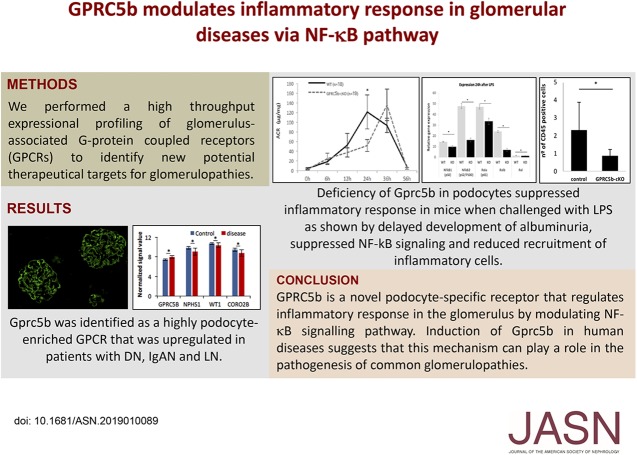
Significance Statement
Mechanisms regulating inflammatory response in glomeruli are poorly understood. In this study, the authors identify a novel G protein–coupled receptor (GPCR) that is highly enriched in podocytes, called Gprc5b. Gprc5b is upregulated in common human glomerular diseases, such as IgA nephropathy, lupus nephritis, and diabetic nephropathy. Studies in knockout animals and human podocytes grown in culture show that Gprc5b promotes glomerular inflammation via regulation of NF-κB pathway in podocytes. Upregulation of Gprc5b in human diseases suggests that this mechanism may play an important role in the pathogenesis of common glomerulopathies.
Keywords: podocyte, inflammation, glomerulus, GPCR
Visual Abstract
Abstract
Background
Inflammatory processes play an important role in the pathogenesis of glomerulopathies. Finding novel ways to suppress glomerular inflammation may offer a new way to stop disease progression. However, the molecular mechanisms that initiate and drive inflammation in the glomerulus are still poorly understood.
Methods
We performed large-scale gene expression profiling of glomerulus-associated G protein–coupled receptors (GPCRs) to identify new potential therapeutic targets for glomerulopathies. The expression of Gprc5b in disease was analyzed using quantitative PCR and immunofluorescence, and by analyzing published microarray data sets. In vivo studies were carried out in a podocyte-specific Gprc5b knockout mouse line. Mechanistic studies were performed in cultured human podocytes.
Results
We identified an orphan GPCR, Gprc5b, as a novel gene highly enriched in podocytes that was significantly upregulated in common human glomerulopathies, including diabetic nephropathy, IgA nephropathy, and lupus nephritis. Similar upregulation of Gprc5b was detected in LPS-induced nephropathy in mice. Studies in podocyte-specific Gprc5b knockout mice showed that Gprc5b was not essential for normal development of the glomerular filtration barrier. However, knockout mice were partially protected from LPS-induced proteinuria and recruitment of inflammatory cells. Mechanistically, RNA sequencing in Gprc5b knockouts mice and experiments in cultured human podocytes showed that Gpr5cb regulated inflammatory response in podocytes via NF-κB signaling.
Conclusions
GPRC5b is a novel podocyte-specific receptor that regulates inflammatory response in the glomerulus by modulating the NF-κB signaling pathway. Upregulation of Gprc5b in human glomerulopathies suggests that it may play a role in their pathogenesis.
Glomerular disease processes are the most common cause of ESRD. Glomerular injury can be caused by systemic diseases, such as in diabetic nephropathy (DN) and lupus nephritis (LN), or more glomerulus-specific disorders, such as IgA nephropathy (IgAN). Patients with glomerulopathies are treated with angiotensin-converting enzyme inhibitors and/or angiotensin receptor II blockers to diminish proteinuria. When disease progresses rapidly, steroids and other immunosuppressive drugs can be used. These pharmaceutical treatment options do not specifically target kidney tissue, and currently we lack molecular targets in the glomerulus to develop more kidney-directed therapeutic options.1
As podocyte loss is a key event in the progression of glomerulopathies, many studies have concentrated on analyzing molecular mechanisms of podocyte death.2 However, podocyte loss is often a late event in the disease and it is likely that targeting earlier changes is a more efficient approach. Glomerular inflammation and recruitment of inflammatory cells is occurring early in glomerulopathies.3 Involvement of inflammation in the progression of glomerulopathies, such as LN and IgAN, is obvious. Similarly, although traditionally DN has been considered a noninflammatory glomerular disease, global transcript profiling studies have shown inflammatory signaling and recruitment of immune cells in diabetic glomeruli.4 The inflammatory signature in glomerulopathies and diabetic glomeruli includes activation of key transcriptional regulator NF-κB and JAK/STAT pathways, which are known to drive cytokine production and influx of inflammatory cells.5−7 The role of podocytes in this process is poorly understood.
G protein–coupled receptors (GPCRs) are a large family of proteins that participate in a wide variety of biologic processes. They have been successfully exploited by the pharmaceutical industry as 20%–30% of the market share of clinically used drugs target members of this family.8,9 To identify potential new pharmaceutical targets in the glomerulus, we recently performed a high-throughput expressional profiling of glomerular GPCRs.10 In this study, we follow up on our previous study and describe an orphan GPCR, Gprc5b, as a novel, highly podocyte-enriched molecule. We show that Gprc5b expression is induced in common human glomerulopathies and that it modulates inflammatory response in podocytes via NF-κB pathway. We speculate that Gprc5b could be a novel molecular target to suppress inflammatory response in glomerular disease processes.
Methods
Human Material
Control human kidney tissue was from kidneys that were removed because of renal carcinoma or from renal biopsy specimens taken from healthy, living, related donor kidneys at Karolinska University Hospital (Stockholm, Sweden). Only kidney tissue that was histologically normal was used. Renal biopsy samples from patients with DN were collected at Karolinska University Hospital (Stockholm, Sweden) and Sahlgrenska University Hospital (Gothenburg, Sweden). The local ethical committees at both sites approved the study (approval no. 2010/579-31/1, Stockholm, Sweden; approval no. 413-09, Gothenburg, Sweden).
Transgenic Mouse Lines
Gene targeting of Gprc5b gene was performed by The European Conditional Mouse Mutagenesis Program (www.eucomm.org). The construct was targeted to surround the exon 2 of the GPRC5b gene, allele Gprc5btm1a(EUCOMM)Wtsi. The mice were in a mixed C57bl/6 and 129Sv background. We crossed these mice with an FLP-deleter line to generate a floxed mouse line, flox Gprc5b (Gprc5b-fl). Gprc5b-fl was crossed with a podocin-cre line to inactivate Gprc5b specifically in podocytes (Gprc5b-cKO). The genotyping was done by PCR using genomic DNA extracted from ear biopsy specimens. Primers for genotyping were as follows: CAS-R1-Term: tcgtggtatcgttatgcgcc; Gprc5b-87598-F: gctggaaggtttctccctct; Gprc5b-87598-R: aagagacaaccaccagacagg.
The Gt(ROSA)26Sortm14(CAG-td-Tomato)Hze/J mice were crossed with a podocin-cre line to activate the td-Tomato expression specifically in podocytes. Breeding and genotyping were done according to standard procedures.
All animal studies were carried out in Preclinical Laboratory (Karolinska Institutet) and were approved by the Ethical Committee on Research Animal Care.
LPS-Induced Proteinuria Mouse Model
Ten-week-old mice were treated by a single intraperitoneal injection of 13 μg/g body wt of LPS (L9641; Sigma). A total of 18 littermate control controls and 19 Gprc5b-cKO animals were included. Urine was collected before the injection and at 6, 12, 24, 36, and 56 hours after the injection. Albumin and creatinine values were measured using Albuwell kit (catalog number 1011; Exocell) and Quantichrome creatinine assay kit (catalog number DICT-500; BioAssay Systems), respectively.
