Introduction
In adult populations, atrial fibrillation (AF) is the major arrhythmia and successful treatment rates are low [1]. In recent years, left atrial appendage occlusion (LAAO) has become an alternative method for stroke prevention in patients in whom oral anticoagulation (OAC) is ineffective or contraindicated or in patients with life-threatening complications [2, 3]. Previous studies have demonstrated that the LAAO procedure is safe and effective in the prevention of thromboembolic events, including in high risk patients [4–10].
In the medical market, there are a number of available devices including endocardial and epicardial devices [4, 5, 9]. However, in some cases, the anatomy of the left atrial appendage (LAA) may constitute a contraindication to implantation of these devices. The LAmbre device is a novel system, designed especially for LAA closure when problematic morphology is present [11, 12].
Aim
Herein, we present the first use of the LAmbre device in Poland in patients with AF.
Material and methods
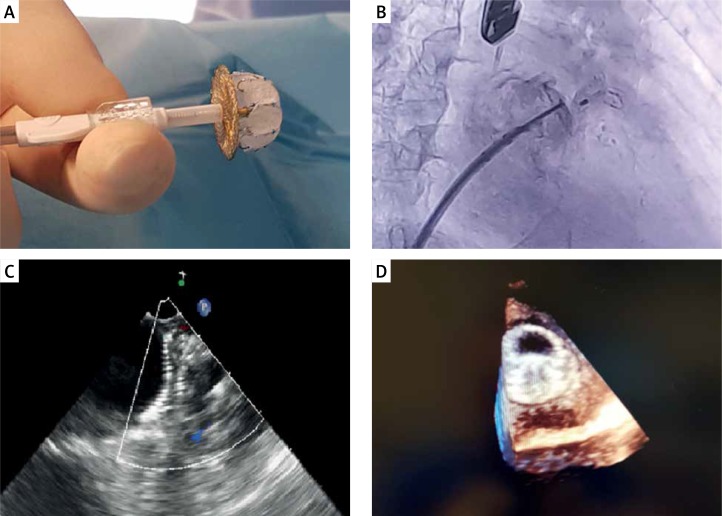
A retrospective, single-center study was performed in 24 consecutive patients with non-valvular AF, who underwent LAAO with the LAmbre device (Lifetech Scientific Corp., Shenzhen, China) between 2016 and 2018 (Figure 1). The LAmbre occluder system was previously described [13]. LAmbre device selection was based on operators’ decision. All procedures were performed under general anesthesia. Patient characteristics are presented in Table I. The LAA anatomy was assessed with computed tomography angiography before each procedure. Oral anticoagulation therapy was discontinued and unfractionated heparin was used during the procedure. After the procedure, aspirin (75 mg/dose/day) and clopidogrel (75 mg/dose/day) for 6 months were recommended in each patient. Leak was defined as the presence of flow from the left atrium to the LAA < 3 mm [14].
Figure 1.
LAmbre occluder connected to the sheath (A), fluoroscopy (B), transesophageal echocardiography (C) and 3D echocardiography (D) of the LAmbre occluder after release and correctly placed in the left atrial appendage
Table I.
Patient characteristics (n = 24)
| Parameter | Results |
|---|---|
| Age [years]: | |
| Mean ± SD | 71.63 ±8.17 |
| Range | 58–85 |
| Female | 33.33% |
| CHADS2 score, mean ± SD | 3.46 ±1.32 |
| CHA2DS2-VASc score, mean ± SD | 4.96 ±1.46 |
| HAS-BLED score, median (Q1;Q3) | 4 (3; 5) |
| Congestive heart failure | 37.5% |
| Hypertension | 100% |
| Diabetes mellitus 2 | 37.5% |
| Previous stroke | 54.17% |
| Vascular disease | 54.17% |
| Alcoholism | 4.17% |
| Indication for LAAO procedure: | |
| Gastrointestinal bleeding: | 37.5% |
| While on NOAC | 16.66% |
| While on VKA | 20.83% |
| CNS bleeding: | 8.32% |
| While on NOAC | 4.17% |
| While on VKA | 4.17% |
| Vitreous bleeding while on NOAC | 4.17% |
| Respiratory tract bleeding while on VKA | 4.17% |
| Urinary tract bleeding on NOAC | 4.17% |
| Thrombus in LAA despite OAC | 4.17% |
| Stroke/TIA despite OAC | 37.5% |
| Pre-procedure anticoagulation: | |
| Vitamin K antagonist: | |
| Warfarin | 33.33% |
| New oral anticoagulant: | |
| Dabigatran | 41.67% |
| Rivaroxaban | 20.83% |
| LMWH | 4.17% |
| LAA measurements [mm]: | |
| LAA length | 26.8 ±5.8 |
| LAA orifice diameter | 23.1 ±4.9 |
| LAA landing zone diameter | 22.9 ±4.8 |
| Follow-up TEE (3 months/6 months): | |
| Device dislodgment | 0%/0% |
| Thrombosis in LA | 0%/0% |
| Pericardial effusion | 0%/0% |
| Residual flow > 3 mm | 0%/0% |
Follow-up visits, including transesophageal echocardiography, were performed at 3 and 6 months post-procedure. Data on mortality, causes of mortality and serious adverse events (SAE) were collected.
