Significance Statement
Surrogate end points are needed to assess whether treatments are effective in the earlier stages of CKD. Measuring the effects of treatments on GFR decline, which leads to kidney failure, might be one way to identify early benefits of CKD treatments. So far regulators have not approved the use of GFR slope, the difference in the change in GFR between treatment groups over time, as an end point in CKD randomized, controlled trials because they are concerned that small treatment effects on GFR may not translate into meaningful clinical benefits. Using a Bayesian individual patient meta-analysis of 47 studies including 60,620 participants, the authors found, that for sufficiently large studies, treatment effects on GFR slope from baseline and from 3-month follow-up of 0.5–1.0 ml/min per 1.73 m2/yr strongly predict benefits on clinical end points such as doubling of serum creatinine, GFR<15 ml/min per 1.73 m2, or ESKD. GFR slope can play a useful role as a surrogate end point for CKD progression in clinical trials.
Keywords: GFR, chronic kidney disease, end stage kidney disease, randomized controlled trials, meta-analysis
Visual Abstract
Abstract
Background
Surrogate end points are needed to assess whether treatments are effective in the early stages of CKD. GFR decline leads to kidney failure, but regulators have not approved using differences in the change in GFR from the beginning to the end of a randomized, controlled trial as an end point in CKD because it is not clear whether small changes in the GFR slope will translate to clinical benefits.
Methods
To assess the use of GFR slope as a surrogate end point for CKD progression, we performed a meta-analysis of 47 RCTs that tested 12 interventions in 60,620 subjects. We estimated treatment effects on GFR slope (mean difference in GFR slope between the randomized groups), for the total slope starting at baseline, chronic slope starting at 3 months after randomization, and on the clinical end point (doubling of serum creatinine, GFR<15 ml/min per 1.73 m2, or ESKD) for each study. We used Bayesian mixed-effects analyses to describe the association of treatment effects on GFR slope with the clinical end point and to test how well the GFR slope predicts a treatment’s effect on the clinical end point.
Results
Across all studies, the treatment effect on 3-year total GFR slope (median R2=0.97; 95% Bayesian credible interval [BCI], 0.78 to 1.00) and on the chronic slope (R2 0.96; 95% BCI, 0.63 to 1.00) accurately predicted treatment effects on the clinical end point. With a sufficient sample size, a treatment effect of 0.75 ml/min per 1.73 m2/yr or greater on total slope over 3 years or chronic slope predicts a clinical benefit on CKD progress with at least 96% probability.
Conclusions
With large enough sample sizes, GFR slope may be a viable surrogate for clinical end points in CKD RCTs.
CKD is common and harmful, causing kidney failure in its late stages, but with few therapies.1 One of the challenges in development and evaluation of therapies for CKD is that randomized, controlled trials (RCTs) to assess efficacy and safety of novel therapies traditionally use kidney failure and doubling of serum creatinine as clinical end points,2 which are late events in the progression of CKD. In order to obtain sufficient end points, RCTs in CKD often require substantial follow-up periods or are restricted to patients with rapidly progressive or late-stage disease, yet some interventions may have a greater effect when applied earlier versus later in the disease course.3 Alternative end points are thus needed to perform RCTs more efficiently, especially in earlier stages of CKD.
Decline in GFR is on the causal pathway to kidney failure, providing strong biologic plausibility for GFR decline as a surrogate end point for CKD progression in RCTs. There are also strong epidemiologic associations of both GFR level and GFR decline with subsequent kidney failure.4–9 However, concerns that relatively small treatment effects on average GFR slope may not translate to treatment effects on clinical end points have complicated regulatory approval of GFR slope as an end point for clinical trials of CKD. Validation of surrogate end points also requires evidence on the basis of randomized comparisons from RCTs that treatment effects on the surrogate end point predict treatment effects on the clinical end point. On the basis of these sorts of analyses, we previously demonstrated that 30% or 40% declines in GFR are valid surrogate end points for CKD progression, but these end points are not appropriate for all populations or interventions, or at early stages of disease. End points on the basis of the mean GFR slope could overcome some of these limitations. However, relatively small differences in mean GFR are generally feasible in follow-up periods for most therapies. Concerns that these seemingly small effects on mean GFR level may not translate to effects on clinical end points have contributed to the reluctance to use GFR slope as an end point in CKD clinical trials. Moreover, patterns of change in GFR after intervention are often nonlinear, with possibly differing direction and rates of changes in early follow-up (herein called acute slope) and longer-term follow-up (herein called chronic slope). The total decline from beginning to the end of the study incorporates both elements (herein called total slope). The frequent occurrence and uncertain implications of acute effects for CKD therapies have also limited the use of GFR slope. Hence, empirical validation of the total and chronic GFR slopes as surrogate end points is necessary before these end points can play a significant role in trials of kidney disease progression.
In March of 2018, the National Kidney Foundation (NKF), Food and Drug Administration (FDA), and European Medicines Agency (EMA) cosponsored a scientific workshop, “Change in Albuminuria and GFR as End Points for Clinical Trials in Early Stages of CKD,” to evaluate surrogate end points for trials of kidney disease progression and to improve understanding of changes in albuminuria and GFR as measures of kidney disease progression in early stages of CKD. For this workshop, we performed an individual patient meta-analysis of 47 RCTs accounting for a total of 60,620 subjects across 12 interventions to provide a comprehensive assessment of total or chronic GFR slope as a surrogate end point for trials of CKD progression. We used Bayesian analyses to examine the agreement between treatment effects on GFR slope and treatment effects on the clinical end point and to inform the use of GFR slope as a surrogate end point in future RCTs.
Methods
A more detailed description of the methods is available in the Supplemental Materials.
Datasets and Analytic Groups
For our prior work investigating surrogate end points, we developed a pooled database of RCTs using a systematic search (see Supplemental Table 1 for search terms and Supplemental Table 2 for complete list of inclusion criteria).10 In December of 2016, we updated this search and identified additional RCTs and requested individual patient data. After eliminating studies that did not have sufficient data, we had a total of 49 RCTs. Risks of bias for each study were assessed using the risk-of-bias tool of the Cochrane collaboration11 (Supplemental Figure 1). For RCTs that evaluated more than one intervention, we included a separate randomized treatment comparison for each independent treatment versus control comparison reported, such that some participants were included in more than one analytic unit.12–16 We then pooled small RCTs that had <100 participants if the disease and intervention were the same and thus had 49 randomized treatment comparisons as the main unit of analysis (herein called studies) (Supplemental Figure 2, Supplemental Table 3).17–29 Tufts Medical Center Institutional Review Board approved this study.
Clinical End Points
The clinical end point was defined as a composite of any of the following events over the full study duration: ESKD (initiation of chronic treatment with dialysis or kidney transplantation), GFR<15 ml/min per 1.73 m2, or sustained doubling of serum creatinine. Of the 49 studies, 47 had sufficient end points for estimation of treatment effects on the clinical end point and were used for the primary analysis.
