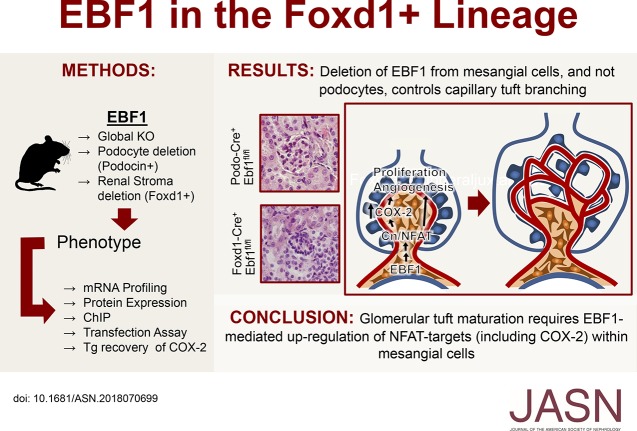
Significance Statement
The specific mechanisms regulating formation of the glomerular tuft during renal development are largely unknown. A previous study showed the transcription factor Early B cell factor 1 (EBF1) is essential for formation of the glomerular tuft. However, the cell types driving glomerular developmental defects in mice lacking EBF1 were not identified. The authors show that deletion of EBF1 from the glomerular mesangium results in impaired glomerular development, whereas deletion of EBF1 from podocytes does not cause developmental abnormalities. They show that EBF1 in the mesangial cells directs glomerular capillary branching through NFAT activation, and consequently COX-2 expression. This is the first evidence that COX-2 inhibition specifically from the mesangial cells impairs renal development, and furthers our understanding of this essential stage of nephrogenesis.
Keywords: genetics and development, capillary tuft, glomerulosclerosis, mesangial cells, podocyte, transcription factors
Visual Abstract
Abstract
Background
We recently showed the transcription factor Early B cell factor 1 (EBF1) is essential for the last stages of metanephric development, and that mice globally deficient in EBF1 display impaired maturation of peripheral glomeruli. EBF1 is present within multiple glomerular cell types, including the glomerular mesangium and podocytes.
Methods
To identify which cell type is driving the glomerular developmental defects in the global EBF1 knockout mice, we deleted EBF1 from the mesangium/pericytes (Foxd1-cre) or podocytes (Podocin-cre) in mice.
Results
Deletion of EBF1 from Foxd1 lineage cells resulted in hypoplastic kidneys, poorly differentiated peripheral glomeruli, and decreased proximal tubular mass in the outer cortex. Renal insufficiency was apparent at P21 when proteinuria presents, fibrosis of both the glomeruli and interstitium rapidly progresses, microthrombi appear, and hematuria develops. Approximately half of the Foxd1+, Ebf1fl/fl mice die before they are 3 months old. Mice with podocyte-targeted deletion of EBF1 exhibited no developmental abnormalities. Mice with Ebf1 deficiency in Foxd1 lineage cells shared characteristics with Ptgs2/COX-2–insufficient models, and mechanistic investigation revealed impaired calcineurin/NFATc1 activation and decreased COX-2 expression. Deletion of COX-2 from the interstitial/mesangial lineage displayed a less severe phenotype than EBF1 deficiency in mice. Overexpressing COX-2 in the EBF1-deficient mice, however, partially restored glomerular development.
Conclusions
The results suggest that EBF1 regulates metanephric development at the last stages of glomerular maturation through its actions in the stromal progenitor (Foxd1+) lineage where it mediates proper regulation of calcineurin/NFAT signaling and COX-2 expression.
Glomerular development involves a complex and orchestrated series of paracrine signaling events between the epithelial podocytes, capillary endothelium, and mesangial stalk. The primitive/presumptive podocytes direct the endothelial precursors into the glomerular cleft of the S-shaped body.1 Migrating endothelial cells and differentiating podocytes next lay down extracellular matrices that fuse to become the glomerular basement membrane (GBM).2−5 The endothelial cells also recruit a stalk of mesangial mesenchyme by secreting PDGF-B. Without a properly formed GBM or recruitment of the mesangium glomerular development is arrested at the single capillary loop stage.6,7 Although it is known that the mesangium controls branching of the initial capillary loop into the subsequent 6–8 loop stage and finally to the mature vascular tuft, there is no known mechanism regulating this procedure. Deletion of ephrin-B2 from the mesangium arrests development at the single capillary loop stage,8 as does inhibition of PDGFR-β signaling.7 Alternately, deletion of various podocyte-specific proteins can arrest tuft development at either the single loop (VEGF, Podl, and Kreisler),9,10 or post the 6–8 loop stage (Foxc2)11 so the mesangium is not the only cell controlling this process.
Early B cell factor 1 (EBF1) is a transcription factor (TF) possessing a unique DNA-binding structure that utilizes an atypical zinc finger in conjunction with an IPT/TIG-like domain.12 Its actions are broader than its name implies, and it is essential for neuronal migration,13 the expression of olfactory receptors,14,15 proper differentiation of adipocytes,16−18 osteoblastogenesis,17 and B cell maturation.19 In the global Ebf1-knockout (Ebf1-KO) model we previously reported that glomerular capillary tuft development is severely inhibited.20 This phenotype of the Ebf1-KOs develops between postnatal day 10 (P10) and postnatal day 14 (P14).20 This is during the very last stages of renal development in mice.
