Abstract
Introduction
Symptomatic anti-Alzheimer's disease (AD) drugs have been commonly used for the treatment of AD. Knowing the natural courses of patients with AD on placebo is highly relevant for clinicians to understand their efficacy and for investigators to design clinical studies.
Methods
The data on rating scales for dementia such as Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-cog) and Severe Impairment Battery were extracted from eight previous Japanese Phase II and III studies. Natural courses of Japanese AD patients in placebo groups were evaluated and statistically analyzed in a pooled and retrospective fashion.
Results
Decreases in ADAS-cog and Severe Impairment Battery was larger at week 22 or 24 than at week 12. Scores of ADAS-cog appeared to deteriorate faster in moderate AD than in mild AD.
Discussion
The present data will provide clinicians following up patients with AD with helpful information on how to manage AD patients and investigators with instruction for clinical study design.
Keywords: Alzheimer's disease, Natural course, Acetylcholine esterase inhibitor, Memantine, ADAS-cog, Mini-Mental State Examination, Severe Impairment Battery
1. Background
Alzheimer's disease (AD) is a devastating progressive neurodegenerative disorder characterized by symptoms of dementia such as impairment in memory and learning, disorientation, deficits in executive function, and behavioral and psychological symptoms of dementia [1], [2]. According to the Alzheimer's Disease International's World Alzheimer Report 2015 (Website: alz.co.uk/research/WorldAlzheimerReport2015.pdf), the global number of patients with dementia was estimated to be 46.8 million in 2015, and the number will almost double every 20 years, with major types of dementia (60–90% of dementia) being AD (Diagnostic and Statistical Manual of Mental Disorders 5) [1]. The number of patients with AD is currently reported to be more than three million in Japan [3], laying not only a significant physical and psychological burden on patients with AD and their caregivers but also increasing economic obligation to the country as a whole.
Acetylcholinesterase inhibitors (AChEIs [i.e., donepezil, galantamine, and rivastigmine] and N-methyl-D-aspartate receptor antagonists [i.e., memantine]) have been approved worldwide for the treatment of AD as symptomatic drugs and are commonly used in current clinical practice in Japan as well as in other countries. However, it is sometimes difficult for prescribers/clinicians to evaluate the efficacy of these drugs. Knowing the natural courses of patients with AD not treated with these drugs is highly relevant for prescribers/clinicians to understand the efficacy of these drugs and explain the importance of these drugs to their patients.
Success rate of development of drugs for AD has been reported to be low, with the lack of understanding of the natural progression of AD being one reason [4], and detailed analyses on the natural courses of AD may also lead to help determine procedures for future clinical studies for AD such as designs of clinical studies and patient selection. However, reports on these subjects have been limited [5], [6], [7], [8], [9], [10], [11], [12]. In this analysis, we extracted data on demographics of Japanese patients and rating scales for dementia such as Mini-Mental State Examination (MMSE), Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-cog), Severe Impairment Battery (SIB), and Clinical Dementia Rating Scales Sum of Boxes (CDR-SB) from eight previous placebo-controlled, double-blind, randomized Japanese clinical trials targeting AD, and report and review the natural courses of Japanese AD patients on placebos.
2. Methods
2.1. Study design and outcomes
Data from Japanese AD patients randomized to the placebo group were collected from eight previous placebo-controlled, double-blind, randomized Japanese clinical trials targeting AD: EIS-161 (Eisai), JPN-3 (Janssen), MA3301 (Daiichi-Sankyo), JPN-5 (Janssen), and D1301 (Novartis/Ono) were designed for mild to moderate AD, and IE2101 (Daiichi-Sankyo), EIS-231 (Eisai), and IE3501 (Daiichi-Sankyo) for severe AD (Table 1). All these trials were conducted for approved symptomatic drugs (i.e., donepezil, galantamine, rivastigmine, and memantine). All the clinical trials were carried out in accordance with the Declaration of Helsinki, and written informed consent was obtained from all participants. Data of AD patients in placebo groups not those of active drugs were extracted in this study. The last trial visit had been set at week 24 for all subjects except for JPN-3 which was set at week 22. Because some data on the registration year and date in D1301 were modified during the anonymization, only the data not affected by this process were utilized for this analysis.
Table 1.