GFR
GFR was estimated using the Chronic Kidney Disease Epidemiology Collaboration 2009 creatinine equation.30 Creatinine was standardized to isotope dilution mass spectroscopy traceable reference methods using direct comparison or was reduced by 5% as has previously been described (Supplemental Table 4).31
Statistical Analyses
Objectives
Our first goal was to evaluate the validity of the chronic and total slopes as surrogate end points by assessing the association between treatment effects on each GFR slope end point and the treatment effects on the clinical end point across studies. Our second goal was to use these results to estimate the probability of clinical benefit associated with treatment effects on GFR slope for application to future studies.
Analyses of the Total and Chronic GFR Slopes (Surrogate End Points)
We used a simplified linear mixed-effects model on the basis of a single slope starting at 3 months postrandomization adjusted for baseline GFR. The Supplemental Material describes in detail how the model accounted for various sources of variation in GFR slopes between and within subjects and treatment arms. Under this model, the differences between the randomized groups in the mean intercepts at 3-month follow-up; the mean slopes after 3 months; and the estimated mean changes from baseline to either 1-, 2-, 3-, or 4-year follow-up factored by the follow-up duration represented the treatment effects on the acute, chronic, and total slopes.
Trial-Level Analysis
The trial-level analysis requires two steps: intent-to-treat estimation of the treatment effects on the surrogate and clinical end points within each RCT and a meta-regression to relate the treatment effects on the surrogate and clinical end points across RCTs. In the first step, treatment effects on GFR slopes were estimated using the shared parametric mixed-effects models described above and were expressed as mean differences in the GFR slopes between the treatment versus control groups, in units of ml/min per 1.73 m2/yr. Treatment effects on the clinical end point were estimated by performing separate Cox proportional hazard regressions to estimate log hazard ratios (HRs) for the treatment in each trial. Summary estimates of treatment effects were obtained by use of random-effects models. In the second step, a Bayesian mixed-effects meta-regression related the estimated treatment effects on the clinical end point to the estimated treatment effects on GFR slope with study as the unit of analysis (details in the Supplemental Material). The model relates the treatment effects on the two end points after accounting for random errors in the estimated effects in each RCT. The meta-regression supports validity of GFR slope as a surrogate end point if (1) the slope of the meta-regression line is statistically significant as defined by 95% Bayesian credible intervals (95% BCIs) that do not cross 0, with a large magnitude; (2) the intercept is close to 0, implying absence of an average effect on the clinical end point when the treatment does not affect GFR slope; (3) the R2 is high, so that treatment effects on GFR slope account for most of the variation in treatment effects on the clinical end point; and (4) the root mean square error (RMSE) is low, assuring low variation in the clinical end point given a fixed treatment effect on GFR slope. We used the designations of low, moderate, and strong trial-level association as defined by R2<0.49, 0.49–0.71, and ≥0.72, respectively.32
Positive Predictive Value
We used positive predictive values (PPVs) to describe the uncertainty in predicting the treatment effect on the clinical end point from the treatment effect on the GFR slope. From the trial-level meta-regression, we computed 95% Bayesian prediction intervals and estimated the probabilities of clinical benefit (defined as HR<1) for an infinite, large, or modest-sized RCT. A large RCT was defined as one in which the treatment effect on GFR slope can be estimated to within an SEM of 0.25, corresponding to a total sample size (N) of about 1900 for RCTs whose average follow-up accorded with the RCTs in the analysis. A modest RCT was defined as having an SEM of 0.4 (N roughly 720). We computed the threshold associated with the smallest observed treatment effect on either the chronic or total slope that would assure a high probability of benefit of the treatment on the clinical end point, which we defined as the treatment effect on the GFR slope end point providing a PPV of 97.5%.
Subgroup and Sensitivity Analyses
We performed the trial-level analysis for the primary analytic dataset overall and by subgroups defined by average study level of baseline albumin-to-creatinine ratio (< or ≥30 mg/g; or < or ≥3.4 mg/mmol), GFR (< or ≥60 ml/min per 1.73 m2), cause (diabetes and diabetic kidney disease, glomerular diseases, or other causes of CKD), and intervention. Because of differences in the ranges of treatment effects, accuracy in predicting the treatment effect on the clinical end point is best compared between subgroups using the RMSE.
Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and R 3.16.1 (R Project for Statistical Computing, www.r-project.org).33
Results
Table 1 summarizes aggregate characteristics of the included studies stratified by disease. The study characteristics of each individual study are reported in Supplemental Tables 5 and 6. Average baseline mean (SD) GFR and median (25th, 75th percentiles) albumin-to-creatinine ratio were 61.7 (26) ml/min per 1.73 m2 and 60 mg/g (13, 554) in the pooled dataset, respectively.
Table 1.
Clinical characteristics of the population stratified by disease cause
| Disease | Studies, N | Patients, N | Age, Mean (SD) | Female, N (%) | Black, N (%) | Diabetes, N (%) | GFR, Mean (SD) | ACR, Median (25, 75th) | Clinical End Points, N (%) | Interventions |
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 47 | 60,620 | 61.4 (11.3) | 22,607 (37.3) | 4601 (7.6) | 45,357 (75.8) | 61.7 (26.4) | 60 (13, 554) | 7115 (11.7) | |
| Diabetes | 12 | 43,481 | 64.0 (8.5) | 15,973 (36.7) | 1484 (3.4) | 43,481 (100) | 69.8 (22.2) | 35 (9, 361) | 2431 (5.6) | RASB versus CCB Intensive BPRASB versus controlSulodexideEmpagliflozin |
| Glomerular disease | 9 | 1389 | 40.8 (12.8) | 497 (35.8) | 19 (1.4) | 5 (0.4) | 73.6 (30.2) | 1317 (838, 2335) | 188 (13.5) | ImmunosuppressionRASB versus control |
| Other CKD | 26 | 15,750 | 56.2 (13.9) | 6137 (39.0) | 3098 (19.7) | 1871 (11.9) | 38.2 (22.1) | 150 (36, 752) | 4496 (28.5) | RASB versus controlRASB versus CCBIntensive BPAlb protocolLow-protein diet |
Other CKD refers to causes of CKD other than glomerular disease or diabetes or cause not specified. Clinical end point defined as the composite of chronic dialysis or kidney transplantation, GFR<15 ml/min per 1.73 m2, or confirmed doubling of serum creatinine. Age is measured in years, follow-up time in months. ACR, albumin-to-creatinine ratio; RASB, renin angiotensin system blocker; CCB, calcium channel blocker; Alb protocol, albuminuria-targeted protocol.