Within the glomerulus EBF1 is present in both mesangial cells and podocytes.20 To identify the causative cell type through which EBF1 regulates development of the capillary tuft we used conditional deletion of Ebf1 from either podocytes or the renal stromal progenitors (to target the mesangium) and report here that deletion from only the stromal progenitors was able to recapitulate the developmental phenotype present in the global Ebf1-KO. Investigation into the mechanism underlying this mesangial-mediated control of capillary development revealed that NFAT/calcineurin signaling and the expression of the prostaglandin synthase enzyme cyclooxygenase-2 (COX-2) is inhibited by the loss of EBF1.
Methods
For the global Ebf1-KO mice, 129×1/SvJ and C57BL/6 mice were obtained from JAX and the National Cancer Institute, respectively, and housed at Yale University School of Medicine. EBF1-KO were originally generated by Rudolph Grosschedl.21 Because of the absence of Ebf1-KO pups on the pure C57BL/6 background, we bred Ebf1+/− mice maintained on the C57BL/6 genetic background to 129×1/SvJ mice, resulting in an F1 generation. The Ebf1+/− F1 mice were then interbred (F2 generation). Ebf1−/− F2 mice were used for all experiments and no further breeding of any F2 generation mice was attempted. Ebf1−/− mice were always compared with age-matched wild-type littermate controls and were genotyped as previously described.22
In the absence of a mesangial-specific cre-driver, we utilized Foxd1-cre mice [Foxd1tm1(GFP/cre)Amc] generated by Andrew McMahon, purchased from JAX (stock no. 012463).23 Podocin-cre (also known as p2.5P-cre), originally generated by L. Holzman, were used to target podocytes specifically.24 Prx1-cre mice target the limb bud and all the descendants of that mesenchyme and were purchased from JAX (stock no. 005584).25 All three lines were mated with animals from R. Grosschedl, where flox sites flank the exons encoding the DNA-binding domain of Ebf1 (Ebf1fl/fl).26 This is the same genetic region that is excised in the global deletion model.21 For lineage tracing, Foxd1-cre+ mice were mated to mT/mG mice (JAX stock no. 007576).27 Triple transgenic animals were also made by mating these to the Ebf1fl/fl animals.
Ptgs2fl/fl mice were purchased from JAX (stock no. 030785).28 Ptgs2-COE and Ptgs2CR mice were originally generated by H. Herschman.28 Ptgs2CR mice were provided tamoxifen (1 mg/ml) in corn oil and injected intraperitoneally with 50 µl per pup on postnatal day 8, 10, and 12. The Yale Medical School Institutional Animal Care and Use Committee approved the purchase and use of all animals (protocol no. 10543).
Mesangial cells were isolated according the methods described previously.29 Because these were mouse glomeruli, they were collected using a 40 µm sieve. Isolated mesangial cells were not passaged, except during the Luciferase assay where a single passage was used to replate cells at the correct density 72 hours after the initial isolation. The NFAT-reporter plasmid used was pGL3-NFAT luciferase, purchased from Addgene.30
Kidneys were bisected and stained for Masson trichrome, H&E, or PAS staining, as required. Glomerular size, number, and locations were quantified in histologic sections using ImageJ. Frozen sections were used for immunofluorescence as described previously.20 Anti-mouse aquaporin 1 antibody was from Abcam (#ab15080). GFR was performed using FITC-labeled inulin infusion. The bladder is accessed and catheterized so that urine samples can be collected. Infusion is mediated through the common carotid artery and a catheter in the external jugular vein records BP continuously, and obtains blood samples. FITC-inulin is infused, after a saline bolus, at approximately 50 µCi/h per mouse, at a volume flow rate of 12 µl/g body wt/h. After the equilibration period, sequential 30-minute urine samples are collected and the volume measured. Blood is sampled (20 µl) at the midpoint of each urine collection. Then, 2 µl of plasma and urine samples are pipetted into a Nanodrop spectrophotometer and then used to calculate the GFR.
Urinary protein was assessed with the Bradford assay, and visualized after SDS-PAGE separation with Coomassie staining. Intact FGF23 was measured using the Quidel Mouse/Rat FGF-23 (Intact) ELISA. BUN was quantified with a colorimetric assay from ThermoFisher (#EIABUN). Creatinine was measured using the kit from Sigma (#MAK080). ELISAs from Cayman Chemical were used to measure PG:PGE metabolites (#514531), 11-dehydro TBXB2 (#519510), PGI metabolites (#501100), 13,14-dihydro-15-keto PGF2α (#516671), and two versions of PGD metabolites (tetranor PDGM, #501001; 11β-PDGF2α #516521).
RNA was collected using the Qiagen miRNeasy kit and reverse transcribed using BioRad iScript. Real-time PCR was completed on an ABI One Step machine with Kapa Universal SYBR fast reagents. In situ hybridization was performed using the HybEZ Hybridization System from Advanced Cell Diagnostics and RNAscopeProbe - Custom Target C1 to mouse EBF1 (#300031). Hybridization was visualized with DAB Peroxidase staining utilizing RNAscope 2.0 HD Reagent Kit - BROWN (#310035). Western blotting was used to visualize COX-2 (#ab52237; Abcam), COX-1 (sc-19998; Santa Cruz Biotechnology), NFATc1 (ab2796; Abcam), and actin (sc-8432; Santa Cruz Biotechnology).
Chromatin immunoprecipitation assay was performed using the anti-EBF1 antibody #ABE1294 (Millipore), and the protocol described previously.31 EBF1 was precipitated from either whole kidney, circulating blood populations, whole spleen, or isolated glomeruli from six kidneys.
