Significance Statement
Randomized clinical trials of treatments to slow CKD progression often require large sample sizes and long follow-up to understand their effects on clinical events. This is especially true in patients with earlier stages of kidney disease who are unlikely to experience ESKD for many years. Surrogate study end points that occur earlier during disease progression could help. To evaluate whether eGFR decline over time may be a good surrogate end point, the authors did a meta-analysis of 14 cohorts. They found that slower eGFR decline was significantly associated with lower risk of ESKD in all populations, including those with better kidney function. The results suggest that change in the slope of eGFR decline may be a good surrogate end point for ESKD in clinical trials, particularly in longer trials with patients with rapidly progressive disease.
Keywords: glomerular filtration rate, end-stage renal disease, chronic kidney disease, progression of chronic renal failure
Abstract
Background
Decline in eGFR is a biologically plausible surrogate end point for the progression of CKD in clinical trials. However, it must first be tested to ensure strong associations with clinical outcomes in diverse populations, including patients with higher eGFR.
Methods
To investigate the association between 1-, 2-, and 3-year changes in eGFR (slope) with clinical outcomes over the long term, we conducted a random effects meta-analysis of 3,758,551 participants with baseline eGFR≥60 ml/min per 1.73 m2 and 122,664 participants with eGFR<60 ml/min per 1.73 m2 from 14 cohorts followed for an average of 4.2 years.
Results
Slower eGFR decline by 0.75 ml/min per 1.73 m2 per year over 2 years was associated with lower risk of ESKD in participants with baseline eGFR≥60 ml/min per 1.73 m2 (adjusted hazard ratio, 0.70; 95% CI, 0.68 to 0.72) and eGFR<60 ml/min per 1.73 m2 (0.71; 95% CI, 0.68 to 0.74). The relationship was stronger with 3-year slope. For a rapidly progressing population with predicted 5-year risk of ESKD of 8.3%, an intervention that reduced eGFR decline by 0.75 ml/min per 1.73 m2 per year over 2 years would reduce the ESKD risk by 1.6%. For a hypothetical low-risk population with a predicted 5-year ESKD risk of 0.58%, the same intervention would reduce the risk by only 0.13%.
Conclusions
Slower decline in eGFR was associated with lower risk of subsequent ESKD, even in participants with eGFR≥60 ml/min per 1.73 m2, but those with the highest risk would be expected to benefit the most.
There are few therapies that slow or prevent CKD progression, particularly in early-stage CKD.1,2 New interventions must be developed and tested in randomized, controlled trials. Unfortunately, clinical trials in persons with earlier stages of CKD can be impractical and costly due to the necessary large sample size and long duration of follow-up, because established clinical end points, such as ESKD and doubling of serum creatinine, are uncommon and occur late in the disease process. In March 2018, the National Kidney Foundation in collaboration with the Food and Drug Administration and the European Medicines Agency sponsored a workshop entitled Change in Albuminuria and GFR as End Points for Clinical Trials in Early Stages of CKD to evaluate the validity of surrogate end points that occur earlier in CKD progression, with the goal of facilitating the development and testing of novel therapies.3–5
This study is one in a series of manuscripts that report analyses undertaken for the workshop that evaluate GFR decline as a surrogate end point for CKD progression in clinical trials.6 A clinical trial designed to show a difference in slope of GFR decline between randomized treatment arms could require a smaller sample size and shorter follow-up than a trial designed to show a difference in the occurrence of clinical end points (e.g., ESKD or even the surrogate end points of 30%–40% decline in GFR, which are sometimes used by the US Food and Drug Administration and the European Medicines Agency), particularly in a population with higher baseline GFR. However, potential surrogates must undergo rigorous evaluation in a variety of settings.6,7 This study was designed to address the first tenet for surrogacy put forth by Prentice,7 namely that a surrogate should have a strong association with the clinical end point. Another manuscript in this series evaluates the evidence for the tenet of Prentice7 that a treatment effect on the surrogate must capture the treatment effect on the clinical end point.8 A third study, a statistical simulation, demonstrates clinical trial settings, which might benefit from the use of GFR slope as an end point while preserving a low risk of false conclusions.9
This study used individual participant data from observational cohorts participating in the CKD Prognosis Consortium (CKD-PC) with the following goals: (1) to quantify the magnitude of association between eGFR decline over 1-, 2-, and 3-year timeframes with subsequent, long-term risk of ESKD; (2) to determine whether the magnitude of association was similar in different subgroups of participants, particularly those with baseline eGFR ≥60 ml/min per 1.73 m2; (3) to consider different methods of estimating individual slope and the implications of using these methods on the associations with the clinical end point; (4) to confirm that associations with death were consistent in direction to those observed with ESKD; and (5) to provide insight on groups of patients in whom GFR slope would (and would not) be a plausible candidate surrogate end point given expected absolute risk reduction.
Methods
Study Design and Data Sources
The CKD-PC is an open, international research group that currently includes >70 cohorts with data on eGFR, albuminuria, and clinical outcomes.10 For this study, we included cohorts that could participate in all of the 1-, 2-, and 3-year baseline periods and had subsequent longitudinal follow-up for ESKD and all-cause mortality. Slope during the baseline period was estimated for all participants with at least two eGFR measures separated by the desired time window, which was defined as 1, 2, or 3 years ±33%, but we required that at least some of the participants have three measures to be able to detect a difference between mixed model and empirical estimates of slope. We stratified analyses by baseline eGFR, conducting separate meta-analyses for individuals with eGFR<60 and ≥60 ml/min per 1.73 m2. Included cohorts could contribute to both meta-analyses if there were sufficient numbers of individuals who developed ESKD (>10 events) within the given eGFR subgroup.
