Summary
Background
Modelling studies have been widely used to inform human papillomavirus (HPV) vaccination policy decisions; however, many models exist and it is not known whether they produce consistent predictions of population-level effectiveness and herd effects. We did a systematic review and meta-analysis of model predictions of the long-term population-level effectiveness of vaccination against HPV 16, 18, 6, and 11 infection in women and men, to examine the variability in predicted herd effects, incremental benefit of vaccinating boys, and potential for HPV-vaccine-type elimination.
Methods
We searched MEDLINE and Embase for transmission-dynamic modelling studies published between Jan 1, 2009, and April 28, 2015, that predicted the population-level impact of vaccination on HPV 6, 11, 16, and 18 infections in high-income countries. We contacted authors to determine whether they were willing to produce new predictions for standardised scenarios. Strategies investigated were girls-only vaccination and girls and boys vaccination at age 12 years. Base-case vaccine characteristics were 100% efficacy and lifetime protection. We did sensitivity analyses by varying vaccination coverage, vaccine efficacy, and duration of protection. For all scenarios we pooled model predictions of relative reductions in HPV prevalence (RRprev) over time after vaccination and summarised results using the median and 10th and 90th percentiles (80% uncertainty intervals [UI]).
Findings
16 of 19 eligible models from ten high-income countries provided predictions. Under base-case assumptions, 40% vaccination coverage and girls-only vaccination, the RRprev of HPV 16 among women and men was 0·53 (80% UI 0·46–0·68) and 0·36 (0·28–0·61), respectively, after 70 years. With 80% girls-only vaccination coverage, the RRprev of HPV 16 among women and men was 0·93 (0·90–1·00) and 0·83 (0·75–1·00), respectively. Vaccinating boys in addition to girls increased the RRprev of HPV 16 among women and men by 0·18 (0·13–0·32) and 0·35 (0·27–0·39) for 40% coverage, and 0·07 (0·00–0·10) and 0·16 (0·01–0·25) for 80% coverage, respectively. The RRprev were greater for HPV 6, 11, and 18 than for HPV 16 for all scenarios investigated. Finally at 80% coverage, most models predicted that girls and boys vaccination would eliminate HPV 6, 11, 16, and 18, with a median RRprev of 1·00 for women and men for all four HPV types. Variability in pooled findings was low, but increased with lower vaccination coverage and shorter vaccine protection (from lifetime to 20 years).
Interpretation
Although HPV models differ in structure, data used for calibration, and settings, our population-level predictions were generally concordant and suggest that strong herd effects are expected from vaccinating girls only, even with coverage as low as 20%. Elimination of HPV 16, 18, 6, and 11 is possible if 80% coverage in girls and boys is reached and if high vaccine efficacy is maintained over time.
Funding
Canadian Institutes of Health Research.
Introduction
Since 2006, two prophylactic human papillomavirus (HPV) vaccines have been widely used worldwide: the bivalent and quadrivalent vaccines. Both vaccines target HPV 16 and 18, which cause about 50% of high-grade cervical lesions, 70% of cervical cancers, and 40–80% of other HPV-related cancers.1–5 The quadrivalent vaccine also targets HPV 6 and 11, which are associated with 80–90% of anogenital wart cases.6 Large randomised controlled clinical trials have shown that both vaccines are highly effective in protecting against vaccine-type persistent HPV infection and precancerous lesions in women and men (vaccine efficacy 93–100%).7,8 More than 65 countries have introduced HPV vaccination programmes.9,10 Most programmes target girls only, with only a handful of countries vaccinating both girls and boys (eg, the USA, Australia, Switzerland, Austria, and Canada).11–17 These decisions have been made with substantial input from mathematical models.11,18–22 Recently, a nonavalent vaccine, which targets HPV 31, 33, 45, 52, and 58 also, has been licensed and recommended for use in the USA23 after reports of strong efficacy and immunogenicity from large trials.24
Mathematical models have consistently predicted that vaccinating girls against HPV is highly cost-effective,25–27 however, the picture is less clear for vaccinating boys.28–35 This is because the cost-effectiveness of vaccinating girls is mainly driven by the direct benefit of HPV vaccination among vaccinated women, which depends on well quantified parameters such as vaccine efficacy and the proportion of cervical cancer due to the vaccine types.27 The incremental effectiveness and cost-effectiveness of vaccinating boys is influenced largely by the magnitude of indirect protection conferred to men by vaccinating girls (herd immunity), which depends on a complex combination of factors (eg, vaccination coverage and sexual behaviour).28,35,36 Modelling studies have shown that if vaccinating girls significantly reduces the burden of HPV-related diseases in men through herd immunity, then vaccinating boys will produce limited additional population-level benefits for heterosexual men and women and thus will not be cost-effective at the same vaccine price.28,30,35 However, it is unclear what vaccination coverage is necessary to achieve substantial herd effects, and whether models are consistent in their predictions.
A systematic review of population-level HPV post-vaccination surveillance data has shown significant reductions in anogenital warts in young men in the first 4 years after girls-only quadrivalent vaccination programmes with high coverage (≥50%).37 In countries with low vaccination coverage (<50%) there was no indication of herd effects.37 In Australia, where quadrivalent vaccination coverage among girls has been consistently higher than 70%, anogenital warts consultations among heterosexual men declined by more than 80% within the first 5 years of the programme (before vaccination of boys).38 These results suggest that HPV vaccination can produce important herd effects for the anogenital warts associated types (HPV 6 and 11), and that elimination of these types might be achievable.
Better understanding the potential long-term population-level effectiveness of HPV vaccination, including herd effects, is crucial to help inform future vaccine policy decisions such as the inclusion of boys in vaccination programmes, incremental impact of increasing vaccination coverage and optimal combinations of HPV vaccination and cervical cancer screening strategies. Mathematical models provide a formal framework to examine these questions, which cannot be answered in trial settings. However, models require many assumptions, which might lead to questions about the validity of predictions and uncertainty for decision makers. A large number of HPV models have been developed over the past decade, but it is still unclear whether the models are giving consistent results and whether we can draw general principles from them. We conducted a systematic review and meta-analysis of model predictions of the long-term population-level effectiveness of HPV vaccination against HPV 6, 11, 16, and 18 infection in women and men, to examine the robustness and variability of predicted herd effects, incremental benefit of vaccinating boys, and potential for HPV vaccine-type elimination.