Immunofluorescence
We used frozen human and mouse samples fixed in cold acetone and blocked with normal goat serum (G9023; Sigma). The primary antibodies, incubated overnight at 4°C, were: Gprc5b (catalog number HPA015247, 1:500; Atlas Antibodies), CD31 (catalog number 303106, 1:2000; Biolegend), PDGFRβ (catalog number MAB1263, 1:1000; R&D System), CD45 (catalog number ab64100, 1:250; Abcam), mouse nephrin (catalog number BP5030, 1:200; Acris). The human nephrin antibody has been described previously.11
Histologic Analysis and Electron Microscopy
For histologic analyses, kidney samples were fixed in 4% paraformaldehyde followed by dehydration and embedding in paraffin. At least eight controls and eight Gprc5b-cKO animals were analyzed in each experiment. Hematoxylin and eosin and periodic acid–Schiff staining were done using standard protocols. Transmission electron microscopy (FEI Tecnai Spirit BioTWIN) was performed on renal cortex samples fixed with glutaraldehyde, following standard procedures.
Quantitative and Conventional PCR
Glomeruli were isolated from human and mouse kidneys as published previously.12,13 All the quantitative PCRs were performed following the standard methods and using SYBR Green (catalog number 1725271; Bio-Rad), and the conventional PCR was performed using HotStarTaq polymerase (catalog number 03643; Qiagen). The primers used were as follows: mGprc5b-F: atgcgggagacagcatttga, mGprc5b-R: ggacagccatttcagtccct; huGprc5b-F: cgtggcatcagagagaaaga, huGprc5b-R: cccagggtccccaggaggaa; Gprc5b exon2–3_F: tgcgggagacagcatttgat, Gprc5b exon2–3_R: gcggagcagttgggatagtc; Gprc5b exon3-_4F: ggactatcccaactgctccg, Gprc5b exon3-_4R: agtttgcaggacgattccgt; Nephrin_F: gagagccccattcaaaggct, Nephrin_R: agaaggagctcacggtttcg; Coro2b_F: aatggaaccccttcatcgac, Coro2b_R: agttggcctcctgcagaaca; wt1_f: gtagccccgactcttgtacg, wt1_r: gtcctggtgtgggtcttcag; Aquaporin3_R: gctggccggtcgtgaagact, hAquaporin3_F: tgtttcgggccccaatggca; mccl2_R: ttctttgggacacctgctgct, mccl2_F: ttaacgccccactcacctgc; huccl2_R: gtgtctggggaaagctagggg, huccl2_F: gaccccaagcagaagtggg; IL-6_F: tacatcctcgacggcatctc, IL-6_R: gctacatttgccgaagagcc; IL-10_F: aactgagacatcagggtggc, IL-10_R: aaggtttctcaaggggctgg; hu28S-F: ttgaaaatccgggggagag, hu28S-R: acattgttccaacatgccag; mGAPDH-F: tgttcctacccccaatgtgt, mGAPDH-R: tgtgagggagatgctcagtg. The values were calculated using 28S or GAPDH as housekeeping gene, and using the 2−ΔΔCTmethod.14
Western Blotting
Western blotting was performed using standard procedures. We used Clarity western ECL substrate (catalog number 1705061; Bio-Rad) and the ChemiDoc touch imaging system (Bio-Rad). Antibodies used were: anti-Gprc5b (Human Protein Atlas, 1:500), anti-P65 (catalog number D14E12, 1:1000; Cell Signaling Technology), anti-pP65 (Ser536) (catalog number 3033, 1:1000; Cell Signaling Technology), anti-FYN (catalog number ab184276, 1:1000; Abcam), anti-pFYN (Y530) (catalog number ab182661, 1:1000; Abcam), pAKT (catalog number 9271, 1:1000; Cell Signaling Technology), pERK (catalog number 4370, 1:1000; Cell Signaling Technology), EGFR (catalog number 4267, 1:1000; Cell Signaling Technology), pEGFR (catalog number 2234, 1:1000; Cell Signaling Technology), pSmad2 (catalog number 8828, 1:1000; Cell Signaling Technology), active β-catenin (catalog number 05–665, 1:400; Millipore), caspase3 (catalog number 966, 1:1000; Cell Signaling Technology), pSTAT3 (catalog number 9131, 1:1000; Cell Signaling Technology), β-actin (catalog number 8227, 1:2000; Abcam).
Podocytes in Cell Culture
Human podocytes were cultured as previously described.15 We developed stable podocyte cell lines expressing Gprc5b by transfecting the cells using Lipofectamine 2000 (Thermo Fisher Scientific). The cells were transfected with p6595 MSCV-IP-N-HA-GPRC5B (catalog number 34894; Addgene) or with an empty vector. Clones were obtained by limited dilution and expanded under puromycin (P8833; Sigma) selection. The overexpression of Gprc5b in different clones was confirmed by Western blotting. Both control and GPRC5b overexpressing cells were deprived overnight with 0.1% FBS medium and treated with 1 μM of LPS (L9641; Sigma), 25 ng/ml of EGF (AF10015A; Peprotech), or 100 ng/ml of Wnt3 (P27467; R&D Systems). To study the role of GPRC5b in Wnt and EGFR pathways, we used the inhibitors IWP-2 (20 nM sc-252928; Santa Cruz Biotechnology) and AG1478 (5 nM 65852; Merck Millipore), respectively. The inhibitors were added 30 minutes before the treatment.
Dual-Luciferase Assay
We used the Dual-Luciferase Reporter Assay System (Promega) to measure NF-κB activation using standard procedures. A vector carrying the Renilla (Renilla reniformis) luciferase reporter gene was used as an internal control. To determinate the changes in NF-κB activity, we transfected the cells with expression vectors encoding human TLR4, human MD-2, and ELAM-1 luciferase reporter plasmid monitoring NF-κB activity.
Next-Generation RNA Sequencing of Mouse Glomeruli
RNA isolated from glomeruli was measured using the Agilent BioAnalyzer 2100. All samples passed the quality criteria by RNA integrity number >8.0, 28S/18S>1.0. RNA samples were then prepared and sequenced by the commercial service of BGI Tech.
All raw sequence reads available in FastQ format was mapped to the mouse genome (mm10), human genome (hg38), and crab-eating macaque (Macaca fascicularis v5.0.92) using Tophat2 with Bowtie2 option,16,17 where adaptor sequences were removed using trim_galore before read mapping. Binary Alignment Map files containing the alignment results were sorted according to the mapping position. Raw read counts for each gene were calculated using featureCounts from Subread package.18
DEseq2 was used to perform the analysis of differential gene expression, where genes with raw counts as input.19 The differentially expressed genes were identified by adjusted P value for multiple testing using Benjamini–Hochberg correction with false discovery rate values <0.1.
Statistical Analyses
Statistical difference between two groups was analyzed by t test. Data were analyzed using the Prism software (GraphPad, San Diego, CA) and was recorded as the mean±SEM, with P<0.05 defined as significant.
