Abstract
Masson tumor (intravascular papillary endothelial hyperplasia) is a rare proliferation of endothelial cells within the wall of a vessel, often thought to represent an aberrant resolution of a thrombosis. We describe the unique case of a 75-year-old man who presented to the clinic with a tender, spontaneous aneurysmal dilation of his left superficial temporal artery (STA). Only 8% of all STA aneurysms are believed to be spontaneous true aneurysms, with the majority being post-traumatic pseudoaneurysms. After successful surgical resection, pathologic examination demonstrated a Masson tumor within an STA aneurysm. This paper describes a case in which both rare entities were discovered and briefly outlines the diagnostic and therapeutic modalities available.
Keywords: Masson tumor, Superficial temporal artery aneurysm
Spontaneous, atraumatic superficial temporal artery (STA) aneurysms are rare, accounting for approximately 8% of all STA aneurysms.1 The majority of aneurysmal dilations of the STA are traumatic pseudoaneurysms after blunt trauma.2, 3 Masson tumor, also known as intravascular papillary endothelial hyperplasia (IPEH), is an uncommon vascular tumor. Masson tumors typically present in the veins or arteries of the head, neck, and upper extremities.4 We describe a unique case of a 75-year-old man who presented to the clinic with a tender, spontaneous aneurysmal dilation of his left STA. The STA aneurysm was excised in the operating room under local anesthesia. Pathologic examination demonstrated a Masson tumor within an STA aneurysm. This case report describes the discovery of both rare entities together. The patient consented to the use and publication of the images and health information.
Case report
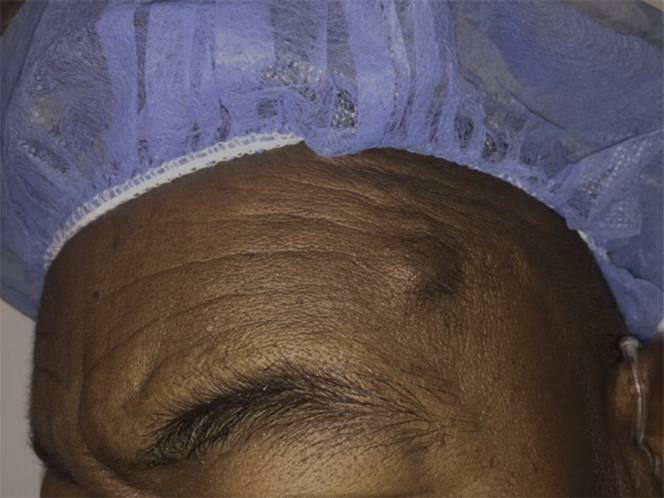
Our patient, a 75-year-old man with a history of type 2 diabetes, hypertension, and end-stage renal disease on dialysis, presented to the vascular surgery office desiring resection of a pulsatile mass in his left temple that was intermittently associated with pain (Fig 1). No further diagnostic imaging was obtained, and the patient was scheduled for outpatient elective resection. In the operating room, the aneurysm measured approximately 1.5 cm in length and 0.7 cm in diameter (Figs 2 and 3). It was carefully dissected free of the surrounding tissue and excised under local anesthesia. The patient was later discharged from the day surgical unit and has had no complications.
Fig 1.
Superficial temporal artery (STA) aneurysm presenting as a tender, pulsatile mass with no previous trauma on our patient's left temple.
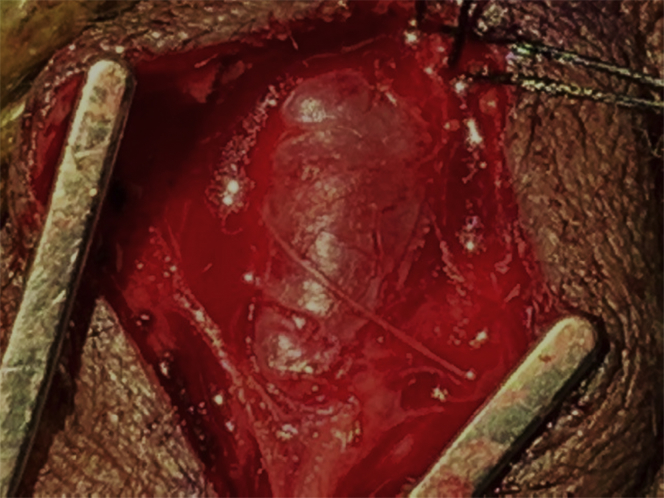
Fig 2.