Methods
Search strategy and selection criteria
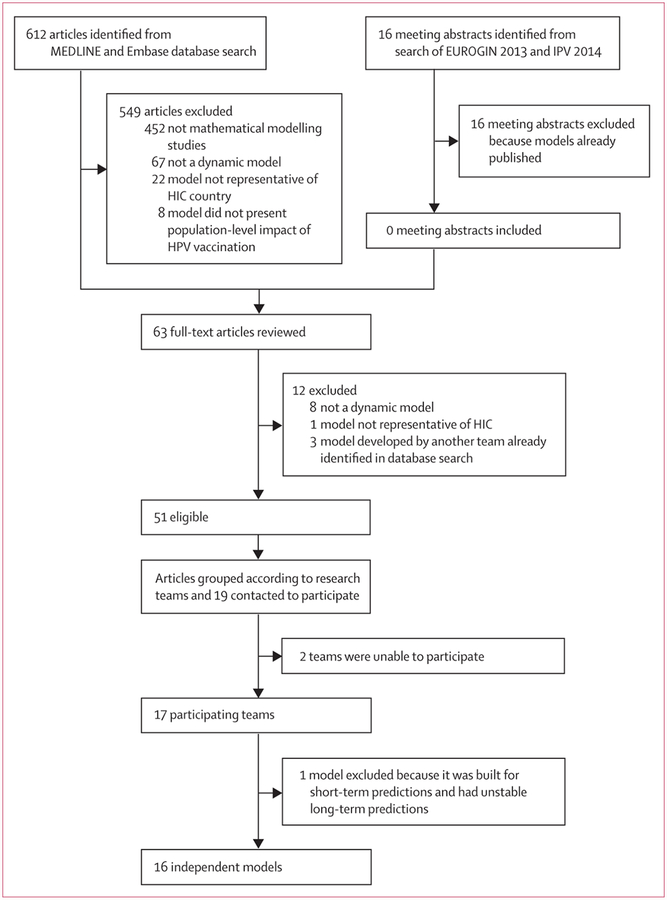
In this systematic review and meta-analysis, we used a three-step systematic process to identify independently developed HPV transmission-dynamic mathematical models from the published literature, which we report in accordance with the PRISMA guidelines.39 First, we systematically reviewed the literature. Modelling studies were eligible for inclusion if the model: (1) was an HPV transmission-dynamic model; (2) produced predictions of the population-level impact of vaccination on HPV 16, 18, 6, or 11 infections among women or men or both; and (3) was developed to examine the impact of HPV vaccination in a high-income country. We searched Medline and Embase databases using a combination of the following MeSH terms, title or abstract words, without restriction on the language of the articles: (“models, theoretical” or “mathematical model” or “models, statistical” or “cost-benefit analysis” or “cost-effectiveness” or “risk-benefit analysis”) and (“papillomavirus vaccines” or “papillomavirus vaccination” or “human papillomavirus vaccine” or “HPV vaccine” or “HPV vaccination”); the exact search for both databases is presented in the appendix p 2. We included models published between Jan 1, 2009, and April 28, 2015, because the authors were likely to use their model to inform future policy decisions. We identified eligible studies through review of titles and abstracts. To identify additional studies, we reviewed the references of selected articles and hand searched the abstracts from the main HPV conferences (Eurogin Congress 2013; Florence, Italy; Nov 3–5; and International Papillomavirus Conference 2014; Seattle, WA, USA; Aug 20–25). MB, ÉB, and MD assessed the eligibility of all studies.
Second, we decided a priori to group the selected studies by research team. Teams were not selected if they used a model previously developed by another team. MB contacted the senior or corresponding authors of all teams to determine whether they were willing to produce new predictions using a standardised-input dataset. The ability to provide new standardised predictions was a prerequisite for inclusion of a model in this meta-analysis, to adequately compare and pool model results. Third, when modelling teams had different versions of their models, we included only their most recent published model in the meta-analysis to ensure independence between model predictions.
Data collection and quality assessment
All eligible and participating teams were asked to fill a standardised form to describe their models in detail. Additionally, MB and ÉB provided the modelling teams with 19 predetermined vaccination scenarios and asked them to transfer these new results using a standardised format (appendix p 3). Our primary outcome was the relative reduction in the overall prevalence (RRprev) of HPV 16, 18, 6, and 11 among heterosexual women and men after 70 years of vaccination (vs no vaccination). We decided, a priori, to stratify outcomes by HPV type and sex. Results were stratified by sex to investigate the herd effects of vaccinating girls only on RRprev among heterosexual men, and were stratified by type to examine if model predictions of herd immunity or elimination differed between HPV vaccine types. Base-case vaccine characteristics used by all groups were 100% vaccine efficacy and lifetime duration of protection. In the sensitivity analyses, we varied vaccine efficacy (to 90%) and duration of protection (to 20 years) for fixed vaccination coverage of 40% and 80%. As a secondary outcome, we present RRprev over time since the introduction of vaccination.
Before contacting modelling teams, MB and ÉB assessed if the studies were of sufficient methodological quality to be included in the pooled analysis. Following recommendations from the International Society for Pharmacoeconomics and Outcomes Research and Society for Medical Decision Making (ISPOR-SMDM) modelling good research practices task force,40 the main quality criteria were that the models had to be transmission-dynamic mathematical models and be calibrated to epidemiological data. Given that an objective of the meta-analysis was to examine herd effects and HPV elimination, the transmission-dynamic model criterion was included as an eligibility criterion. Finally, once model results were obtained from the participating modelling teams, MB and ÉB assessed the construct validity of predictions.
Data analysis
We derived pooled predictions of HPV vaccination population-level impact by calculating the median and 10th, 25th, 75th, and 90th percentiles of the predictions of the models, and present RRprev with 80% uncertainty intervals (80% UI; the 10th and 90th percentiles). These pooled results illustrate the central tendency and variability or robustness of model predictions, with all models having equal weight. These pooled results are different to summary estimates from classical meta-analysis of empirical studies, which are pooled with weights based on sample size or variance. Incremental RRprev were obtained by subtracting the RRprev of girls-only vaccination from the RRprev of girls and boys vaccination.