Results
GPRC5b Is Highly Enriched in Podocytes
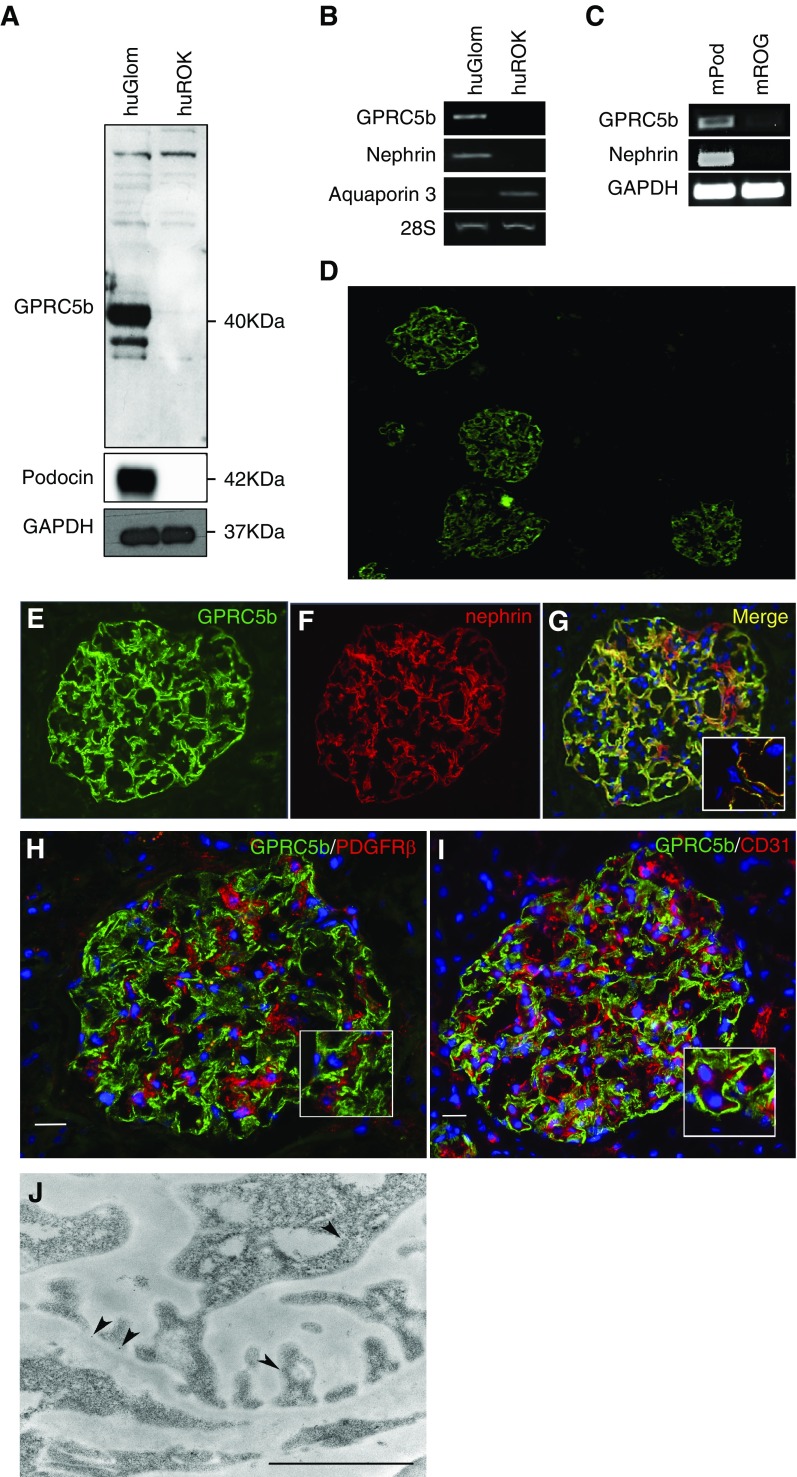
We identified Gprc5b through a large-scale transcript profiling where we compared the expression of 378 GPCRs in human glomerulus to rest of cortex tissue (ROK).10 In that study, Gprc5b was one of the highly glomerular-enriched GPCRs. To validate this, we performed Western blotting and conventional PCR on samples obtained from human glomerulus and ROK, and in mouse podocytes isolated from a transgenic mouse line expressing td-tomato in podocytes. Western blotting showed a strong band, sized approximately 40 kDa in human glomeruli (Figure 1A), whereas this protein was not detected in the ROK fraction. The size of the protein was in line with published data.20 By PCR, only the glomerular fraction generated a product when Gprc5b-specific primers were used (Figure 1B). The purity of the human glomerular fraction was verified by analyzing podocin and nephrin expression, whereas aquaporin 3 was used as a tubular marker (Figure 1, A and B). In mice, podocytes isolated from dt-tomato animals showed an enrichment of Gprc5b when compared with the rest of the glomerulus (Figure 1C). Nephrin was used to validate the purity of podocytes.
Figure 1.
GPRC5b is enriched in human and mouse podocytes. (A) In human kidney, Western blotting for Gprc5b detects a 40 kDa protein only in the glomerulus (huGlom) and not in rest of the kidney (huROK). Podocin was used to show the purity of the glomerular fraction and GAPDH as a loading control. (B) Gprc5b transcript shows strong enrichment in the glomerulus when compared with the rest of the kidney as detected by conventional PCR. Nephrin and aquaporin 3 were used as glomerular and rest of kidney fraction markers, respectively, whereas 28S gene was used as a loading control. (C) The PCR for Gprc5b in FACS-isolated mouse podocytes (mPod) and rest of glomerulus (mROG) shows an enrichment in podocytes. Nephrin was used to validate the purity of podocyte fractions and GAPDH as a loading control. (D) Immunofluorescence staining for GPRC5b (green) in human kidney cortex shows strong immunoreactivity in glomeruli and no significant signal in extraglomerular areas. (E–G) Double staining of Gprc5b (green) and podocyte foot process marker nephrin (red) shows nearly complete colocalization (yellow). DAPI (blue) was used as a nucleus marker. (H and I) Double labeling with the mesangial marker PDGFRβ (red) or with the endothelial marker CD31 (red) does not show significant overlapping reactivity. (J) Immunoelectron microscopy shows gold label for Gprc5b (arrowheads) on podocyte plasma membrane. Magnifications: ×40 in (D), ×200 in (E–G), ×400 in (H and I). Scale bar, 250 nm in (J).
The location of Gprc5b in human glomeruli was evaluated by double-immunofluorescence staining of Gprc5b with nephrin, PDGFRβ, and CD31, markers for podocytes, mesangial cells, and endothelial cells, respectively. Gprc5b colocalized with the podocyte foot process marker nephrin, whereas no overlap was detected between PDGFRβ and CD31 (Figure 1, D–I). To localize Gprc5b in foot processes, we performed immunoelectron microscopic analysis. The signal for Gprc5b was restricted to podocytes and enriched in the apical membrane of foot processes (Figure 1J). Taken together, our data indicate that Gprc5b is a novel highly podocyte-enriched molecule localized at the apical plasma membrane.
Gprc5b Expression Is Induced in DN and Other Common Glomerulopathies
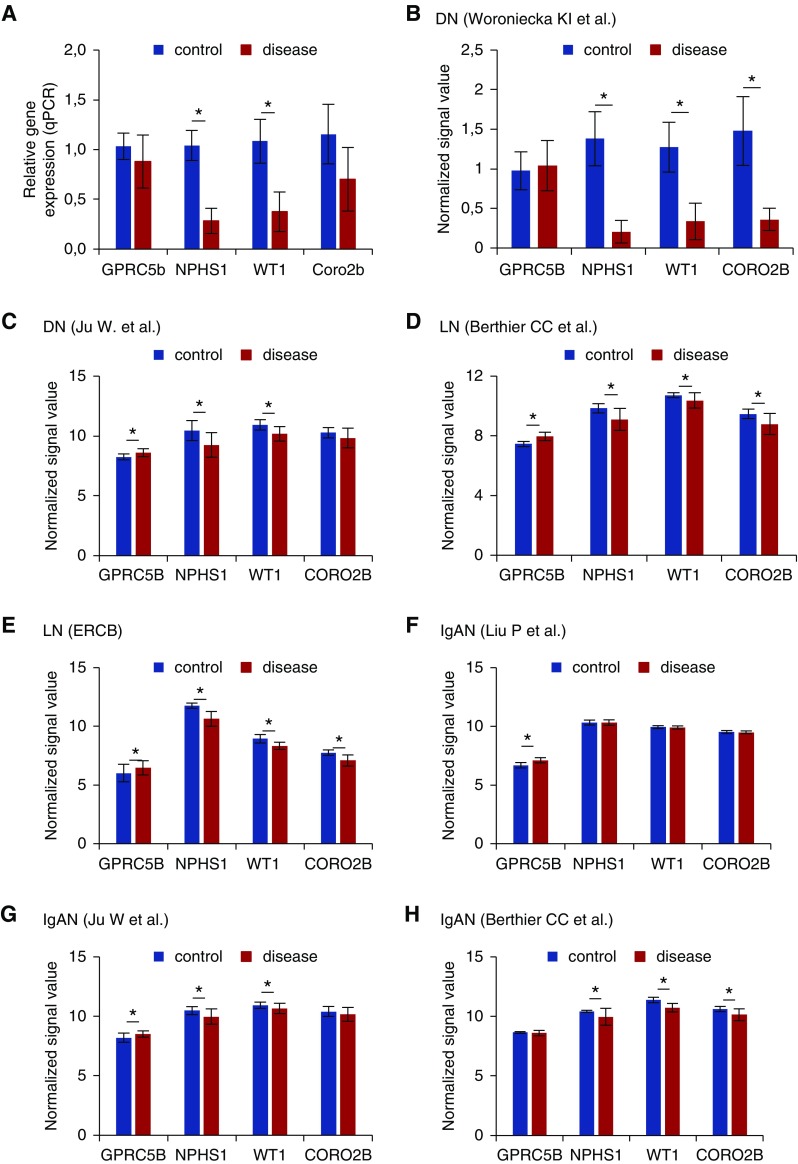
To determine whether Gprc5b is involved in the pathogenesis of human glomerular diseases, we analyzed its expression in different glomerulopathies. First, we performed qPCR on glomeruli isolated from DN and healthy controls. In diabetic glomeruli, podocyte-specific genes Nphs1, Wt1, and Coro2b showed a significant downregulation, which was probably secondary to a loss of podocytes (Figure 2A). In contrast to this, Gprc5b levels were not significantly altered, suggesting that Gprc5b expression was induced in remaining podocytes.
