Abstract
Background:
The objective of this study was to estimate the number of years after onset of a quadrivalent HPV vaccination program before notable reductions in genital warts and cervical intraepithelial neoplasia (CIN) will occur in teenagers and young adults in the United States.
Methods:
We applied a previously published model of HPV vaccination in the United States and focused on the timing of reductions in genital warts among both sexes and reductions in CIN 2/3 among females. Using different coverage scenarios, the lowest being consistent with current 3-dose coverage in the United States, we estimated the number of years before reductions of 10%, 25%, and 50% would be observed after onset of an HPV vaccination program for ages 12–26 years.
Results:
The model suggested female-only HPV vaccination in the intermediate coverage scenario will result in a 10% reduction in genital warts within 2–4 years for females aged 15–19 years and a 10% reduction in CIN 2/3 among females aged 20–29 years within 7–11 years. Coverage had a major impact on when reductions would be observed. For example, in the higher coverage scenario a 25% reduction in CIN2/3 would be observed with 8 years compared with 15 years in the lower coverage scenario.
Conclusions:
Our model provides estimates of the potential timing and magnitude of the impact of HPV vaccination on genital warts and CIN 2/3 at the population level in the United States. Notable, population-level impacts of HPV vaccination on genital warts and CIN 2/3 can occur within a few years after onset of vaccination, particularly among younger age groups. Our results are generally consistent with early reports of declines in genital warts among youth.
Keywords: Papillomavirus, Vaccination, Models, Cervical neoplasms, Genital warts
1. Introduction
Human papillomavirus (HPV) vaccination is expected to reduce the burden of HPV-associated disease in the United States [1]. However, mathematical modeling studies have suggested that reductions in HPV-associated cancers could take decades to achieve through vaccination [2–9]. In contrast, notable reductions in genital warts and high grade cervical intraepithelial neoplasia (CIN) are expected to occur much sooner after onset of HPV vaccination than the reductions in HPV-associated cancers [5–7]. As such, plans to monitor vaccine impact in the United States and other developed countries include HPV-associated outcomes with shorter expected time frames to observe vaccine impact (such as CIN and genital warts) in addition to longer-term outcomes such as cervical cancer [10,11]. In fact, there is preliminary, ecological evidence of a population-level impact of HPV vaccine on genital warts and high-grade cervical abnormalities among teenagers and young adults in the United States and Australia [12–17].
The purpose of this project was to estimate the number of years after onset of a quadrivalent HPV vaccination program before notable reductions in CIN 2/3 and genital warts will occur among teenagers and young adults in the United States. These estimates can help to illustrate plausible scenarios of HPV vaccine impact over time.
2. Methods
2.1. Description of model
We used a deterministic, dynamic population-based HPV model to estimate the timing of reductions in genital warts and CIN 2/3 after onset of quadrivalent HPV vaccination in the United States [6]. The model, described in more detail elsewhere [6], is a simplified compartmental model in which each age cohort was divided into four classes (“susceptible, not vaccinated”; “infected, not vaccinated”, “vaccinated, not infected”, and “vaccinated, infected”) according to type-specific HPV infection. Specifically, the model calculations were performed separately for HPV 16, HPV 18, and HPV 6/11, and the impact of quadrivalent vaccination was estimated based on the combined results of these calculations.
The model first takes into account the incidence of HPV-associated health outcomes in the absence of HPV vaccination, obtained primarily from surveillance data and medical claims data. Then, reductions in the incidence of these adverse outcomes are estimated based on vaccine coverage, vaccine efficacy, and the percentage of the adverse health outcomes attributable to the HPV vaccine types. To do so, we apply annual, sex- and age-specific probabilities of acquiring a specific HPV type. Vaccination is assumed to reduce the annual probability of acquiring HPV vaccine types according to the vaccine efficacy assumptions below. To reflect indirect effects of vaccination (herd immunity), these sex and age-specific HPV acquisition probabilities are adjusted each year to reflect reductions in HPV in the population. Rather than explicitly modeling the transition from HPV acquisition to HPV-associated outcomes, we estimate vaccine impact by assuming the percentage reduction in health outcomes attributable to a given HPV type was equal to the percentage reduction in cumulative lifetime exposure to that HPV type.
In this study, we applied the model exactly as described previously [6], with three exceptions. First, we used different (updated) coverage scenarios as described in Table 1. Second, we modified the sensitivity analyses as described in Table 2 to focus on different scenarios of vaccine impact over time. Finally, we applied lower values of the annual probability of acquiring HPV 6/11 for ages 10–14 years so that the burden of HPV 6/11 in this age group relative to that of ages 15–19 years would be consistent with the corresponding relative burden of genital warts for these two age groups based on insurance claims data [18].