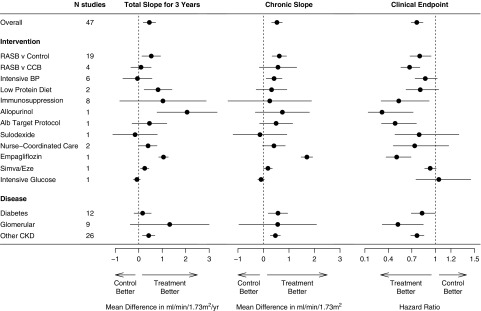
For most studies, the mean total slope at 1, 2, 3, and 4 years and chronic slope were slightly attenuated in the treatment arm compared with the control arm (Supplemental Table 7). For example, the pooled mean total slope at 3 years was −3.49 (95% confidence intervals −4.04, −2.93) ml/min per 1.73 m2/yr in the control arm and −2.94 (−3.45, −2.43) ml/min per 1.73 m2/yr in the treatment arm. The mean treatment effect on the total slope at 3 years (0.45 [0.19, 0.72] ml/min per 1.73 m2/yr) was similar to that of the chronic slope (0.53 [0.32, 0.74]) ml/min per 1.73 m2/yr) with apparent variation by intervention (Figure 1, Supplemental Figure 3, A–E, Supplemental Table 8).
Figure 1.
Treatment effect on total slope at 3 years, chronic slope, and on the clinical end point. Shown are treatment effects on total slope at 3 years (left), on chronic slope (middle), and treatment effects on clinical end point (right). Treatment effects on GFR slope are expressed as mean difference in treatment minus control and are expressed in ml/min per 1.73 m2/yr. The clinical end point is defined as ESKD, GFR<15 ml/min per 1.73 m2, or doubling of serum creatinine. Treatment effect on the clinical end point is expressed as HR. The circles represent the estimated treatment effect and the horizontal line its 95% confidence interval. Data for all studies are shown in Supplemental Figure 3, A and D, and Supplemental Figure 4. Alb, albuminuria; CCB, calcium channel blocker; RASB, renin angiotensin system blockers.
A total of 7115 patients reached the composite clinical end point across the 47 studies (Supplemental Table 9). Across all interventions, the active treatment led to a reduction in risk for the clinical end point (HR, 0.76; 95% confidence interval, 0.69 to 0.84), with similar results across subgroups (Figure 1, right panel; Supplemental Figure 4).
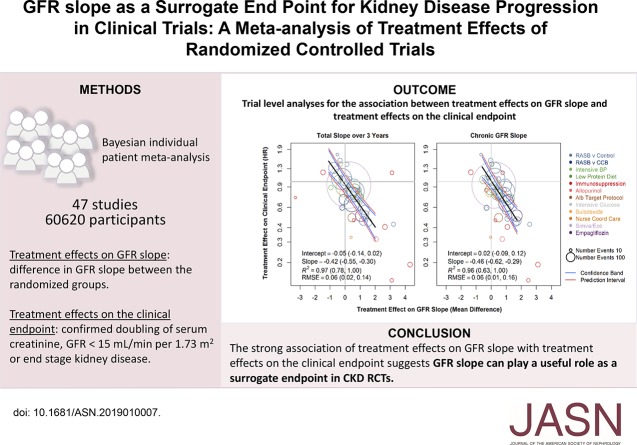
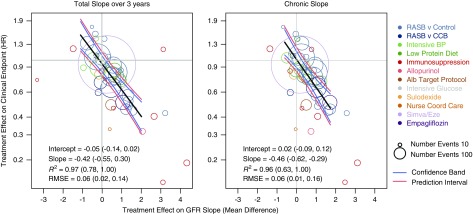
There was strong agreement between the treatment effects on the total slope at 3 years and those of the clinical end point (Figure 2, Supplemental Table 10). The slope of the meta-regression line was −0.42 (95% BCI, −0.55 to −0.30 per ml/min per 1.73 m2/yr), which indicates that each 0.75 ml/min per 1.73 m2/yr greater treatment effect on the total GFR slope was associated with an average 27% lower hazard for the clinical end point (95% BCI, 20% to 34%). The intercept of the regression line was −0.05 (95% BCI, −0.14 to 0.02), indicating that when the treatment had no effect on the total GFR slope at 3 years, there was a low probability of having a substantial treatment effect on the clinical end point. The median estimate for R2 was 0.97 (95% BCI, 0.78 to 1.00), with Bayesian probabilities of 0.1%, 0.9%, and 99% for the R2 values falling into low, moderate, or high ranges for the strength of a surrogate end point. Similar results are shown for the chronic slope (R2 0.96), with Bayesian probabilities of 0.6%, 5%, and 94% for the R2 values falling into low, moderate, or high ranges for the strength of a surrogate end point.32 Results were weaker when the total slope was computed over shorter durations (Supplemental Figure 5, Supplemental Table 10).
Figure 2.
Trial-level analyses for the association between treatment effects on GFR slope and treatment effects on the clinical end point. Left panel: Total slope at 3 years. Right panel: Chronic slope. Shown is the relationship between estimated treatment effects on the clinical end point (ESKD, GFR<15 ml/min per 1.73 m2, or doubling of serum creatinine) on the vertical axis and estimated treatment effects on the GFR slope on the horizontal axis. Treatment effects on GFR slope are expressed as mean difference in treatment minus control and are expressed in ml/min per 1.73 m2/yr. The clinical end point is defined as treated kidney failure, doubling of creatinine, or GFR<15 ml/min per 1.73 m2. Treatment effect on the clinical end point is expressed as HR. The colors indicate intervention type. Each circle is a separate intervention with the size of the circle proportional to the number of events. The black line is the line of regression through the studies. The blue line is the confidence band. The pink lines are the prediction bands computed from the model. Alb, albuminuria; CCB, calcium channel blocker; RASB, renin angiotensin system blockers.
No clear evidence of significant differences in RMSE was found in comparisons of summary results obtained from subgroups stratified by GFR or cause of disease, but credible intervals were wide for some groups (Supplemental Figure 6, Supplemental Table 11, Table 2).
Table 2.