We used univariate linear meta-regressions to identify potential sources of heterogeneity among the different models’ predictions for HPV 16, giving equal weight to all models. We looked at model characteristics in univariate analysis and examined the potential interactions between each characteristic and vaccination coverage (40% and 80%), and between each characteristic and vaccination strategy (girls only and girls and boys). Given the number of models, it was not possible to perform multivariate analysis. We verified that our results were similar when using HPV 18. We used SAS version 9.4 for all analyses.
Role of the funding source
The funders of the study had no role in the study design, data collection, analysis and interpretation, or writing of the report. MB, ÉB, and MD had full access to all the data in the study and MB had final responsibility for the decision to submit for publication.
Results
Our search led to the identification of 51 articles published by 19 different research teams (figure 1). Of the 19 teams contacted to participate in the meta-analysis, 17 provided new standardised model predictions. All models met the main methodological quality criteria. However, one model41 was excluded because it was built for short-term predictions, and demographic change assumptions produced unstable long-term predictions. The 16 models included in the meta-analysis vary in terms of type (deterministic or stochastic), structure (assumptions about sexual activity, partnership formation and dissolution, transmission, and natural immunity), and baseline HPV prevalence, and were developed in ten countries (Australia, Canada, Finland, Germany, Ireland, Italy, Netherlands, Norway, UK, and USA; appendix pp 4–5). HPV 16, 18, 6, and 11 were included in 16, 13, five, and three models, respectively.
Figure 1: Model selection.
HPV=human papillomavirus. HIC=high-income country. EUROGIN=Eurogin International Multidisciplinary Congress. IPV=International Papillomavirus Conference.
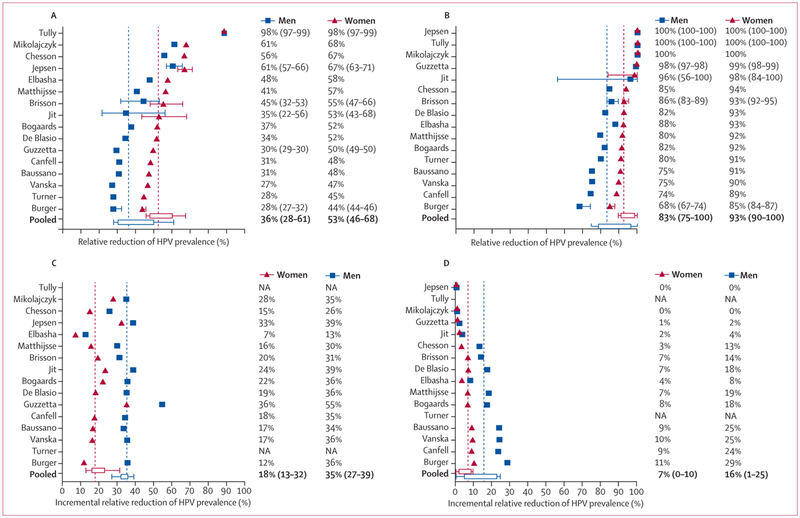
Among women after 70 years of girls-only vaccination, the overall predicted prevalence of HPV 16 decreased by 53% (RRprev 0·53 [80% UI 0·46–0·68]) assuming 40% coverage, and by 93% (RRprev 0·93 [80% UI 0·90–1·00]) assuming 80% coverage (figure 2). At 40% coverage, the corresponding RRprev for HPV 6, 11, and 18 were 7 to 28 percentage points greater than HPV 16, and at 80% coverage these types were eliminated (HPV 11) or close to elimination (HPV 18 and 6; figure 3A, appendix pp 6–13). Given that population-level effectiveness (RRprev) was substantially greater than vaccination coverage for all HPV vaccine-targeted types, these results indicate that girls-only vaccination is expected to produce substantial herd effects for unvaccinated women even at low coverage, with greater herd immunity effects for HPV 18, 6, and 11 than for HPV 16 (figure 3A, appendix p 14).
Figure 2: Population-level impact of HPV vaccination of girls only (A, B) and boys and girls (C, D).
Predicted relative reduction in the prevalence (RRprev) of HPV 16 among women and men after 70 years of girls-only vaccination, assuming 40% (A) and 80% (B) vaccination coverage; and predicted incremental relative reduction in the prevalence of HPV 16 among women and men after 70 years by vaccinating boys in addition to girls only, assuming 40% (C) and 80% (D) vaccination coverage. The pooled estimates are medians and 80% uncertainty intervals (10% and 90% percentile) of predictions. Models with error bars provided uncertainty intervals (10th and 90th percentile) around their median model predictions. When a model’s results includes a median estimate and uncertainty range, the pooled results used the median value. HPV=human papillomavirus. NA=not available.
Figure 3: Pooled predictions of the vaccine-type-specific population-level impact of HPV vaccination.
Relative reduction of HPV prevalence among women and men after70 years of girls-only vaccination (A), and incremental relative reduction in HPV prevalence among women and men after 70 years by vaccinating boys in addition to girls only (B). Shown here are median (line) and 25th and 75th percentiles (box) and 10th and 90th percentiles (whiskers) of the predictions of the models. HPV 11 results have a different presentation due to the few models that include this outcome. See appendix pp 6–9 for forest plots of model predictions for types HPV 16, 18, 6, and 11; and appendix pp 10–13 for values of pooled estimates and uncertainty intervals. HPV=human papillomavirus.
Important herd effects were also predicted in men after girls-only vaccination for both low and high coverage scenarios (figures 2, 3). Among men, the overall prevalence of HPV 16 decreased by 36% after 70 years of girls-only vaccination assuming 40% coverage (RRprev 0·36 [80% UI 0·28–0·61]), and by 83% assuming 80% coverage (0·83 [0·75–1·00]; figure 2). The RRprev of HPV 18, 6, and 11 were again greater than that of HPV 16 (figure 3A, appendix p 14).