All values are expressed as mean±SEM. We evaluated differences between groups through ANOVA. Significance is indicated when P≤0.05. Analysis of large ranked sum groups was performed using Mann–Whitney U test and significance was indicated when P≤0.01. Survival curves were compared using a log-rank (Mantel–Cox) test and significance is indicated when P≤0.05.
Results
EBF1 Regulates Glomerular Maturation through Actions in the Stromal-Progenitor Lineage
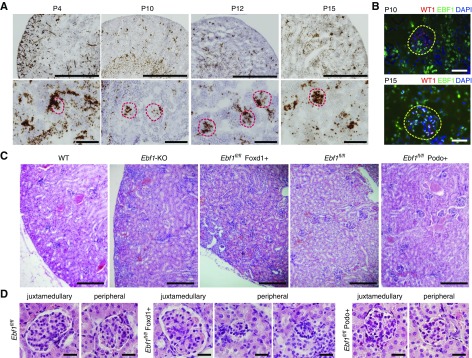
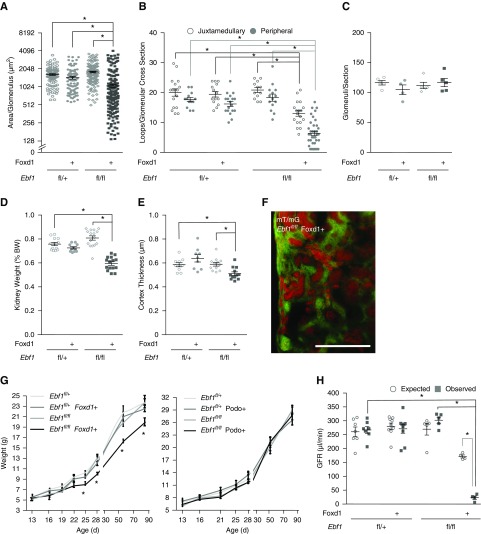
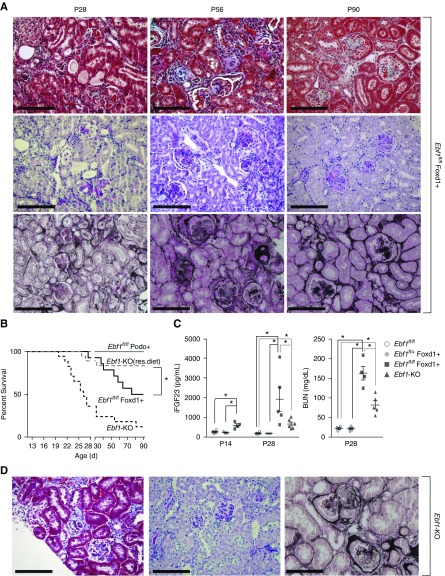
During our original characterization of the global Ebf1-KO mouse, we observed that the expression of EBF1 is temporally and spatially regulated in the developing kidney.32 EBF1 appears in the mesangial cells before P10 but expands to the podocytes by P14, and maintains this expression into the adult (Figure 1, A and B). To determine the cell type through which EBF1 is exerting its developmental effects on the glomerulus, renal stroma descendants (including the mesangium) or the podocytes were targeted, respectively, using mice harboring transgenic cre-recombinase under the control of either the Foxd123 or Podocin24 promoters in the presence of a floxed version of Ebf1 (Ebf1fl/fl).33 Although no developmental phenotype was apparent in the Ebf1fl/fl,Podo+ animals, the Ebf1fl/fl,Foxd1+ mice displayed markedly impaired development of the outer cortex (Figure 1C). Like the global Ebf1-KO, Ebf1fl/fl,Foxd1+ mice also displayed immature glomeruli and pockets of undifferentiated mesenchyme (Figure 1, C and D). Decreased glomerular cross-sectional area and a paucity of capillary loop development within the peripheral structures was more severe than in the juxtamedullary corpuscles (Figure 2, A and B). Branching does not appear to be affected as there were an equal number of glomeruli per section (Figure 2C). Kidneys from the Ebf1fl/fl,Foxd1+ mice were also hypoplastic, and exhibited a thinned cortex (Figure 2, D and E). Heterozygous animals were developmentally normal. Lineage tracing, using an mT/mG reporter mouse that marks cells where recombination occurred by changing them from expressing tdTomato (no recombination) to GFP-positive (recombination),27 also demonstrated that the nontubular mesenchyme that persists in Ebf1fl/fl,Foxd1+ mice arises from the stromal lineage as these cells are GFP-positive on this background (Figure 2F).
Figure 1.
Foxd1 lineage–specific deletion of EBF1 reproduces the congenital renal malformations of global EBF1 deletion. (A) In situ hybridization of EBF1 from postnatal day 4 (P4) to postnatal day 15 (P15). Low-magnification bar, 1000 µm; high-magnification bar, 100 µm. (B) Immunofluorescence of EBF1 and WT1 in glomeruli at P10 and P14. Bar, 25 µm. (C) H&E-stained kidney sections from 1-month-old mice are shown for the global Ebf1-KO mice, podocyte-targeted conditional KO (Ebf1fl/fl,Podo+), and renal stroma–targeted knockouts (Ebf1fl/fl,Foxd1+). Floxed control (without any recombination, Ebf1fl/fl) are also shown. Bar, 200 µm. (D) Glomerular development from representative juxtamedullary and peripheral corpuscles are shown for each deletion model. Bar, 25 µm.