Trial-level analysis by subgroup
| Group | Subgroup | N Studies (N Interv) | N patients (N Events) | Meta-Regression Slope | Intercept | R2 | RMSE |
|---|---|---|---|---|---|---|---|
| Total slope at 3 yr | |||||||
| Overall | 47 (12) | 60,620 (7115) | −0.42 (−0.55 to −0.30) | −0.05 (−0.14 to 0.02) | 0.97 (0.78 to 1.00) | 0.06 (0.02 to 0.14) | |
| GFR | <60 | 34 (10) | 28,633 (6375) | −0.39 (−0.62 to −0.13) | −0.06 (−0.18 to 0.05) | 0.86 (0.18 to 0.99) | 0.06 (0.02 to 0.16) |
| ≥60 | 13 (6) | 31,987 (740) | −0.41 (−0.57 to −0.28) | −0.10 (−0.26 to 0.06) | 1.00 (0.87 to 1.00) | 0.05 (0.01 to 0.22) | |
| ACR | <30 | 6 (4) | 30,234 (622) | −0.50 (−0.77 to −0.20) | −0.07 (−0.26 to 0.10) | 0.98 (0.38 to 1.00) | 0.05 (0.01 to 0.31) |
| ≥30 | 41 (10) | 30,386 (6493) | −0.41 (−0.57 to −0.26) | −0.06 (−0.16 to 0.04) | 0.95 (0.63 to 1.00) | 0.07 (0.02 to 0.16) | |
| Disease | Diabetes | 12 (6) | 43,481 (2431) | −0.52 (−0.73 to −0.32) | −0.05 (−0.15 to 0.05) | 0.98 (0.72 to 1.00) | 0.04 (0.01 to 0.16) |
| Glomerular | 9 (2) | 1389 (188) | −0.29 (−0.50 to −0.09) | −0.26 (−0.62 to 0.10) | 0.99 (0.33 to 1.00) | 0.06 (0.01 to 0.47) | |
| Other CKD | 26 (8) | 15,750 (4496) | −0.35 (−0.59 to −0.09) | −0.08 (−0.22 to 0.05) | 0.87 (0.16 to 0.99) | 0.07 (0.02 to 0.18) | |
| Chronic slope | |||||||
| Overall | 47 (12) | 60,620 (7115) | −0.46 (−0.62 to −0.29) | 0.02 (−0.09 to 0.12) | 0.96 (0.63 to 1.00) | 0.06 (0.01 to 0.16) | |
| GFR | <60 | 34 (10) | 28,633 (6375) | −0.42 (−0.74 to −0.11) | 0.00 (−0.18 to 0.15) | 0.89 (0.13 to 0.99) | 0.06 (0.01 to 0.16) |
| ≥60 | 13 (6) | 31,987 (740) | −0.50 (−0.71 to −0.32) | 0.10 (−0.13 to 0.31) | 0.99 (0.70 to 1.00) | 0.06 (0.01 to 0.31) | |
| ACR | <30 | 6 (4) | 30,234 (622) | −0.48 (−0.77 to −0.21) | 0.16 (−0.06 to 0.41) | 0.98 (0.34 to 1.00) | 0.06 (0.01 to 0.34) |
| ≥30 | 41 (10) | 30,386 (6493) | −0.45 (−0.71 to −0.21) | 0.00 (−0.16 to 0.14) | 0.94 (0.39 to 1.00) | 0.06 (0.02 to 0.18) | |
| Disease | Diabetes | 12 (6) | 43,481 (2431) | −0.48 (−0.69 to −0.28) | 0.15 (−0.01 to 0.31) | 0.98 (0.62 to 1.00) | 0.04 (0.01 to 0.18) |
| Glomerular | 9 (2) | 1389 (188) | −0.33 (−0.63 to −0.09) | −0.42 (−0.78 to −0.06) | 0.99 (0.35 to 1.00) | 0.06 (0.01 to 0.46) | |
| Other CKD | 26 (8) | 15,750 (4496) | −0.49 (−0.82 to −0.22) | −0.01 (−0.15 to 0.14) | 0.96 (0.48 to 1.00) | 0.04 (0.01 to 0.13) |
Units of GFR are ml/min per 1.73 m2. Units of ACR are mg/g. Results for slope, intercept, R2, and RMSE are presented as median and 2.5–97.5 Bayesian credible intervals. N Interv, number of types of intervention; ACR, albumin-to-creatinine ratio.
For application of GFR slope as a surrogate end point in future RCTs, Table 3 shows the predicted HRs and 95% prediction intervals for the treatment effects on the clinical end point as well as the corresponding PPVs. For example, for future large trials, the model predicts that a mean difference between the treatment and control groups of 0.54 or 0.48 ml/min per 1.73 m2/yr in total GFR slope over either 2 or 3 years, respectively, and 0.62 ml/min per 1.73 m2/yr for chronic slope, confers a 97.5% probability of a nonzero clinical benefit. For modest-sized trials, a study would be required to have observed treatment effects of 0.72 or 0.74 ml/min per 1.73 m2/yr mean difference in total GFR slope over either 2 or 3 years, respectively, and 0.85 ml/min per 1.73 m2/yr mean difference for chronic GFR slope, to confer 97.5% probability of clinical benefit. The predicted probabilities for a clinical benefit for total slope over 1 year are lower.
Table 3.
Application of GFR slope as surrogate end point in new RCT: predicted treatment effect on clinical end point and PPV
| GFR Slope | Observed Treatment Effect on Change in GFR Slope | Infinite Sample Size in New RCT | Large RCT | Modest RCT | |||
|---|---|---|---|---|---|---|---|
| Median HR and 95% Prediction Interval | PPV | Median HR and 95% Prediction Interval | PPV | Median HR and 95% Prediction Interval | PPV | ||
| Total slope over 4 yr | 0.5 | 0.78 (0.69 to 0.87) | 1.00 | 0.78 (0.59 to 1.01) | 0.97 | 0.78 (0.52 to 1.15) | 0.90 |
| 0.75 | 0.69 (0.61 to 0.78) | 1.00 | 0.69 (0.52 to 0.89) | 1.00 | 0.69 (0.46 to 1.02) | 0.97 | |
| 1.0 | 0.61 (0.53 to 0.7) | 1.00 | 0.61 (0.46 to 0.8) | 1.00 | 0.61 (0.4 to 0.91) | 0.99 | |
| Threshold for treatment effect on GFR slope to assure PPV≥97.5% | 0.20 | 0.52 | 0.79 | ||||
| Total slope over 3 yr | 0.5 | 0.77 (0.64 to 0.90) | 1.00 | 0.77 (0.59 to 0.99) | 0.98 | 0.77 (0.53 to 1.11) | 0.93 |
| 0.75 | 0.69 (0.58 to 0.81) | 1.00 | 0.69 (0.52 to 0.89) | 1.00 | 0.69 (0.47 to 1.00) | 0.98 | |
| 1.0 | 0.62 (0.52 to 0.74) | 1.00 | 0.62 (0.47 to 0.80) | 1.00 | 0.62 (0.42 to 0.90) | 1.00 | |
| Threshold for treatment effect on GFR slope to assure PPV≥97.5% | 0.24 | 0.48 | 0.74 | ||||
| Total slope over 2 yr | 0.5 | 0.75 (0.56 to 0.98) | 0.98 | 0.75 (0.54 to 1.01) | 0.97 | 0.75 (0.51 to 1.07) | 0.95 |
| 0.75 | 0.7 (0.52 to 0.91) | 0.99 | 0.7 (0.5 to 0.94) | 0.99 | 0.69 (0.47 to 0.99) | 0.98 | |
| 1.0 | 0.65 (0.48 to 0.85) | 1.00 | 0.65 (0.46 to 0.87) | 1.00 | 0.64 (0.43 to 0.92) | 0.99 | |
| Threshold for treatment effect on GFR slope to assure PPV ≥97.5% | 0.42 | 0.54 | 0.72 | ||||
| Total slope over 1 yr | 0.5 | 0.74 (0.49 to 1.1) | 0.94 | 0.74 (0.49 to 1.11) | 0.94 | 0.74 (0.48 to 1.11) | 0.93 |
| 0.75 | 0.72 (0.47 to 1.06) | 0.96 | 0.72 (0.47 to 1.07) | 0.95 | 0.72 (0.47 to 1.07) | 0.95 | |
| 1.0 | 0.69 (0.46 to 1.03) | 0.97 | 0.69 (0.45 to 1.04) | 0.97 | 0.69 (0.45 to 1.04) | 0.96 | |
| Threshold for treatment effect on GFR slope to assure PPV≥97.5% | 1.26 | 1.32 | 1.31 | ||||
| Chronic slope | 0.5 | 0.8 (0.66 to 0.95) | 0.99 | 0.8 (0.6 to 1.05) | 0.95 | 0.8 (0.54 to 1.17) | 0.88 |
| 0.75 | 0.72 (0.59 to 0.86) | 1.00 | 0.72 (0.54 to 0.94) | 0.99 | 0.72 (0.48 to 1.05) | 0.96 | |
| 1.0 | 0.65 (0.53 to 0.78) | 1.00 | 0.65 (0.48 to 0.85) | 1.00 | 0.65 (0.42 to 0.94) | 0.99 | |
| Threshold for treatment effect on GFR slope to assure PPV≥97.5% | 0.37 | 0.62 | 0.85 | ||||
Units of GFR are ml/min per 1.73 m2. Treatment effect on GFR slope is expressed as mean difference and in units of ml/min per 1.73 m2/yr. Treatment effect on the clinical end point is expressed as HR. PPVs are defined as the 97.5% probabilities for clinical benefit, defined as HR<1 for an infinite, large, or modest-sized RCT. A large RCT was defined as one in which the treatment effect on GFR slope can be estimated to within an SEM of 0.25, corresponding to a total sample size (N) of about 1900 for RCTs whose average follow-up accorded with the RCTs in the analysis. A modest RCT was defined as having SEM of 0.4 (N roughly 720).