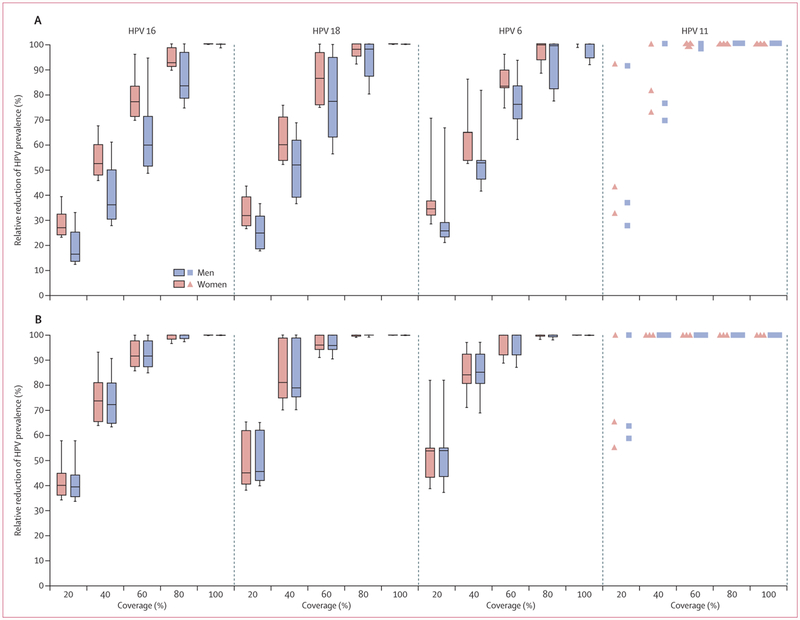
Post-vaccination reduction in HPV prevalence over time after girls-only vaccination was faster for women than men, for types HPV 6 and 11 compared with HPV 16 and 18, and at higher coverage than at lower coverage (appendix pp 15–16).
Vaccinating 40% of boys in addition to 40% of girls (girls and boys strategy) resulted in incremental reductions of HPV 16 prevalence of 18% among women (incremental RRprev 0·18 [80% UI 0·13–0·32]) and 35% among men (0·35 [0·27–0·39]) after 70 years (figure 2C). Given the important herd effects following girls-only vaccination with 80% coverage, vaccinating 80% of boys in addition to girls produced small incremental reductions in HPV 16 prevalence (incremental RRprev of 0·07 [80% 0·00–0·10] for women and 0·16 [80% 0·01–0·25] for men), after 70 years (figure 2D). The incremental population-level effectiveness of vaccinating boys in addition to girls was similar between the HPV vaccine types when assuming 40% coverage, but was substantially higher for HPV 16 (vs HPV 18, 6, 11) when assuming 80% coverage (figure 3B). Vaccinating boys in addition to girls produced a slightly faster decline in vaccine-type-specific prevalence among women and men (appendix pp 15–16).
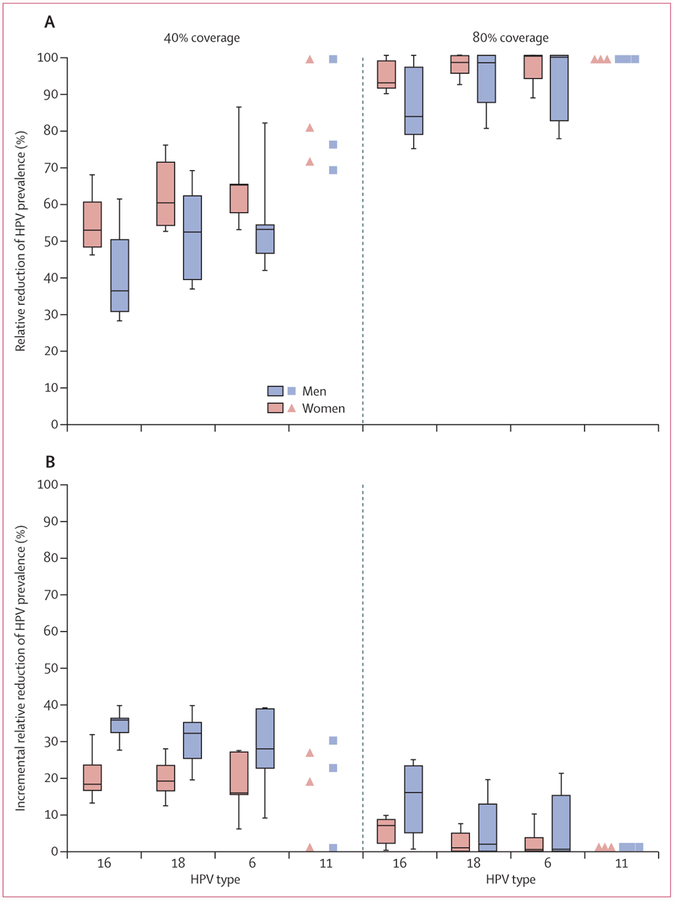
For the same number of additional vaccinated individuals, increasing coverage in a girls-only strategy was predicted to provide greater population-level benefits than was including boys in a vaccination programme. The models predicted that increasing girls-only vaccination coverage by 40% (from 40% to 80%) reduced HPV 16, 18, 6, and 11 prevalence in women by an additional 40%, 38%, 35%, and 19%, respectively; higher than the incremental benefits of vaccinating 40% of boys in addition to 40% of girls (figure 4; appendix pp 10–13, 15–16). The same increase in girls-only vaccination coverage from 40% to 80% was also more effective in reducing HPV 16, 18, and 6 prevalence in men than the incremental benefit of vaccinating 40% of boys in addition to 40% of girls, and the strategies were equally effective for HPV 11 (figure 4, appendix pp 10–13).
Figure 4: Pooled predictions according to vaccination coverage and vaccine type.
Relative reduction of HPV prevalence among women and men after girls-only vaccination (A) and after vaccination of boys in addition to girls (B). Shown here are median (line) and 25th and 75th percentiles (box) and 10th and 90th percentiles (whiskers) of the predictions of the models. HPV 11 results have a different presentation due to the limited number of models that include this outcome. See appendix pp 6–9 for forest plots of model predictions for types HPV 16, 18, 6, and 11; and appendix pp 10–13 for values of pooled estimates and uncertainty intervals. HPV=human papillomavirus.