A total of 14 cohorts had the requisite data and agreed to participate (Supplemental Appendix 1). From these 14 cohorts, we included participants ages ≥18 years old without ESKD that developed during or before the baseline period. The Johns Hopkins Bloomberg School of Public Health Institutional Review Board approved this study.
Estimating GFR Slope
Outpatient serum creatinine values were converted to eGFR using the Chronic Kidney Disease Epidemiology Collaboration equation.11 Clinical trials have generally estimated GFR slope using mixed models to reduce the variance derived from unreliable estimates. Thus, as a primary exposure, we used linear mixed models with an unstructured variance-covariance matrix, random intercept, and random slope for each individual to estimate slope (mixed model slope).12 Models took the form eGFRi(t)=b0+b0i+(b1+b1i)×t+ei, where t is time; b0 and b1 are the fixed intercept and slope, respectively; and b0i and b1i are the random intercept and slope, respectively. For comparison, slope was also empirically estimated for each individual using least squares linear regression of all measures of eGFR on time (least squares slope) during the given baseline period (1, 2, or 3 years). The 2-year slopes were considered the primary exposure of interest.
Outcome
The primary outcome was ESKD, which was defined as the initiation of kidney replacement therapy (Supplemental Appendix 1). The secondary outcome was all-cause mortality. Time at risk for both outcomes began on the date of the last creatinine used in the eGFR slope estimate.
Statistical Analyses
Individual cohort characteristics were summarized using means and SDs for continuous variables and proportions for categorical variables. These characteristics—age, sex, race (black or nonblack), baseline eGFR, systolic BP, diabetes mellitus status, history of cardiovascular disease, smoking status, and total cholesterol—were measured within 1 year before the first serum creatinine used in the slope estimation, and they were used as adjustment variables in subsequent models. Cox models related individual eGFR slope estimates during the baseline period to risk of ESKD and mortality thereafter, with eGFR slope modeled as a two-piece linear spline with a knot at 0 ml/min per 1.73 m2 per year. This was repeated within strata of age; sex; and diabetes, hypertension, and cardiovascular disease status. Within-cohort coefficients for each spline component of eGFR slope were combined using random effects meta-analysis. Heterogeneity in estimates was evaluated visually through forest plots and quantified using the I2 statistic. In sensitivity analyses, we assessed the effect of varying numbers of eGFR measurements by focusing on study participants with five or more measures of eGFR. We compared estimates of the eGFR slope-ESKD association using all eGFR measures with those that assessed slope using only three measures.
We estimated the absolute risk reduction of ESKD for an individual assuming a difference in eGFR slope of 0.75 ml/min per 1.73 m2 per year by applying hazard ratios from the mixed model slopes to baseline subhazard of ESKD risk (estimated by the method of Fine and Gray and using mortality as a competing event).13 The scenarios consisted of baseline eGFR =75 ml/min per 1.73 m2, fixed levels of covariates (chosen to reflect the mean value across cohorts), and predicted eGFR slopes of −1, −3, and −5 ml/min per 1.73 m2 per year over 2 years for baseline urine albumin-to-creatinine ratios of 10, 30, and 100 mg/g, assuming no change in albuminuria. We then estimated the absolute risk reduction of ESKD for a hypothetical population by assuming variation (SD of 4 ml/min per 1.73 m2 per year) around the mean eGFR slopes of −1, −3, and −5 ml/min per 1.73 m2 per year over 2 years on the basis of the same covariates. We also examined the proportion of individuals reaching 30% and 40% reduction in eGFR during the baseline period. Analyses were performed using Stata/MP 14.2 software for Windows (www.stata.com).
Results
Baseline Characteristics of Included Cohorts
Across the 14 cohorts, there were 3,353,210 individuals included in the 1-year eGFR slope analysis, 3,881,215 in the 2-year analysis, and 3,943,212 in the 3-year analysis (Table 1). There were 12 cohorts in the eGFR<60 ml/min per 1.73 m2 analyses and seven in the eGFR≥60 ml/min per 1.73 m2 analyses (five of the cohorts were in both). In the 2-year eGFR <60 ml/min per 1.73 m2 analyses, average age was 71 years old, 56% were women, and 3% were black. Mean eGFR was 47 ml/min per 1.73 m2, and 28% had diabetes mellitus. In the 2-year eGFR ≥60 ml/min per 1.73 m2 analyses, average age was 56 years old, 24% were women, and 11% were black. Mean eGFR was 89 ml/min per 1.73 m2, and 21% had diabetes mellitus. Cohort characteristics were fairly similar in the 1- and 3-year observation periods (Supplemental Tables 1 and 2).
Table 1.