Summary of study designs
| Trial name | Study design | AD severity | Drugs and doses | Treatment period | Main inclusion criteria | Primary endpoint | Other efficacy endpoints |
|---|---|---|---|---|---|---|---|
| EIS-161 | Randomized, placebo-controlled, parallel-group, double-blind | Mild to moderate | Donepezil 5 mg/day, placebo | 24 weeks |
|
ADAS-Cog CGIC | MENFIS, CDR, Caregiver-rated modified Crichton scale |
| JPN-3 | Randomized, placebo-controlled, parallel-group, double-blind | Mild to moderate | Galantamine 16 mg/day, galantamine 24 mg/day, placebo | 22 weeks |
|
ADAS-Cog CIBIC Plus | DAD, BEHAVE-AD, MENFIS |
| MA3301 | Randomized, placebo-controlled, parallel-group, double-blind | Mild to moderate | Memantine 10 mg/day, memantine 20 mg/day, placebo | 24 weeks |
|
ADAS-Cog CIBIC Plus | DAD, Crichton Geriatric Behavioral Rating Scale, MMSE, CDR |
| JPN-5 | Randomized, placebo-controlled, parallel-group, double-blind | Mild to moderate | Galantamine 16 mg/day, galantamine 24 mg/day, placebo | 24 weeks |
|
ADAS-Cog CIBIC Plus | DAD, BEHAVE-AD, MENFIS |
| D1301 | Randomized, double-blind, placebo-controlled, dose-finding | Mild to moderate | Rivastigmine 9 mg/5 cm2 patch, rivastigmine 18 mg/10 cm2 patch, placebo | 24 weeks |
|
ADAS-Cog CIBIC Plus | Secondary endpoint
|
| IE2101 | Randomized, placebo-controlled, parallel-group, double-blind | Severe | Memantine 10 mg/day, memantine 20 mg/day, placebo | 24 weeks |
|
SIB ADCS-ADL | CIBIC Plus NPI MMSE FAST |
| EIS-231 | Randomized, placebo-controlled, parallel-group, double-blind | Severe | Donepezil 5 mg/day, donepezil 10 mg/day, placebo | 24 weeks |
|
SIB CIBIC Plus | ADCS-ADL, BEHAVE-AD |
| IE3501 | Randomized, placebo-controlled, parallel-group, double-blind | Severe | Memantine 20 mg/day, placebo | 24 weeks |
|
SIB CIBIC Plus | NA |
Abbreviations: AD, Alzheimer's disease; ADAS-cog, Alzheimer's Disease Assessment Scale-cognitive subscale; SIB, Severe Impairment Battery; DSM, Diagnostic and Statistical Manual of Mental Disorders; MMSE, Mini-Mental State Examination; CDR, Clinical Dementia Rating; CGIC, Clinical Global Impression of Change; CIBIC plus, Clinician's Interview-Based Impression of Change plus caregiver input; DAD, Disability Assessment for Dementia; BEHAVE-AD, Behavioral pathology in Alzheimer's Disease; MENFIS, Mental Function Impairment Scale; NPI, Neuropsychiatric Inventory; ADCS-ADL, Alzheimer's disease Cooperative Study-ADL scale; FAST, Functional assessment staging; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke & the Alzheimer's Disease and Related Disorders Association; NA, not applicable.
Table 2 presents a summary of the obtained data from these trials. The data on ADAS-cog for cognitive function (score range 0–70, higher scores correlated with worse cognitive function), SIB for cognitive function (range 0–100, higher scores with better cognitive function), MMSE for cognitive function (range 0–30, higher scores with better cognitive function), CDR-SB for severity of dementia (range 0–18, higher scores with more severe dementia), Clinician's Interview-Based Impression of Change plus caregiver input (CIBIC plus) for comprehensive global measure of detectable change in function, behavior and cognition (range 0–7 for each domain, change of higher scores with change of worse symptoms), and its subdomains (Disability Assessment for Dementia for activity of daily living [ADL], Behavioral pathology in Alzheimer's Disease [BEHAVE-AD] for behavioral and psychological symptoms of dementia, Mental Function Impairment Scale for cognitive function, and Alzheimer's Disease Cooperative Study-ADL scale [ADCS-ADL] for ADL) were extracted as test batteries for evaluating the state of dementia from all trials where available.
Table 2.
Summary of patients in placebo groups from eight placebo-controlled randomized trials targeting AD
| AD severity |
Mild to moderate |
Severe |
||||||
|---|---|---|---|---|---|---|---|---|
| Trial name | EIS-161 | JPN-3 | MA3301 | JPN-5 | D1301 | IE2101 | EIS-231 | IE3501 |
| Company name | Eisai | Janssen | Daiichi-Sankyo | Janssen | Novartis/Ono | Daiichi-Sankyo | Eisai | Daiichi-Sankyo |
| Total number of patients | 129 | 136 | 180 | 194 | 268 | 107 | 102 | 208 |
| Trial period | 1996–1998 | 2001–2003 | 2003–2007 | 2006–2008 | 2007–2008 | 2002–2004 | 2003–2004 | 2005–2008 |
| Visit (weeks) | −4, 0, 4, 8, 12, 16, 20, 24 | −4, 0, 12, 22 | −4, 0, 4, 12, 24 | −4, 0, 8, 12, 16, 24 | 0, 8, 16, 24 | 0, 4, 12, 24 | 0, 8, 16, 24 | 0, 4, 12, 24 |
| ADAS-cog | + | + | + | + | + | − | − | − |
| SIB | - | - | - | - | - | + | + | + |
| MMSE | + | + | + | + | + | + | + | - |
| CDR | + | - | + | - | - | - | - | - |
| CIBIC plus | - | + | + | + | + | + | + | + |
| DAD | - | + | + | + | + | + | - | - |
| BEHAVE-AD | - | + | - | + | + | + | + | + |
| MENFIS | + | + | - | + | + | + | - | + |
| ADCS-ADL | - | - | - | - | - | + | + | - |
| Laboratory data | + | + | + | + | + | + | + | + |
| Vital sign | + | - | + | - | + | + | + | + |
Abbreviations: AD, Alzheimer's disease; ADAS-cog, Alzheimer's Disease Assessment Scale-cognitive subscale; SIB, Severe Impairment Battery; MMSE, Mini-Mental State Examination; CDR, Clinical Dementia Rating; CIBIC plus, Clinician's Interview-Based Impression of Change plus caregiver input; DAD, Disability Assessment for Dementia; BEHAVE-AD, Behavioral pathology in Alzheimer's Disease; MENFIS, Mental Function Impairment Scale; ADCS-ADL, Alzheimer's disease Cooperative Study-ADL scale; +, present; -, absent.