Table 1.
Vaccine coverage scenarios: annual probability of initiating HPV vaccine series among females, by age.
| Coverage scenario | Age 12 years | Ages 13–18 years | Ages 19–26 years |
|---|---|---|---|
| Lower | 0.20 | 0.10 | 0.01 |
| Intermediate | 0.28 | 0.17 | 0.02 |
| Higher | 0.70 | 0.60 | 0.35 |
The annual probabilities of vaccination reflect the probability of receiving all three vaccine doses among females not vaccinated in previous years. The probabilities shown above for vaccination at age 12 years were applied in every year of the vaccine program except year 1. In year 1, the probability of initiating the vaccine series at age 12 was reduced by 50% to allow for phase-in of the vaccine program. In scenarios of male vaccination, we applied the same annual probabilities of vaccination for males as females, except in the lower coverage scenario in which we assumed the probabilities of vaccination for males would be one-half that of females. A complete description of the model and a full description of all model parameter values and references are available elsewhere [6].
Table 2.
Parameters varied in the sensitivity analyses: base case values and values in the pessimistic and optimistic scenarios.
| Parameter varied | Base case | Pessimistic scenario | Optimistic scenario |
|---|---|---|---|
| Vaccine efficacy against HPV 6/11/16/18, females | 95% | 85% | 100% |
| Vaccine efficacy against HPV 6/11/16/18, males | 90% | 80% | 100% |
| Percent of CIN 2/3 attributable to HPV 16/18 | 59% | 52% | 67% |
| Percent of genital warts attributable to HPV 6/11 | 90% | 70% | 100% |
| Peak age of HPV acquisition (years) | 20 | 16 | 24 |
Parameter values and ranges were obtained from a previously published model [6], except the lower bound values for vaccine efficacy and the lower- and upper-bound values of the peak age of HPV infection, which were assumed.
2.2. Time horizon
We examined the first 25 years of a vaccine program. In each year, a new cohort of 8-year-olds entered the model and a cohort of 99-year-olds exited the model. In each year, those aged 12–26 years who were eligible for vaccination but who had not been vaccinated previously were subject to the annual probability of receiving the vaccine.
2.3. Vaccine characteristics and coverage
Vaccine efficacy against HPV 6, 11, 16, and 18 was assumed to be 95% in females and 90% in males [19–24], with lifelong duration of efficacy. We assumed no cross-protection against other, non-vaccine HPV types.
We assumed that all those who initiated the vaccine series would receive all three doses. We examined three coverage scenarios: lower, intermediate, and higher (Table 1). In the intermediate coverage scenario, the annual probability of receiving the 3-dose vaccine series was 28% at age 12, 17% for ages 13–18 years, and 2% for ages 19–26 years. The annual probabilities of receiving vaccination for the lower and intermediate scenarios were selected such that the resulting coverage after the first few years of the vaccine program would be consistent with estimates of vaccine coverage in the United States. Specifically, under the coverage assumptions described in Table 1, the average coverage of 13- to 17-year-olds in year 6 of the vaccine program would be about 37% in the lower coverage scenario and about 52% in the intermediate coverage scenario, consistent with estimated HPV vaccine coverage rates of 35% (three doses) and 53% (vaccine initiation) of females aged 13–17 years in the United States based on 2011 data from the National Immunization Survey-Teen [25]. The higher coverage scenario was included to illustrate the potential impact of HPV vaccination when coverage of targeted age groups exceeds 70%, as has been achieved among females in the United Kingdom and Australia [16,26–28].
We examined six vaccination scenarios in which the three coverage levels described above (lower, intermediate, and higher) were applied to each of two vaccination strategies (vaccination of females aged 12–26 years, and vaccination of both sexes aged 12–26 years). When we included male vaccination, we assumed that male vaccination would begin 5 years after female vaccination, as the recommendation for routine HPV vaccination of males in the United States was established about 5 years after the recommendation for routine vaccination of females [29]. After onset of male vaccination, the annual probability of vaccination for males was assumed to be the same as females (Table 1), except in the lower coverage scenario in which the annual probability of vaccination for males was assumed to be one-half that of females.