Baseline characteristics of individuals in cohorts participating in the 2-year observation period for change in eGFR over time stratified by baseline eGFR
| Cohort | N | Age, yr | Women, % | Black, % | eGFR, ml/min per 1.73 m2 | SBP, mm Hg | Diabetes, % | History of CVD, % | Current smoker, % | Former smoker, % | TC, mmol/L |
|---|---|---|---|---|---|---|---|---|---|---|---|
| eGFR<60 | |||||||||||
| AASK | 744 | 54 (11) | 39 | 100 | 42 (11) | 150 (24) | 0 | 53 | 44 | 28 | 5.5 (1.1) |
| BC CKD | 8950 | 70 (13) | 46 | 0 | 32 (11) | 136 (23) | 44 | 24 | 2.6 | 6.1 | NAa |
| CCF | 18,873 | 72 (11) | 55 | 12 | 47 (10) | 131 (19) | 25 | 25 | 0.2 | 2.3 | 4.7 (1.1) |
| Geisinger | 19,200 | 73 (12) | 62 | 0.8 | 47 (11) | 134 (20) | 28 | 42 | 8.4 | 27 | 5.0 (1.1) |
| KP Hawaii | 5468 | 71 (11) | 53 | 0 | 47 (10) | 137 (22) | 52 | 35 | 7.4 | NA | 4.7 (1.1) |
| Maccabi | 29,211 | 74 (11) | 50 | 0 | 49 (10) | 134 (19) | 32 | 47 | 1.09 | 19 | 4.8 (1.1) |
| MASTERPLAN | 513 | 61 (12) | 31 | 0 | 36 (11) | 136 (20) | 24 | 30 | 21 | 53 | 4.8 (1.1) |
| MDRD | 591 | 52 (12) | 38 | 6.6 | 35 (11) | 132 (18) | 3.9 | 13 | 10 | NA | 5.6 (1.1) |
| NZDCS | 1913 | 71 (9) | 57 | 0 | 48 (10) | 142 (21) | 100 | 1.7 | 8.1 | 33 | 5.3 (1.2) |
| RENAAL | 1139 | 60 (7) | 37 | 14 | 38 (11) | NA | 100 | NA | 17.2 | NA | NA |
| SCREAM | 35,049 | 69 (10) | 61 | 0 | 48 (10) | NA | 15 | 36 | NA | NA | 5.2 (1.2) |
| Sunnybrook | 1013 | 70 (13) | 42 | 0 | 35 (12) | NA | 52 | 16 | 7.2 | 19 | NA |
| Subtotal | 122,664 | 71 (11) | 56 | 3 | 47 (10) | 134 (20) | 28 | 37 | 4.2 | 18 | 5.0 (1.2) |
| eGFR 60+ | |||||||||||
| ADVANCE | 8457 | 66 (6) | 40 | 0.4 | 83 (13) | 144 (21) | 100 | 24 | 16 | 27 | 5.2 (1.2) |
| Geisinger | 138,682 | 55 (15) | 56 | 1.7 | 92 (17) | 128 (18) | 16 | 15 | 17 | 24 | 5.1 (1.0) |
| KP Hawaii | 15,140 | 58 (13) | 49 | 0 | 86 (16) | 135 (20) | 67 | 16 | 13 | NA | 4.8 (1.2) |
| Maccabi | 720,012 | 47 (16) | 59 | 0 | 101 (17) | 124 (17) | 9 | 9 | 2.1 | 23 | 5.0 (1.0) |
| NZDCS | 7093 | 59 (13) | 49 | 0.11 | 86 (16) | 138 (19) | 100 | 0.54 | 16 | 30 | 5.4 (1.1) |
| RCAV | 2,408,814 | 61 (13) | 5.9 | 16.8 | 83 (15) | 134 (18) | 27 | 20 | NA | NA | NA |
| SCREAM | 460,353 | 48 (15) | 54 | 0 | 97 (17) | NA | 6.2 | 8.7 | NA | NA | 5.4 (1.1) |
| Subtotal | 3,758,551 | 56 (15) | 24 | 11 | 89 (18) | 132 (18) | 21 | 16 | 5.0 | 23 | 5.0 (1.1) |
| Total | 3,881,215 | 57 (15) | 25 | 11 | 87 (19) | 132 (19) | 21 | 17 | 4.9 | 23 | 5.0 (1.1) |
CVD, cardiovascular disease; SBP, systolic BP; TC, total cholesterol.
Variables missing >50% were marked not available (NA).
Summary of eGFR Slope within Cohorts
Over the 2-year baseline period, the within-cohort median number of serum creatinine measurements ranged from three to 13 in the eGFR<60 ml/min per 1.73 m2 cohorts and from three to five in the eGFR≥60 ml/min per 1.73 m2 cohorts. Subsequent to this baseline period, there were 6083 ESKD events and 44,135 deaths over a mean follow-up of 3.3 years in the eGFR<60 ml/min per 1.73 m2 cohorts and 6552 ESKD events and 520,061 deaths over 4.2 years in the eGFR≥60 ml/min per 1.73 m2 cohorts (Supplemental Table 3). Median number of serum creatinine measurements, subsequent follow-up time, and number of events for the 1- and 3-year baseline periods are shown in Supplemental Tables 4 and 5. The 2-year mixed model mean slope ranged from −4.92 to 0.27 ml/min per 1.73 m2 per year and from −3.71 to −1.06 ml/min per 1.73 m2 per year in the eGFR<60 and ≥60 ml/min per 1.73 m2 cohorts, respectively (Supplemental Table 6). SDs of eGFR slopes were smaller with longer observation periods (medians for ≥60 ml/min per 1.73 m2 cohorts: 6.0, 3.7, and 3.2 for 1-, 2-, and 3-year slopes, respectively), and mean slopes were generally more modest. Even among rapid progressors with 2-year eGFR slope <−3 ml/min per 1.73 m2 per year, few clinical end points of 30% or 40% change occurred, particularly at higher eGFR: 15.6% and 5.6%, respectively, among eGFR≥60 ml/min per 1.73 m2 and 48.2% and 23.8%, respectively, among eGFR<60 ml/min per 1.73 m2.