2.2. Statistical analyses
The distribution of demographic and baseline characteristics for the placebo groups of the eight trials are summarized in the descriptive statistical values and frequency table. The means from baseline to 22 or 24 weeks for each trials were calculated in ADAS-cog, SIB, CDR-SB, Disability Assessment for Dementia, BEHAVE-AD, Mental Function Impairment Scale, and ADCS-ADL.
For the value of change from the baseline for ADAS-cog, the pooled analysis in five studies for mild to moderate AD (EIS-161, JPN-3, MA3301, JPN-5, and D1301) was performed using a mixed effect model for repeated measures with time points, subgroup factor, studies, and interaction of subgroup factor with time points. The least square mean by time points and the P value of interaction test were drawn as figures. In pooled analysis, subgroup factor was defined by gender (male or female), age (<70, 70–79 or >79 years), disease duration (≤1, 1<−3 or ≥3 years), age at onset (<70, 70–75 or ≥75 years), MMSE (≥18 or <18, mild and moderate AD, respectively), ADAS-cog (<28 or ≥28), and presence of rehabilitation (no or yes). These cutoff values were divided as the tertiles of the data for the age, disease duration, and age at onset. The cutoff value of ADAS-cog was converted from that of MMSE using the formulation (ADAS-cog = 60.9–1.85*MMSE) described in the previous report [7]. Moreover, the pooled analysis for SIB stratified by caregivers' relationship to patients was estimated in three studies for severe AD (IE2101, EIS-231, and IE3501).
All analyses were performed by using SAS software, version 9.4 (SAS Institute, Cary, North Carolina).
3. Results
3.1. Demographics and characteristics of the studies
Visit timing ranged from baseline to 22 or 24 weeks, and the years when the studies took place ranged from 1996 to 2008 (almost 10-year interval) (Table 2). All the enrolled patients were Japanese and living in Japan.
Table 3 displays patients' demographics and other characteristics in each trial. Females were more frequent than males in all studies. There was no patient with prior use of AChEI (i.e., donepezil) in EIS-161 because no AChEI had been approved before this trial, but patients with previous use of AChEI occurred in other trials (except for D1301 whose data were not available because of the aforementioned anonymization reason). The data on education, apolipoprotein E (APOE) genotype status, and biomarkers such as the amyloid β (Aβ) and tau were unavailable in the present study. Baseline values of representative test batteries of each study are shown in Table 4.
Table 3.
Demographics of patients in placebo groups from eight placebo-controlled randomized trials targeting AD
| AD severity |
Mild to moderate |
Severe |
||||||
|---|---|---|---|---|---|---|---|---|
| Trial name | EIS-161 | JPN-3 | MA3301 | JPN-5 | D1301 | IE2101 | EIS-231 | IE3501 |
| Total number of patients∗ | 129 | 136 | 180 | 194 | 268 | 107 | 102 | 208 |
| Gender, N (%) | ||||||||
| Female | 84 (65.1) | 96 (70.6) | 111 (61.7) | 135 (59.6) | 182 (67.9) | 76 (71.0) | 84 (82.4) | 135 (64.9) |
| Male | 45 | 40 | 69 | 59 | 86 | 31 | 18 | 73 |
| Age (years), Mean (SD) | 69.5 (8.88) | 74.6 (8.46) | 72.5 (9.06) | 75.6 (7.62) | - | 73.6 (8.87) | 79.5 (7.25) | 74.9 (8.44) |
| Disease duration (months), Mean (SD) | 40.78 (22.13) | 16.04 (21.91) | 16.73 (18.45) | 39.63 (24.94) | - | 54.84 (32.26) | 67.38 (41.84) | 54.35 (30.32) |
| Age at onset (years) | ||||||||
| Number | 128 | 136 | 180 | 194 | - | 107 | 96 | 208 |
| Mean (SD) | 65.7 (8.83) | 72.7 (8.71) | 71.1 (9.13) | 71.8 (7.97) | 69.0 (9.28) | 73.8 (7.86) | 70.4 (8.81) | |
| MMSE, N (%) | - | |||||||
| ≥18 | 54 (41.9) | 51 (37.5) | 94 (52.2) | 76 (39.2) | 132 (49.3) | 0 (0.0) | 0 (0.0) | |
| <18 | 75 (58.1) | 85 (62.5) | 85 (47.2) | 118 (60.8) | 136 (50.7) | 107 (100.0) | 102 (100.0) | |
| Unknown | 0 (0.0) | 0 (0.0) | 1 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Caregiver, N (%) | ||||||||
| Presence | 129 (100.0) | - | - | - | 263 (98.1) | 107 (100.0) | 102 (100.0) | 208 (100.0) |
| Absence | 0 (0.0) | 5 (1.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Caregivers' relationship with patients, N (%) | ||||||||
| Wife | 42 (32.6) | - | - | - | - | 26 (24.3) | 13 (12.7) | 63 (30.3) |
| Husband | 45 (34.9) | 24 (22.4) | 13 (12.7) | 60 (28.8) | ||||
| Child | 25 (19.4) | 48 (44.9) | 31 (30.4) | 73 (35.1) | ||||
| Others | 17 (13.2) | 9 (8.4) | 45 (44.1) | 12 (5.8) | ||||
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||||
| History of rehabilitation, N (%) | ||||||||
| Absence | 123 (95.3) | 97 (71.3) | 141 (78.3) | 180 (92.8) | - | 50 (46.7) | 81 (79.4) | 89 (42.8) |
| Presence | 6 (4.7) | 39 (28.7) | 39 (21.7) | 14 (7.2) | 57 (53.3) | 21 (20.6) | 119 (57.2) | |
| AChEIs as prior treatment, N (%) | ||||||||
| Presence | 0 (0.0) | 66 (48.5) | 97 (53.9) | 106 (54.6) | - | 45 (42.1) | 0 (0.0) | 141 (67.8) |
| Absence | 129 (100.0) | 70 (51.5) | 83 (46.1) | 88 (45.4) | 62 (57.9) | 102 (100.0) | 67 (32.2) | |
Abbreviations: AD, Alzheimer's disease; -, absent data; MMSE, Mini-Mental State Examination; AChEIs, acetylcholinesterase inhibitors; SD, standard deviation.