Discussion
There is strong biologic plausibility and epidemiologic support for GFR slope as a measure of kidney disease progression. This report provides the addition of trial-level analyses to support GFR slope as a surrogate end point performed for a scientific workshop cosponsored by the NKF, FDA, and EMA. We found that with computation of GFR slope using a robust method, treatment effects on both the total slope at 3 years and the chronic slope have strong associations with the treatment effect on clinical end points, with similar results across key subgroups, including patients with higher baseline GFR, with weaker associations observed for total slope of shorter duration, especially at 1 year. We provide thresholds for minimum effects on change in GFR slope that provide high confidence for significant treatment effects on the clinical end point, providing guidance as to how to interpret treatment effects on GFR slope in future RCTs. This, together with the two companion papers, supports the validity and utility of GFR slope as a surrogate end point in RCTs of CKD progression.34,35
The results provide strong general support for the validity of both total slope over 3 years and chronic slope as surrogate end points in CKD RCTs. The treatment effects on both the chronic and total slopes over 3 years accounted for at least an estimated 96% of the variation between studies in treatment effects on the clinical end point, with Bayesian probabilities of at least 90% that the R2s exceed 0.72, a threshold suggested for a strong surrogate end point.34 The strength of this trial-level association compares favorably with widely used surrogate end points in other fields.36–38 Our analyses imply that, although an effective treatment may reduce mean GFR decline by what might appear to be a small magnitude over the typical duration of RCTs, treatment effects in the range of 0.5–1.00 ml/min per 1.73 m2/yr can have high predictive values of >98% for benefit on the clinical end point. The companion meta-analysis of observational studies that examined the association between GFR slope and subsequent ESKD incidence in more than 1 million individuals demonstrated results consistent with our results.34
The frequently encountered presence of acute effects in therapies for CKD progression has limited the use of GFR slope, and, when used, there are often questions as to how GFR slope should be computed—should we use the total slope, which incorporates both the acute and chronic periods, or just the chronic slope?14 As we demonstrated, use of total slope with a follow-up time over 3 years or more limits the effect of the acute effect, but long trials are often challenging to accomplish. A greater effect of varying acute effects likely explains the deterioration in the trial-level association when the total slope is computed over shorter time intervals. The effect of the acute effect may also be reduced by using the chronic slope as the primary end point, and our results are in support. There has been reluctance to use the chronic slope as a primary outcome because it is defined by change in GFR from a postbaseline time point at which the GFR has already been modified by the treatment, incurring risk of bias due to attenuation of the acute effect or early discontinuation of the study medication.36 Future work should guide us on how to minimize bias with the use of chronic slope, such as innovative designs employing off-treatment GFR measurements and application of different prerandomization baseline measurements for the treatment and control arms after introduction of the treatment in a run-in phase, as seen in the recent studies evaluating tolvaptan in polycystic kidney disease.37
There are several implications of these results. First, in phase 3 studies, GFR slope could be considered as a candidate primary end point. However, as shown in the simulations companion paper, total or chronic GFR slope confers the advantage of statistical power only under select circumstances.35 For example, the advantage of total slope over time to event time points is clearly demonstrated when there is no acute effect. The effect of an acute effect on the performance of the total slope is greater when the acute effect is large, the time interval for calculating total slope is short, or the control group progression rate is slow. Hence, the time interval required for good performance of the total slope in specific RCTs is likely to depend on each of these factors. The simulations also show that chronic slope has substantially greater power than the clinical end point when the acute effect is negative, but there is a risk of false conclusions for benefit. Thus, sponsors or investigators who consider GFR slope as an end point should do so after consideration of the entire set of design parameters. Second, trials that utilize clinical end points will be most sensitive to fast progressors. GFR slope can be used to demonstrate whether there is similar or different benefit among slow progressors. GFR slope can be used to explore heterogeneous effects among subgroups for trials that are powered for the clinical end point. Similarly, GFR slope might be an appropriate end point for confirmatory studies in a subsequent study after initial RCT showed benefit on the clinical end point but there is interest in demonstrating benefit of a drug in a population or with a study design where the clinical end point is not practical. Third, GFR slope is likely to have great value in phase 2 studies, which are shorter and smaller than phase 3 studies and cannot be powered for benefit on the clinical end point. Thus, overall, use of GFR slope can be considered a potential end point in the design and implementation of multiple phases within a drug development pathways.