Associations of eGFR Slope with Subsequent ESKD within Cohorts
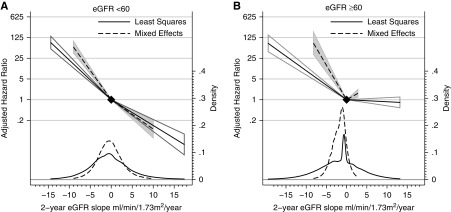
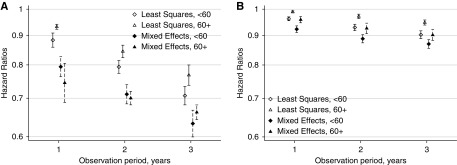
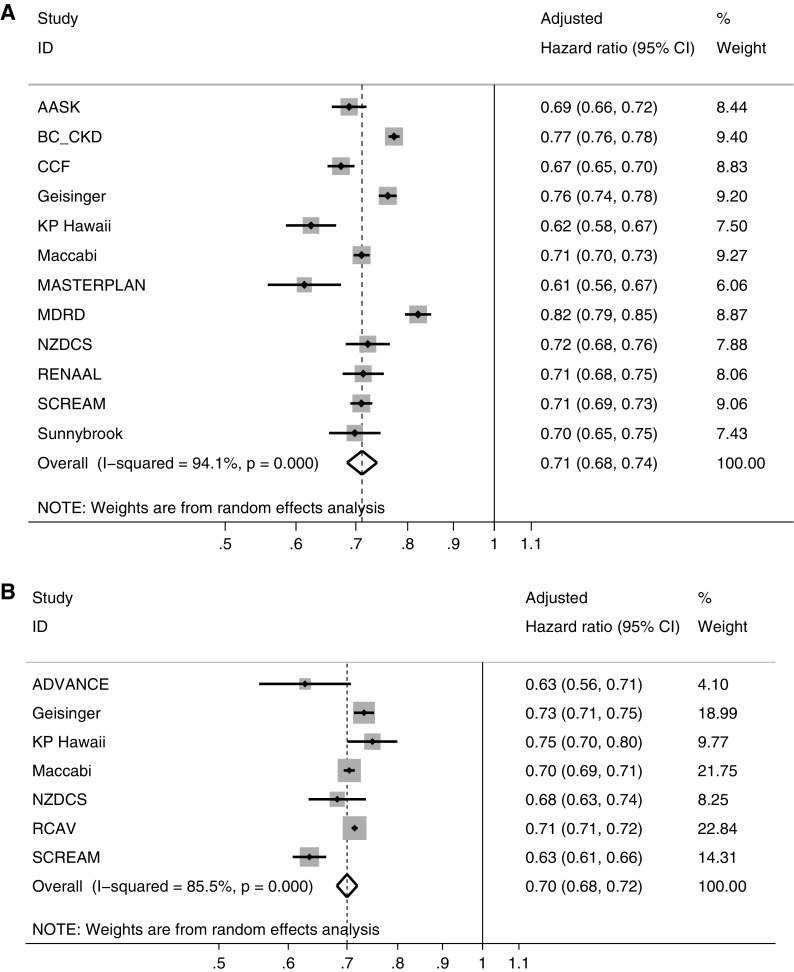
In both unadjusted and covariate-adjusted analyses, a steeper eGFR decline over a 2-year observation period was associated with higher risk of subsequent ESKD. This association was statistically significant in the meta-analysis within both strata of eGFR and over all observation periods (Figure 1, Supplemental Figure 1). A lesser eGFR decline by 0.75 ml/min per 1.73 m2 per year was associated with lower risk of ESKD in all individual cohorts (Supplemental Table 7) and within strata of age; sex; and diabetes, hypertension, and cardiovascular disease status (Supplemental Figures 2–6). Associations were stronger when estimated over a longer baseline period and weaker when estimated using least squares (Figure 2, left panel). Conditioned on having a complete 2-year baseline period, the association between 2-year slope and ESKD was not stronger when eGFR decline was estimated using five or more eGFR measurements compared with eGFR decline on the basis of three eGFR measurements. Overall, a reduction in mixed model slope of eGFR decline by 0.75 ml/min per 1.73 m2 per year over 2 years was associated with 29% and 30% lower risks of subsequent ESKD in participants with baseline eGFR <60 and ≥60 ml/min per 1.73 m2, respectively, with some quantitative but little qualitative heterogeneity in associations across cohorts (Figure 3). Findings were similar when stratified by use of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker at the beginning and the end of the baseline period (Supplemental Figure 7).
Figure 1.
Meta-analyzed adjusted hazard ratios show a strong association between 2-year eGFR decline and subsequent ESKD in participants with eGFR <60 ml/min per 1.73 m2 (A) and ≥60 ml/min per 1.73 m2 (B), with stronger associations when using mixed effects models to estimate slope.
Figure 2.
Meta-analyzed adjusted hazard ratios show the protective effect of a slower eGFR decline by 0.75 ml/min per 1.73 m2 per year on subsequent ESKD (A) and death (B) in participants with eGFR <60 ml/min per 1.73 m2 and ≥60 ml/min per 1.73 m2, with stronger associations when slope is estimated over longer time periods and when using mixed effect models.
Figure 3.
A slower eGFR decline by 0.75 ml/min per 1.73 m2 per year over two years is consistently protective for ESKD across cohorts and participants with eGFR <60 ml/min per 1.73 m2 (A) and ≥60 ml/min per 1.73 m2 (B).