Figure 2.
Foxd1 lineage–specific deletion of EBF1 impairs development of the glomerular capillary tuft. (A) Glomeruli in transverse kidney sections were counted and displayed as the number visible per cross section. Mice with either a single floxed Ebf1 allele (Ebf1fl/+) or two floxed alleles (Ebf1fl/fl) were assessed in the absence or presence of Foxd1-driven cre. (B) Thin sections were analyzed from 1-month-old mice and the number of capillary loops present in each glomerular cross section was quantified. Glomeruli form the periphery were tallied separately from the juxtamedullary structures. (C) The average area of each glomerulus present in transverse kidney sections was quantified. (D) Kidney weight expressed as a percentage of body weight in 1-month-old mice. (E) Cortex thickness measured from transverse sections of kidney. (F) Kidney cortex from 1-month-old Ebf1fl/fl,Foxd1+ mouse. Green indicates Foxd1 lineage, red indicates all other cells. Bar, 100 µm. (G) Growth curves were established for each conditional knockout for the first 3 months of life. (H) GFR was measured using inulin infusion (observed values). Expected values were calculated from body weight of the animals using an equation for allometric scaling of the predicted value to meet the metabolic needs of mammals.34 *P≤0.05.
Functionally, Ebf1fl/fl,Foxd1+ mice exhibit renal insufficiency. It is important to note that Ebf1fl/fl,Foxd1+ mice are runted from P24 on, whereas Ebf1fl/fl,Podo+ mice are not (Figure 2G). The GFR in 6- to 7-week-old Ebf1fl/fl,Foxd1+ animals was reduced by 80% of the expected filtration load (when allometrically scaled for body size34), and by 89% compared with controls (Figure 2H). Thus, conditional deletion of EBF1 from the renal stroma results in an animal with congenital low nephron endowment resulting from the failure of the glomeruli to properly form capillary tufts.
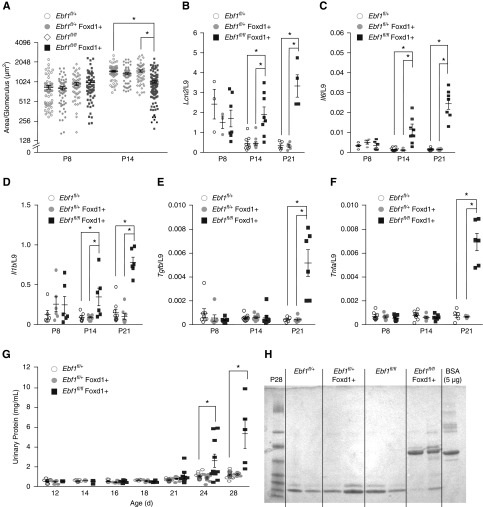
Our original observations in the global Ebf1-KO revealed that renal development fails to progress between P10 and P14.20 We confirmed that glomerular maturation arrests at the same developmental stage in the Ebf1fl/fl,Foxd1+ mice by measuring glomerular size at P8 and P14 (Figure 3A). Tubular damage, supported by the induction of N-GAL/Lcn2 mRNA from the kidneys, was also evident at P14 (Figure 3B), as was renal production of the inflammatory cytokines IL-6 and IL-1β (Figure 3, C and D). TNFα and TGFβ took longer to be expressed (Figure 3, E and F). We reported before that the global Ebf1-KO animals presented with albuminuria at P14.20 Ebf1fl/fl,Foxd1+ mice displayed albuminuria by P21 (Figure 3, G and H). Proteinuria was never observed in Ebf1fl/fl,Podo+ animals (Supplemental Figure 1).
Figure 3.
Conditional deletion models and the onset of renal dysfunction. (A) The average area of each glomerulus in transverse kidney sections were quantified for each genotype at the ages indicated. (B) RNA expression of the nonspecific injury marker Lcn2/N-GAL from whole kidney. (C) RNA expression of IL-6 from whole kidneys. (D) RNA expression of IL-1β from whole kidneys. (E) RNA expression of TGFβ from whole kidneys. (F) RNA expression of TNFα from whole kidneys. (G) Proteinuria was measured in mice with conditional deletion of EBF1 from the stromal lineage from postnatal day 12–28. (H) Urine from Ebf1fl/fl,Foxd1+ mice and controls was collected at P21, separated by SDS-PAGE, and stained with Coomassie. BSA is used as a loading control. *P≤0.05.
CKD Progresses More Rapidly in Foxd1 Lineage-Targeted Conditional Knockouts than the Global Ebf1-KO Mice
In our previous characterization of the global Ebf1-KO mice it was noted that despite severely reduced GFR, the mice were slow to accumulate fibrotic damage.20 Histologic examination of Ebf1fl/fl,Foxd1+ mice demonstrated that this is not true in the conditional knockout despite having a similar defect in glomerular and cortical development. Although albuminuria was apparent a week later in the Ebf1fl/fl,Foxd1+ mice, they accumulate glomerulosclerosis within 1 week of the onset of proteinuria (Figure 4A). Additionally, urine dipstick testing from Ebf1fl/fl,Foxd1+ mice showed strong leukocyturia (plus two, data not shown) at P24 and hematuria at 4–6 weeks of age. Capillary congestion and microthrombi are also visible in the glomeruli by P28 (Figure 1D). By contrast, congestion and hematuria do not appear positive in the global Ebf1-KO mice until they are 2–3 months old,20 whereas leukocytes remain negative or plus one (data not shown).
