offers a novel therapy effective against intracranial glioblastoma. Cancer Res. 2014;74(19):5364–70. Epub 2014/08/12. doi: 10.1158/0008-5472.CAN-14-0386. PubMed PMID: 25106428; PMCID: PMC4528677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foucquier J, Guedj M. Analysis of drug combinations: current methodological landscape. Pharmacol Res Perspect. 2015;3(3):e00149. Epub 2015/07/15. doi: 10.1002/prp2.149. PubMed PMID: 26171228; PMCID: PMC4492765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greco WR, Bravo G, Parsons JC. The search for synergy: a critical review from a response surface perspective. Pharmacol Rev. 1995;47(2):331–85. Epub 1995/06/01. PubMed PMID: 7568331. [PubMed] [Google Scholar]
- 9.Loewe S The problem of synergism and antagonism of combined drugs. Arzneimittelforschung. 1953;3(6):285–90. Epub 1953/06/01. PubMed PMID: 13081480. [PubMed] [Google Scholar]
- 10.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. Epub 1984/01/01. PubMed PMID: 6382953. [DOI] [PubMed] [Google Scholar]
- 11.Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, Baylin SB, Herman JG. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343(19):1350–4. Epub 2000/11/09. doi: 10.1056/NEJM200011093431901. PubMed PMID: 11070098. [DOI] [PubMed] [Google Scholar]
- 12.Iwadate Y, Matsutani T, Hasegawa Y, Shinozaki N, Oide T, Tanizawa T, Nakatani Y, Saeki N, Fujimoto S. Selection of chemotherapy for glioblastoma expressing O(6)-methylguanine-DNA methyltransferase. Exp Ther Med. 2010;1(1):53–7. Epub 2010/01/01. doi: 10.3892/etm_00000009. PubMed PMID: 23136592; PMCID: PMC3490398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viel T, Monfared P, Schelhaas S, Fricke IB, Kuhlmann MT, Fraefel C, Jacobs AH. Optimizing glioblastoma temozolomide chemotherapy employing lentiviral-based anti-MGMT shRNA technology. Mol Ther. 2013;21(3):570–9.Epub 2013/01/16. doi: 10.1038/mt.2012.278. PubMed PMID: 23319055; PMCID: PMC3589165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen SH, Kuo CC, Li CF, Cheung CH, Tsou TC, Chiang HC, Yang YN, Chang SL, Lin LC, Pan HY, Chang KY, Chang JY. O(6) -methylguanine DNA methyltransferase repairs platinum-DNA adducts following cisplatin treatment and predicts prognoses of nasopharyngeal carcinoma. Int J Cancer. 2015;137(6):1291–305. Epub 2015/02/20. doi: 10.1002/ijc.29486. PubMed PMID: 25693518. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka S, Kobayashi I, Utsuki S, Oka H, Yasui Y, Fujii K. Down-regulation of O6-methylguanine-DNA methyltransferase gene expression in gliomas by platinum compounds. Oncol Rep. 2005;14(5):1275–80. Epub 2005/10/08. PubMed PMID: 16211296. [PubMed] [Google Scholar]
- 16.Tanaka S, Akimoto J, Kobayashi I, Oka H, Ujiie H. Individual adjuvant therapy for malignant gliomas based on O6-methylguanine-DNA methyltransferase messenger RNA quantitation by real-time reverse-transcription polymerase chain-reaction. Oncol Rep. 2008;20(1):165–71. Epub 2008/06/26. PubMed PMID: 18575733. [PubMed] [Google Scholar]
- 17.Rothenberg ML. Irinotecan (CPT-11): recent developments and future directions--colorectal cancer and beyond. Oncologist. 2001;6(1):66–80. Epub 2001/02/13. PubMed PMID: 11161230. [DOI] [PubMed] [Google Scholar]
- 18.Kuo CC, Liu JF, Chang JY. DNA repair enzyme, O6-methylguanine DNA methyltransferase, modulates cytotoxicity of camptothecin-derived topoisomerase I inhibitors. J Pharmacol Exp Ther. 2006;316(2):946–54. Epub 2005/11/01. doi: 10.1124/jpet.105.095919. PubMed PMID: 16258022. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto R, Takano H, Okamura T, Park JS, Tanimoto K, Sekikawa T, Yamamoto W, Sparreboom A, Verweij J, Nishiyama M. O(6)-methylguanine-DNA methyltransferase (MGMT) as a determinant of resistance to camptothecin derivatives. Jpn J Cancer Res. 2002;93(1):93–102. Epub 2002/01/23. PubMed PMID: 11802813; PMCID: PMC5926864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao VT, Jung TY, Jung S, Jin SG, Moon KS, Kim IY, Kang SS, Park CS, Lee KH, Chae HJ. The correlation and prognostic significance of MGMT promoter methylation and MGMT protein in glioblastomas. Neurosurgery. 2009;65(5):866–75; discussion 75. Epub 2009/10/17. doi: 10.1227/01.NEU.0000357325.90347.A1. PubMed PMID: 19834398. [DOI] [PubMed] [Google Scholar]