Associations of eGFR Slope with Subsequent Mortality within Cohorts
A lesser eGFR decline by 0.75 ml/min per 1.73 m2 per year was associated with lower risk of subsequent mortality, although the magnitude of this association was small compared with the association with ESKD and was not statistically significant in every cohort (Supplemental Table 8). Associations were also stronger when slopes were observed over longer baseline periods (Figure 2, right panel).
Absolute Risk Reduction of ESKD Associated with a Lesser Slope of eGFR Decline
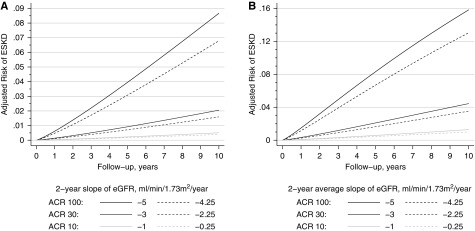
The expected reduction in absolute risk of ESKD associated with a 0.75 ml/min per 1.73 m2 per year smaller slope of eGFR decline was greatest in individuals with a greater predicted eGFR decline (Figure 4A). For a hypothetical population with mean eGFR of 75 ml/min per 1.73 m2 and mean (SD) eGFR decline of −5 (4) ml/min per 1.73 m2 per year, the predicted 5-year risk of ESKD was 8.3%. A treatment effect that reduced eGFR decline by 0.75 ml/min per 1.73 m2 per year would be expected to reduce the 5-year ESKD risk to 6.7% (Figure 4B). However, the expected reduction in absolute risk of ESKD would be much lower in a population with slower decline: the same intervention would reduce 5-year ESKD risk from 0.58% to 0.45% in a population with a mean eGFR decline of −1 (4) ml/min per 1.73 m2 per year.
Figure 4.
The expected decrease in absolute risk associated with slowing eGFR decline by 0.75 ml/min per 1.73 m2 per year over two years increases with longer follow-up time and higher risk. The panels show the expected absolute risks of ESKD for 2-year eGFR decline of −5, −3, and −1 ml/min per 1.73 m2 per year (solid lines) and a 0.75-ml/min per 1.73 m2 per year slower eGFR decline (dotted lines) for (A) a person with baseline eGFR of 75 ml/min per 1.73 m2 and albuminuria-to-creatinine ratio (ACR) as shown and (B) a population of people with mean eGFR decline of −5, −3, and −1 ml/min per 1.73 m2 per year, SD of 4 ml/min per 1.73 m2 per year, and the same covariates.
Discussion
In this global, individual participant data meta-analysis spanning >3 million participants, we provide evidence that short-term eGFR decline exhibits a strong and robust association with risk of subsequent ESKD, with a reduction in slope of eGFR decline by 0.75 ml/min per 1.73 m2 per year over 2 years associated with a 30% lower risk of subsequent ESKD. These results add to the analyses of clinical trials—where a treatment effect of 0.75 ml/min per 1.73 m2 per year in the total slope was associated with a 27% lower hazard for the treatment effect on the clinical end point—by quantifying the associations between slope measured over the relatively short term and the subsequent long-term risk of clinical events and by demonstrating consistency, even in populations with eGFR≥60 ml/min per 1.73 m2. Coupled with simulation studies, however, they also suggest that eGFR slope as a surrogate end point may not be useful in slowly progressing populations or short-term studies, particularly when a treatment has an acute effect on eGFR.
Theoretically, treating patients early in the disease course, before substantial GFR decline occurs, may be more beneficial than a treatment delivered in advanced disease, when little is preventable. Some causes of CKD, such as polycystic kidney disease, diabetes mellitus, and diseases with severely increased albuminuria, are considered high risk for progression to ESKD, even with higher GFR. However, drug development in a high-risk population with early disease is challenging in the absence of accepted surrogate biomarkers. When the expected timeframe for developing clinical events is long, such as is the case for ESKD or even a 30%–40% decrease in GFR, trials may be prohibitively expensive. For example, an individual with baseline eGFR of 75 ml/min per 1.73 m2 and a rapid eGFR decline of 5 ml/min per 1.73 m2 per year would take 4.5 years to experience a 30% decrease in eGFR. In our study, even in the subpopulation with 2-year mixed model slopes <−3 ml/min per 1.73 m2, only 15.6% of participants with eGFR≥60 ml/min per 1.73 m2 experienced a 30% decrease in eGFR (5.6% reached a 40% decrease), providing limited power for a putative clinical trial.
Slope of GFR decline is a biologically plausible surrogate, because it is on the path to ESKD, and there are previous trials that have used eGFR slope as a primary or secondary end point.3,4,14–19 Although our results provide support for the use of difference in eGFR slope by treatment arms as an end point in clinical trials, we note several points of caution. First, our analyses only evaluated efficacy with respect to ESKD and death. Specific interventions may have associated harms, and no matter the magnitude of those risks, the risk-to-benefit ratio increases when the expected benefit is diminished. Second, although simulation studies have shown that the use of eGFR slope as a surrogate will provide greater efficiency in trials with high mean baseline GFR, our results suggest that a reduction in slope has likely benefit with respect to preventing kidney outcomes only in rapidly progressive populations. Third, a substantial portion of the study population had positive slopes, and an intervention that may improve GFR by increasing single-nephron GFR rather than reducing nephron loss may not result in long-term benefit. Fourth, a surrogate end point will likely not be useful for clinical trials in populations with high risks of competing events, such as death.