Figure 2.
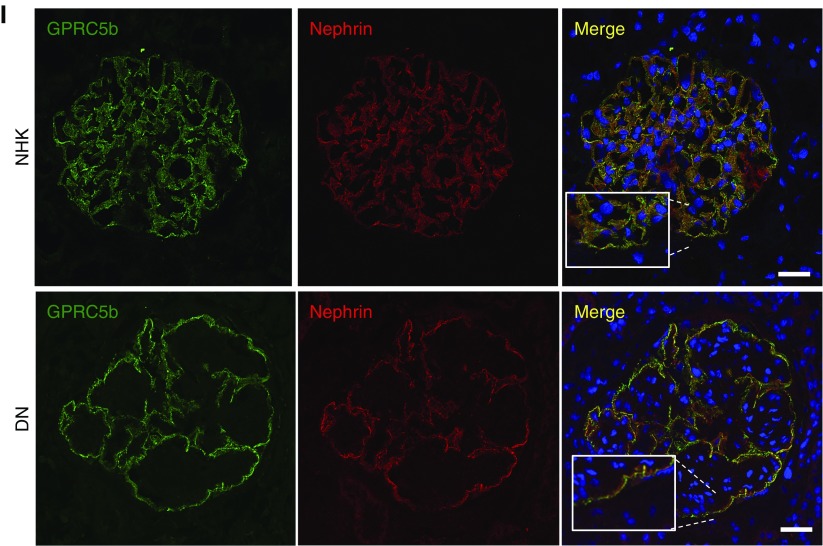
Glomerular Gprc5b expression is induced in common human glomerulopathies. (A) Quantitative PCR analysis of Gprc5b in isolated glomeruli shows that the expression is not significantly altered in DN, whereas podocyte genes Nphs1 and Wt1 are downregulated. *P<0.001. (B) Analysis of microarray data generated by Woroniecka et al.7 in glomeruli isolated from DN and control patients. The expression of Gprc5b is unchanged whereas other podocyte genes are strongly downregulated in DN. *P<0.001. (C) In the data set generated by Ju et al.,5 the expression of Gprc5b is upregulated in DN glomeruli, whereas other podocyte genes are either downregulated or unchanged. *P<0.001. (D) In the data set generated by Berthier et al.,21 the expression of Gprc5b is induced in LN glomeruli, whereas other podocyte genes are either downregulated or not changed. *P<0.001. (E) In the data set generated by European Renal cDNA Bank (ERCB), the expression of Gprc5b is induced in LN glomeruli, whereas other podocyte genes are either downregulated or not changed. *P<0.001. (F) In the data set generated by Liu et al.,22 the expression of Gprc5b is upregulated in IgAN glomeruli, whereas other podocyte genes are not changed. *P<0.001. (G) In the data set generated by Ju et al.5 the expression of Gprc5b is induced in IgAN glomeruli, whereas other podocyte genes are downregulated or unchanged. *P<0.001. (H) In the data set generated by Berthier et al.,21 the expression of Gprc5b is unchanged in IgAN glomeruli, whereas other podocyte genes are downregulated. *P<0.001. (I) Immunofluorescence staining for Gprc5b (green) and nephrin (red) shows colocalization in human DN glomeruli. No staining for Gprc5b is detected in other cell types than podocytes. DAPI (blue) was used as a nucleus marker. Magnifications: ×200.
To validate our finding, we analyzed publicly available transcriptome data on isolated glomeruli in human DN. In the data set by Woroniecka et al.,7 Gprc5b expression was not significantly changed in DN glomeruli, although other podocyte genes analyzed were strongly downregulated (Figure 2B). This downregulation is probably due to loss of podocytes, as glomeruli in this study were collected from patients with advanced disease (mean GFR 31 mL/min per 1.73 m2). In the data set generated from isolated DN glomeruli by Ju et al.5 Gprc5b expression was significantly upregulated, whereas other podocyte genes were either downregulated or not significantly changed (Figure 2C). Taken together, Gprc5b expression seems to be induced in podocytes during the progression of DN.
To see whether this upregulation was specific to DN or a shared molecular mechanism in glomerulopathies, we investigated the expression of Gprc5b in previously published transcriptome data on isolated glomeruli in LN and IgAN. In the two LN data sets analyzed,5,21 Gprc5b was significantly induced in the glomerulus, whereas other podocyte markers were downregulated (Figure 2, D and E). In the three IgAN data sets analyzed,5,6,22 Gprc5b was similarly either significantly induced or unaltered in the glomerulus, whereas other podocyte markers were either downregulated or unaltered (Figure 2, F–H).
To exclude the possibility that Gprc5b was expressed in glomerulopathies by some other cell type(s) than podocytes, we performed immunofluorescence staining in biopsies collected from five patients with DN. No significant expression was detected outside podocytes as demonstrated by double labeling with nephrin (Figure 2I). To summarize, Gprc5b expression seems to be induced in podocytes in three common human glomerulopathies, DN, LN, and IgAN.