Initial exposure of the aneurysmal degeneration of the left superficial temporal artery (STA).
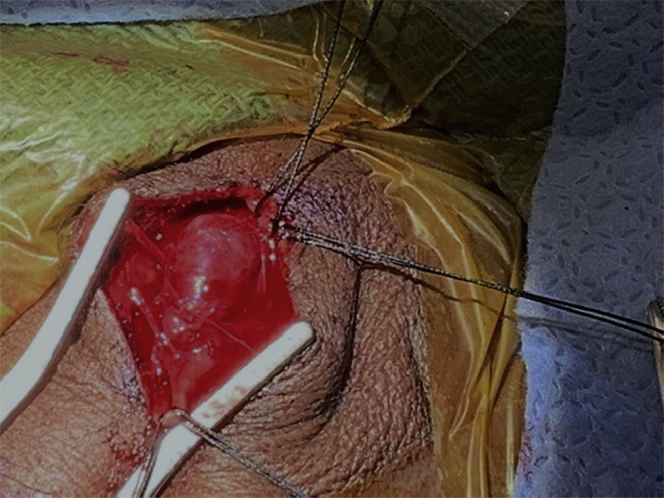
Fig 3.
Meticulous dissection and ligation of proximal and distal vasculature to superficial temporal artery (STA) aneurysm.
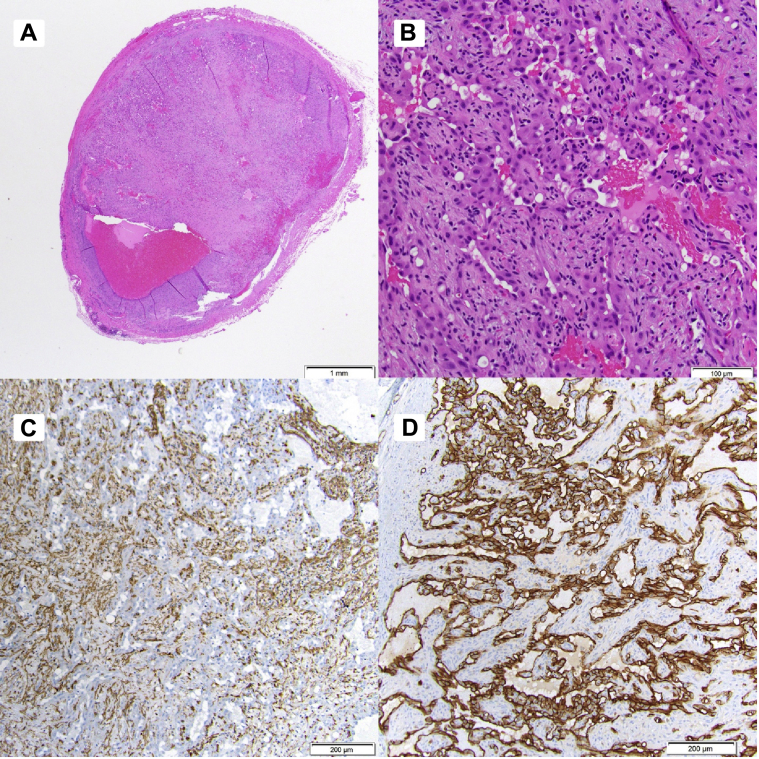
The pathologist noted endothelial reaction with mixed features of a Masson tumor (Fig 4, A and B). The tumor tested positive for the smooth muscle cell marker actin (Fig 4, C and D) and endothelial cell membrane marker (CD31), both of which are consistent with the diagnosis of Masson tumor. Margins were negative.
Fig 4.