An interesting question in the design of clinical trials is how often and how long to assess eGFR during an intervention. Data from our study suggest stronger associations with the clinical end point with longer periods of observation. Although we found no difference in associations between 2-year slope and ESKD when using three or greater than or equal to five measures of creatinine, this analysis required that a participant be observed for the full 2 years. Clinical trialists cannot know which participants will complete the trial at enrollment; thus, a study design with infrequent recordings of eGFR risks losing information on participants who are lost to follow-up. Analyses of clinical trials and simulations suggest that a longer duration of follow-up helps mitigate bias from any acute effect of an intervention.8,9 Additional studies are needed to test whether adding additional filtration markers, such as cystatin C or other low molecular weight serum proteins, or measurement of GFR using clearance of filtration markers may help further reduce measurement error in slope estimation.
Strengths of this study include the large sample size; the rigorous individual participant–level data meta-analysis; evaluation of association within strata of age, sex, eGFR, diabetes, and other comorbidities; and investigation of different observation periods to estimate GFR slope. However, some limitations must also be mentioned. We evaluate differences in slopes between persons, not a response to therapy within a person or between therapeutic groups. The reason that individuals have different slopes is unknown, and thus, we extrapolate in the application to clinical trials and the effect of an intervention. We did not model nonlinearity in slope or changes in therapy during the observation period, preferring to focus on the approach with the fewest assumptions and most closely resembling an intention-to-treat analysis. Nonetheless, recent studies do suggest that eGFR trajectories during 1–4 years among clinical trial participants are most often compatible with linearity.20 Finally, the estimates of absolute risk reduction reflect a scenario in which there is no change in albuminuria in the baseline period.13
In summary, results from this global, individual participant–level meta-analysis demonstrate a consistent association between eGFR slope and subsequent development of ESKD, even when the slope difference is small and observed over only 1–3 years. Results were consistent with those estimated from clinical trials8 and suggest the validity of GFR slope as a surrogate end point in clinical trials designed to slow kidney disease progression. Our results were robust in individuals with eGFR>60 ml/min per 1.73 m2, an important population in whom early therapy may be most effective to prevent long-term outcomes but time to event analyses have little power. However, eGFR slopes do not address safety, and they are unlikely to be useful in the short term for a treatment with an acute effect or a population with low risk of CKD progression. In conjunction with recent work in clinical trials and statistical simulation,8,9 these results may support the validity of eGFR slope as a surrogate end point in select clinical trials.
Funding
The CKD Prognosis Consortium (CKD-PC) Data Coordinating Center is funded in part by a program grant from the US National Kidney Foundation (which in turn, receives support from industry) and National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK100446-01. A variety of sources have supported enrollment; data collection, including laboratory measurements; and follow-up in the collaborating cohorts of the CKD-PC. These funding sources include government agencies, such as national institutes of health and medical research councils as well as foundations and industry sponsors listed in Supplemental Appendix 3.
Disclosures
Dr. Matsushita reports funding and personal fees from Kyowa Hakko Kirin and personal fees from Akebia outside of the submitted work. Dr. Inker reports grants from the National Institute for Health (NIH) and grants from the National Kidney Foundation (NKF) during the conduct of the study. In addition, Dr. Inker has a patent pending (“Precise estimation of GFR from multiple biomarkers” to Drs. Inker, Levey, and Coresh). Dr. Inker reports funding to Tufts Medical Center for research and contracts with the NIH, NKF, Retrophin, Omeros Corp., Reata Pharmaceuticals, and Dialysis Clinic, Inc. She has consulting agreements with Tricida Inc. and Omeros Corp. Dr. Kovesdy reports personal fees from Amgen, personal fees from Sanofi-Aventis, personal fees from Fresenius Medical Care, personal fees from Keryx, grants from Shire, personal fees from Bayer, personal fees from Abbott, personal fees from Abbvie, personal fees from Dr. Schar, and personal fees from Astra-Zeneca outside the submitted work. Dr. Wetzels reports grants from Sanofi, grants from Pfizer, grants from Amgen, grants from Achillion, other from Shire, and other from Vifor Fresenius outside the submitted work. Dr. Woodward reports personal fees from Amgen and personal fees from Kirin outside the submitted work. Dr. Levey reports grants from the NKF and grants from the NIH during the conduct of the study and other from Siemens outside the submitted work. In addition, Dr. Levey has a patent pending (“Precise estimation of GFR from multiple biomarkers” to Dr. Inker, Dr. Levey, and Dr. Coresh). Dr. Coresh reports grants from the NIH and grants from the NKF during the conduct of the study.
Supplementary Material
Acknowledgments
The planning and operations committee of the National Kidney Foundation in collaboration with the Food and Drug Administration and the European Medicines Agency Workshop on Change in Albuminuria and GFR as End Points for Clinical Trials in Early Stages of CKD contributed to the design and critical review of these analyses.