2.4. Assessment of vaccine impact on genital warts and high grade CIN over time
We estimated the reductions in genital warts over time among two age groups (15–19 years and 20–29 years) separately for both sexes. We estimated the reductions in CIN 2/3 among women in one age group (20–29 years). In each year of our model, the makeup of the age groups change as the oldest birth cohort ages out and a younger birth cohort ages in. For example, at the end of each year the cohort of 19-year-olds turns 20 years old, leaves the 15- to 19-year age group, and joins the 20- to 29-year age group. Similarly, at the end of each year, the 14-year-old cohort turns 15 years old and enters the 15- to 19-year age group.
2.5. Sensitivity analyses
We examined how the model results changed when we simultaneously varied several key assumptions in the model. In order to summarize the most extreme model results across various scenarios, we examined an optimistic scenario and a pessimistic scenario in which we simultaneously varied five key parameters as described in Table 2. In the optimistic scenario, values for the five parameters which maximized the estimated impact of vaccination were selected. Specifically, in the optimistic scenario we applied upper-bound values for vaccine efficacy and the percent of health outcomes attributable to the HPV vaccine types. We also assumed a later age at which HPV incidence peaks. In our model, vaccine impact (in terms of the percent decrease in genital warts and CIN among younger age groups) is greater when the peak age of HPV acquisition is later because a higher percent of those vaccinated will be naïve to the HPV vaccine types at the time of vaccination. In contrast, for the pessimistic scenario, we applied lower-bound values for vaccine efficacy and the percent of health outcomes attributable to the HPV vaccine types, and assumed that HPV incidence peaks at a younger age. We examined the optimistic and pessimistic scenarios under all three of the coverage scenarios described in Table 1.
3. Results
Reductions in selected HPV-associated outcomes over the first 25 years of the HPV vaccine program are shown in Figs. 1–3. Results are also summarized in Table 3, which shows the number of years after onset of HPV vaccination until certain thresholds of vaccine impact (reductions in genital warts and CIN 2/3 of at least 10%, 25%, and 50%) are observed in the selected age groups. The results in parentheses in Table 3 are those from the sensitivity analyses and show how the time frame changes under the assumptions in the optimistic and the pessimistic scenarios, respectively.
Fig. 1.
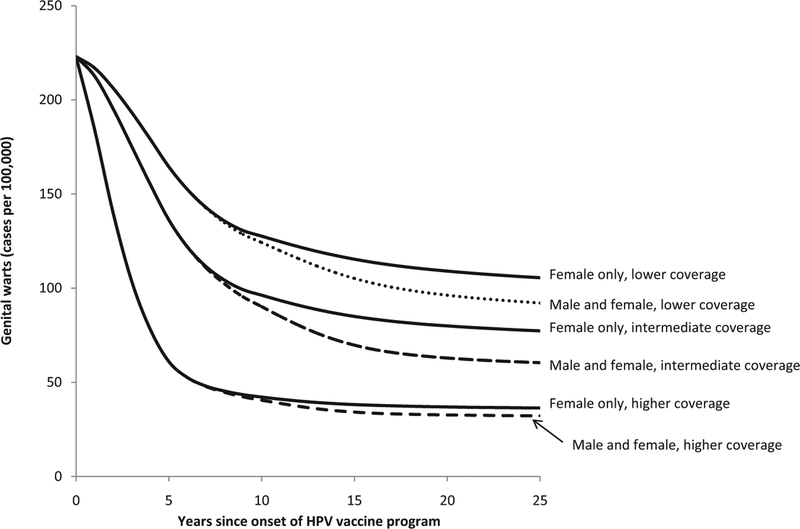
The estimated decline in genital warts among females aged 15–19 years following onset of a quadrivalent HPV vaccination program under 6 vaccination scenarios. Two vaccination strategies (“Female-only vaccination” shown with solid lines and “Male and female vaccination” shown with dashed lines) for ages 12–26 years were modeled under three coverage levels (lower, intermediate, and higher) as described in Table 1.
Fig. 3.
