Mesangial cells play a critical role in the development of glomeruli, acting in concert with podocytes and endothelial cells to form a functional filtration unit. In this issue of JASN, two papers1,2 identify transcription factors that are necessary for mesangial cell function and consequently, glomerular tuft development.
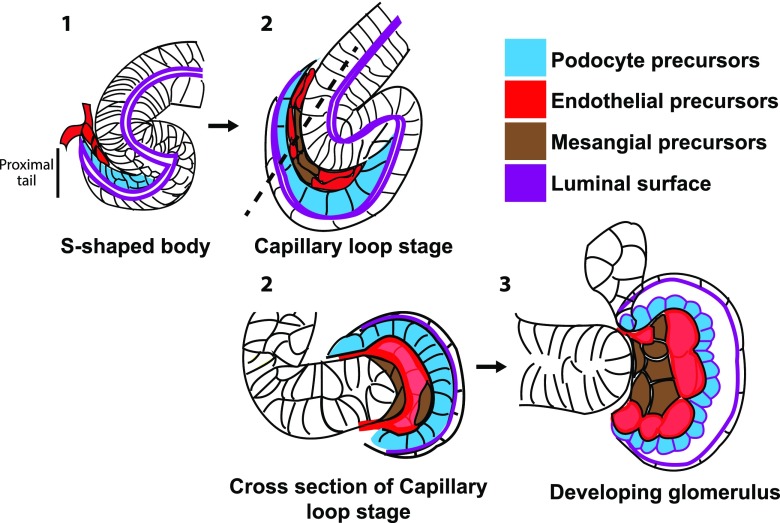
Glomerulogenesis begins at the onset of nephrogenesis. Nephron progenitor cells are progressively recruited to create an epithelial structure called the renal vesicle. Recent data suggest that the timing and position of cell recruitment are critical: progenitors that are recruited last and proximal to the renal vesicle are fated to become podocyte and parietal epithelial precursors.3 As this structure matures into an s-shaped tubule, the podocyte and parietal epithelial precursors become its proximal tail (Figure 1). Endothelial “tip cell” progenitors migrate into the proximal cleft of the tail from the surrounding capillary plexus (a process called sprouting angiogenesis) to form the first glomerular capillary tube. FoxD1+ mesangial precursors also migrate into the cleft. It is widely accepted that VEGFA secreted by podocyte precursors recruits endothelial precursors, which secrete PDGFB to recruit mesangial precursors (reviewed in ref. 4). As the proximal s-shaped tubule widens, the capillary tube does as well and ultimately, becomes an intricate capillary tuft.
Figure 1.
Schematic of glomerular development showing capillary loop formation. (A) Endothelial and mesangial precursors migrate into the proximal cleft of the s-shaped tubule called the s-shaped body. (B) At the capillary loop stage, the endothelia have formed a single continuous tube that is sandwiched between basal lamina of the podocyte precursors and mesangial cells. (C) The capillary undergoes more extensive looping as glomerular development proceeds.