A, Standard hematoxylin-eosin stain shows proliferation of endothelial cells in a papillary architecture (low power). B, High-power hematoxylin-eosin slide demonstrating pericytes and endothelial cells of intravascular papillary endothelial hyperplasia (IPEH). No atypia is present. C, Smooth muscle actin immunohistochemistry stain highlights the pericytes around the endothelial cells. Pericytes are contractile cells that envelop endothelial cells on blood vessels.12D, The endothelial cells are spotlighted with the CD31 immunohistochemistry stain.
Discussion
Spontaneous, atraumatic STA aneurysms are a rare entity, representing approximately 8% of all STA aneurysms.1 Aneurysmal dilation of the STA is typically associated with trauma, to which the vessel is predisposed because of its superficial course within connective tissue. Typically, post-traumatic aneurysms develop 2 to 6 weeks after blunt head trauma.2, 3 The majority of these are pseudoaneurysms.2 True aneurysmal dilation is rare. A comprehensive literature review conducted in 2014 identified just 32 cases of spontaneous STA aneurysm.1 Among these, a pulsatile mass was the symptom leading to presentation, with pain a somewhat less common complaint occurring in 21.9% of patients.1
Pain, increasing size, cosmetic reasons, and overlying skin changes are all accepted indications for resection with no further diagnostic imaging required if the results will not affect clinical management.2 Some have proposed imaging with duplex ultrasound, computed tomography angiography, and magnetic resonance angiography. Open ligation and resection are curative and safe.5
A 2009 case report described a 4-cm STA aneurysm proximal to the development of a temporal porocarcinoma. The authors postulated that hemodynamic stress caused by increased vascular demands by the scalp tumor may have led to the aneurysmal dilation.6
Masson tumor (IPEH) is an uncommon vascular tumor that typically presents in the head, neck, and upper extremities.4 A literature review dating back 35 years noted that fewer than 250 cases have been described involving the head and neck, of which <5% involved the forehead.6 We found a single report describing an IPEH within an aneurysmal dilation in a branch of the STA presenting as an asymptomatic enlarging mass.7
These tumors are typically painless, slow-growing masses, more common in female patients of any age, and are histologically classified into three main types. The primary type is typically associated with a dilated vein but may less commonly be associated with an artery. The secondary type resides within pre-existing vascular abnormalities. The extravascular type, which is the least common, arises in extravascular hematomas.7, 8 Our patient would be classified as secondary type (arising in STA aneurysm).
The IPEH was first described in 1923 by the French pathologist Pierre Masson within a thrombosed hemorrhoid. He thought the disorder was a benign neoplasm. Masson theorized that the proliferation of benign cells led to hemorrhoid thrombus formation.9, 10 The opposite causality is now believed to be the etiology of IPEH. Inflammation and vascular stasis related to thrombus formation, typically within a vein, stimulate macrophage release of endothelial basic fibroblast growth factor, which in turn can support formation of IPEH.8 In the rare instances in which arterial involvement has been reported, IPEH tends to be associated with aneurysmal degeneration of the vessel in both the peripheral and intracranial circulations.9 These tumors are typically positive for CD31, CD34, smooth muscle actin, and factor VIII-related antigen. CD105 (a marker for primary endothelial neoplasms) is typically negative and can help differentiate a Masson tumor from an angiosarcoma.8 Complete resection is believed to be curative and should be meticulously undertaken as residual tumor can aggressively recur.10 If complete resection is not feasible, the tumor's growth can stabilize or undergo short-term regression if it is treated with adjuvant radiotherapy or chemotherapy. Gamma knife radiotherapy may also be of benefit.10
Differential diagnosis of pulsatile lesions must include malignant vascular neoplasms, most importantly angiosarcoma, to prevent inadequate treatment.11 Clinical examination is performed to prompt surgical resection if the lesion is easily accessible. These lesions can be difficult to differentiate even with preoperative imaging, and the final diagnosis is confirmed with pathologic evaluation. Masson tumors and angiosarcoma are differentiated histologically. Masson tumors, unlike angiosarcomas, are confined to the vessel and immunohistochemistry marker CD105 negative.8, 11
Conclusions
This case is one of a rare vascular tumor arising within a spontaneous aneurysm of the STA. We believe this case is unique because of both the unusual diagnosis of Masson tumor and the presentation as a painful pulsatile mass within a rarely seen spontaneous STA aneurysm. It is likely that thrombus formation within the aneurysm and the resultant inflammatory response encouraged growth of this unusual neoplasm.