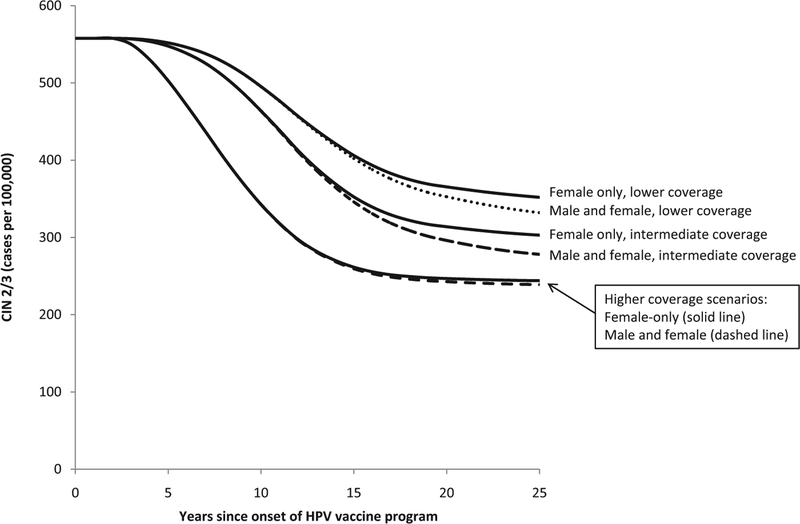
The estimated decline in cervical intraepithelial neoplasia (CIN) 2/3 among females aged 20–29 years following onset of a quadrivalent HPV vaccination program under 6 vaccination scenarios. Two vaccination strategies (“Female-only vaccination” shown with solid lines and “Male and female vaccination” shown with dashed lines) for ages 12–26 years were modeled under three coverage levels (lower, intermediate, and higher) as described in Table 1.
Table 3.
Estimated number of years after onset of HPV vaccination until reductions of at least 10%, 25%, and 50% are observed in genital warts and cervical intraepithelial neoplasia (CIN) grade 2/3 in the selected age groups, for three coverage scenarios.
| Outcome/sex and age group (years) | Lower coverage scenario | Intermediate coverage scenario | Higher coverage scenario |
|---|---|---|---|
| Panel A: female-only vaccination | |||
| Number of years until a 10% reduction | |||
| Genital warts, females 15–19 | 3 (2–5) | 2 (2–4) | 1 (1–2) |
| Genital warts, males 15–19 | 6 (5–12) | 5 (4–9) | 3 (3–6) |
| Genital warts, females 20–29 | 7 (6–10) | 6 (5–8) | 3 (2–5) |
| Genital warts, males 20–29 | 11 (8–16) | 9 (7–14) | 7 (4–11) |
| CIN 2/3, females 20–29 | 10 (9–12) | 9 (7–11) | 6 (4–7) |
| Number of years until a 25% reduction | |||
| Genital warts, females 15–19 | 5 (4–17) | 4 (3–8) | 2 (2–4) |
| Genital warts, males 15–19 | 11 (8 to >25) | 8 (7 to >25) | 5 (4–10) |
| Genital warts, females 20–29 | 10 (8–16) | 8 (7–12) | 5 (3–8) |
| Genital warts, males 20–29 | 16 (12 to >25) | 13 (10 to >25) | 9 (6–16) |
| CIN 2/3, females 20–29 | 15 (12 to >25) | 12 (10–18) | 8 (6–12) |
| Number of years until a 50% reduction | |||
| Genital warts, females 15–19 | 18 (8 to >25) | 7 (5 to >25) | 3 (2–12) |
| Genital warts, males 15–19 | >25 (>25 to >25) | >25 (15 to >25) | 9 (6 to >25) |
| Genital warts, females 20–29 | 16 (12 to >25) | 12 (10 to >25) | 7 (5–15) |
| Genital warts, males 20–29 | >25 (18 to >25) | 21 (14 to >25) | 14 (9 to >25) |
| CIN 2/3, females 20–29 | >25 (>25 to >25) | >25 (15 to >25) | 14 (9 to >25) |
| Panel B: male and female vaccination | |||
| Number of years until a 10% reduction | |||
| Genital warts, females 15–19 | 3 (2–5) | 2 (2–4) | 1 (1–2) |
| Genital warts, males 15–19 | 6 (5–9) | 5 (4–8) | 3 (3–6) |
| Genital warts, females 20–29 | 7 (6–10) | 6 (5–8) | 3 (2–5) |
| Genital warts, males 20–29 | 10 (8–14) | 9 (7–12) | 6 (4–9) |
| CIN 2/3, females 20–29 | 10 (9–12) | 9 (7–11) | 6 (4–7) |
| Number of years until a 25% reduction | |||
| Genital warts, females 15–19 | 5 (4–14) | 4 (3–8) | 2 (2–4) |
| Genital warts, males 15–19 | 9 (8–20) | 7 (6–11) | 5 (4–8) |
| Genital warts, females 20–29 | 10 (8–15) | 8 (7–12) | 5 (3–8) |
| Genital warts, males 20–29 | 14 (11–21) | 12 (9–16) | 8 (6–12) |
| CIN 2/3, females 20–29 | 15 (12 to >25) | 12 (10–17) | 8 (6–12) |
| Number of years until a 50% reduction | |||
| Genital warts, females 15–19 | 14 (8 to >25) | 7 (5 to >25) | 3 (2–11) |
| Genital warts, males 15–19 | 20 (12 to >25) | 10 (8 to >25) | 7 (6–12) |
| Genital warts, females 20–29 | 16 (12 to >25) | 12 (10 to >25) | 7 (5–15) |
| Genital warts, males 20–29 | 20 (15 to >25) | 15 (12 to >25) | 11 (8–18) |
| CIN 2/3, females 20–29 | >25 (20 to >25) | 25 (15 to >25) | 14 (9 to >25) |
“>25” indicates that the given percentage reduction was not reached over the 25-year analytic horizon of the model. The two results in parentheses are from the sensitivity analyses and show the number of years needed to achieve the listed outcome in the optimistic and pessimistic scenarios, respectively.