The paramount importance of mesangial cells for the formation of the glomerular capillary tuft has been established through mouse genetic models lacking mesangial progenitor cell ingress or mesangial proliferation. These mice have glomeruli with a single dilated capillary loop/sac or reduced capillary tuft complexity.4 The lack of ingress may be due to defects in mesangial cell specification, migration, or recruitment. The prevailing model for how the mesangial cells promote tuft formation is via “splitting” or intussusceptive angiogenesis, in which mesangial cells insert cellular processes through the lumen of a broadening capillary loop, subdividing it into a plexus. Although direct evidence of this process during mammalian glomerulogenesis is lacking, histology consistent with this mechanism has been shown for plexus formation in chick kidney development5 and capillary repair after Thy1.1 nephritis in rats.6
Mesangial cells have been notoriously difficult to study for several reasons. The histologic parameters used to assess mesangial defects are not easily quantified, and functional assays are lacking. Isolated mesangial cells quickly dedifferentiate in culture, and available immortalized mesangial cell lines also are undifferentiated. Furthermore and perhaps most importantly, there is a lack of in vivo tools, such as mesangial cell–specific Cre lines in mice, that allow cell type–specific modification/observation in vivo. Although single-cell RNAseq experiments have been performed in the developing and mature kidney, genes expressed uniquely in mesangial cells that could be suitable for a Cre or fluorescent reporter line have not been described yet. Attempts to study gene function in mesangial cells often use the FoxD1-cre mouse line. FoxD1+ cells constitute a population of progenitor cells that give rise to renal stroma, pericytes, vascular smooth muscle cells, and mesangial cells. Typically, the genes being conditionally deleted are present in all or some of the FoxD1+ progenitor cells and their derivatives, which poses a problem when trying to assign cell type–specific roles. In particular, cortical stromal cells are instrumental in promoting nephron differentiation,7 and pericytes are required for microvascular integrity.8,9 Thus, deletion of a gene from FoxD1+ cells may potentially cause reduced nephron number, nephron tubular abnormalities, vascular hemorrhage and/or peritubular capillary rarefaction that could secondarily cause defects in glomeruli and the mesangium. For example, a significant reduction in nephron number and kidney mass will cause hyperfiltration of remaining glomeruli, which ultimately, can lead to glomerulosclerosis. In addition, FoxD1 is expressed in some podocytes as early as late stages of glomerulogenesis, further complicating data interpretation.10,11 Despite these shortcomings, when carefully executed, studies of gene function using the FoxD1-cre (or other stromal Cre line) can reveal interesting facets of mesangial cell function.
In the work by Grigorieva et al.,1 the authors examine the role of GATA3, a transcription factor expressed by the ureteric bud and FoxD1+-stromal cell progenitors during development and their derivatives in adulthood. Homozygous GATA3 loss is known to cause renal agenesis in mice.12 This defect, which has been attributed to its role in the ureteric bud, precludes study of GATA3 at later developmental steps and in additional cell types. In this study, the authors find that haploinsufficiency of GATA3 in mice leads to small glomeruli, a defect that they find is due to reduced ingress and proliferation of mesangial cells into developing glomeruli. The glomeruli consequently have reduced numbers of capillary loops. Interestingly, the number of mesangial cells remains reduced in adult glomeruli. A previous study has demonstrated that mesangial cell injury and loss in adults can be corrected through repopulation by cells recruited from the juxtaglomerular apparatus,13 which apparently does not occur in these mutants. Thus, GATA3’s function in mesangial ingress and/or proliferation must persist into adulthood and/or in the juxtaglomerular apparatus–derived progenitor cells. Alternatively, there may be a critical time period for mesangial ingress and their ability to promote normal capillary looping.
Another significant finding from Grigorieva et al.1 is that GATA3 is a robust marker of healthy and diseased mesangial nuclei in both mouse and human glomeruli. This nuclear localization allows for facile quantification of mesangial cell number, avoiding issues of cell segmentation that beleaguer efforts using cytoplasmic and membrane markers. Accurate mesangial cell quantification has great potential in clinical applications, because it could be used to better assess developmental defects as well as acquired renal diseases with increased mesangial cells or mesangial expansion. A final intriguing finding is that GATA3 expression is increased in the majority of proliferating mesangial cells in experimental mesangial proliferative GN and patient biopsies of IgA nephropathy. Future experiments to uncover the role of GATA3 in proliferation or response to injury will be of great interest.
In the work of Nelson et al.,2 the authors studied the transcription factor EBF1 in glomerular development. Their prior studies had demonstrated that EBF1 knockout mice have small kidneys with glomerulosclerosis and reduced capillary complexity.14 Because EBF1 is produced in FoxD1+ progenitor cells, mesangial cells, and podocytes, they generated mice with conditional deletion of EBF1 using FoxD1-cre and Podocin-cre. Only deletion using the FoxD1-cre leads to mice with small kidneys and reduced filtration. These mutants have an expanded interstitium and small sclerotic glomeruli with fewer capillary loops, the latter consistent with a role for EBF1 in mesangial cells. Exploration of the underlying mechanism using mesangial cells isolated from mutant mice revealed that prostenoids and COX2 expression are reduced via an indirect mechanism. Furthermore, they found that inducible expression of COX2 partially rescued the phenotype of the EBF1 mutants, thereby increasing the size of glomeruli. Additional mechanistic and functional studies will be needed to understand this interesting finding and dissect the roles of prostenoids and COX2 in glomerular development.