Figure 4.
Rapid progression to glomerulosclerosis in the Ebf1fl/fl,Foxd1+ mice. (A) Masson trichrome, PAS, and Jones staining of Ebf1fl/fl,Foxd1+ knockout mice at 1–3 months of age. Bar, 100 µm. (B) Survival curves were calculated for the global KOs and each conditional KO model during the first 3 months of life. Global deficient mice are shown by two curves. Those on normal chow (Ebf1-KO) and on a high-fat calorie rescue diet to counteract their lipodystrophy/starvation [Ebf1-KO(res. diet)]. (C) Circulating intact FGF-23 protein expression in EDTA plasma from Ebf1fl/fl,Foxd1+ and Ebf1-KO at P14 and P28. BUN content in EDTA plasma of Ebf1fl/fl,Foxd1+ and Ebf1-KO mice at P28. (D) Masson trichrome, PAS, and Jones staining of Ebf-KO mice at 3 months of age. Bar, 100 µm *P≤0.05 by ANOVA.
Global Ebf1-KO are extremely sick from their congenital renal disease combined with a wasting lipodystrophy attributable to the pleiotropic actions of EBF1 throughout the body.20,35 Without intervention, 90% of the global Ebf1-KO mice die before they are 3 months old. A rescue diet focused on countering the lipodystrophy improves survival in Ebf1-KO mice up to 75% (Figure 4B). By contrast, restricted deletion of EBF1 from only the Foxd1 lineage manifests with 50% mortality over the same period, and further supports a more aggressive progression of renal disease in the conditional knockout mice (Figure 4B). We confirmed that Foxd1-cre is does not drive recombination in adipose tissue (Supplemental Figure 2). Comparison of the sclerosis present in Ebf1fl/fl,Foxd1+ mice with global Ebf1-KO on a rescue diet shows markedly advanced sclerosis in the Ebf1fl/fl,Foxd1+ animals (Figure 4, A and D).
Biomarker analysis also demonstrated a more fulminant disease in the Ebf1fl/fl,Foxd1+ animals. FGF-23 expression stratifies with remnant GFR.36,37 Circulating intact FGF-23 was elevated in Ebf1fl/fl,Foxd1+ mice and the global Ebf1-KO mice, but was consistently higher in the targeted deletion model (Figure 4C). Kidney function, measured by the accumulation of BUN, also indicated a more advanced disease in the Foxd1-targeted mice than in age-matched global knockouts (Figure 4C).
Loss of EBF1 Abrogates Renal COX-2 Expression
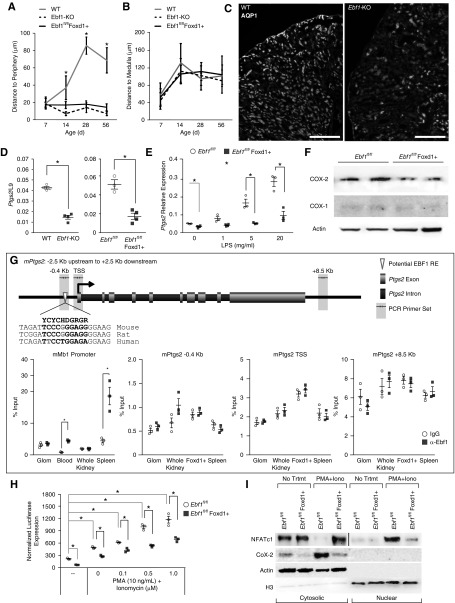
The peripheral nature of the defect in glomerular development present in the Ebf1-KO phenotype is rare, but reminiscent of inhibited COX-2/Ptgs2 signaling. Knockout, hypomorph, and chemical inhibition of COX-2 activity all result in thinning of the cortex, immature peripheral glomerular maturation, reduced density of the outer proximal tubule mantle with glomeruli that reside against the capsule, and persistence of mesenchymal islands.38−41 Ebf1fl/fl,Foxd1+ mice recreate all of these including thinning of the proximal tubular mantle and compression of the glomeruli against the capsule (Figure 5, A–C).
Figure 5.
Reduced COX-2 expression in EBF1-deficient mesangial cells. (A) Distance from the Bowman’s capsule of the most peripheral glomeruli in the cortex from the kidney capsule at the ages indicated for global Ebf1-KO and Ebf1fl/fl,Foxd1+ mice. (B) Distance from the Bowman’s capsule of the most juxtamedullary glomeruli from the outer medulla at the ages indicated. (C) Aquaporin 1 (Aqp1) staining to mark the proximal tubule in P21 kidney from Ebf1-KO mice and controls. (D) Relative expression of Ptgs2 mRNA in glomeruli of P12 Ebf1-KO mice, Ebf1fl/fl,Foxd1+ mice, and their controls. (E) Expression of Ptgs2 mRNA in mesangial cells from P12 Ebf1fl/fl,Foxd1+ mice and controls when stimulated with the indicated concentration of LPS for 6 hours in vitro. (F) COX-1 and COX-2 protein expression in EBF1-deficinent mesangial cells from P12. (G) ChIP assay for EBF1 occupancy around the Ptgs2 gene. Primers used are indicated by arrows shaded in gray. The sequence for the best EBF-like response element is shown at the −0.4 kb site with the species conservation in rat and human also shown. Bases that vary from the consensus sequence are highlighted in gray. To prove that the ChIP antibody can pull down EBF1 and associated DNA, we included results from a known EBF1 binding site within B cells, the Mb1 promoter, within circulating B cells in the blood and resident B cells of the spleen. The transcriptional start sites (TSS) of Ptgs2 and a negative control region 8.5 kb downstream of the TSS (that did not contain any sequences that looked similar to an EBF response element) were also chosen for amplification. (H) Mesangial cells from Ebf1fl/fl,Foxd1+ mice and controls were transfected with an NFAT-regulated luciferase reporter and treated with phorbol 12-myristate 13-acetate (PMA) and Ionomycin at the indicated concentrations. (I) Isolated mesangial cells from P12 kidneys were serum starved for 2 hours and then treated with vehicle or PMA+ionomycin for 4 hours. Protein was collected separated into nuclear and cytosolic fractions and then blotted for NFATc1 and COX-2. *P≤0.05 by ANOVA.