GPRC5b Is Not Essential for the Normal Development of the Glomerulus Filtration Barrier
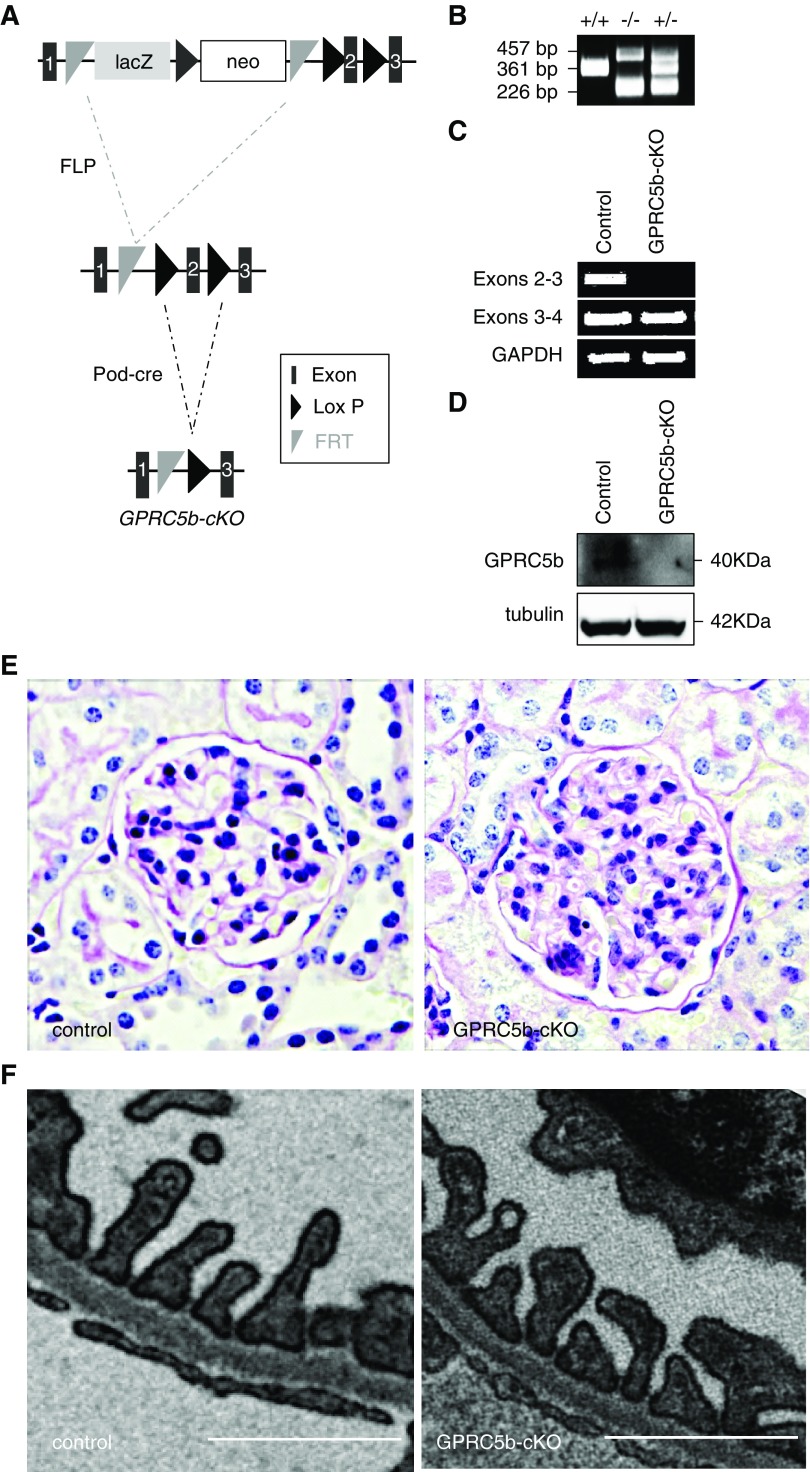
To understand the biologic role of Gprc5b in the kidney, we generated a podocyte-specific knockout mouse line (Gprc5b-cKO). We deleted exon 2 of the mouse Gprc5b gene by a strategy summarized in Figure 3A. To validate the genotype of our mice we used primers that amplified a 361 bp wild-type band, whereas the knockout allele generated 226 and 457 bp products (Figure 3B). The success of the knockout strategy was tested using PCR in glomeruli isolated from Gprc5b-cKO and control mice. We designed specific primers for the regions between the exons 2–3 and 3–4. In Gprc5b-cKO mice the band for exons 2–3 is missing, but not the band for exons 3–4, indicating that exon 2 is depleted but Gprc5b mRNA is still generated (Figure 3C). To evaluate whether this mRNA is stable and able to generate a protein, we performed Western blotting for GPRC5b in isolated glomeruli. The Gprc5b-cKO mice showed no protein expression (Figure 3D), indicating the successful inactivation of Gprc5b gene in podocytes.
Figure 3.
Podocytes and the glomerulus mature normally in podocyte-specific knockout (KO) animals. (A) Strategy to generate podocyte-specific KO mice for Gprc5b. Mice carrying a targeting cassette for exon 2 of Gprc5b gene was crossed with a FLP-deleter line to generate a floxed allele, followed by crossing with podocin-cre line to remove exon 2 specifically in podocytes. (B) Genotyping of wild-type (+/+), heterozygote (+/−), and Gprc5b-cKO (−/−) mice. The wild-type band is 361 bp and bands for the floxed allele are 226 and 457 bp, respectively. (C) PCR in mouse glomeruli isolated from Gprc5b-cKO and control mice using specific primers for exons 2–3 and exons 3–4. In Gprc5b-cKO glomeruli no product is amplified when using primers for exons 2–3. (D) Western blotting for Gprc5b in mouse glomerulus shows a band around 40 kDa in control mice, whereas this protein is missing in glomeruli isolated from Gprc5b-cKO mice. Tubulin was used as a loading control. (E) No significant differences were observed in light microscopic examination of Gprc5b-cKO kidneys when compared with control kidneys. (F) In electron microscopic analysis, no morphologic abnormalities were detected in the glomerular filtration barrier in Gprc5b-cKO mice. Magnifications: ×200 in (E). Scale bar, 250 nm in (F).
The depletion of Gprc5b in podocytes did not have any notable effect on the development of the glomerulus, as shown by standard light microscopy (Figure 3E). The ultrastructure of the filtration barrier as shown by electron microscopy was also unaffected, including the presence of regularly interdigitating foot processes and normal slit diaphragms (Figure 3F). Functionally, the filtration barrier seemed intact as no albuminuria was detected in animals aged between 1 week and 1 year (data not shown).
LPS-Induced Glomerulopathy in Mouse Phenocopies Upregulation of Gprc5b in Human Glomerulopathies
Next, we wanted to challenge Gprc5b-cKO mice with a disease model that would copy the molecular phenotype detected in human glomerulopathies, i.e., induction of Gprc5b expression in glomeruli. We analyzed previously published23−27 and in-house transcriptome data sets generated from isolated glomeruli. We detected no induction of Gprc5b in DN models db/db and OVE26 (Supplemental Figure 1). Similarly, no significant change in Gprc5b expression was detected in adriamycin-induced or other nephropathy models. In contrast to this, LPS-induced glomerulopathy, characterized by rapidly developing transient proteinuria, showed over five-fold induction of Gprc5b expression in isolated glomeruli (Supplemental Figure 1).23 Similarly, quantitative PCR analysis of Gprc5b in glomeruli isolated from streptozotocin-induced diabetic mice showed a significant induction of Gprc5b expression (Supplemental Figure 1). However, as Gprcb-cKO mice have B6 background that is resistant to streptozotocin induced kidney injury, we challenged the Gprcb-cKO mice with LPS.
Gprc5b Deficiency in Podocytes Delays Response to LPS by Modulating the Activation of NF-κB Pathway
As albuminuria levels in LPS-induced nephropathy are changing rapidly, we used a rather large animal cohort in our study, including a total of 18 control and 19 Gprc5b-cKO mice. The control mice, as described previously,23 developed a peak of albuminuria 24 hours after LPS injection (Figure 4A). Interestingly, the Gprc5b-cKO animals showed a significant delay in the response, presenting the peak of albuminuria at 36 hours, 12 hours after the control mice (P<0.05). Both controls and Gprc5b-cKOs recovered completely from albuminuria at 56 hours after the LPS injection (Figure 4A). Morphologic analysis in animals euthanized 36 hours after the injection demonstrated no obvious changes at light microscopic level (Supplemental Figure 2A), whereas electron microscopy showed foot process effacement that was similar in both animal groups (Supplemental Figure 2B).
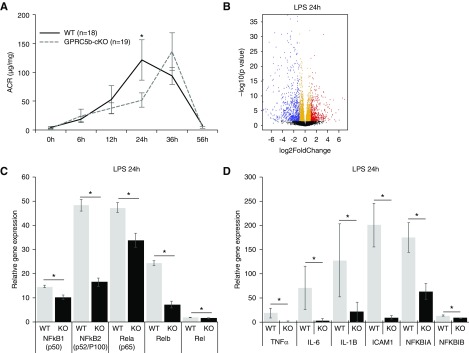
Figure 4.
Gprc5b-cKO mice show a delay in response to LPS challenge and significant transcriptional changes in NF-κB pathway. (A) Urinary albumin-to-creatinine ratio (ACR) at different time points after the LPS injection. In Gprc5b-cKO mice the peak of albuminuria is delayed by 12 hours when compared with control mice. Values are expressed as mean ±SD; *P<0.05. (B) RNA sequencing of isolated glomeruli was performed 24 hours after LPS injection. Volcano plot shows upregulated (red) and downregulated (blue) genes. Differentially expressed genes are defined as genes with P<0.001 and false discovery rate <0.1, when compared Gprc5b-cKO versus controls. (C) The expression of five family members of NF-κB were significantly downregulated in Gprc5b-cKO mice when compared with control glomeruli as shown by RNA sequencing. (D) The expression of downstream targets of NF-κB activation TNF-α, IL-6, IL-1B, and ICAM1 were robustly downregulated in Gprc5b mice glomeruli. *P<0.05.