The main drawback to using GFR slope as an end point in a pivotal trial is that it does not directly indicate the magnitude of the treatment benefit on the clinical end point. Some clinicians might question whether a small difference in mean GFR slope between treatment arms is sufficient evidence to adopt the intervention. Our results as well as those of our companion paper linking changes in GFR slope over a 1-, 2-, or 3-year period provide quantifiable data on the implications of such differences in GFR slope for longer term outcome.34
Strengths of this study include a systematic literature search to include all available studies, resulting in a large and diverse collection of RCTs, and a rigorous evaluation using individual patient data. Because we analyzed patient-level data, we were able to characterize agreement between the GFR slope and clinical end points after adjusting for spurious correlations in sampling error that resulted from inclusion of the same GFR measurements in the GFR slope and clinical end points. Our application of a robust method for analysis of GFR slope that accounted for informative censoring and multiple potential sources of variability in GFR measurements over time allowed us to apply a uniform analysis of GFR slope across all RCTs. Our use of a Bayesian meta-regression model with diffuse prior distributions allowed us to rigorously account for multiple sources of uncertainty and to translate treatment effects on the surrogate end points to probabilities of benefit on the clinical end point.
There are several limitations. First, because ascertainment of clinical end points was limited to the follow-up period of each trial, we were able to evaluate only the association between the treatment effects on the surrogate and clinical end points during the RCTs, and could not determine whether treatment effects on the surrogate end points predicted the longer term effects of the treatment on future clinical end points. The first companion paper evaluates epidemiologic associations over a longer period of follow-up.34,38 Second, because we used the same slope model for each RCT, somewhat different results might be obtained if the model for slope were tailored to each RCT, including trial-specific strategies for informative censoring and designating the timing of the acute effect.39 Third, because most included trials were not designed as short trials we cannot be certain about the effect of lesser follow-up time on the results, nor could we consider the effect of increased measurement frequencies on such shorter trials. Fourth, our results are dependent on the specific RCTs available to us. Hence, application of these results to future trials with different characteristics to those included here must be done with caution, particularly in trials with larger magnitude of acute effects, or lower rates of GFR decline. Fifth, our analyses do not address the risk that slope-based analyses would lead to false positive conclusions under the null hypothesis of no effect of the treatment on the clinical end point, nor do they evaluate the specific conditions in which analyses of GFR slope outcomes provide superior statistical power than analyses of the clinical end point. Our companion paper uses simulations to address the latter three questions.35
In summary, the results presented here, together with our companion papers, suggest that total and chronic GFR slope are strong surrogate end points and may be used as end points for RCTs of kidney disease progression in certain circumstances in both early and late CKD. Future work is required to optimize the design of RCTs to deploy slope-based end points to increase efficiency while preserving a sufficiently low risk of false positive conclusions.
FUNDING
The study was funded by the National Kidney Foundation. A variety of sources have supported the randomized, controlled trials included in the Chronic Kidney Disease Epidemiology Collaboration. These funding sources include government agencies such as the National Institutes of Health and medical research councils as well as foundations and industry sponsors listed in Supplemental Appendix 2.
Disclosures
Dr. Inker reports funding to Tufts Medical Center for research and contracts with the National Institutes of Health (NIH), National Kidney Foundation (NKF), Retrophin, Omeros, Reata Pharmaceuticals, and Dialysis Clinic, Inc. She has consulting agreements with Tricida Inc. and Omeros Corp. Tufts Medical Center, John Hopkins University and Metabolon Inc. have a collaboration agreement to develop a product to estimate GFR from a panel of markers. Dr. Levey reports grants from the NIH and the NKF during the conduct of the study, and funding from Siemens outside of the submitted work. Dr. Coresh has grants from the NIH and the NKF related and unrelated to this research. Dr. Inker, Dr. Levey, and Dr. Coresh have a patent Precise estimation of GFR from multiple biomarkers pending to Dr. Coresh, Dr. Inker, and Dr. Levey; and Tufts Medical Center, John Hopkins University, and Metabolon Inc. have a collaboration agreement to develop a product to estimate GFR from a panel of markers. Dr. Heerspink reports grants and other from Abbvie, other from Astellas, grants and other from AstraZeneca, grants and other from Boehringer Ingelheim, grants and other from Janssen, other from Fresenius, other from Gilead, and other from Merck, outside of the submitted work. Dr. Wanner reports personal fees from Boehringer Ingelheim during the conduct of the study, and personal fees from Lilly, personal fees from AstraZeneca, and personal fees from MSD outside of the submitted work. Dr. Floege has received consultancy honoraria and/or speaker fees from Alnylam, Amgen, Bayer, Calliditas, Chugai, Fresenius, Omeros Corp., and Vifor. Dr. Perkovic reports personal fees for Advisory Boards or Scientific Presentations from Retrophin, Janssen, Merck, and Servier. He has served on Steering Committees for trials funded by Abbvie, Boehringer Ingelheim, GlaxoSmithKline, Janssen, and Pfizer; and participated in Scientific Presentations/Advisory boards with Abbvie, Astellas, Astra Zeneca, Bayer, Baxter, Bristol-Myers Squibb, Boehringer Ingelheim, Dimetrix, Durect, Eli Lilly, Gilead, GlaxoSmithKline, Novartis, Novo Nordisk, Pfizer, Pharmalink, Relypsa, Sanofi, Tricida, and Vitae, with fees paid to his institution. Dr. Vonesh served as a paid biostatistics consultant for the NKF for the expressed purpose of developing statistical models for use in the estimation and comparison of GFR slopes as a surrogate end point in CKD randomized, controlled trials. He is also serving as a biostatistics consultant to Prometic and Tricida, Inc., in which some of the work entails consulting on the design and analysis of clinical trials in patients with CKD. Dr. Greene reports grants from the NKF during the conduct of the study, and personal fees from DURECT Corporation, Janssen Pharmaceuticals, and Pfizer Inc., outside of the submitted work. Dr. Beck, Dr. Gansevoort, Dr. Ying, Dr. Tighiouart, Dr. Li, and Dr. Simon have no conflicts to report.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the help of Juhi Chaudhari in preparation of the manuscript and Shiyuan Miao in preparation of the figures.
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The corresponding author had full access to all of the data in the study and final responsibility for the decision to submit for publication.
Dr. Inker, Dr. Greene, Dr. Heerspink, Dr. Coresh, Dr. Levey, and Dr. Gansevoort conceived of the study concept and design. The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) investigators/collaborators listed below acquired the data. Dr. Tighiouart, Dr. Simon, Dr. Greene, and Dr. Inker analyzed the data. All authors took part in the interpretation of the data. Dr. Inker and Dr. Greene drafted the manuscript, and all authors provided critical revisions of the manuscript for important intellectual content. All collaborators shared data and were given the opportunity to comment on the manuscript. Dr. Inker, Dr. Greene, Dr. Heerspink, and Dr. Levey obtained funding for the CKD-EPI and individual cohort and collaborator support is listed in Supplemental Appendix 2.