We investigated whether COX-2 signaling was abrogated in Ebf1-deficient tissue by first noting production of Ptgs2 mRNA from glomeruli of P12 mice was reduced in vivo (Figure 5D). Cultured isolated mesangial cells from Ebf1fl/fl,Foxd1+ mice also exhibited fewer Ptgs2 transcripts and a blunted induction after LPS treatment in vitro (Figure 5E). Loss of EBF1 in the Ebf1fl/fl,Foxd1+ mice reduced COX-2 protein production from cultured mesangial cells as well (Figure 5F).
We used chromatin immunoprecipitation to determine if EBF1 was directly regulating Ptgs2 expression, but few potential response elements for EBF1 exist in or around the Ptgs2 locus, and no evidence of EBF1 recruitment was observed (Figure 5G). Ptgs2 does not appear to be a direct transcriptional target of EBF1.
Ptgs2 is regulated through the cooperative actions of many TFs, including NFATs.42 Animal models of calcineurin Aα deletion also phenocopy the loss of COX-2 in renal development by limiting NFAT signaling in those kidneys.43 Mesangial cells were transfected with an NFAT-driven Luciferase reporter and found to have blunted activation of NFAT signaling at baseline, and also in response to PMA and ionomycin when EBF1 was deleted (Figure 5H). Furthermore, activation and movement of NFATc1 into the nucleus was reduced in mesangial cells in vitro (Figure 5I). In these same cells, COX-2 failed to be expressed at the protein level in a manner that reflected NFAT mobilization to the nucleus. Taken together these data indicate that EBF1 works within the mesangial cells during glomerular development where it does not directly inhibit production of Ptgs2 transcription, but instead modulates activation of NFAT signaling though a primary or secondary mechanism. In the absence of EBF1, proper induction of NFAT-regulated target genes, including Ptgs2, is inhibited. Consequently, mesangial deficiency of EBF1 phenocopies abrogation of the COX-2 signaling axis.
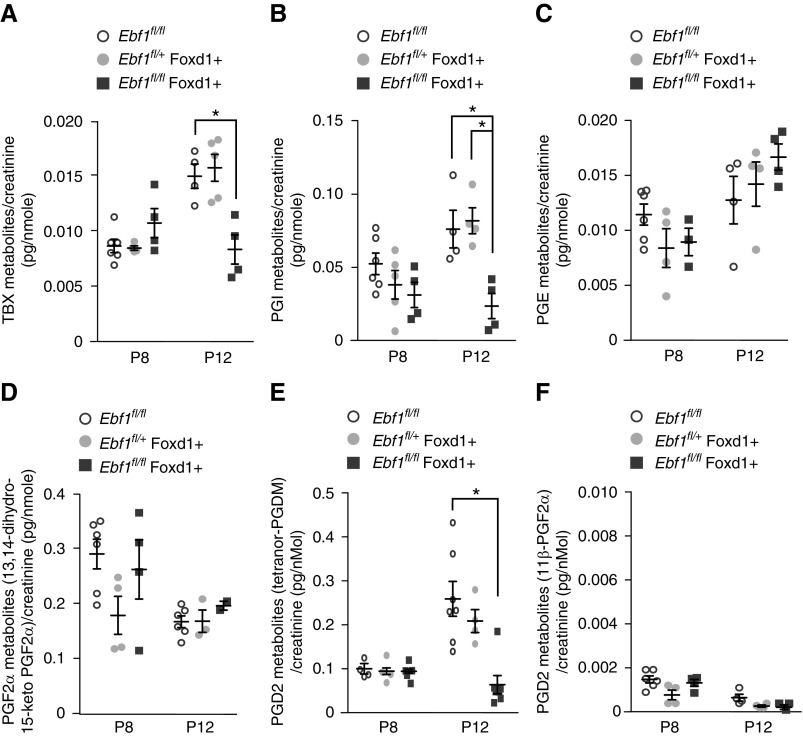
COX-2 catalyzes the initial step in conversion of AA to biologically active prostanoids. Urine was collected from Ebf1fl/fl,Foxd1+ mice in the early postnatal period to identify the prostanoids that are involved in glomerular development. Metabolites of all five main prostanoid pathways were detectable in the urine. PGD2 metabolites were the most highly abundant, and also were significantly reduced after Ebf1 deletion (Figure 6A). Thromboxane and PG I metabolites were also significantly impaired by the loss of EBF1. No change in the production of PGE or PGF2α pathway metabolites were observed (Figure 6, B–F). These results indicate that during glomerular development the production of prostanoid PGD > PGI > thromboxane family members, and all three are impaired in the absence of EBF1.
Figure 6.