Statistical analysis
Data are expressed as mean ± standard deviation or median (interquartile range; Q1 – 25th percentile and Q3 – 75th percentile), unless otherwise stated. Categorical variables were expressed as counts and percentages.
Results
All procedures were successfully completed with no perioperative complications. The LAAO procedure or device related mortality was 0%. The mean time for the procedure was 62.92 ±14.21 min. Eleven different sizes of occluder were implanted during the procedures, depending on the size and shape of the left atrial appendage. The choice of device size was made by the operator during the procedure based on intraprocedural transesophageal echocardiography (TEE) examination. There was a 100% success rate with no complications. No post-procedural leaks were observed. Half of the patients were discharged from hospital on the second or third day following the procedure.
The overall follow-up was 349 months. During the follow-up period, there were 4 (16.7%) deaths; 1 case with acute exacerbation of chronic renal failure complicated by heart failure (5 months after the procedure); 1 case of out-of-hospital cardiac arrest (5 months after the procedure), 1 case of post neurosurgery complications due to cerebral artery aneurysm (15 months after the procedure) and in 1 case, the cause of death was unknown. There were no deaths connected to the procedure. Gastrointestinal bleeding were observed in 2 (8.34%) cases. There was 1 (4.17%) case of transient ischemic attack and 1 (4.17%) case of stroke, 16 and 3 months after the procedure, respectively. In both cases control TEE examination showed no device thrombus. In the remaining patients follow-up TEE showed no device thrombi or LAA leaks (Table I).
Discussion
We present the first results in Poland of the LAAO procedure with LAmbre devices, with a 100% success rate and with no perioperative complications. From our initial experience, implantation is associated with a high success rate and good clinical outcomes.
Our results are similar to the most popular endocardial devices such as the Watchman or Amplatzer [4, 15]. Surprisingly, in our study, there was a larger number of postprocedural bleeding episodes, which were observed in 8.34% of patients, compared to other endocardial trials [4, 15]. However, in our study, patients had a very high risk of bleeding (HAS-BLED score 4) and, in more than 60% of patients, the indication for LAAO was previous bleeding episodes. Of note, all bleeding episodes were among patients who were receiving antiplatelet therapy, and none were receiving OAC.
The observed mortality rate (16.6%) was also higher than that reported in other endocardial device trials [4, 15]. However, none of the deaths were related to the procedure. Boersma et al. observed a 9.8% mortality rate at 12 months of observation in a Watchman device trial [4]. Importantly, all deceased patients were free of thrombus on the occluder and from postprocedural leak at 3-month and 6-month visits. Additionally, no device thrombi were observed, despite not receiving OAC, even in transient ischemic attack (TIA) and stroke patients. Similar data were obtained by Huang et al. [14].
The LAA morphology, including the LAA shape, ostium width and depth, plays a critical role in the choice of device. The most popular devices such as the Watchman, with its umbrella-like shape, should be avoided in shallow or multilobar LAAs. The second most commonly used device, the Amplatzer ACP, should be avoided in shallow LAAs because of the relatively proximal position of LAA implantation [13]. The LAmbre device is available in a larger range of device sizes (16–36 mm) compared to the Watchman (21–30 mm) and ACP (16–34 mm) devices. It is also highly adaptive to many LAA sizes due to its smaller umbrellas with larger covers. Therefore, the larger choice of sizes and favorable device properties may make the LAmbre device more suitable for complex LAA anatomies, such as chicken wing or shallow LAA [13].