To understand the molecular mechanisms of how Gprc5b deficiency in podocytes diminishes albuminuria after LPS injection, we performed RNA sequencing in glomeruli isolated at 24 hours, the time point when the levels of albuminuria were maximally different between the groups. A total of 4440 genes were differentially expressed between knockout and control glomeruli at 24 hours (Figure 4B, Supplemental Appendix 1). Pathway analysis revealed 164 pathways to be significantly affected. Among the top affected pathways we saw signaling events that associate with inflammatory response, including “Cytokine-cytokine receptor interaction,” “Chemokine signalling pathway,” and “NF-κB signalling pathway” (Supplemental Figure 3). As Gprc5b has been linked to NF-κB signaling in adipose tissue,11 we analyzed this pathway further. The gene expression of all five NF-κB family members were significantly downregulated in Gprc5b-cKO glomeruli compared with controls (Figure 4C). This was observed together with a downregulation of well known downstream targets of NF-κB activation, such as TNF-α, IL-6, IL-1B, and ICAM1 (Figure 4D). Taken together, the results indicate that control mice present a strong activation of NF-κB in glomeruli 24 hours after LPS injection, whereas Gprc5b-cKO mice are protected from this activation. Of note, to validate that LPS induced NF-κB signaling pathway in wild-type animals, we compared RNA sequencing data at 0 and 24 hours after the injection of LPS in control glomeruli. RNA sequencing data showed a robust activation of NF-κB signaling pathway as both components of this pathway and down-stream effectors were induced (Supplemental Figure 4, Supplemental Appendix 2).
Gprc5b Modulates the Recruitment of Inflammatory Cells to Glomeruli
To analyze downstream effects of suppressed NF-κB activation in Gprc5b-cKO, we analyzed whether this affected the production of chemotactic molecules that play a key role in recruitment of inflammatory cells. A large number of the chemoattractants were downregulated in Gprc5b-cKO glomeruli when compared with controls, as shown by RNA sequencing data (Figure 5A). The downregulation of ccl2 was validated using quantitative PCR (Figure 5B). To analyze whether this influenced the recruitment of inflammatory cells to glomeruli, we counted the number of CD45-positive glomerular cells in LPS-treated animals. Interestingly, we detected less CD45-positive cells in Gprc5b-cKO glomeruli (Figure 5, C and D), indicating that Gprc5b expression in podocytes mediated the recruitment of immune cells to glomerular tufts under pathologic stimuli.
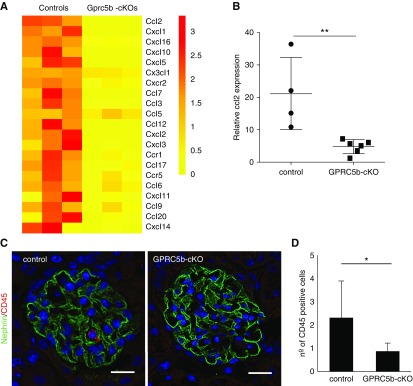
Figure 5.
Expression of chemokines and recruitment of inflammatory cells is suppressed in Gprc5b-cKO glomeruli. (A) The expression of 21 chemokines is significantly downregulated in Gprc5b-cKO glomeruli as shown by a heat map generated from RNA sequencing data. (B) Validation of the downregulation of ccl2 expression by quantitative PCR. GAPDH was used as a housekeeping gene. **P<0.01. (C and D) Immunofluorescence analysis of CD45-positive cells in glomeruli 36 hours after LPS treatment shows significantly less immune cells in Gprc5b-cKO glomeruli when compared with controls. Magnifications: ×200. *P<0.05.
Overexpression of Gprc5b Causes a Constitutive Activation of NF-κB
To validate the results obtained in mice, we carried out in vitro experiments using immortalized human podocytes.16 As podocytes in culture often lose the expression of genes enriched in podocytes,28 it was not surprising that Gprc5b was not expressed in this cell line (Figure 6A). Therefore, we generated a stable human podocyte cell line that overexpressed Gprc5b and mimicked the in vivo situation by challenging the cells with 10 μM LPS for 4 and 24 hours. In control cells, the treatment with LPS induced an increase in the phosphorylation levels of P65, one of the family members of NF-κB, at 4 and 24 hours (Figure 6, A and B). In contrast, the cells overexpressing Gprc5b showed a constitutive activation of P65, which was further exacerbated by LPS treatment. The levels of total P65 did not differ between two groups or different conditions (Figure 6, A and B). No significant differences were detected in pEGFR/tEGFR and pβ-catenin/tβ-catenin ratios (Figure 6, A, C, and D). To validate that Gprc5b also modulates downstream targets of NF-κB signaling, we measured levels of the proinflammatory cytokines ccl2, IL6, and M-CSF1 by quantitative PCR. A strong elevation of these known NF-κB effectors was detected in Gprc5b-expressing podocytes (Figure 6E).
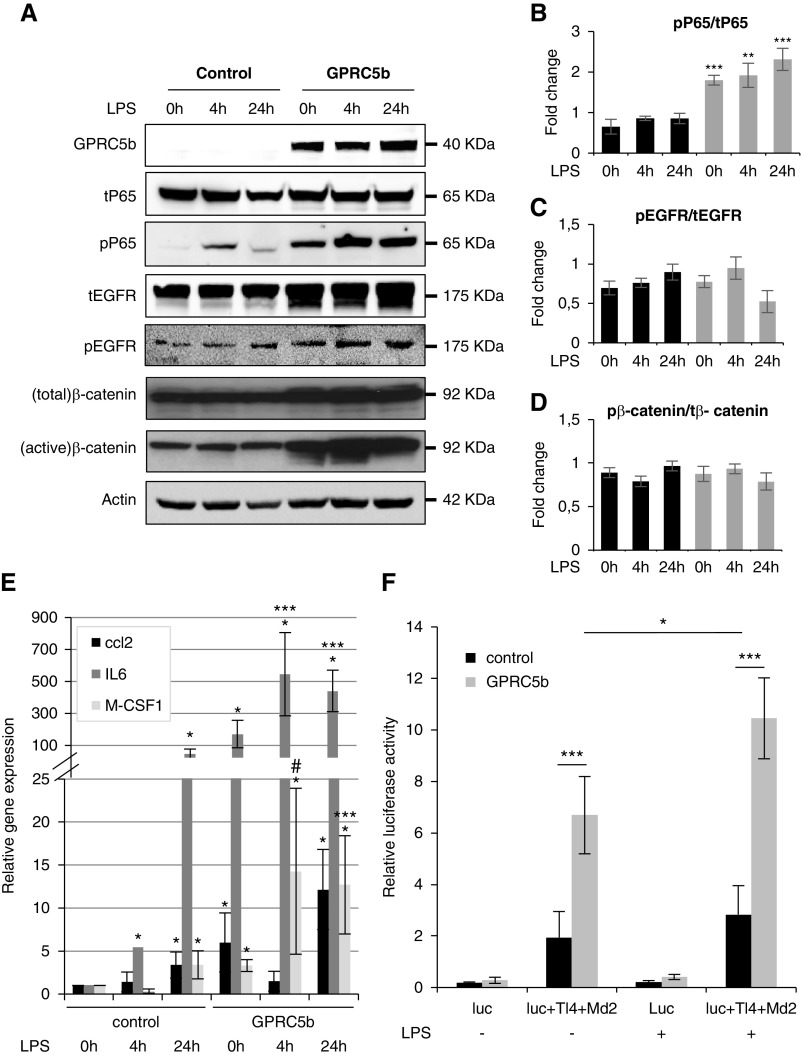
Figure 6.
Overexpression of Gprc5b in cultured human podocytes causes a constitutive activation of NF-κB. (A) In cultured podocytes, a stable overexpression of Gprc5b promotes the activation of NF-κB after treatment with LPS as shown by increased phosphorylation of P65 (pP65). In the presence of Gprc5b, even untreated cells show P65 activation. No change in total levels of P65 was detected between different samples. Similarly, Gprc5b expression promotes the activation of EGFR and β-catenin as detected by increased phosphorylation of the proteins. The total amount of these proteins was also increased. Actin was used as a loading control. (B–D) Ratios of pP65/tP65, pEGFR/tEGFR, and pβ-catenin/tβ-catenin. (E) Quantitative PCR analysis of ccl2, IL-6, and M-CSF1 demonstrates the induction of these NF-κB downstream targets in Gprc5b expressing cells. *P<0.001 compared with control 0 hours; ***P<0.001 compared with GPRC5b at 0 hours. (F) Activation of NF-κB using a luciferase reporter system in cultured podocytes with and without 4 hours LPS exposure. Expression of Gprc5b results in overactivation of NF-κB in both untreated and LPS treated cells. Values for all the experiments are expressed as mean±SD of three independent experiments. *P<0.05; **P<0.01; ***P<0.001. Luc, luciferase reporter of NF-κB pathway; Md2, cofactor of Tlr4; Tl4, toll like receptor 4.