Increasing coverage considerably improved population-level effectiveness up to 80% for girls-only vaccination and 60% for girls and boys vaccination, after which, increasing coverage had very little marginal benefit (figure 4). Substantial herd effects were predicted with girls-only vaccination coverage as low as 20% (figure 4, appendix pp 10–13). Interestingly, 19% (three of 16), 46% (six of 13), 60% (three of five), and 100% (three of three) of models predicted that HPV 16, 18, 6, and 11, respectively, would be eliminated among heterosexual populations if girls-only vaccination reaches 80% coverage (figure 4, appendix p 14). The girls and boys strategy substantially increased the predicted potential for elimination; 64%, 92%, 80%, and 100% of models predicted that HPV 16, 18, 6, and 11 would be eliminated with 80% coverage, and a few models predicted elimination of types HPV 18, 6, and 11 with 60% coverage (figure 4, appendix p 14).
Predicted population-level effectiveness and herd effects were much lower and more variable across the models when assuming that vaccine duration of protection was 20 years compared with lifelong protection (appendix p 17). However, reducing vaccine efficacy from 100% to 90% had little impact on the pooled predictions (appendix p 17).
In the meta-regression analysis, vaccination coverage and vaccination strategies were the main sources of heterogeneity between the predictions of the models (data not shown). However, the meta-regression results are presented separately by vaccination coverage and vaccination strategy, given that significant interactions were observed between these variables and the other model characteristics (appendix pp 18–19). Across all vaccination scenarios, the main source of heterogeneity in predictions for women and men was whether the transmission-dynamic models included (or not) different sexual activity risk groups (appendix pp 18–19). Models that did not include risk groups for sexual activity predicted significantly higher population-level HPV vaccination effectiveness and herd effects. Population-level effectiveness was also significantly higher among models that assume lower natural immunity among women (mostly when vaccine protection is assumed lifelong), or include the natural history of cervical cancer (when vaccine protection is 20 years). These results were similar when examining model predictions for HPV 18.
Discussion
The findings from our systematic review and meta-analysis suggest that HPV vaccination will produce strong herd effects leading to substantial long-term reductions in HPV infection and related diseases in unimmunised women and men. Herd effects are predicted even with vaccination coverage as low as 20%, and to be greater for HPV 18, 6, and 11 types than for HPV 16. Given the substantial herd effects of girls-only vaccination when coverage is moderate to high, the incremental benefit of vaccinating boys is predicted to be small. However, most models predict that vaccinating boys in addition to girls could eliminate HPV 16, 18, 6, and 11, as long as 80% coverage is achieved in both sexes and the vaccine confers long-term protection.
Our findings have important policy implications. First, because of the important herd effects from girls-only vaccination, our models predict that increasing HPV vaccination coverage among girls has greater incremental benefit for both male and female individuals than adding boys to a vaccination programme. The herd effects for heterosexual men are predicted to be about the same magnitude as the level of girls-only vaccination coverage. Given this, the epidemiological and economic considerations about vaccinating boys should focus on the following issues: the feasibility and incremental marginal costs of increasing vaccination coverage among girls versus introducing a gender-neutral programme;42 whether the price of the vaccine can be reduced for boys for the strategy to be cost-effective; and the importance placed on achieving equal protection for men who have sex with men (MSM).
Second, our models suggest that elimination of HPV 6, 11, and 18 is likely if vaccination coverage of girls and boys reaches 80% and the vaccine provides long-term protection. However, vaccination coverage might have to be slightly higher than 80% to eliminate HPV 16, the type responsible for most of the HPV-related burden. These results also have implications for the recently licensed nonavalent vaccine. Given their prevalence and biological characteristics, the additional types are expected to behave similarly to type HPV 18 and thus be easier to eliminate than HPV 16. It should also be noted that the time to elimination might be shorter than was predicted in the meta-analysis (appendix pp 15–16), because we did not include scenarios with catch-up programmes. Policy makers might want to examine whether to set objectives to eliminate HPV vaccine types in their jurisdictions and set vaccination coverage targets.
Third, post-vaccination reduction of HPV 6 and 11 infections was predicted to be steeper than for HPV 16 and 18 infections, which is due to the shorter durations of infectiousness and lower transmissibility of these low oncogenic risk types.43 Hence, HPV 6 and 11-related disease (eg, anogenital warts) trends are a poor proxy of change in HPV 16 and 18 and related diseases, and should not be directly extrapolated to inform policy decisions regarding prevention of HPV-related cancers and its precursors.
Fourth, the models predicted substantial herd effects even at relatively lower coverage (40%). However, a recent meta-analysis found evidence of significant herd effects only in higher-coverage settings.37 Our study findings suggest this difference is likely to be because herd effects take longer to become evident where coverage is lower. Early programme impact data should not be used to rule out herd effects in settings where coverage is low.
This study addresses a key source of concern when using mathematical model results for decision making: the robustness and validity of predictions. By systematically identifying published HPV transmission-dynamic models that were independently developed from one another and presenting the distribution of predictions, our analysis provides decision makers with a measure of uncertainty and robustness surrounding model predictions. Reassuringly, the key conclusions of this systematic review were consistent among the models, even though the models were built to represent different countries and varied in structure and assumptions.