Patient numbers of each category is the same as the total number of patients in the third column unless otherwise indicated.
Table 4.
Baseline values of representative rating scales for dementia patients in placebo groups from eight placebo-controlled randomized trials targeting AD
| AD severity |
Mild to moderate AD |
Severe AD |
||||||
|---|---|---|---|---|---|---|---|---|
| Trial name | EIS-161 | JPN-3 | MA3301 | JPN-5 | D1301 | IE2101 | EIS-231 | IE3501 |
| Total number of patients | 129 | 136 | 180 | 194 | 268 | 107 | 102 | 208 |
| CDR severity | ||||||||
| Number | ||||||||
| 1 | 78 | - | 130 | - | - | - | - | - |
| 2 | 51 | - | 50 | - | - | - | - | - |
| CDR-SB | ||||||||
| Number | 129 | - | 180 | - | - | - | - | - |
| Mean (SD) | 7.68 (2.43) | - | 7.20 (2.54) | - | - | - | - | - |
| ADAS-cog | ||||||||
| Number | 126 | 136 | 180 | 194 | 266 | - | - | - |
| Mean (SD) | 26.73 (9.89) | 24.04 (7.52) | 20.91 (8.71) | 26.41 (7.10) | 24.86 (9.48) | - | - | - |
| SIB | ||||||||
| Number | - | - | - | - | - | 107 | 101 | 206 |
| Mean (SD) | - | - | - | - | - | 72.57 (17.84) | 67.03 (22.96) | 70.05 (18.66) |
| MMSE | ||||||||
| Number | 129 | 136 | 179 | 194 | 268 | 107 | 102 | - |
| Mean (SD) | 16.54 (3.85) | 16.47 (3.26) | 17.46 (3.51) | 16.51 (3.16) | 16.75 (2.85) | 10.42 (2.91) | 7.99 (3.33) | - |
| DAD | ||||||||
| Number | - | 136 | 180 | 194 | 268 | 107 | - | - |
| Mean (SD) | - | 60.26 (22.24) | 63.72 (22.43) | 64.49 (20.03) | 66.54 (20.03) | 33.92 (18.73) | - | - |
| BEHAVE-AD | ||||||||
| Number | - | 136 | - | 194 | 268 | 107 | 102 | 208 |
| Mean (SD) | - | 5.65 (5.57) | - | 5.31 (4.91) | 4.86 (4.50) | 7.64 (6.12) | 8.24 (6.09) | 6.96 (5.93) |
| MENFIS | ||||||||
| Number | 129 | 136 | - | 194 | 268 | 107 | - | 208 |
| Mean (SD) | 30.30 (9.32) | 27.46 (11.82) | - | 26.66 (9.93) | 23.29 (11.21) | 40.86 (10.66) | - | 36.33 (11.33) |
| ADCS-ADL | ||||||||
| Number | - | - | - | - | - | 107 | 102 | - |
| Mean (SD) | - | - | - | - | - | 31.59 (10.12) | 26.43 (11.50) | - |
Abbreviations: AD, Alzheimer's disease; CDR, Clinical Dementia Rating; CDR-SB, Clinical Dementia Rating Scales Sum of Boxes; ADAS-cog, Alzheimer's Disease Assessment Scale-cognitive subscale; SIB, Severe Impairment Battery; MMSE, Mini-Mental State Examination; DAD, Disability Assessment for Dementia; BEHAVE-AD, Behavioral pathology in Alzheimer's Disease; MENFIS, Mental Function Impairment Scale; ADCS-ADL, Alzheimer's disease Cooperative Study-ADL scale; -, absent data; SD, standard deviation.
3.2. Longitudinal changes of test batteries
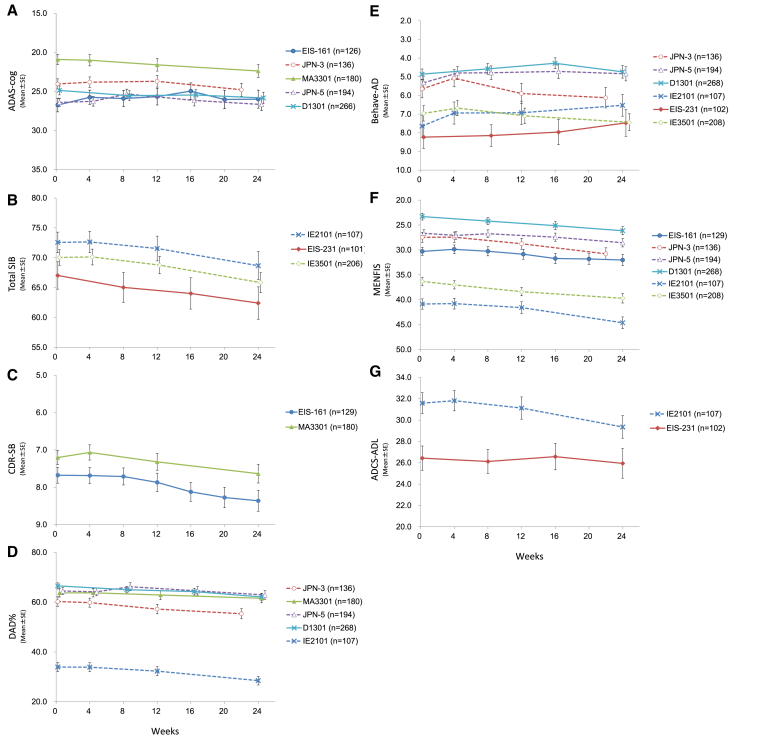
Fig. 1 illustrates longitudinal changes of each test battery, and the scores of those batteries worsened over time except for BEHAVE-AD (Fig. 1E). The degree of deterioration was greater at week 22 or 24 than at week 12.