The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
The CKD Prognosis Consortium (CKD-PC) investigators/collaborators (cohort acronyms/abbreviations are listed in Supplemental Appendix 2) are as follows. AASK: Dr. Astor (University of Wisconsin School of Medicine and Public Health), Lawrence J. Appel (Johns Hopkins University), Tom Greene (University of Utah), and Teresa Chen (Johns Hopkins Medicine). ADVANCE: John Chalmers, Min Jun, Toshiaki Ohkuma (The George Institute for Global Health, University of Sydney), and Dr. Woodward (Johns Hopkins University and The George Institute for Global Health, University of Oxford and University of New South Wales). BC CKD: Dr. Levin (British Columbia Provincial Renal Agency and University of British Columbia) and Ognjenka Djurdjev (British Columbia Provincial Renal Agency). CCF: Dr. Schold, Joseph Nally (Cleveland Clinic), Sankar Navaneethan (Baylor College of Medicine), and Susana Arrigain (Cleveland Clinic). Geisinger: Dr. Chang, H. Lester Kirchner, Jamie Green, and Kevin Ho (Geisinger Health System). KP Hawaii: Dr. Lee (Kaiser Permanente Hawaii). Maccabi: Dr. Shalev (Maccabi Healthcare Services, Tel Aviv University), Cheli Melzer Cohen, Inbal Goldshtein (Maccabi Healthcare Services), and Gabriel Chodick (Maccabi Healthcare Services and Tel Aviv University). MASTERPLAN: Dr.Wetzels, Jan van de Brand (Radboud University Medical Center), Peter J. Blankestijn, and Arjan van Zuilen (University Medical Center Utrecht). MDRD: Mark Sarnak and Dr. Inker (Tufts Medical Center). NZDCS: Dr. Kenealy, C. Raina Elley, Simon Moyes (University of Auckland), and John F. Collins (Auckland City Hospital). RCAV: Dr. Kovesdy (University of Tennessee Health Science Center and Memphis Veterans Affairs Medical Center), Praveen K. Potukuchi, Keiichi Sumida (University of Tennessee Health Science Center), Miklos Z. Molnar (University of Tennessee Health Science Center and Methodist University Hospital Transplant Institute), and Kamyar Kalantar-Zadeh (University of California, Irvine). RENAAL: Dick de Zeeuw, Dr. Pena, and Hiddo J. L. Heerspink (University Medical Center Groningen). SCREAM: Dr. Carrero, Hong Xu, and Marco Trevisan (Karolinska Institutet). Sunnybrook: Dr. Naimark (University of Toronto) and Navdeep Tangri (University of Manitoba).
CKD-PC Steering Committee: Dr. Coresh (Chair) (Johns Hopkins University), Dr. Gansevoort (University Medical Center Groningen), Dr. Grams (Johns Hopkins University), Stein Hallan, Dr. Kovesdy (University of Tennessee Health Science Center and Memphis Veterans Affairs Medical Center), Dr. Levey (Tufts Medical Center), Dr. Matsushita (Johns Hopkins University), Dr. Shalev (Maccabi Healthcare Services and Tel Aviv University), and Dr. Woodward (Johns Hopkins University and The George Institute for Global Health, University of Oxford and University of New South Wales).
CKD-PC Data Coordinating Center: Dr. Ballew (Assistant Project Director), Jingsha Chen (Programmer), Dr. Coresh (Principal Investigator), Dr. Grams (Director of Nephrology Initiatives), Lucia Kwak (Programmer), Dr. Matsushita (Director), Ms. Sang (Lead Programmer), Aditya Surapeneni (Programmer) (Johns Hopkins University), and Dr. Woodward (Senior Statistician) (Johns Hopkins University and The George Institute for Global Health, University of Oxford and University of New South Wales).
Planning and Operations Committees: Dr. Levey (Chair) (Tufts Medical Center), Dr. Gansevoort (University Medical Center Groningen), Dr. Coresh (Johns Hopkins University), Dick de Zeeuw (University Medical Center Groningen), Kai-Uwe Eckardt (Friedrich-Alexander-Universität Erlangen-Nürnberg and Charité-Universitätsmedizin Berlin), Hrefna Gudmundsdottir (Icelandic Medicines Agency and Landspitali-The National University Hospital of Iceland), Dr. Levin (British Columbia Provincial Renal Agency and University of British Columbia), Romaldas Maciulaitis (European Medicines Agency and Lithuanian University of Health Sciences), Tom Manley (US National Kidney Foundation), Vlado Perkovic (The George Institute for Global Health, University of Sydney), Kimberly Smith, Norman Stockbridge, Aliza Thompson (US Food and Drug Administration), Thorsten Vetter (European Medicines Agency), Kerry Willis (US National Kidney Foundation), and Luxia Zhang (Peking University First Hospital).
Some of the data reported here have been supplied by the US Renal Data System.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019010008/-/DCSupplemental.
Supplemental Appendix 1. Data analysis overview and analytic notes for some of the individual studies.
Supplemental Appendix 2. Acronyms or abbreviations for studies included in this report and their key references linked to the web references.
Supplemental Appendix 3. Acknowledgements and funding for collaborating cohorts.
Supplemental Figure 1. Adjusted hazard ratios and density of 1-year (upper row) and 3-year (lower row) eGFR slopes.
Supplemental Figure 2. Forest plot of adjusted hazard ratios for ESKD associated with a 0.75-ml/min per 1.73 m2 per year change in eGFR over 2 years estimated using linear mixed models and stratified by baseline eGFR and age.
Supplemental Figure 3. Forest plot of adjusted hazard ratios for ESKD associated with a 0.75-ml/min per 1.73 m2 per year change in eGFR over 2 years estimated using linear mixed models and stratified by baseline eGFR and sex.
Supplemental Figure 4. Forest plot of adjusted hazard ratios for ESKD associated with a 0.75-ml/min per 1.73 m2 per year change in eGFR over 2 years estimated using linear mixed models and stratified by baseline eGFR and diabetes status.
Supplemental Figure 5. Forest plot of adjusted hazard ratios for ESKD associated with a 0.75-ml/min per 1.73 m2 per year change in eGFR over 2 years estimated using linear mixed models and stratified by baseline eGFR and hypertension status.
Supplemental Figure 6. Forest plot of adjusted hazard ratios for ESKD associated with a 0.75-ml/min per 1.73 m2 per year change in eGFR over 2 years estimated using linear mixed models and stratified by baseline eGFR and history of cardiovascular disease status.