The results were further validated using a luciferase reporter for NF-κB activation. In the luciferase assay, the cells overexpressing Gprc5b presented significantly more NF-κB activation when compared with control cells (Figure 6F). Similarly, after LPS treatment, Gprc5 cells showed more reporter activity than control cells (Figure 6F). Taken together, the in vitro experiments supported our results in knockout mice that Gprc5b modulates the activation of NF-κB signaling pathway in podocytes.
Activation of NF-κB by Gprc5b Is Not Mediated by EGFR– or β-Catenin–Dependent Signaling Pathways
As several molecular pathways can activate NF-κB in podocytes,29 we analyzed the phosphorylation levels of several molecular triggers upstream of NF-κB activation, such as EGFR, ERK, STAT3, Smad2, FYN, and β-catenin. Whereas no difference in the activation of FYN, STAT3, Smad2, ERK, AKT, and caspase 3 were detected in Gprc5b-expressing cells (Supplemental Figure 5), we observed a significant elevation in total and phosphorylated forms of EGFR and β-catenin (Figure 6A). As this suggested that the activation of NF-κB might be downstream of EGFR and/or β-catenin, we tested this hypothesis by treating Gprc5b-overexpressing cells with IWP-2 (β-catenin inhibitor) and AG1478 (EGFR inhibitor). EGF treatment caused an activation of EGFR in both control and Gprc5b cells and, as expected, AG1478 prevented this activation (Supplemental Figure 5, B and C). However, the activation of EGFR did not happen simultaneously with the phosphorylation of P65 in control or in Gprc5b cells, and the treatment of Gprc5b cells with only AG1478 did not reduce the phosphorylation levels of P65. Thus, EGFR did not mediate activation of P65 in this cell model. Similarly, for the β-catenin pathway, after activation of the pathway with Wnt3, inhibition with IWP-2 did not affect the phosphorylation status of P65, although it strongly suppressed β-catenin activation (Supplemental Figure 5, D and E). To summarize, Gprc5b-mediated activation of NF-κB pathway in podocytes does not seem to be mediated by EGFR or β-catenin.
Discussion
We describe a novel, highly podocyte-enriched GPCR, Gprc5b, the expression of which is upregulated in common human glomerulopathies. Studies in podocyte-specific knockout mice show that the induction of Gprc5b expression modulates inflammatory response in the glomerulus. We suggest that this orphan GPCR can offer a new way to suppress inflammation in a glomerulus-targeted fashion.
RNA sequencing of knockout glomeruli revealed the NF-κB signaling pathway as a downstream target of Gprc5b. This association was validated in cell culture, where overexpression of Gprc5b in podocytes promoted the activation of NF-κB. The NF-κB pathway has been shown to be activated in glomeruli isolated from patients with DN, IgAN, and LN.5−7 As we detected induction of Gprc5b in these common glomerulopathies, we speculate that Gprc5b regulates the activation of NF-κB signaling in human glomerular disorders and thus plays a pathogenic role in disease progression. Data generated in animal models suggest that inhibiting NF-κB signaling can have protective effects in the glomerulus. In passive Heymann nephritis rats, activation of NF-κB is detected in podocytes.30 Treatment with the NF-κB inhibitor pyrrolidine dithiocarbamate suppressed this activation and reduced albuminuria levels.30 In a rat crescentic GN model, inhibition of NF-κB with decoy oligonucleotides had similar beneficial effects by reducing proteinuria and histologic damage.31 In podocytes, the importance of NF-κB pathway has been shown in a transgenic mouse line in which the suppression of NF-κB exclusively in podocytes ameliorated the development of glomerulopathy.32
As abnormal NF-κB activation is a central event in the pathogenesis of many human diseases, therapeutic targeting of the pathway has been investigated intensively for over two decades.33 However, these efforts have failed to provide specific inhibitors for clinical use, mainly because of dose-limiting toxicities caused by global suppression of NF-κB. Recently, efforts have been concentrated on finding ways to manipulate the signaling pathway in a more cell-/tissue-selective fashion. In the kidney, Gprc5b, being a highly podocyte-enriched molecule, could offer a therapeutic window to target NF-κB in a cell-specific fashion.
Gprc5b has previously been investigated in pancreas, brain, and adipose tissue. In the pancreas Gprc5b was suggested to be a negative modulator of insulin secretion,34 whereas in the spinal cord the downregulation of Gprc5b in neurons is associated with neuropathic pain.35 In adipose tissue, conventional Gprc5b knockout mice showed resistance to inflammation induced by a high-fat diet, which is a key event in the development of insulin resistance.20 Interestingly, this protective effect seems to be due to lack of NK-κB pathway activation in adipocytes, similarly to the situation observed in our podocyte-specific knockout animals.
One limitation of our study was the lack of available mouse models as LPS-induced glomerulopathy was the only one that molecularly phenocopied the situation in patients with the induction of Gprc5b expression. LPS injection causes endotoxemia with an increase in serum creatinine levels, and is used as a model of AKI.36 However, it is also a well established model to study glomerular disease processes as it induces albuminuria and podocyte damage.23 Obviously, studies in clinically more relevant mouse models are indicated to explore further the involvement of Gprc5b in glomerulopathies.
Gprc5b belongs to “Retinoic Acid-Inducible G-protein-coupled receptors” that comprise four orphan receptors (Gprc5a–d). Recently, we showed that Gprc5a was also highly enriched in podocytes, where it contributed to the progression of DN by modulating EGFR signaling.10 In cell culture, we have detected that Gprc5a inhibits NF-κB signaling (Ma X, Patrakka J et al. unpublished data). Thus, Gprc5a and Gprc5b seem to have opposite effects in podocytes. It is interesting that both of these two structurally closely related receptors are highly enriched in podocytes. To explore the functional relation of Gprc5a to Gprc5b, we are generating a double-knockout mouse line in which both receptors are inactivated in podocytes.
We used cultured podocytes to validate the connection between Gprc5b and NF-κB. Overexpression studies showed the activation of NF-κB signaling pathway using multiple readouts. Moreover, we detected an activation of both EGFR and β-catenin, two known upstream effectors of NF-κB. This prompted us to test whether Gprc5b-mediated activation of NF-κB was mediated by one of these pathways. However, studies using EGFR– and β-catenin–specific inhibitors failed to verify this link. As we did not detect significant changes in EGFR and β-catenin pathways in our RNA sequencing analysis in vivo, we believe that these pathways are not centrally involved in mediating Gprc5b signaling in podocytes.
To summarize, we identified Gprc5b as a highly podocyte-enriched GPCR that modulates inflammatory response via NF-κB pathway. Because of its induction in common human glomerulopathies, we suggest GPRC5b as a new attractive therapeutic target to suppress glomerular inflammation. More studies are warranted to understand the molecular connection between Gprc5b and NF-κB activation in podocytes.
Disclosures
Dr. Lal is employed by AstraZeneca. Nyström has a consulting agreement with AstraZeneca. Dr. Patrakka’s research is supported by AstraZeneca. All of the remaining authors have nothing to disclose.
Funding
Dr. Patrakka’s laboratory was supported by grants from Karolinska Institutet/AstraZeneca ICMC, Swedish Diabetes Foundation, Marianne and Marcus Wallenberg Foundation, Westman Foundation, Swedish Kidney Foundation, and through the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and the Karolinska Institute. Dr. Nyström was supported by The IngaBritt and Arne Lundberg Research Foundation, the Swedish Research Council, the Swedish Kidney Foundation, and through the regional agreement on medical training and clinical research (ALF) between Västra Götaland County and Sahlgrenska University Hospital.