Fig. 1.
Longitudinal changes of test batteries in eight studies; scores of test batteries (A: ADAS-cog; B: total SIB; C: CDR-SB; D: DAD; E: BEHAVE-AD; F: MENFIS; and G: ADCS-ADL) over time. N shows the number of patients in each group. X axis exhibits treatment period (week). Abbreviations: ADAS-cog, Alzheimer's Disease Assessment Scale-cognitive subscale; SIB, Severe Impairment Battery; CDR-SB, Clinical Dementia Rating Scales Sum of Boxes; DAD, Disability Assessment for Dementia; BEHAVE-AD, Behavioral pathology in Alzheimer's Disease; MENFIS, Mental Function Impairment Scale; ADCS-ADL, Alzheimer's disease Cooperative Study-ADL scale.
The ADAS-cog score at baseline was 24.54 ± 8.86 (mean ± standard deviation [SD]) in the five studies for mild to moderate AD (EIS-161, JPN-3, MA3301, JPN-5, and D1301). Changes of ADAS-cog scores from baseline showed very small worsening or even slight improvement in some studies at week 12 (Fig. 1A). Worsening patterns of EIS-161 and JPN-5 in ADAS-cog targeting mild to moderate AD were very similar although all patients in EIS-161 were AChEI-naive (i.e., donepezil-naive), and both trials were conducted by different sponsors at separate times (there was a difference of almost 10 years between the initiation of EIS-161 and that of JPN-5). Furthermore, somewhat transient improvement of ADAS-cog scores were observed at around week 8 in these two studies, which appears to be due to placebo effect. Change of mean ADAS-cog values from baseline to week 22 or week 24 in the five studies was 1.11 ± 5.39 (mean ± SD, P < .001).
Scores of SIB in the studies for severe AD also deteriorated over time (more largely at week 24 than at week 12) and exhibited no distinct improvement (no placebo effect) during the study period (Fig. 1B).
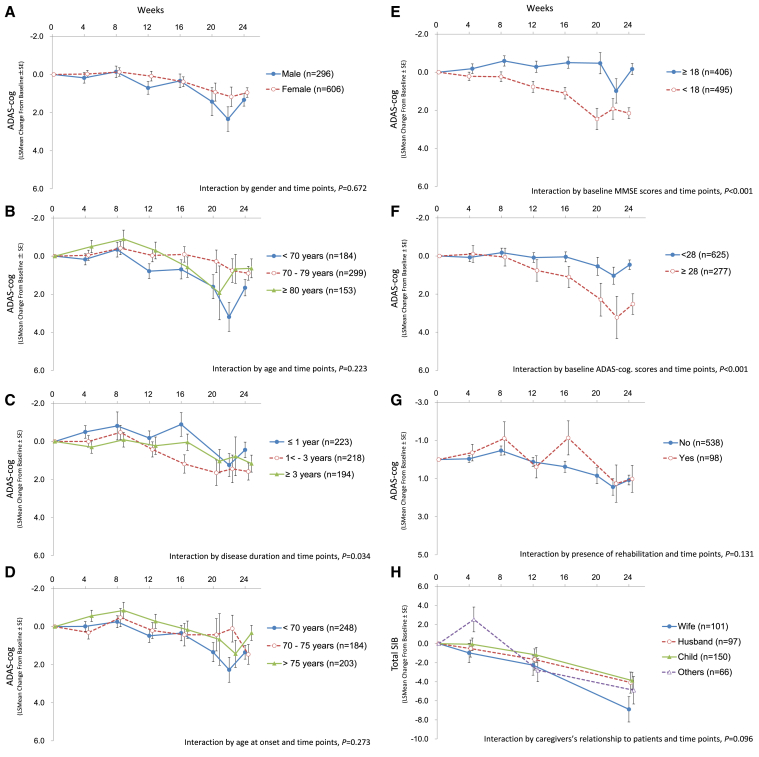
3.3. Longitudinal change of ADAS-cog by subgroup
Longitudinal changes of ADAS-cog by subgroup for gender, age, disease duration, age at onset, baseline MMSE scores, baseline ADAS-cog scores, and presence of rehabilitation are displayed in Fig. 2. No clear trend was observed after stratification by gender, age, age at onset, and presence of rehabilitation. However, the interaction by disease duration and time points had significant difference (P = .034) (Fig. 2C). Because the disease duration was related with the MMSE and the ADAS-cog scores at baseline (P < .001 in the trend tests), this interaction was thought to be a reflection of cognitive function but not a direct relationship. The subgroup by baseline MMSE scores demonstrated that ADAS-cog worsens more rapidly in the group of MMSE<18 (moderate AD) than in that of MMSE≥18 (mild AD) (Fig. 2E). In a similar fashion, the data stratified by baseline ADAS-cog scores deteriorated faster in the group of ADAS-cog≥28 (moderate AD) compared with that of ADAS-cog<28 (mild AD) (Fig. 2F).