According to good modelling practice guidelines, model validation should include between-model corroboration using independent models and, if possible, external or predictive validation.44 Our systematic review shows high consistency in HPV model predictions of population-level reductions in HPV vaccine types among women and men when vaccination coverage is 80% or higher for girls only and 60% or higher for girls and boys vaccination. The models also consistently predict that post-vaccination reductions will be smaller for HPV 16 than the other vaccine types. However, there is substantial variability in predictions when vaccination coverage is between 40–60%, and when duration of vaccine protection is 20 years. Our meta-regression identified two key model choices or parameters that explain this variability: inclusion of sexual activity risk groups in the model and the level of natural immunity among women. Sexual activity has been shown to be very heterogeneous in populations (eg, skewed distribution of number of partners), leading most models built for prediction of interventions against sexually transmitted diseases to include sexual risk groups. Furthermore, a recent meta-analysis found that the proportion of women who develop natural immunity against a subsequent HPV 16 or 18 infection is greater than 35% when controlling for potential confounders or using neutralising assays.45 When considering only the predictions of models that include different sexual activity risk groups and that assume that the proportion of women who develop natural immunity is greater than 35%, the variability in predictions is substantially reduced with little impact on the median pooled estimates (eg, at 40% vaccination coverage, the RRprev of HPV 16 among women after girls-only vaccination is 0·52 [80% UI 0·45–0·58] vs 0·53 [0·46–0·68] when considering all predictions (appendix pp 20–21). Finally, although external or predictive validation was beyond the scope of the paper, the descriptive results of the pooled analysis are in line with results from a recent meta-analysis of post-HPV vaccination data,37 showing strong herd effects shortly after vaccination in countries with high vaccination coverage and very little evidence of herd effects in this timeframe where coverage is low. In addition, models included in the systematic review have previously shown that they reproduce the reduction in HPV 16 and 18 infection46,47 and anogenital warts consultations observed in the first 5 years after HPV vaccination introduction.48
Our main findings are likely generalisable to most high-income countries given that they are based on models from ten of these countries and very little heterogeneity remains when results are stratified by vaccination coverage, vaccination strategy, and key model characteristics. In addition, there are similarities in sexual behaviour,49 HPV type distribution,50,51 and age profile of HPV prevalence52 among high-income countries. However, our predicted herd effects and potential for elimination should be extrapolated to low-income and middle-income countries (LMIC) with caution, because there are important differences between high-income countries and LMICs in sexual behaviour and potential cofactors of HPV such as high HIV prevalence.49,53,54
The study has two main limitations that should be considered, in addition to those inherent in meta-analysis.55,56 First, although the models were built independently from one another and showed variability in their structures, similarities remain in key structural assumptions, which could contribute to the consistency of findings. None of the models included MSM, incorporated multisite infection or transmission (transmission between oral, anal, and genital sites), or the possibility of reactivation. Modelling HPV transmission between men would be unlikely to have an effect on predictions about the herd effects of girls-only vaccination, but could slightly impact predictions about elimination. Including higher risk groups or MSM, who have greater HPV prevalence (and higher R0), would make it more difficult to eliminate HPV, and therefore the coverage required for elimination could be higher than estimated by the models in the systematic review. On the other hand, recent modelling studies suggest that adding multisite transmission or reactivation would probably have very little impact on long-term predictions about the population-level effectiveness of HPV vaccination.57,58 Second, we purposely decided to examine the predicted impact of vaccination on HPV infection rather than disease endpoints, as the main focus of the paper is on herd effects and elimination of HPV vaccine types. Additional uncertainty in HPV model predictions, when examining the impact of vaccination on disease endpoints, will be in the absolute reduction of HPV burden of disease (related to country specific pre-vaccination burden of disease and proportion due to the vaccine types) and the timing of these benefits (related to assumptions about progression from infection to lesions or cancer).
To our knowledge, our study includes a greater number of models than any published comparison of infectious disease models, reflecting the influential role that modelling has played in HPV vaccination policy decisions. Furthermore, this is the first model comparison study in which systematic review methodology was used to identify all potentially eligible mathematical models (reducing possible selection bias); independence of models was controlled for by including only one model per team and producing predictions for predetermined and standardised scenarios without prior harmonisation of model structures or natural history parameters; and meta-analytic techniques were used to describe the central tendency and variability of predictions and identify sources of heterogeneity in results. Finally, our study shows that the HPV models developed over the past decade give consistent results in terms of population-level effectiveness against infection, herd effects, and the possibility of elimination, which could be reassuring for decision makers using these models for vaccination policy decisions. We also identified key HPV modelling choices (ie, inclusion of sexual risk groups, natural history of cervical cancer, and natural immunity) that have the greatest influence on model predictions, which should guide the future development of models.
In conclusion, the results of this study indicate strong promise for the long-term population-level impact of HPV vaccination programmes. However, it will be important to continue to validate HPV model predictions and to compare them to post-vaccination surveillance data, as many policy decisions about HPV vaccination (ie, number of doses, which vaccine to use, and vaccination of boys) and cervical cancer screening remain.
Supplementary Material
Research in context.
Evidence before this study
Many models have been developed over the past decade to understand HPV epidemiology and to help guide policy decisions concerning HPV vaccination. However, it is unknown whether these models are giving consistent results in terms of HPV vaccination population-level effectiveness, herd effects, incremental benefit of vaccinating boys in addition to girls, and elimination. To examine this question, we conducted a systematic review and meta-analysis of HPV transmission-dynamic model predictions of the long-term population-level effectiveness of HPV vaccination against HPV 6, 11, 16, and 18 infection in women and men. To our knowledge, this is the first meta-analysis of HPV model predictions, and is the first to examine the potential for elimination.
Added value of the study
Our study shows that the HPV models give generally consistent results, which can be reassuring for decision makers using these models for vaccination policy decisions. Results suggest that HPV vaccination will produce strong herd effects leading to substantial long-term reductions in HPV infection and related diseases in unimmunised women and men. Herd effects are predicted even with vaccination coverage as low as 20%. Given the substantial herd effects of girls-only vaccination when coverage is moderate to high, the incremental benefit of vaccinating boys is predicted to be small. To our knowledge, our study is the first to suggest that elimination of vaccine-targeted HPV types is possible if vaccination coverage of girls and boys reaches 80%. Finally, our study includes a greater number of modelsthan any published comparison of infectious disease models, reflecting the influential role modelling has played in HPV vaccination policy decisions.
Implications of available evidence
HPV vaccination is likely to have a strong direct and indirect impact across different countries and coverage levels. The case for vaccinating boys could mainly depend on other issues besides the predicted additional benefit for heterosexual males, such as cost of the vaccine for boys, feasibility of increasing coverage in girls, and equity for men who have sex with men. Although early post-HPV vaccination surveillance data do not show herd effects in settings with low coverage, our study suggests that this is probably because herd effects take longer to become evident when coverage is lower. Policy makers might want to examine whether to set objectives to eliminate HPV vaccine types in their jurisdictions.