CKD-EPI investigators/collaborators (study acronyms/abbreviations are listed in Supplemental Appendix 1 with other abbreviations):
AASK: Dr. Greene, ABCD: Robert W. Schrier, Raymond O. Estacio, ADVANCE: Dr. Perkovic, AIPRI: Giuseppe Maschio, Francesco Locatelli, ALTITUDE: Hans-Henrik Parving, DMSc, Bari: Francesco Paolo Schena, Manno Carlo, Bologna: Pietro Zucchelli, Boston: Barry M. Brenner, canPREVENT: Brendan Barrett, MB, FRCPC, Copenhagen: Anne-Lise Kamper, DMSc, Svend Strandgaard, CSG: Roger A. Rodby, Richard D. Rohde Julia B. Lewis, Edmund Lewis, EMPA-REG OUTCOME: Dr. Wanner, Maximilian von Eynatten, Fukuoka: Ritsuko Katafuchi, Groningen: Paul E. de Jong, G.G. van Essen, Guangzhou: Fan Fan Hou, Di Xie, HALT-PKD: Ronald D. Perrone, Vicente Torres, Arlene Chapman, Godela Brosnahan, HKVIN: Philip Li, FRCP, C.B. Leung, FRCP, C.C. Szeto, FRCP, K.M. Chow, MRCP, IDNT: Edmund Lewis, Lawrence G. Hunsicker, Julia B. Lewis, Jamie P. Dwyer, Lecco: Francesco Locatelli, Lucia Del Vecchio, Simeone Andrulli, Claudio Pozzi, Leuven: Bart Maes, LNCS: Julia B. Lewis, Jamie Dwyer, Edmund Lewis, John M. Lachin, ScD, MADRID: Marian Goicoechea, Eduardo Verde, Ursula Verdalles, Jose Luño, Madrid: Manuel Praga, Fernando Caravaca, Eduardo Gutierrez, Angel Sevillano, MASTERPLAN: Jack F.M. Wetzels, Peter J Blankestijn, Arjan D. van Zuilen, Jan van den Brand, MDRD: Dr. Beck, Dr. Greene, John Kusek, Saulo Klahr, Milan: Claudio Ponticelli Montagnino, Patrizia Passerini, Gabriella Moroni, Giuseppe Montogrino, New York: Gerald B. Appel, Gershon Frisch, ORIENT: Fumiaki Kobayashi, Hirofumi Makino, Sadayoshi Ito, Enyu Imai, Hong Kong Lupus Nephritis: Tak Mao Chan, REIN 1: Giuseppe Remuzzi, FRCP, Piero Ruggenenti, REIN 2: Giuseppe Remuzzi, FRCP, Piero Ruggenenti, RENAAL: Dick De Zeeuw, Dr. Heerspink, Barry M. Brenner, William Keane, ROAD: Fan Fan Hou, Rochester: James Donadio, Fernando C. Fervenza, SHARP: Martin Landray, FRCP, Will Herrington, Natalie Staplin, Colin Baigent, FRCP, STOP-IgAN RWTH Aachen—Jürgen Floege, Thomas Rauen, Christina Fitzner, Ralf-Dieter Hilgers, Strasbourg: Thierry P. Hannedouche, SUN-MACRO: Julia B. Lewis, Jamie P. Dwyer, Edmund J. Lewis, Texas: Robert D. Toto, Victoria: Gavin J Becker, Benno U. Ihle, Priscilla S. Kincaid-Smith, DSc.
The planning and operations committee of the National Kidney Foundation, Food and Drug Administration, and European Medicines Agency Scientific Workshop on Change in Albuminuria and GFR as End Points for Clinical Trials in Early Stages of CKD contributed to the design and critical review of these analyses. Planning and Operations Committees: Dr. Levey (Chair), Dr. Gansevoort, Dr. Coresh, Dick de Zeeuw, Kai-Uwe Eckardt, Hrefna Gudmundsdottir, Adeera Levin, Romaldas Maciulaitis, Tom Manley, Dr. Perkovic, Kimberly Smith, Norman Stockbridge, Aliza Thompson, Thorsten Vetter, Kerry Willis, and Luxia Zhang.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Clinical Trial Data Sharing: The Time Is Now,” on pages 1556–1558.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019010007/-/DCSupplemental.
Supplemental Appendix 1: Abbreviations, units, and terms.
Supplemental Appendix 2: Study funding sources.
Protocol.
Background and rationale.
Dataset development.
Datasets and analytic groups.
Data management.
Clinical end points.
Estimated GFR.
GFR slope.
Analyses.
Trial-level model for relating treatment effects on the clinical end point to treatment effects on GFR slope.
Prediction intervals and positive predictive value.
Supplemental Table 1. Search terms.
Supplemental Table 2. Study inclusion criteria.
Supplemental Table 3. Studies pooled by intervention.
Supplemental Table 4. Description of studies.
Supplemental Table 5. Patient characteristics by study.
Supplemental Table 6. Distribution of the maximum visit time for each person by duration.
Supplemental Table 7. Slopes (95% confidence intervals) by treatment arm for each intervention.
Supplemental Table 8. Treatment effects by intervention.
Supplemental Table 9 End points used by study.
Supplemental Table 10. Trial-level analysis for GFR slope overall and by different duration.
Supplemental Table 11. Summary of trial-level analyses for GFR slope by subgroup.
Supplemental Figure 1. Evaluation of bias.
Supplemental Figure 2. Flowchart.
Supplemental Figure 3. Treatment effect on GFR slope.
Supplemental Figure 3A. Chronic slope.
Supplemental Figure 3C. Total slope at 2 year.
Supplemental Figure 3D. Total slope at 3 year.
Supplemental Figure 3E. Total slope at 4 year.
Supplemental Figure 4. Forest plot for clinical end point.
Legend for Supplemental Figures 5 and 6.
Supplemental Figure 5. Trial-level analyses for the association between treatment effects on total GFR slope by varying duration and treatment effect on the clinical end point.
Supplemental Figure 6. Trial-level analyses for the association between treatment effects on GFR slope and treatment effects on the clinical end point by level of eGFR.
Supplemental Figure 6A. Total GFR slope over 3 Year.
Supplemental Figure 6B. Chronic GFR slope.