Supplementary Material
Acknowledgments
Dr. Zambrano was responsible for the design of the study, the majority of animal and cell culture work, the analysis of experimental data, and wrote the first draft of the manuscript. Dr. Möller-Hackbarth was responsible for animal work, analysis of experimental data, and manuscript revision. Dr. Li was responsible for computational analysis of the RNA sequencing experiment and manuscript revision. Dr. Charrin was responsible for animal work, analysis of experimental data, and manuscript revision. Ms. Schwarz was responsible for animal work, analysis of experimental data, and manuscript revision. Dr. Rodriguez was responsible for the design of the study, generation of the knockout mouse line, and manuscript revision. Dr. Nyström provided tissue material and was responsible for manuscript revision. Dr. Wernerson provided tissue material and was responsible for manuscript revision. Dr. Lal was responsible for analysis of experimental data and manuscript revision. Dr. Patrakka was responsible for development and design of the study, analysis of experimental data, manuscript revision, and overall planning and execution of the study.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019010089/-/DCSupplemental.
Supplemental Figure 1. Gprc5b expression in various mouse models of glomerulopathy.
Supplemental Figure 2. Histological evaluation of kidneys 36 hours after LPS injection.
Supplemental Figure 3. Table of the most significantly affected pathways in Gprc5b-cKO glomeruli when compared with control glomeruli collected 24 hours after the LPS injection.
Supplemental Figure 4. Comparison of glomerular transcriptomes between 0 and 24 hours after LPS injection in control animals.
Supplemental Figure 5. Analysis of different pathways upstream of NF-κB activation in Gprc5b-expressing cells.
Supplemental Appendix 1. RNA sequencing data in Gprc5b-cKO and control glomeruli 24 hours after the injection of LPS.
Supplemental Appendix 2. RNA sequencing data in wild-type glomeruli 0 and 24 hours after the injection of LPS.
References
- 1.Lal MA, Patrakka J: Understanding podocyte biology to develop novel kidney therapeutics. Front Endocrinol (Lausanne) 9: 409, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tharaux PL, Huber TB: How many ways can a podocyte die? Semin Nephrol 32: 394–404, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Holdsworth SR, Tipping PG: Leukocytes in glomerular injury. Semin Immunopathol 29: 355–374, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Reidy K, Kang HM, Hostetter T, Susztak K: Molecular mechanisms of diabetic kidney disease. J Clin Invest 124: 2333–2340, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ju W, Greene CS, Eichinger F, Nair V, Hodgin JB, Bitzer M, et al.: Defining cell-type specificity at the transcriptional level in human disease. Genome Res 23: 1862–1873, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ju W, Nair V, Smith S, Zhu L, Shedden K, Song PXK, et al.: ERCB, C-PROBE, NEPTUNE, and PKU-IgAN Consortium : Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med 7: 316ra193, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woroniecka KI, Park AS, Mohtat D, Thomas DB, Pullman JM, Susztak K: Transcriptome analysis of human diabetic kidney disease. Diabetes 60: 2354–2369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wacker D, Stevens RC, Roth BL: How ligands illuminate GPCR molecular pharmacology. Cell 170: 414–427, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roth BL, Irwin JJ, Shoichet BK: Discovery of new GPCR ligands to illuminate new biology. Nat Chem Biol 13: 1143–1151, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma X, Schwarz A, Sevilla SZ, Levin A, Hultenby K, Wernerson A, et al.: Depletion of Gprc5a promotes development of diabetic nephropathy. J Am Soc Nephrol 29: 1679–1689, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruotsalainen V, Patrakka J, Tissari P, Reponen P, Hess M, Kestilä M, et al.: Role of nephrin in cell junction formation in human nephrogenesis. Am J Pathol 157: 1905–1916, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takemoto M, He L, Norlin J, Patrakka J, Xiao Z, Petrova T, et al.: Large-scale identification of genes implicated in kidney glomerulus development and function. EMBO J 25: 1160–1174, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto T: Isolation and Enrichment of Glomeruli Using Sieving Techniques. In: Renal and Urinary Proteomics: Methods and Protocols, edited by Thongboonkerd V, Germany, Wiley-VCH Verlag GmbH & Co, 2009, pp 1–7 [Google Scholar]
- 14.Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Saleem MA, O’Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, et al.: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Langmead B, Salzberg SL: Fast gapped-read alignment with Bowtie 2. Nat Methods 9: 357–359, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL: TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14: R36, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao Y, Smyth GK, Shi W: featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30: 923–930, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Love MI, Huber W, Anders S: Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YJ, Sano T, Nabetani T, Asano Y, Hirabayashi Y: GPRC5B activates obesity-associated inflammatory signaling in adipocytes. Sci Signal 5: ra85, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Berthier CC, Bethunaickan R, Gonzalez-Rivera T, Nair V, Ramanujam M, Zhang W, et al.: Cross-species transcriptional network analysis defines shared inflammatory responses in murine and human lupus nephritis. J Immunol 189: 988–1001, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu P, Lassén E, Nair V, Berthier CC, Suguro M, Sihlbom C, et al.: Transcriptomic and proteomic profiling provides insight into mesangial cell function in IgA nephropathy. J Am Soc Nephrol 28: 2961–2972, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Y, He L, Takemoto M, Patrakka J, Pikkarainen T, Genové G, et al.: Glomerular transcriptome changes associated with lipopolysaccharide-induced proteinuria. Am J Nephrol 29: 558–570, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Doné SC, Takemoto M, He L, Sun Y, Hultenby K, Betsholtz C, et al.: Nephrin is involved in podocyte maturation but not survival during glomerular development. Kidney Int 73: 697–704, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Ding M, Cui S, Li C, Jothy S, Haase V, Steer BM, et al.: Loss of the tumor suppressor Vhlh leads to upregulation of Cxcr4 and rapidly progressive glomerulonephritis in mice. Nat Med 12: 1081–1087, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Reiniger N, Lau K, McCalla D, Eby B, Cheng B, Lu Y, et al.: Deletion of the receptor for advanced glycation end products reduces glomerulosclerosis and preserves renal function in the diabetic OVE26 mouse. Diabetes 59: 2043–2054, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ratelade J, Arrondel C, Hamard G, Garbay S, Harvey S, Biebuyck N, et al.: A murine model of Denys-Drash syndrome reveals novel transcriptional targets of WT1 in podocytes. Hum Mol Genet 19: 1–15, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Shankland SJ, Pippin JW, Reiser J, Mundel P: Podocytes in culture: Past, present, and future. Kidney Int 72: 26–36, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Rangan G, Wang Y, Harris D: NF-kappaB signalling in chronic kidney disease. Front Biosci 14: 3496–3522, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Mudge SJ, Paizis K, Auwardt RB, Thomas RJ, Power DA: Activation of nuclear factor-kappa B by podocytes in the autologous phase of passive Heymann nephritis. Kidney Int 59: 923–931, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Tomita N, Morishita R, Tomita S, Gibbons GH, Zhang L, Horiuchi M, et al.: Transcription factor decoy for NFkappaB inhibits TNF-alpha-induced cytokine and adhesion molecule expression in vivo. Gene Ther 7: 1326–1332, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Brähler S, Ising C, Hagmann H, Rasmus M, Hoehne M, Kurschat C, et al.: Intrinsic proinflammatory signaling in podocytes contributes to podocyte damage and prolonged proteinuria. Am J Physiol Renal Physiol 303: F1473–F1485, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Freitas RHCN, Fraga CAM: NF-κB-IKKβ pathway as a target for drug development: Realities, challenges and perspectives. Curr Drug Targets 19: 1933–1942, 2018 [DOI] [PubMed] [Google Scholar]
- 34.Soni A, Amisten S, Rorsman P, Salehi A: GPRC5B a putative glutamate-receptor candidate is negative modulator of insulin secretion. Biochem Biophys Res Commun 441: 643–648, 2013 [PubMed] [Google Scholar]
- 35.Chung HJ, Kim JD, Kim KH, Jeong NY: G protein-coupled receptor, family C, group 5 (GPRC5B) downregulation in spinal cord neurons is involved in neuropathic pain. Korean J Anesthesiol 66: 230–236, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colbert JF, Ford JA, Haeger SM, Yang Y, Dailey KL, Allison KC, et al.: A model-specific role of microRNA-223 as a mediator of kidney injury during experimental sepsis. Am J Physiol Renal Physiol 313: F553–F559, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.