Vaccine coverage of 12-year-old girls is 20% in the lower scenario, 28% in the intermediate scenario, and 70% in the higher scenario. We also assumed annual probabilities of initiating the vaccination program through age 26 years. See Table 1 for details of coverage assumptions.
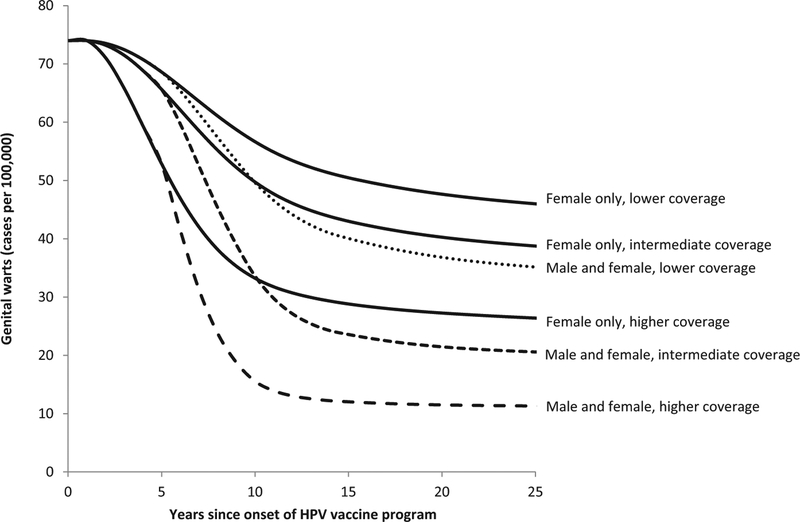
Of the outcomes and age groups we examined, the earliest impact of HPV vaccination was found for genital warts among ages 15–19 years. For example, in the intermediate coverage scenario, female-only vaccination was predicted to reach the threshold of a 10% reduction in genital warts in females aged 15–19 years within 2 years of onset of vaccination and to reach the threshold of a 25% reduction within 4 years (Fig. 1 and Table 3). Also in the intermediate coverage scenario, reductions of 10% in genital warts in males aged 15–19 years were predicted within 5 years under a female-only vaccination program (Fig. 2 and Table 3). Reductions of 10% in CIN 2/3 among women aged 20–29 years were predicted within 9 years in the intermediate coverage scenario (Fig. 3 and Table 3).
Fig. 2.
The estimated decline in genital warts among males aged 15–19 years following onset of a quadrivalent HPV vaccination program under 6 vaccination scenarios. Two vaccination strategies (“Female-only vaccination” shown with solid lines and “Male and female vaccination” shown with dashed lines) for ages 12–26 years were modeled under three coverage levels (lower, intermediate, and higher) as described in Table 1.
As expected, reductions of 25–50% in these outcomes will take longer to occur than reductions of 10%, just as reductions in the lower coverage scenario will take longer to achieve than equivalent reductions in the higher coverage scenario (Figs. 1–3 and Table 3). For some of the outcomes we examined, reductions of 25% and 50% were not reached over the 25-year analytic horizon of the model. For example, 50% reductions in CIN 2/3 among women aged 20–29 years were not predicted to be achieved over the 25-year analytic horizon in the lower coverage scenario with female-only vaccination. As another example, in the pessimistic scenario, reductions of 25% in genital warts in males aged 15–19 years were not predicted to be achieved over the 25-year analytic horizon in the lower and intermediate coverage scenarios for female-only vaccination.