Fig. 2.
Longitudinal change of ADAS-cog after stratification. The data show ADAS-cog changes after stratification by gender (A), age (B), disease duration (C), age at onset (D), baseline MMSE scores (E), baseline ADAS-cog scores (F), and presence of rehabilitation (G). The data for SIB stratified by caregivers' relationship to patients are shown in (H). N shows the number of patients in each group. X axis exhibits treatment period (week). Abbreviations: MMSE, Mini-Mental State Examination; ADAS-cog, Alzheimer's Disease Assessment Scale-cognitive subscale; SIB, Severe Impairment Battery.
Relationships between total SIB and types of caregivers were examined in the three studies targeting severe AD as shown in Fig. 2H. However, no clear trend in change of SIB scores was observed by difference in caregiver types (Fig. 2H).
4. Discussion
This is the first analysis report on natural courses of Japanese patients with AD in placebo groups using data from placebo-controlled, double-blind, randomized clinical trials. Scores of almost all scales including ADAS-cog and SIB evaluating cognitive function worsened over time, and the degree was larger at week 22 or 24 than at week 12.
Changes of ADAS-cog scores from baseline showed very small worsening or even slight improvement in some studies at week 12. Worsening patterns of EIS-161 and JPN-5 in ADAS-cog targeting mild to moderate AD were very similar although all patients in EIS-161 were AChEI-naive (i.e., donepezil-naive), and both trials were conducted by different sponsors at separate times (EIS-161 was conducted almost 10 years earlier than JPN-5). This fact suggests similar changes of scales in well-designed studies, regardless of prior use of AChEIs and the year of conducted trials; however, it is still debatable whether ADAS-cog deterioration is slower in recent trials than in past trials [7]. Furthermore, somewhat transient improvement of ADAS-cog scores were observed at around week 8 in these two studies (EIS-161 and JPN-5), which appears to be due to placebo effect as suggested in previous reports [7], [8]. This placebo effect in the present report may be caused by a learning effect because of frequent visits for ADAS-cog evaluation (i.e., at 0, 4, and 8 weeks).
Change of mean ADAS-cog values from baseline to week 22 or week 24 in the five studies for mild to moderate AD (EIS-161, JPN-3, MA3301, JPN-5, and D1301) was 1.11 ± 5.39 (mean ± SD, P < .001). This change is smaller (approximately 2.2/year if calculated per year) than the previous report (5.5 ± 0.229/year, mean ± standard error) [7] despite similar ADAS-cog score at baseline between the present study (24.54 ± 8.86, mean ± SD) and in the previously mentioned report (25.4, mean) [7], suggesting that the difference in the ADAS-cog change from baseline is possibly not associated with difference in disease severity at baseline. Therefore, this may be related with shorter treatment periods in our five studies (22–24 weeks) than in the previous report [7], suggesting that studies with longer treatment periods tend to show greater decline of ADAS-cog [5]. Otherwise, this smaller change may also be related with placebo response, and the natural course of AD outside the clinical trials may be more severe than what is shown in this study. Regardless of the reason, these data on ADAS-cog decline are helpful for prescribers/clinicians to predict and explain future decline of cognitive function and effectiveness of AD drugs for their patients.
Scores of SIB also deteriorated over time (more largely at week 24 than at week 12) and exhibited no distinct improvement (no placebo effect) during the studies. No placebo effect may be associated with disease severity (i.e., severe AD) and/or less frequent visits compared with the previously mentioned two studies (EIS-161 and JPN-5). A previous report analyzed a total of 499 moderate to severe AD patients on placebo from three randomized controlled trials using memantine or placebo conducted outside of Japan [13]. MMSE and SIB of the 499 patients on placebo at baseline were 9.8 (3.2) and 75.4 (18.5) shown as mean ± SD, respectively, and similar to those in the present study. In this report, SIB continuously declined over time in patients on placebo as the present study, but SIB in patients on memantine displayed transient improvement at weeks 4 and 12. These findings demonstrate that SIB in patients in placebo groups do not show transient improvement due to the placebo effect, but the group on memantine has demonstrated improved SIB scores compared to the placebo group. Considering these findings, SIB scores are unlikely to improve in the natural course of AD, making improvement of those to be a good indicator for drug efficacy. Further evaluation will be required because this is the first report to show longitudinal changes of SIB in three studies in parallel.
Analyses on the presence of rehabilitation and caregiver's relationship to patients were adopted because the use of the nursing-care services leads to an inappropriate rating of CIBIC plus due to the reduction in the time spent on nursing care and in the opportunity for observation of the patient's activities of daily living by the caregiver resulting from the use of the nursing-care services [14]. However, no clear impact of presence of rehabilitation or caregiver's relationship to patients on assessment of neuropsychological tests was discerned.