Prostanoid production from mesangial cells. (A–F) Prostanoid metabolites were measured in urine from Ebf1fl/fl,Foxd1+ mice and their controls at postnatal day 8 and 12. There are five primary fates of the PGG2 that COX-2 generates from AA, but because of the transient half-lives of several of these we looked for the appropriate metabolites of each fate: (A) thromboxanes, (B) PGI, (C) PGE, (D) PGF, and (E) PGD. (F) An additional variant of the PGD pathway that was specifically noted to be absent from mouse urine, 11β-PGF2α, was used as a negative control to identify the background. All values were normalized to creatinine. *P≤0.05.
Mesangial COX-2 Overexpression Partially Restores Glomerular Development in EBF1-Deficient Mice
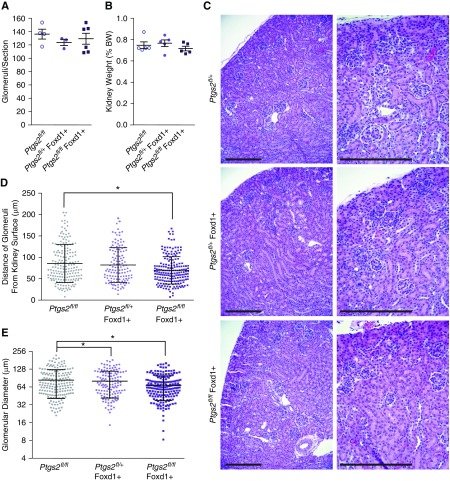
Although hypomorphic and global knockout genetic analysis of Ptgs2 inhibition have been modeled in mice, no reports on a targeted-deletion model exist. We utilized Ptgs2fl/fl mice and the Foxd1-cre to eliminate EBF1 from the kidney stroma lineage. Like the Ebf1fl/fl,Foxd1+ mice these animals did not display any defect in glomerular branching (Figure 7A). Although the kidneys trended slightly smaller than their littermates, there was not a significant decreased in kidney weight (Figure 7B). Unlike the models of EBF1 deletion and the previous reports of global COX-2 inhibition, islands of undifferentiated mesenchyme did not persist in the cortex of Ptgs2fl/fl,Foxd1+ animals (Figure 7C). The glomeruli of the Ptgs2fl/fl,Foxd1+ animals did, however, reside in closer proximity to the capsule than in their control littermates (Figure 7D). The glomerular diameters were also decreased in Ptgs2fl/fl,Foxd1+ mice, but to a lesser degree than was observed in the Ebf1-deficient models (Figure 7, C and E). Together, these data indicate that deletion of Ptgs2 specifically from the stromal lineage partially reconstitutes the phenotype of a global Ptgs2 deletion and the Ebf1 deletion models.
Figure 7.
Foxd1-targeted deletion of COX-2 exhibits decreased glomerular maturation. (A) Glomeruli in transverse kidney sections were counted and displayed as the number visible per cross section. Mice with either a single floxed Ptgs2 allele (Ptgs2fl/+) or two floxed alleles (Ptgs2fl/fl) were assessed in the absence or presence of Foxd1-driven cre. (B) Kidney weight expressed as a percentage of body weight in P21 mice. (C) H&E-stained kidney sections from P21 Ptgs2fl/fl,Foxd1+ mice are shown. Bar, 200 µm. (D) Proximity of the most superficial glomeruli to the kidney capsule was quantified across a transverse histology section. (E) Glomerular diameter was measured for all glomeruli present in transverse histology sections. *P≤0.05.
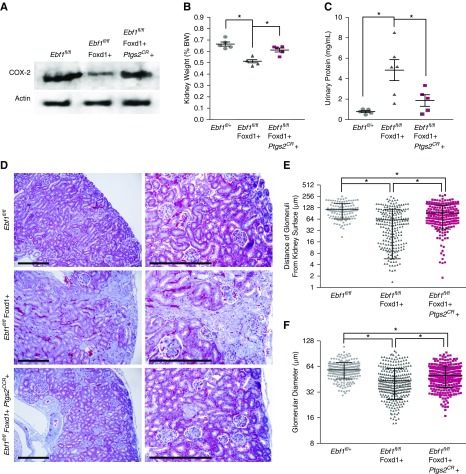
We next used overexpression of COX-2 on the Ebf1-deficient background to determine if it could rescue renal development. We first tried to use a floxed constitutive overexpression model (Ptgs2 COE), restricted to the Foxd1 lineage, but were unable to birth any pups that were positive for both the cre-expression and transgenic COX-2 production, indicating that this was embryonic lethal. We instead switched to a tamoxifen-regulated Ptgs2 model (Ptgs2CR). Tamoxifen-induced expression of COX-2 was confirmed in lysate of glomeruli from P13 animals (Figure 8A). Kidneys were less hypoplastic with overexpression of COX-2 during this critical period of glomerular development (Figure 8B). Proteinuria was lessened at 1 month, through the addition of COX-2 (Figure 8C). Glomeruli increased in their distance from the kidney capsule and the glomerular diameter was also increased (Figure 8, D–F). These data indicate that transiently increasing expression of COX-2 during the last stages of renal development can partially restore glomerular development from EBF1-deficient kidneys.
Figure 8.