Acknowledgments
The main funder of this study was the Canadian Institutes of Health Research. We received support from the Canada Research Chairs programme (support for MB), an operating grant from the Canadian Institutes of Health Research (number MOP-119427), and a foundation scheme grant from the Canadian Institutes of Health Research (number FDN-143283). IB is supported by the European Community’s Seventh Framework Programme (FP7-HEALTH-2013; grant number 603019) and the Bill & Melinda Gates Foundation (number OPP1053353), and is an Honorary Research Fellow at the School of Public Health, Imperial College, London, UK. MRJ was supported by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Immunisation at the London School of Hygiene & Tropical Medicine in partnership with Public Health England. JB is supported from the European Community’s Seventh Framework Programme (FP7-HEALTH-2013; grant no. 603019). JJK and EAB are supported by the US National Cancer Institute of the National Institutes of Health (number R01CA160744). KC is supported by a NHMRC Career Development Fellowship Grant (number AP1082989). The views expressed here are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health or Public Health England nor the Centers for Disease Control and Prevention.
Declaration of interests
MB has received an unrestricted grant from Merck (for herpes zoster; project is finished). MD has consulted for GlaxoSmithKline (herpes zoster vaccine). JAB has received consultancy fees from GlaxoSmithKline and Merck (fees were collected by his employer). AP and MP are employees of Merck. All other authors declare no competing interests.
Footnotes
See Online for appendix
References
- 1.Clifford GM, Smith JS, Aguado T, Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer 2003; 89: 101–05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348: 518–27. [DOI] [PubMed] [Google Scholar]
- 3.De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer 2009; 124: 1626–36. [DOI] [PubMed] [Google Scholar]
- 4.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 2005; 14: 467–75. [DOI] [PubMed] [Google Scholar]
- 5.Guan P, Howell-Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV-positive women: A meta-analysis from cervical infection to cancer. Int J Cancer 2012; 131: 2349–59. [DOI] [PubMed] [Google Scholar]
- 6.Garland SM, Steben M, Sings HL, et al. Natural history of genital warts: analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis 2009; 199: 805–14. [DOI] [PubMed] [Google Scholar]
- 7.Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374: 301–14. [DOI] [PubMed] [Google Scholar]
- 8.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356:1928–43. [DOI] [PubMed] [Google Scholar]
- 9.Brotherton J, Bloem P. HPV Vaccination: Current Global Status. Curr Obstet Gynecol Rep 2015; 4: 220–33. [Google Scholar]
- 10.World Health Organization. Countries with HPV vaccine in the national immunization programme and planned introductions. World Health Organization/IVB Database; January 2014. http://www.who.int/immunization/diseases/hpv/decision_implementation/en/ (accessed Sept 15, 2014). [Google Scholar]
- 11.Centers for Disease Control and Prevention. Recommendations on the use of quadrivalent human papillomavirus vaccine in males. Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011; 60: 1705–08. [PubMed] [Google Scholar]
- 12.Georgousakis M, Jayasinghe S, Brotherton J, Gilroy N, Chiu C, Macartney K. Population-wide vaccination against human papillomavirus in adolescent boys: Australia as a case study. Lancet Infect Dis 2012; 12: 627–34. [DOI] [PubMed] [Google Scholar]
- 13.Switzerland Federal Office of Public Health. Human papillomavirus. http://www.bag.admin.ch/themen/medizin/00682/00684/03853/index.html?lang=en (accessed Dec 18, 2015).
- 14.Department of Health and Wellness. Prince Edward Island. Immunization schedule http://www.gov.pe.ca/health/immunizationschedule (accessed Dec 18, 2015).
- 15.Alberta Health Services. Alberta prevents cancer. http://albertapreventscancer.ca/reduce-your-risk/prevent-hpv-infections/ (accessed Dec 18, 2015).
- 16.Ministère de la Santé et des Services Sociaux du Québec (MSSS). Vaccin contre les infections par les virus du papillome humain (VPH). http://sante.gouv.qc.ca/conseils-et-prevention/vaccin-contre-les-infections-par-les-virus-du-papillome-humain-vph/ (accessed Dec 18, 2015).
- 17.Austrian Ministry of Health. HPV vaccination. http://www.bmg.gv.at/home/Timeline/Timeline/?timeline_mode=list&yat-table1-filters-tags=Pr%C3%A4vention (accessed Feb 26, 2016).
- 18.Markowitz LE, Dunne EF, Saraiya M, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rec 2014; 63: 1–30. [PubMed] [Google Scholar]
- 19.Comité sur l’immunisation du Québec (CIQ) et Comité scientifique ad hoc VPH. La Vaccination contre les VPH au Québec: Mise à jour des connaissances et propositions du comité d’experts. Direction des risques biologiques et de la santé au travail, Institut national de santé publique Québec (INSPQ), 2012: chapter 2. [Google Scholar]
- 20.Canadian Immunization Committee. Recommendations on a human papillomavirus immunization program. Ontario: Canadian Immunization Committee, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.JCVI. Human papillomavirus vaccines to protect against cervical cancer. London: Joint Committee on Vaccination and Immunisation, 2008. [Google Scholar]
- 22.World Health Organization. Evidence based recommendations on Human Papilloma Virus (HPV) vaccines schedules: Background paper for SAGE discussions; March 11, 2014. http://www.who.int/immunization/sage/meetings/2014/april/1_HPV_Evidence_based_recommendationsWHO_with_Appendices2_3.pdf (accessed July 23, 2014). [Google Scholar]
- 23.Advisory Committee on Immunization Practices (ACIP). Meeting of the Advisory Committee on Immunization Practices (ACIP) October 29–30, 2014—Summary Report. Atlanta: Centers for Disease Control and Prevention (CDC), 2015. [Google Scholar]
- 24.Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 2015; 372: 711–23. [DOI] [PubMed] [Google Scholar]
- 25.Brisson M, Van de Velde N, Boily MC. Economic evaluation of human papillomavirus vaccination in developed countries. Public Health Genomics 2009; 12: 343–51. [DOI] [PubMed] [Google Scholar]