When these tumors involve the arterial system as opposed to the more common venous presentation, they are almost universally noted to present with some associated aneurysmal dilation. A degree of clinical suspicion is warranted in patients presenting with otherwise unexplained spontaneous aneurysm formation in the intracranial, head, neck, and extremity vasculature. As these tumors have been noted to rarely occur de novo within extravascular hematomas, meticulous surgical technique should be employed during resection to avoid spilling any of the aneurysm-associated thrombus because of the theoretical risk of extravascular recurrence. When it can be successfully accomplished, as with this case, complete resection with clear margins is curative.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Kawai H., Hamasaki T., Imamura J., Tomonori N., Odashiro T., Yamahata H. Three cases of spontaneous superficial temporal artery aneurysm with literature review. Neurol Med Chir (Tokyo) 2014;54:854–860. doi: 10.2176/nmc.cr2013-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riaz A.A., Ismail M., Sheikh N., Ahmed N., Atkin G., Richman P. Spontaneously arising superficial temporal artery aneurysms: a report of two cases and review of the literature. Ann R Coll Surg Engl. 2004;86:W38–W40. doi: 10.1308/147870804128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peick A.L., Nichols W.K., Curtis J.J., Silver D. Aneurysms and pseudoaneurysms of the superficial temporal artery caused by trauma. J Vasc Surg. 1988;8:606–610. doi: 10.1067/mva.1988.avs0080606. [DOI] [PubMed] [Google Scholar]
- 4.Kim O.H., Kim Y.M., Choo H.J., Lee S.J., Kim Y.M., Yi J.H. Subcutaneous intravascular papillary endothelial hyperplasia: ultrasound features and pathological correlation. Skeletal Radiol. 2016;45:227–233. doi: 10.1007/s00256-015-2281-7. [DOI] [PubMed] [Google Scholar]
- 5.Kim E. True aneurysms of the superficial temporal artery: diagnosis and treatment. Clin Neurol Neurosurg. 2014;126:64–68. doi: 10.1016/j.clineuro.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Kawabori M., Kuroda S., Nakayama N., Kenmotsu Y., Shimizu H., Tanino M. Spontaneous giant aneurysm of the superficial temporal artery: case report. Neurol Med Chir (Tokyo) 2009;49:198–201. doi: 10.2176/nmc.49.198. [DOI] [PubMed] [Google Scholar]
- 7.Shah H.C., Mittal D.H., Shah J.K. Intravascular papillary endothelial hyperplasia (Masson's tumor) of the scalp with intracranial extension. J Pediatr Neurosci. 2014;9:260–262. doi: 10.4103/1817-1745.147584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akdur N.C., Donmez M., Gozel S., Ustun H., Hucumenoglu S. Intravascular papillary endothelial hyperplasia: histomorphological and immunohistochemical features. Diagn Pathol. 2013;8:167. doi: 10.1186/1746-1596-8-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMahon G.S., Mikhail H.M., Molyneux A.J., Thomas D.V., Hicks R.C. "Masson's pseudoangiosarcoma" in a popliteal aneurysm: tumor or thrombus? Cause or effect? Ann Vasc Surg. 2010;24 doi: 10.1016/j.avsg.2009.05.014. 257.e1-3. [DOI] [PubMed] [Google Scholar]
- 10.Velazquez C., Font J., Torres F., Araji O., Barquero J. Masson tumor as humeral artery aneurysm. Ann Vasc Surg. 2008;22:127–129. doi: 10.1016/j.avsg.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Espinosa A., Gonzalez J., Garcia-Navas F. Intravascular papillary endothelial hyperplasia at foot level: a case report and literature review. J Foot Ankle Surg. 2017;56:72–74. doi: 10.1053/j.jfas.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Bergers G., Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]