References
- 1.Levey AS, Coresh J: Chronic kidney disease. Lancet 379: 165–180, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Levey AS, Inker LA, Matsushita K, Greene T, Willis K, Lewis E, et al. : GFR decline as an end point for clinical trials in CKD: A scientific workshop sponsored by the national kidney foundation and the US Food and drug administration. Am J Kidney Dis 64: 821–835, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Schievink B, Kröpelin T, Mulder S, Parving HH, Remuzzi G, Dwyer J, et al. : Early renin-angiotensin system intervention is more beneficial than late intervention in delaying end-stage renal disease in patients with type 2 diabetes. Diabetes Obes Metab 18: 64–71, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, et al. ; CKD Prognosis Consortium: Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: A collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 3: 514–525, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, et al. ; Chronic Kidney Disease Prognosis Consortium: Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 79: 1331–1340, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, et al. ; Chronic Kidney Disease Prognosis Consortium: Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 80: 93–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turin TC, Coresh J, Tonelli M, Stevens PE, de Jong PE, Farmer CK, et al. : Change in the estimated glomerular filtration rate over time and risk of all-cause mortality. Kidney Int 83: 684–691, 2013 [DOI] [PubMed] [Google Scholar]
- 8.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, et al. ; Chronic Kidney Disease Prognosis Consortium: Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 79: 1341–1352, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, et al. : Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 311: 2518–2531, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inker LA, Lambers Heerspink HJ, Mondal H, Schmid CH, Tighiouart H, Noubary F, et al. : GFR decline as an alternative end point to kidney failure in clinical trials: A meta-analysis of treatment effects from 37 randomized trials. Am J Kidney Dis 64: 848–859, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Higgins J, Green S: The Cochrane Collaboration. In: Cochrane Handbook for Systematic Reviews of Interventions, Chichester, John Wiley & Sons, 2011: Available at: www.cochrane-handbook.org. [Google Scholar]
- 12.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. ; Collaborative Study Group: Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Wright JT Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, et al. ; African American Study of Kidney Disease and Hypertension Study Group: Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK trial. JAMA 288: 2421–2431, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, et al. ; Modification of Diet in Renal Disease Study Group: The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med 330: 877–884, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Estacio RO, Jeffers BW, Gifford N, Schrier RW: Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care 23[Suppl 2]: B54–B64, 2000 [PubMed] [Google Scholar]
- 16.Torres VE, Abebe KZ, Chapman AB, Schrier RW, Braun WE, Steinman TI, et al. ; HALT-PKD Trial Investigators: Angiotensin blockade in late autosomal dominant polycystic kidney disease. N Engl J Med 371: 2267–2276, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Praga M, Gutiérrez E, González E, Morales E, Hernández E: Treatment of IgA nephropathy with ACE inhibitors: A randomized and controlled trial. J Am Soc Nephrol 14: 1578–1583, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Li PK, Leung CB, Chow KM, Cheng YL, Fung SK, Mak SK, et al. ; HKVIN Study Group: Hong Kong study using valsartan in IgA nephropathy (HKVIN): A double-blind, randomized, placebo-controlled study. Am J Kidney Dis 47: 751–760, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Ponticelli C, Passerini P, Salvadori M, Manno C, Viola BF, Pasquali S, et al. : A randomized pilot trial comparing methylprednisolone plus a cytotoxic agent versus synthetic adrenocorticotropic hormone in idiopathic membranous nephropathy. Am J Kidney Dis 47: 233–240, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Ponticelli C, Zucchelli P, Passerini P, Cagnoli L, Cesana B, Pozzi C, et al. : A randomized trial of methylprednisolone and chlorambucil in idiopathic membranous nephropathy. N Engl J Med 320: 8–13, 1989 [DOI] [PubMed] [Google Scholar]
- 21.Ponticelli C, Altieri P, Scolari F, Passerini P, Roccatello D, Cesana B, et al. : A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J Am Soc Nephrol 9: 444–450, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Ponticelli C, Zucchelli P, Passerini P, Cesana B; The Italian Idiopathic Membranous Nephropathy Treatment Study Group: Methylprednisolone plus chlorambucil as compared with methylprednisolone alone for the treatment of idiopathic membranous nephropathy. N Engl J Med 327: 599–603, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Maes BD, Oyen R, Claes K, Evenepoel P, Kuypers D, Vanwalleghem J, et al. : Mycophenolate mofetil in IgA nephropathy: Results of a 3-year prospective placebo-controlled randomized study. Kidney Int 65: 1842–1849, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Frisch G, Lin J, Rosenstock J, Markowitz G, D’Agati V, Radhakrishnan J, et al. : Mycophenolate mofetil (MMF) vs placebo in patients with moderately advanced IgA nephropathy: A double-blind randomized controlled trial. Nephrol Dial Transplant 20: 2139–2145, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Pozzi C, Andrulli S, Del Vecchio L, Melis P, Fogazzi GB, Altieri P, et al. : Corticosteroid effectiveness in IgA nephropathy: Long-term results of a randomized, controlled trial. J Am Soc Nephrol 15: 157–163, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Pozzi C, Andrulli S, Pani A, Scaini P, Del Vecchio L, Fogazzi G, et al. : Addition of azathioprine to corticosteroids does not benefit patients with IgA nephropathy. J Am Soc Nephrol 21: 1783–1790, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pozzi C, Andrulli S, Pani A, Scaini P, Roccatello D, Fogazzi G, et al. : IgA nephropathy with severe chronic renal failure: A randomized controlled trial of corticosteroids and azathioprine. J Nephrol 26: 86–93, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Katafuchi R, Ikeda K, Mizumasa T, Tanaka H, Ando T, Yanase T, et al. : Controlled, prospective trial of steroid treatment in IgA nephropathy: A limitation of low-dose prednisolone therapy. Am J Kidney Dis 41: 972–983, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Manno C, Torres DD, Rossini M, Pesce F, Schena FP: Randomized controlled clinical trial of corticosteroids plus ACE-inhibitors with long-term follow-up in proteinuric IgA nephropathy. Nephrol Dial Transplant 24: 3694–3701, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skali H, Uno H, Levey AS, Inker LA, Pfeffer MA, Solomon SD: Prognostic assessment of estimated glomerular filtration rate by the new chronic kidney disease epidemiology collaboration equation in comparison with the modification of diet in renal disease study equation. Am Heart J 162: 548–554, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Prasad V, Kim C, Burotto M, Vandross A: The strength of association between surrogate end points and survival in oncology: A systematic review of trial-level meta-analyses. JAMA Intern Med 175: 1389–1398, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Viechtbauer W: Conducting meta-analyses in R with the metafor package. J Stat Softw 36: 1–48, 2010 [Google Scholar]
- 34.Grams M, Sang Y, Ballew S, Matsushita K, Astor BC, Carrero JJ, et al. : Evaluating glomerular filtration rate slope as a surrogate end point for ESKD in clinical trials: an individual participant meta-analysis of observational data. J Am Soc Nephrol 30: 1746–1755, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greene T, Ying J, Vonesh EF, Tighiouart H, Levey AS, Coresh J, et al. : Performance of GFR slope as a surrogate endpoint for kidney disease progression in clinical trials: A statistical simulation. J Am Soc Nephrol 30: 1756–1769, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gassman JJ, Greene T, Wright JT Jr, Agodoa L, Bakris G, Beck GJ, et al. : Design and statistical aspects of the African American Study of Kidney Disease and Hypertension (AASK). J Am Soc Nephrol 14[Suppl 2]: S154–S165, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, et al. ; TEMPO 3:4 Trial Investigators: Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coresh J, Heerspink HLJ, Sang Y, Matsushita K, Arnlov J, Astor BC, et al. ; Chronic Kidney Disease Prognosis Consortium and Chronic Kidney Disease Epidemiology Collaboration: Change in albuminuria and subsequent risk of end-stage kidney disease: An individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol 7: 115–127, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vonesh E, Tighiouart H, Ying J, et al.: Mixed-effects models for slope-based endpoints in clinical trials of chronic kidney disease. Stat Med 2019, In Press. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.