3.1. Sensitivity analyses: optimistic and pessimistic scenarios
The degree to which results in the optimistic scenario differed from those of the pessimistic scenario varied across the health outcomes and age groups that we examined. For example, in the intermediate coverage scenario for female-only vaccination, there was little difference across the optimistic and pessimistic scenarios regarding the timing of reductions of 10% in genital warts in females aged 15–19 years. Reductions of 10% in genital warts were expected within 2 years in the optimistic scenario and within 4 years in the pessimistic scenario. In contrast, in this same intermediate-coverage scenario for female-only vaccination, there were substantial differences across the optimistic and pessimistic scenarios regarding the timing of reductions of 50% in genital warts in males aged 20–29 years. Such reductions in genital warts were predicted within 15 years in the optimistic scenario but not within 25 years in the pessimistic scenario.
4. Discussion
Typically, post-licensure vaccine monitoring in the United States includes the assessment of vaccine coverage and implementation, monitoring vaccine safety, and evaluation of the vaccine’s impact on disease outcomes [11]. Current plans for monitoring the impact of HPV vaccine on health outcomes include the following outcomes: HPV-associated cancers, CIN2/3 and adenocarcinoma in situ (AIS), Pap test abnormalities, non-cervical precancer lesions, genital warts, and HPV prevalence [11,30,31]. As expected, our estimates suggest that HPV vaccine coverage is an important determinant of the timing and magnitude of the population-level impact of vaccination.
Although we assumed that all those who initiated vaccination would complete the series, in reality the completion rate among those who start the vaccine series is about 70% [25]. Our lower coverage scenario is consistent with 3-dose HPV vaccine coverage among females in the United States and our intermediate coverage scenario is consistent with ≥ 1-dose coverage, based on estimated vaccine coverage in 2011 [25]. Thus, if there is high vaccine efficacy with less than 3 doses [32], our intermediate coverage scenario might be the most applicable of our three scenarios in predicting the timing of HPV vaccine impact in the United States, at least in the short term.
4.1. Limitations
Our model is relatively simple compared to other models that have estimated the potential impact of HPV vaccination on various HPV-associated health outcomes [7,33–36], as discussed in more detail elsewhere [6]. One key simplification is that we assumed 100% lifelong, type-specific natural immunity against re-infection. All else equal, the estimated impact of HPV vaccination is generally greater in models with lower levels of natural immunity than in models such as ours with higher levels of natural immunity [36]. Similarly, the impact of HPV vaccination would be more substantial and might be observed sooner than we estimated if there is cross-protection against non-vaccine HPV types.
Another key simplification is that our model assesses the impact of vaccination over a sequence of discrete, 1-year time steps instead of continuously as in most of the more complex models. Our use of discrete time steps might underestimate the speed and degree to which HPV vaccination might impact HPV transmission dynamics, particularly in the first few years after vaccination. Therefore the application of our model, which was developed to assess the cost-effectiveness of vaccination over a 100-year time horizon, to short-term scenarios might lead us to over-estimate the time needed to achieve notable, population level-reductions in HPV-associated health outcomes.
Finally, we estimated the potential impact of HPV vaccination on the incidence of genital warts and CIN 2/3 assuming that all other factors that influence these outcomes (e.g., sexual behavior, cervical cancer screening, and availability of prevention resources) remained constant over time. Given the simplifications described above, our model results are best interpreted as a somewhat conservative illustration of possible post-vaccination impact in the United States and allow comparisons across different coverage scenarios.
4.2. Comparison to early reports of vaccine impact
Although our modeling predictions cannot be interpreted as precise estimates, it is useful to compare our modeling results with post-vaccination surveillance data to assess the plausibility of our model predictions. Our results are generally consistent with early reports of a potential population-level vaccine impact on genital warts in the United States. Based on medical claims data in the United States, Flagg et al. reported declines in genital warts prevalence of about 38% among females aged 15–19 years from 2006 to 2010 [13]. Our model predicted declines in genital warts among females aged 15–19 years of 25% within 4 years (and 50% within 7 years) of female-only vaccination in the intermediate coverage scenario. Based on administrative claims data in California, where 1-dose HPV vaccine coverage among adolescent females aged 13–17 years was 56% in 2010, reductions in genital warts from 2007 to 2010 were estimated at 35% for females under age 21 years, 19% for males under age 21 years, 10% for females aged 21–25 years, and 11% for males aged 21–25 years [12]. For the intermediate coverage scenario and higher coverage scenario, our model results are reasonably consistent with these estimated reductions in genital warts, at least among females.