The ADAS-cog data stratified by baseline MMSE or ADAS-cog scores showed that the scores of ADAS-cog worsen faster in the group (MMSE<18 or ADAS-cog≥28) corresponding to moderate AD than that of mild AD (MMSE≥18 or ADAS-cog<28), being compatible with previous reports [8], [15]. This suggests the possibility of moderate AD as a more appropriate AD population compared with mild AD in clinical studies in terms of displaying efficacy of symptomatic anti-AD drugs over placebo. The data also help to properly calculate the effect size or sample size in future clinical studies. The previous report analyzed a total of 2882 AD patients from nine randomized controlled trials and one Alzheimer's Disease Neuroimaging Initiative [16]. Similar to the present data, this report showed greater rate of progression on the ADAS-cog in patients with lower MMSE scores at baseline compared to those with higher scores although patients on both placebo and active drugs are included in this analysis. This report suggest that enrichment of more severe AD based on MMSE status at baseline has a small effect on the annual rate of change of ADAS-cog and causes the expense of excluding a large number of patients, requiring longer recruitment period of patients. This fact leads us to consider both well-balanced patient populations (i.e., ratio of mild to moderate) and recruitment period when designing future clinical trials.
Previous reports indicated potential several covariates such as age, gender, and APOE genotype status affecting disease progression [8], [9], [10], [11], [12], whereas the present data showed no clear trend on age and gender. Further evaluation may be required to know whether these covariates are truly relevant.
The present study has some limitations. Numbers of collected trials are relatively small (five for mild to moderate and three for severe AD). The data on education, APOE genotype status, and biomarkers were unavailable. The data on the present study are extracted from placebo-controlled, double-blind, clinical trials, and the natural course of AD outside the clinical trials may be more severe than what is shown in this study.
5. Conclusions
The present study is the first instance of a study showing natural courses of Japanese patients with AD in placebo groups (i.e., untreated with currently approved symptomatic drugs for AD). This study provides prescribers/clinicians following up patients with AD with helpful information on how to manage patients with AD (i.e., explanation of disease courses and efficacy of anti-AD drugs to their patients) and use these drugs according to patients' disease stages. This will also help create design of future clinical studies such as criteria for cognitive function at baseline, primary endpoint, and treatment period. It should be noted that placebo effect is highly likely to be observed at around week 8 (in particular, in mild to moderate AD). Furthermore, to relevantly show efficacy of symptomatic anti-AD drugs over placebo (larger effect size), moderate AD appears to be more appropriate than mild AD and week 24 more appropriate than week 12 as treatment period although details depend on the objectives and conditions of the target studies.
Research in context.
-
1.
Systematic review: The author searched literature using sources such as PubMed and Embase. This was conducted with various combinations of search terms including Alzheimer's disease (AD), longitudinal, and placebo to understand that the data in previous reports are similar to the present Japanese data regarding the decline of rating scale scores in Alzheimer's disease.
-
2.
Interpretation: Decline of Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-cog) appeared to be slower in the present Japanese data compared with the previous ones reported outside of Japan. Furthermore, the present data demonstrated that decreases in ADAS-cog and Severe Impairment Battery are larger at week 22 or 24 that at week 12, and scores of ADAS-cog deteriorate faster in moderate AD than in mild AD.
-
3.
Future directions: The present data will provide clinicians following up AD patients with helpful information on how to manage AD patients and investigators with instruction for clinical study design (more beneficial at week 22 or 24 as evaluation time points and moderate AD as target AD severity). Further studies using Japanese as well as non-Japanese data are required to replicate and consolidate the present data.
Acknowledgments
The authors would especially like to thank the following contributors for providing the data to this study: Daiichi Sankyo Company, Limited, Eisai Co., Ltd., Janssen Pharmaceutical K.K, Novartis Pharma, Ono Pharmaceutical Co., Ltd. They also appreciate the kind support of Dr. Anthony Swain and Ms. Yuka Namikawa for revising the manuscript and language editing.
The contents of the manuscript are based on the personal opinions of the authors and unrelated with positions of their institutes/companies.
Funding Sources: This study was funded by Japanese Society of Scaling Keys of Evaluation Techniques for CNS Disorders Heterogeneity. This society is partially supported by Astellas Pharma Inc., Biogen Japan Ltd., Daiichi Sankyo Company, Limited, Eisai Co., Ltd., EP-SOGO Co., Ltd., FUJIFILM Toyama Chemical Co., Ltd., Janssen Pharma K.K., Kowa Company Ltd., Linical Co., Ltd., MedAvante-Prophase, Inc, Micron Japan, Ltd., Mitsubishi Tanabe Pharma Corporation, MSD K.K., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., Sumitomo Dainippon Pharma Co., Ltd., and Takeda Pharmaceutical Limited.
Footnotes
Y.N. has received consultant fees from Astellas Pharma Inc., Biogen Japan Ltd., Daiichi Sankyo Company, Limited, Eisai Co., Ltd., Eli Lilly Japan K.K., FUJIFILM Toyama Chemical Co., Ltd., GE Healthcare Japan, Janssen Pharma K.K., Kowa Company Ltd., Meiji Seika Pharma Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Nihon Medi-Physics Co., Ltd., Mochida Pharmaceutical Co., Ltd., MSD K.K., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Ono Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., Shionogi & Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Tsumura Yakuhin Sangyo Co., Ltd., and Yoshitomiyakuhin Corporation. Y.Y. has no conflict of interest. A.H. has received consultancy fees from Eisai Co., Ltd., Daiichi Sankyo Company, Limited, Janssen Pharmaceutical K.K., Novartis Pharma K.K., Ono Pharmaceutical Co., Ltd., Takeda Pharmaceutical Limited, and Kyowa-Kirin Co., Ltd. M.W. is an employee of Nippon Boehringer Ingelheim Co., Ltd., H.K. and N.K. with Eisai Co., Ltd., H.M. with Astellas Pharma Inc., Y.T. with Biogen Japan Ltd., K.N with Ono Pharmaceutical Co., Ltd., M.K. with Daiichi Sankyo Company, Limited, T. Saito and M.T. with Janssen Pharma K.K, T. Sato with Sumitomo Dainippon Pharma Co., Ltd., and K.T. with Novartis Pharma K.K.