- 21.Melguizo C, Prados J, Gonzalez B, Ortiz R, Concha A, Alvarez PJ,Madeddu R, Perazzoli G, Oliver JA, Lopez R, Rodriguez-Serrano F, Aranega A. MGMT promoter methylation status and MGMT and CD133 immunohistochemical expression as prognostic markers in glioblastoma patients treated with temozolomide plus radiotherapy. J Transl Med. 2012;10:250. Epub 2012/12/19. doi: 10.1186/1479-5876-10-250. PubMed PMID: 23245659; PMCID: PMC3551841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uno M, Oba-Shinjo SM, Camargo AA, Moura RP, Aguiar PH, Cabrera HN, Begnami M, Rosemberg S, Teixeira MJ, Marie SK. Correlation of MGMT promoter methylation status with gene and protein expression levels in glioblastoma. Clinics (Sao Paulo). 2011;66(10):1747–55. Epub 2011/10/21. PubMed PMID: 22012047; PMCID: PMC3180167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Becker A BE, McElroy J, Cui T, Geurts M, Liu Z, Haque SJ, Robe P, Chakravarti A. MGMT protein expression adds prognostic value beyond MGMT promoter methylation and stratifies survival prognoses of un-methylated glioblastoma patients. Int J Radiat Oncol Biol Phys. 2018;102(3):S47. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Cytotoxicity of alisertib and carboplatin or irinotecan in additional glioblastoma cells. Cells were seeded, treated with drugs for 72 hr and cultured an additional 3–4 days. Drug concentrations were chosen as multiples of approximate IC50s for colony formation. A-B. CFAs of T98 and GB30 cells treated with alisertib, carboplatin or alisertib and carboplatin. C-D. CFAs of T98 and GB30 cells treated with alisertib, irinotecan or alisertib and irinotecan. All experiments were performed twice. The average percent survival of both experiments is shown.
Figure S2: Overexpression of exogenous MGMT leads to synergy between alisertib and carboplatin in cell lines with low endogenous MGMT. A. U251 cells transfected with MGMT or an empty vector were seeded, and treated for 4 days with alisertib (200 nM), carboplatin (15 μM), irinotecan (4 μM), alisertib + carboplatin or alisertib + irinotecan, and western blotting was performed to determine expression of MGMT and apoptosis markers cleaved PARP and cleaved caspase-3. B-C. CFAs of U251 cells stably transfected with MGMT or empty vector (EV) were performed with alisertib and carboplatin alone and in combination. D. Stably transfected GB30 cells (MGMT or empty vector) were seeded and treated with alisertib (25 nM), carboplatin (3 μM), irinotecan (0.5 μM), alisertib + carboplatin or alisertib + irinotecan for 3 days. Average percent survival of drug-treated cells compared to untreated MGMT or empty vector controls are presented. Mean ± S.D. are shown. *p = 0.0065 and **p = 0.032.
Table S1: Potency of antineoplastic agents in glioblastoma cells in vitro. IC50 values found for each drug and cell line used in CFA experiments are shown.
Table S2: Statistical analyses for potential synergy between A. alisertib and carboplatin, and B. alisertib and irinotecan. Values less than 1.0 indicate synergy in both the Bliss and Chou-Talalay models and are highlighted in bold. Confidence intervals less than 1.0 suggest a higher degree of statistical significance.
Table S3: Glioblastoma cell line mutations.
Table S4: p Values for annexin V binding assays.
Table S5: Statistical analyses for potential synergy between alisertib and carboplatin in U251 cells with exogenous MGMT overexpression versus empty vector.