In both studies, the defect in mesangial cells leads to impaired development of the capillary tuft. How mesangial cells truly induce capillary plexus formation and looping remains an outstanding question in the field. Furthermore, the chemotactic and adhesive interactions that could drive these processes are unclear. Presumably, mesangial cell protrusions could anchor to the glomerular basement membrane. Indeed, mice with mutations in laminin subunit α5 have reduced glomerular capillary looping among other defects, suggesting that laminin mediates mesangial cell adhesion.15 Additionally, after the initial plexus forms, there are likely subsequent steps to create the extensively looped capillary tuft that may involve extensive remodeling of mesangial-GBM and mesangial-endothelial interactions. Future studies that characterize the three-dimensional structure of glomerular loop development and mesangial arborization and the molecular cues that drive these processes will likely reveal novel aspects of glomerular developmental disorders.
Disclosures
None.
FUNDING
Kidney research in the Marciano laboratory is supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases grants R01DK118032 and R01DK099478.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Grigorieva IV, Oszwald A, Grigorieva EF, Schachner H, Neudert B, Ostendorf T, et al. : A novel role for GATA3 in mesangial cells in glomerular development and injury. J Am Soc Nephrol 30: 1641–1658, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson T, Velazquez H, Troiano N, Fretz JA: Early B Cell Factor 1 (EBF1) regulates glomerular development by controlling mesangial maturation and consequently COX-2 expression. J Am Soc Nephrol 30: 1559–1572, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindström NO, De Sena Brandine G, Tran T, Ransick A, Suh G, Guo J, et al. : Progressive recruitment of mesenchymal progenitors reveals a time-dependent process of cell fate acquisition in mouse and human nephrogenesis. Dev Cell 45: 651–660.e4, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaughan MR, Quaggin SE: How do mesangial and endothelial cells form the glomerular tuft? J Am Soc Nephrol 19: 24–33, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Makanya AN, Stauffer D, Ribatti D, Burri PH, Djonov V: Microvascular growth, development, and remodeling in the embryonic avian kidney: The interplay between sprouting and intussusceptive angiogenic mechanisms. Microsc Res Tech 66: 275–288, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Notoya M, Shinosaki T, Kobayashi T, Sakai T, Kurihara H: Intussusceptive capillary growth is required for glomerular repair in rat Thy-1.1 nephritis. Kidney Int 63: 1365–1373, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Das A, Tanigawa S, Karner CM, Xin M, Lum L, Chen C, et al.: Stromal-epithelial crosstalk regulates kidney progenitor cell differentiation. Nat Cell Biol 15: 1035–1044, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindahl P, Johansson BR, Levéen P, Betsholtz C: Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277: 242–245, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Kramann R, Wongboonsin J, Chang-Panesso M, Machado FG, Humphreys BD: Gli1+ pericyte loss induces capillary rarefaction and proximal tubular injury. J Am Soc Nephrol 28: 776–784, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi A, Mugford JW, Krautzberger AM, Naiman N, Liao J, McMahon AP: Identification of a multipotent self-renewing stromal progenitor population during mammalian kidney organogenesis. Stem Cell Reports 3: 650–662, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyle SC, Liu Z, Kopan R: Notch signaling is required for the formation of mesangial cells from a stromal mesenchyme precursor during kidney development. Development 141: 346–354, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim KC, Lakshmanan G, Crawford SE, Gu Y, Grosveld F, Engel JD: Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nat Genet 25: 209–212, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Hugo C, Shankland SJ, Bowen-Pope DF, Couser WG, Johnson RJ: Extraglomerular origin of the mesangial cell after injury. A new role of the juxtaglomerular apparatus. J Clin Invest 100: 786–794, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fretz JA, Nelson T, Velazquez H, Xi Y, Moeckel GW, Horowitz MC: Early B-cell factor 1 is an essential transcription factor for postnatal glomerular maturation. Kidney Int 85: 1091–1102, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kikkawa Y, Virtanen I, Miner JH: Mesangial cells organize the glomerular capillaries by adhering to the G domain of laminin alpha5 in the glomerular basement membrane. J Cell Biol 161: 187–196, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]