References
- 1.Roehr B. American Psychiatric Association explains DSM-5. BMJ. 2013;346:f3591. doi: 10.1136/bmj.f3591. [DOI] [PubMed] [Google Scholar]
- 2.Kumar K., Kumar A., Keegan R.M., Deshmukh R. Recent advances in the neurobiology and neuropharmacology of Alzheimer's disease. Biomed Pharmacother. 2017;98:297–307. doi: 10.1016/j.biopha.2017.12.053. [DOI] [PubMed] [Google Scholar]
- 3.Asada T. Health Labour Sciences Research Grant (Comprehensive Research Project on Measures against Dementia). “Incidence Rate of Dementia in the Urban Area, and Response to Living Functional Disability Caused by Dementia”. Compr Res Rep. 2011 to 2012 (in Japanese) [Google Scholar]
- 4.Cummings J., Lee G., Mortsdorf T., Ritter A., Zhong K. Alzheimer's disease drug development pipeline: 2017. Alzheimers Dement (N Y) 2017;3:367–384. doi: 10.1016/j.trci.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irizarry M.C., Webb D.J., Bains C., Barrett S.J., Lai R.Y., Laroche J.P. Predictors of placebo group decline in the Alzheimer's disease Assessment Scale-cognitive subscale (ADAS-Cog) in 24 week clinical trials of Alzheimer's disease. J Alzheimers Dis. 2008;14:301–311. doi: 10.3233/jad-2008-14304. [DOI] [PubMed] [Google Scholar]
- 6.Ashford J.W., Schmitt F.A. Modeling the time-course of Alzheimer dementia. Curr Psychiatry Rep. 2001;3:20–28. doi: 10.1007/s11920-001-0067-1. [DOI] [PubMed] [Google Scholar]
- 7.Ito K., Ahadieh S., Corrigan B., French J., Fullerton T., Tensfeldt T. Disease progression meta-analysis model in Alzheimer's disease. Alzheimer's Dement. 2010;6:39–53. doi: 10.1016/j.jalz.2009.05.665. [DOI] [PubMed] [Google Scholar]
- 8.Ito K., Corrigan B., Romero K., Anziano R., Neville J., Stephenson D. Understanding placebo responses in Alzheimer's disease clinical trials from the literature meta-data and CAMD database. J Alzheimers Dis. 2013;37:173–183. doi: 10.3233/JAD-130575. [DOI] [PubMed] [Google Scholar]
- 9.Ito K., Corrigan B., Zhao Q., French J., Miller R., Soares H. Disease progression model for cognitive deterioration from Alzheimer's Disease Neuroimaging Initiative database. Alzheimer's Dement. 2011;7:151–160. doi: 10.1016/j.jalz.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Rogers J.A., Polhamus D., Gillespie W.R., Ito K., Romero K., Qiu R. Combining patient-level and summary-level data for Alzheimer's disease modeling and simulation: a beta regression meta-analysis. J Pharmacokinet pharmacodynamics. 2012;39:479–498. doi: 10.1007/s10928-012-9263-3. [DOI] [PubMed] [Google Scholar]
- 11.Samtani M.N., Farnum M., Lobanov V., Yang E., Raghavan N., Dibernardo A. An improved model for disease progression in patients from the Alzheimer's disease neuroimaging initiative. J Clin Pharmacol. 2012;52:629–644. doi: 10.1177/0091270011405497. [DOI] [PubMed] [Google Scholar]
- 12.William-Faltaos D., Chen Y., Wang Y., Gobburu J., Zhu H. Quantification of disease progression and dropout for Alzheimer's disease. Int J Clin Pharmacol Ther. 2013;51:120–131. doi: 10.5414/CP201787. [DOI] [PubMed] [Google Scholar]
- 13.Mecocci P., Bladstrom A., Stender K. Effects of memantine on cognition in patients with moderate to severe Alzheimer's disease: post-hoc analyses of ADAS-cog and SIB total andsingle-item scores from six randomized, double-blind, placebo-controlled studies. Int J Geriatr Psychiatry. 2009;24:532–538. doi: 10.1002/gps.2226. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura Y., Homma A. Does the use of nursing-care services reduce the information about dementia patients provided by their caregivers? Dement Geriatr Cogn Dis Extra. 2011;1:139–149. doi: 10.1159/000329158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tchalla A.E., Clement J.P., Saulnier I., Beaumatin B., Lachal F., Gayot C. Predictors of rapid cognitive decline in patients with mild-to-moderate Alzheimer disease: a prospective cohort study with 12-month follow-up performed in memory clinics. Dement Geriatr Cogn Disord. 2018;45:1–10. doi: 10.1159/000487938. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy R.E., Cutter G.R., Wang G., Schneider L.S. Using baseline cognitive severity for enriching Alzheimer's disease clinical trials: how does mini-mental state examination predict rate of change? Alzheimers Dement (N Y) 2015;1:46–52. doi: 10.1016/j.trci.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]