- 26.Fesenfeld M, Hutubessy R, Jit M. Cost-effectiveness of human papillomavirus vaccination in low and middle income countries: a systematic review. Vaccine 2013; 31: 3786–804. [DOI] [PubMed] [Google Scholar]
- 27.Jit M, Brisson M, Portnoy A, Hutubessy R. Cost-effectiveness of female human papillomavirus vaccination in 179 countries: a PRIME modelling study. Lancet Glob Health 2014; 2: e406–14. [DOI] [PubMed] [Google Scholar]
- 28.Brisson M, van de Velde N, Franco EL, Drolet M, Boily MC. Incremental impact of adding boys to current human papillomavirus vaccination programs: role of herd immunity. J Infect Dis 2011; 204: 372–76. [DOI] [PubMed] [Google Scholar]
- 29.Choi YH, Jit M, Gay N, Cox A, Garnett GP, Edmunds WJ. Transmission dynamic modelling of the impact of human papillomavirus vaccination in the United Kingdom. Vaccine 2010; 28: 4091–102. [DOI] [PubMed] [Google Scholar]
- 30.Bogaards JA, Kretzschmar M, Xiridou M, Meijer CJ, Berkhof J, Wallinga J. Sex-specific immunization for sexually transmitted infections such as human papillomavirus: insights from mathematical models. PLoS Med 2011; 8: e1001147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JJ, Goldie SJ. Cost effectiveness analysis of including boys in a human papillomavirus vaccination programme in the United States. BMJ 2009; 339: b3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chesson HW, Ekwueme DU, Saraiya M, Dunne EF, Markowitz LE. The cost-effectiveness of male HPV vaccination in the United States. Vaccine 2011; 29: 8443–50. [DOI] [PubMed] [Google Scholar]
- 33.Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine 2010; 28: 6858–67. [DOI] [PubMed] [Google Scholar]
- 34.Ben Hadj Yahia MB, Jouin-Bortolotti A, Dervaux B. Extending the human papillomavirus vaccination programme to include males in high-income countries: a systematic review of the cost-effectiveness studiess. Clin Drug Investig 2015; 35: 471–85. [DOI] [PubMed] [Google Scholar]
- 35.Smith MA, Lew JB, Walker RJ, Brotherton JM, Nickson C, Canfell K. The predicted impact of HPV vaccination on male infections and male HPV-related cancers in Australia. Vaccine 2011; 29: 9122–22. [DOI] [PubMed] [Google Scholar]
- 36.Bogaards JA, Wallinga J, Brakenhoff RH, Meijer CJ, Berkhof J. Direct benefit of vaccinating boys along with girls against oncogenic human papillomavirus: bayesian evidence synthesis. BMJ 2015; 350: h2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drolet M, Benard E, Boily MC, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2015; 15: 565–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali H, Donovan B, Wand H, et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data. BMJ 2013; 346: f2032. [DOI] [PubMed] [Google Scholar]
- 39.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pitman R, Fisman D, Zaric GS, et al. Dynamic Transmission Modeling: A Report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-5. Med Decis Making 2012; 32: 712–21. [DOI] [PubMed] [Google Scholar]
- 41.Shafer LA, Jeffrey I, Elias B, Shearer B, Canfell K, Kliewer E. Quantifying the impact of dissimilar HPV vaccination uptake among Manitoban school girls by ethnicity using a transmission dynamic model. Vaccine 2013; 31: 4848–55. [DOI] [PubMed] [Google Scholar]
- 42.Ryser MD, McGoff K, Herzog DP, Sivakoff DJ, Myers ER. Impact of coverage-dependent marginal costs on optimal HPV vaccination strategies. Epidemics 2015; 11: 32–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brisson M, Van de Velde N, Boily MC. Different population-level vaccination effectiveness for HPV types 16, 18, 6 and 11. Sex Transm Infect 2011; 87: 41–43. [DOI] [PubMed] [Google Scholar]
- 44.Weinstein MC, O’Brien B, Hornberger J, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices—Modeling Studies. Value Health 2003; 6: 9–17. [DOI] [PubMed] [Google Scholar]
- 45.Beachler DC, Jenkins G, Safaeian M, Kreimer AR, Wentzensen N. Natural acquired immunity against subsequent genital human papillomavirus infection: a systematic review and meta-analysis. J Infect Dis 2016; 213: 1444–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith MA, Canfell K. Testing previous model predictions against new data on human papillomavirus vaccination program outcomes. BMC Res Notes 2014; 7: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elfstrom KM, Lazzarato F, Franceschi S, Dillner J, Baussano I. Human papillomavirus vaccination of boys and extended catch-up vaccination: effects on the resilience of programs. J Infect Dis 2016; 213: 199–205. [DOI] [PubMed] [Google Scholar]
- 48.Brisson M, Van de Velde N, Drolet M, et al. HPV-advise: technical appendices. http://www.marc-brisson.net/HPVadviseCEA.pdf (accessed Aug 2, 2016).
- 49.Wellings K, Collumbien M, Slaymaker E, et al. Sexual behaviour in context: a global perspective. Lancet 2006; 368: 1706–28. [DOI] [PubMed] [Google Scholar]
- 50.de Sanjose S, Diaz M, Castellsague X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis 2007; 7: 453–59. [DOI] [PubMed] [Google Scholar]
- 51.Clifford GM, Smith JS, Plummer M, Munoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer 2003; 88: 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruni L, Diaz M, Castellsague X, Ferrer E, Bosch FX, de Sanjose S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis 2010; 202: 1789–99. [DOI] [PubMed] [Google Scholar]
- 53.Adler DH, Kakinami L, Modisenyane T, et al. Increased regression and decreased incidence of HPV-related cervical lesions among HIV-infected women on HAART. AIDS 2012; 26: 1645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baussano I, Lazzarato F, Brisson M, Franceschi S. Human papillomavirus vaccination at a time of changing sexual behavior. Emerg Infect Dis 2016; 22: 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greenland S Can meta-analysis be salvaged? Am J Epidemiol 1994; 140: 783–87. [DOI] [PubMed] [Google Scholar]
- 56.Bailar JC 3rd. The promise and problems of meta-analysis. N Engl J Med 1997; 337: 559–61. [DOI] [PubMed] [Google Scholar]
- 57.Lemieux-Mellouki P, Drolet M, Jit M, Brisson M. Modelling multi-site transmission of HPV and its impact on vaccine effectiveness against HPV. 30th International Papillomavirus Conference; Lisbon, Portugal; Sept 17–21, 2015. [Google Scholar]
- 58.Korostil IA, Regan DG. The potential impact of HPV-16 reactivation on prevalence in older Australians. BMC Infect Dis 2014; 14: 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.