Our results are not as consistent with early reports of a potential population-level vaccine impact on genital warts in Australia. Donovan et al. reported declines in genital warts of about 60% among vaccine-eligible females (ages 12–26) within 3 years of onset of an HPV vaccination that achieved 70% 3-dose coverage in school-based programs [16]. Read et al. reported declines in genital warts of about 90% among males and females under age 21 years within 5 years of onset of HPV vaccination, with smaller declines among men and women aged 21–29 years [15]. Our model does not predict reductions in genital warts of 90% in the younger age groups within 5 years after female-only HPV vaccination. However, under the most optimistic assumptions in the higher coverage scenario for female-only vaccination, our model does predict substantial reductions in genital warts among both sexes within 6 years of onset of a vaccination program. For example, our model shows possible reductions of 50% within 2 years for females aged 15–19 years, within 6 years for males aged 15–19 years, and within 5 years for females aged 20–29 years, and reductions of 25% for males aged 20–29 years within 6 years (Table 3, higher coverage scenario, lower bound values).
Summary
Our model provides useful, potentially conservative estimates of the potential timing and magnitude of the impact of HPV vaccination on genital warts and CIN 2/3 at the population level in the United States. Our results are generally consistent with early reports of declines in genital warts among youth after onset of HPV vaccination programs, particularly if there is vaccine efficacy with less than a full 3-dose series.
Acknowledgement
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Conflict of interest statement: None.
References
- [1].Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007;56(RR-2):1–24. [PubMed] [Google Scholar]
- [2].Barnabas RV, Laukkanen P, Koskela P, Kontula O, Lehtinen M, Garnett GP. Epidemiology of HPV 16 and cervical cancer in Finland and the potential impact of vaccination: mathematical modelling analyses. PLoS Med 2006;3(5): e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Garnett GP, Kim JJ, French K, Goldie SJ. Modelling the impact of HPV vaccines on cervical cancer and screening programmes. Vaccine 2006;24(Suppl. 3):S178–86. [DOI] [PubMed] [Google Scholar]
- [4].Kohli M, Ferko N, Martin A, Franco EL, Jenkins D, Gallivan S, et al. Estimating the long-term impact of a prophylactic human papillomavirus 16/18 vaccine on the burden of cervical cancer in the UK. Br J Cancer 2007;96(1):143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Brisson M, Van d V, De WP, Boily MC. The potential cost-effectiveness of prophylactic human papillomavirus vaccines in Canada. Vaccine 2007;25(July (20)):5399–408. [DOI] [PubMed] [Google Scholar]
- [6].Chesson HW, Ekwueme DU, Saraiya M, Dunne EF, Markowitz LE. The cost-effectiveness of male HPV vaccination in the United States. Vaccine 2011;29(October (26)):8443–50. [DOI] [PubMed] [Google Scholar]
- [7].Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis 2007;13(1):28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Taira AV, Neukermans CP, Sanders GD. Evaluating human papillomavirus vaccination programs. Emerg Infect Dis 2004;10(November (11)):1915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Goldie SJ, Kohli M, Grima D, Weinstein MC, Wright TC, Bosch FX, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst 2004;96(8):604–15. [DOI] [PubMed] [Google Scholar]
- [10].Wong CA, Saraiya M, Hariri S, Eckert L, Howlett RI, Markowitz LE, et al. Approaches to monitoring biological outcomes for HPV vaccination: challenges of early adopter countries. Vaccine 2011;29(5):878–85. [DOI] [PubMed] [Google Scholar]
- [11].Markowitz LE, Hariri S, Unger ER, Saraiya M, Datta SD, Dunne EF. Post-licensure monitoring of HPV vaccine in the United States. Vaccine 2010;28(30): 4731–7. [DOI] [PubMed] [Google Scholar]
- [12].Bauer HM, Wright G, Chow J. Evidence of human papillomavirus vaccine effectiveness in reducing genital warts: an analysis of California public family planning administrative claims data, 2007–010. Am J Public Health 2012;102(5):833–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Flagg EW, Schwartz R, Weinstock H. Prevalence of anogenital warts among participants in private health plans in the United States, 2003–010: potential impact of HPV vaccination. Am J Public Health 2013:e1–8, 10.2105/AJPH.2012.301182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fairley CK, Hocking JS, Gurrin LC, Chen MY, Donovan B, Bradshaw C. Rapid decline in presentations for genital warts after the implementation of a national quadrivalent human papillomavirus vaccination program for young women. Sex Transm Infect 2009;85:499–502. [DOI] [PubMed] [Google Scholar]