Conclusions
The LAAO procedure with the LAmbre device is associated with a high success rate and good short term clinical results.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 2.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2016;50:e1–88. doi: 10.1093/ejcts/ezw313. [DOI] [PubMed] [Google Scholar]
- 3.Litwinowicz R, Konstanty-Kalandyk J, Goralczyk T, et al. Dabigatran level monitoring prior to idarucizumab administration in patients requiring emergent cardiac surgery. J Thromb Thrombolysis. 2018;45:9–12. doi: 10.1007/s11239-017-1587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boersma LV, Ince H, Kische S, et al. Efficacy and safety of left atrial appendage closure with WATCHMAN in patients with or without contraindication to oral anticoagulation: 1-year follow-up outcome data of the EWOLUTION trial. Heart Rhythm. 2017;14:1302–8. doi: 10.1016/j.hrthm.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 5.Litwinowicz R, Bartus M, Ceranowicz P, et al. Stroke risk reduction after LAA occlusion in elderly patients with atrial fibrillation: long-term results. Pol Arch Intern Med. 2018;128:327–9. doi: 10.20452/pamw.4264. [DOI] [PubMed] [Google Scholar]
- 6.Bartus K, Myc J, Bartus M, et al. Rapid left atrial appendage thrombus formation in epicardial percutaneous LAA suture ligation with LARIAT. Adv Interv Cardiol. 2018;14:435–7. doi: 10.5114/aic.2018.79876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartus K, Litwinowicz R, Dziewierz A, et al. Coronary artery bypass grafting after left atrial appendage ligation – is anti-inflammatory treatment recommendation post LARIAT effective? Adv Interv Cardiol. 2018;14:438–9. doi: 10.5114/aic.2018.79877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Litwinowicz R, Bartus M, Burysz M, et al. Long term outcomes after left atrial appendage closure with the LARIAT device – stroke risk reduction over five years follow-up. PLoS One. 2018;13:e0208710. doi: 10.1371/journal.pone.0208710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S, Chun KJ, Bordignon S, et al. Left atrial appendage occlusion using LAmbre Amulet and Watchman in atrial fibrillation. J Cardiol. 2019;73:299–306. doi: 10.1016/j.jjcc.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Litwinowicz R, Bartus M, Ceranowicz P, et al. Left atrial appendage occlusion for stroke prevention in diabetes mellitus patients with atrial fibrillation: long-term results. J Diabetes. 2019;11:75–82. doi: 10.1111/1753-0407.12824. [DOI] [PubMed] [Google Scholar]
- 11.Chen S, Schmidt B, Bordignon S, et al. Feasibility of percutaneous left atrial appendage closure using a novel LAmbre occluder in patients with atrial fibrillation: initial results from a prospective cohort registry study. J Cardiovasc Electrophysiol. 2018;29:291–7. doi: 10.1111/jce.13385. [DOI] [PubMed] [Google Scholar]
- 12.Park JW, Sievert H, Kleinecke C, et al. Left atrial appendage occlusion with lambre in atrial fibrillation: initial European experience. Int J Cardiol. 2018;265:97–102. doi: 10.1016/j.ijcard.2018.02.120. [DOI] [PubMed] [Google Scholar]
- 13.Reinsch N, Ruprecht U, Buchholz J, et al. Initial experience of percutaneous left atrial appendage closure using the LAmbre device for thromboembolic prevention. J Cardiovasc Med (Hagerstown) 2018;19:491–6. doi: 10.2459/JCM.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 14.Huang H, Liu Y, Xu Y, et al. Percutaneous left atrial appendage closure with the lambre device for stroke prevention in atrial fibrillation: a prospective, multicenter clinical study. JACC Cardiovasc Interv. 2017;10:2188–94. doi: 10.1016/j.jcin.2017.06.072. [DOI] [PubMed] [Google Scholar]
- 15.Landmesser U, Schmidt B, Nielsen-Kudsk JE, et al. Left atrial appendage occlusion with the AMPLATZER Amulet device: periprocedural and early clinical/echocardiographic data from a global prospective observational study. Eurointervention. 2017;13:867–76. doi: 10.4244/EIJ-D-17-00493. [DOI] [PubMed] [Google Scholar]