Supplemental Figure 7. Adjusted hazard ratios for ESKD associated with a 0.75-ml/min per 1.73 m2 per year change eGFR over 2 years estimated using linear mixed models, stratified by baseline eGFR, and including an interaction term for category of ACE/ARB use at first and last visits (e.g., new use corresponds to no use at the first visit and use at the last visit of the baseline period used to calculate the slope of eGFR decline).
Supplemental Table 1. Baseline characteristics of individuals in cohorts participating in the 1-year observation period for change in eGFR over time stratified by baseline eGFR.
Supplemental Table 2. Baseline characteristics of individuals in cohorts participating in the 3-year observation period for change in eGFR over time stratified by baseline eGFR.
Supplemental Table 3. Median number of creatinine measurements within individuals in cohorts participating in the 2-year observation period for change in eGFR over time, with subsequent follow-up and number of events.
Supplemental Table 4. Median number of creatinine measurements within individuals in cohorts participating in the 1-year observation period for change in eGFR over time, with subsequent follow-up and number of events.
Supplemental Table 5. Median number of creatinine measurements within individuals in cohorts participating in the 3-year observation period for change in eGFR over time, with subsequent follow-up and number of events.
Supplemental Table 6. Mean and SD of change in eGFR over time in cohorts separately by 1-, 2-, and 3-year observation periods stratified by baseline eGFR and estimated using linear mixed models and linear regression.
Supplemental Table 7. Hazard ratios for ESKD associated with a 0.75-ml/min per 1.73 m2 per year change in eGFR over time separately by 1-, 2-, and 3-year observation periods stratified by baseline eGFR and estimated using linear mixed models and linear regression.
Supplemental Table 8. Hazard ratios for all-cause mortality associated with a 0.75-ml/min per 1.73 m2 per year improvement in eGFR decline over time separately by 1-, 2-, and 3-year observation periods stratified by baseline eGFR and estimated using linear mixed models and linear regression.
References
- 1.Himmelfarb J: Chronic kidney disease and the public health: Gaps in evidence from interventional trials. JAMA 297: 2630–2633, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Strippoli GF, Craig JC, Schena FP: The number, quality, and coverage of randomized controlled trials in nephrology. J Am Soc Nephrol 15: 411–419, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Thompson A, Cattran DC, Blank M, Nachman PH: Complete and partial remission as surrogate end points in membranous nephropathy. J Am Soc Nephrol 26: 2930–2937, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson A, Lawrence J, Stockbridge N: GFR decline as an end point in trials of CKD: A viewpoint from the FDA. Am J Kidney Dis 64: 836–837, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Inker LA, Mondal H, Greene T, Masaschi T, Locatelli F, Schena FP, et al.: Early change in urine protein as a surrogate end point in studies of IgA nephropathy: An individual-patient meta-analysis. Am J Kidney Dis 68: 392–401, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Stevens LA, Greene T, Levey AS: Surrogate end points for clinical trials of kidney disease progression. Clin J Am Soc Nephrol 1: 874–884, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Prentice RL: Surrogate endpoints in clinical trials: Definition and operational criteria. Stat Med 8: 431–440, 1989 [DOI] [PubMed] [Google Scholar]
- 8.Inker LA, Heerspink HJ, Tighiouart H, Levey AS, Coresh J, Gansevoort R, et al. : GFR slope as a surrogate end point for kidney disease progression in clinical trials: A meta-analysis of treatment effects of randomized controlled trials. J Am Soc Nephrol 30: 1735–1745, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greene T, Ying J, Vonesh E, Tighiouart H, Levey AS, Coresh J, et al. : Performance of GFR slope as a surrogate endpoint for kidney disease progression in clinical trials: A statistical simulation. J Am Soc Nephrol 30: 1756–1769, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsushita K, Ballew SH, Astor BC, Jong PE, Gansevoort RT, Hemmelgarn BR, et al.: Chronic Kidney Disease Prognosis Consortium : Cohort profile: The chronic kidney disease prognosis consortium. Int J Epidemiol 42: 1660–1668, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al.: CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson GK: That BLUP is a good thing: The estimation of random effects. Stat Sci 6: 15–32, 1991 [Google Scholar]
- 13.Coresh J, Heerspink HJL, Sang Y, Matsushita K, Arnlov J, Astor BC, et al.: Chronic Kidney Disease Prognosis Consortium and Chronic Kidney Disease Epidemiology Collaboration : Change in albuminuria and subsequent risk of end-stage kidney disease: An individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol 7: 115–127, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agodoa LY, Appel L, Bakris GL, Beck G, Bourgoignie J, Briggs JP, et al. ; African American Study of Kidney Disease and Hypertension (AASK) Study Group : Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: A randomized controlled trial. JAMA 285: 2719–2728, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Wright JT Jr., Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, et al.: African American Study of Kidney Disease and Hypertension Study Group : Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK trial. JAMA 288: 2421–2431, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, et al.: Modification of Diet in Renal Disease Study Group : The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med 330: 877–884, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, et al.: TEMPO 3:4 Trial Investigators : Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schrier RW, Abebe KZ, Perrone RD, Torres VE, Braun WE, Steinman TI, et al.: HALT-PKD Trial Investigators : Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med 371: 2255–2266, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, et al.: REPRISE Trial Investigators : Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med 377: 1930–1942, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Weldegiorgis M, de Zeeuw D, Li L, Parving HH, Hou FF, Remuzzi G, et al.: Longitudinal estimated GFR trajectories in patients with and without type 2 diabetes and nephropathy. Am J Kidney Dis 71: 91–101, 2018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.