- [15].Read TR, Hocking JS, Chen MY, Donovan B, Bradshaw CS, Fairley CK. The near disappearance of genital warts in young women 4 years after commencing a national human papillomavirus (HPV) vaccination programme. Sex Transm Infect 2011;87(7):544–7. [DOI] [PubMed] [Google Scholar]
- [16].Donovan B, Franklin N, Guy R, Grulich AE, Regan DG, Ali H, et al. Quadrivalent human papillomavirus vaccination and trends in genital warts in Australia: analysis of national sentinel surveillance data. Lancet Infect Dis 2011;11(l):39–44. [DOI] [PubMed] [Google Scholar]
- [17].Brotherton JM, Fridman M, May CL, Chappell G, Saville AM, Gertig DM. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet 2011;377(9783):2085–92. [DOI] [PubMed] [Google Scholar]
- [18].Hoy T, Singhal PK, Willey VJ, Insinga RP. Assessing incidence and economic burden of genital warts with data from a US commercially insured population. Curr Med Res Opin 2009;25(10):2343–51. [DOI] [PubMed] [Google Scholar]
- [19].Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007;356(19): 1915–27. [DOI] [PubMed] [Google Scholar]
- [20].Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 2005;6(5):271–8. [DOI] [PubMed] [Google Scholar]
- [21].Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007;356(19):1928–43. [DOI] [PubMed] [Google Scholar]
- [22].Munoz N, Manalastas R Jr, Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet 2009;373(9679):1949–57. [DOI] [PubMed] [Google Scholar]
- [23].Palefsky J, Giuliano A. Efficacy of the quadrivalent HPV vaccine against HPV 6/11/16/18-related genital infection in young men. Nice, France: European Research Organization on Genital Infection and Neoplasia (EUROGIN) 2008; 2008. [Google Scholar]
- [24].Giuliano AR, Palefsky J, Goldstone S, Moreira ED, Penny ME, Arand C, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med 2011;364(5):401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dorell C, Stokley S, Yankey D, Jeyarajah J, MacNeil J, Markowitz L. National and state vaccination coverage among adolescents aged 13 through 17 years – United States, 2011. MMWR Morb Mortal Wkly Rep 2012;61(34): 671–7. [PubMed] [Google Scholar]
- [26].Gertig DM, Brotherton JM, Saville M. Measuring human papillomavirus (HPV) vaccination coverage and the role of the National HPV Vaccination Program Register, Australia. Sex Health 2011;8(2):171–8. [DOI] [PubMed] [Google Scholar]
- [27].Jit M, Chapman R, Hughes O, Choi YH. Comparing bivalent and quadrivalent human papillomavirus vaccines: economic evaluation based on transmission model. Br Med J 2011;343:d5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kessels SJ, Marshall HS, Watson M, Braunack-Mayer AJ, Reuzel R, Tooher RL Factors associated with HPV vaccine uptake in teenage girls: a systematic review. Vaccine 2012;30(24):3546–56. [DOI] [PubMed] [Google Scholar]
- [29].Centers for Disease Control Prevention. Recommendations on the use of quadrivalent human papillomavirus vaccine in males – Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011;60(50):1705–8. [PubMed] [Google Scholar]
- [30].Chesson HW, Flagg EW, Koutsky L, Hsu K, Unger ER, Shlay JC, et al. Modeling the impact of quadrivalent HPV vaccination on the incidence of Pap test abnormalities in the United States. Vaccine 2013. 10.1016/j.vaccine.2013.04.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Markowitz LE, Hariri S, Lin C, Dunne EF, Steinau M, McQuillan G, et al. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis 2013. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [32].Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, et al. Proof-of-principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst 2011;103(19):1444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kim JJ, Goldie SJ. Cost effectiveness analysis of including boys in a human papillomavirus vaccination programme in the United States. Br Med J 2009;339:b3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine 2010;28(42):6858–67. [DOI] [PubMed] [Google Scholar]
- [35].Jit M, Choi YH, Edmunds WJ. Economic evaluation of human papillomavirus vaccination in the United Kingdom. Br Med J 2008;337:a769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Van de Velde N, Brisson M, Boily MC. Understanding differences in predictions of HPV vaccine effectiveness: a comparative model-based analysis. Vaccine 2010;28(33):5473–84. [DOI] [PubMed] [Google Scholar]