COX-2 overexpression partially rescues EBF1-deficient glomerular development. (A) Western blot COX-2 expression in of glomeruli at P12 from control (Ebf1fl/fl), Ebf1fl/fl,Foxd1+ conditional deletion, and conditional deletion mice with tamoxifen-induced COX-2 expression (Ebf1fl/fl,Foxd1+,Ptgs2CR). (B) Kidney weight expressed as a percentage of body weight in 1-month-old mice. (C) Urinary protein in 1-month-old mice. (D) Masson trichrome–stained sections from 1-month-old control (Ebf1fl/fl), Ebf1fl/fl,Foxd1+ conditional deletion, and conditional deletion mice with tamoxifen-induced COX-2 expression (Ebf1fl/fl,Foxd1+,Ptgs2CR). Bar, 200 µm. (E) Proximity of the most superficial glomeruli to the kidney capsule was quantified across a transverse histology section. (F) Glomerular diameter was measured for all glomeruli present in transverse histology sections. *P≤0.05.
Discussion
In the glomerulus both the epithelial podocytes and the stromal mesenchyme-derived mesangial cells express EBF1. In our original article on the global EBF1 knockout20 we hypothesized, on the basis of the timing of the upregulation of EBF1, and the defective maturation of the podocytes in those mice, that EBF1 was directly regulating glomerular development through transcriptional control within the podocytes. We reveal here, that it is not the podocytes but the glomerular mesangium within which EBF1 regulates capillary branching and tuft development. Although the Podo-cre–mediated deletion of EBF1 has no changes in glomerular maturation, loss of EBF1 from the Foxd1 lineage recapitulated the entire phenotype of the global knockout mice.
Structural differences in peripheral versus juxtaglomerular nephrons and varied responses of the glomeruli attached to these nephrons to stress and compensatory nephron load have been noted for years.44 Glomerular hypertrophy stratifies through cortex depth for multiple CKD risk factors, including advanced age, increased body surface area, and race.45 Cortex location also shows stratification in laboratory models of renal injury and during development.46−48 We have demonstrated here that the importance of EBF1 in glomerular maturation has a similar effect, in that the formation of peripheral glomeruli is hypersensitive to the absence of this TF within the mesangial cells. Why these are more sensitive is unclear. All mesangial cells express EBF1, regardless of cortex depth. We have shown before that EBF2 is also expressed in kidney, although in a more ubiquitous pattern than EBF1.20 Because these proteins homo- and heterodimerize, it is possible that subtle changes in relative expression of the EBF family within the mesangial cells of varying cortex depth may possibly make the loss of EBF1 more detrimental to the cells of periphery. This explanation does not, however, explain why inhibition of COX-2 activity also displays a similar depth-dependent phenotype.
Apart from a few observations we do not know the identities of paracrine signals mesangial cells produce to direct capillary branching. Defects in Notch signaling result in failure of mesangial recruitment and glomeruli without a developed capillary bed displaying microaneurism.49−51 Likewise, mesangial deletion of ephrin-B2 arrests development at the single capillary loop stage.8 Although COX-2 appears to be central to the mechanism regulating glomerular capillary branching, the promoter for Ptgs2 does not appear to be a direct transcriptional target of EBF1. We have revealed here that NFAT-mediated transcription is inhibited in EBF1-deficient cells. We are currently interrogating the participants of calcium-calcineurin signaling that influence the ultimate activation of NFAT proteins in mesangial cells to identify the point(s) in signal transduction that is/are abrogated in our knockout cells, and identify the direct target(s) of EBF1 regulated in this process. Importantly, all the COX-2 inhibition studies that have been done and shown a similar glomerular phenotype have been either chemically induced or global hypomorphs/deletion models. None have traced these actions back to the stromal lineage cells specifically until now.
The more-mild phenotype of the Ptgs2fl/fl,Foxd1+ mice was unexpected. It may be that enough paracrine prostanoids are still produced in these animals to partially compensate for the targeted deletion. That, however, should also be true in the EBF1-deficient models, where restricted expression from this lineage is enough to inhibit glomerular development. It is also possible that this is a combinatory effect of multiple NFAT-regulated targets that are misregulated in addition to COX-2 that control the phenotype that has been observed. In the EBF1-deficient mice, NFAT signaling and prostanoid production are impeded, whereas in the Ptgs2fl/fl,Foxd1+ mice only prostanoid production was blocked. This may be why COX-2 overexpression partially corrected deletion of EBF1, but did not fully compensate for it; alternatively, we simply may not have increased prostanoid production to perfectly match the timing and expression that occurs during renal development. Parsing out of these potential mechanisms is the focus of our ongoing research.
It is worth note that progression of CKD is more rapid in Ebf1fl/fl,Foxd1+ mice than in global knockouts. One explanation for this might be a protective absence of B cells in the global knockouts arising from the essential role of EBF1 in lymphopoiesis. Alternately, there are several different cell types in the kidney that express EBF1, including the podocytes. It is possible that the absence of EBF1 in one of these other cell types is protective. Some evidence for a protective benefit of the loss of EBF family proteins in podocytes and the Drosophila nephrocyte has already been demonstrated in animals without EBF2 and Collier (the Drosophila homolog) expression.52 We have preliminary evidence to suggest the same is true with EBF1 deletion (data not shown). Clarifying this effect and understanding the mechanisms involved in this process is an area of ongoing investigation.
In summary, we have demonstrated that the absence of EBF1 from the stromal lineage of the kidney results in inhibition of glomerular tuft formation. The mesangial cells of these glomeruli are inhibited in their expression of the inducible cyclooxygenase COX-2. This occurs not through direct regulation of Ptgs2 by EBF1, but instead via a mechanism that involves reduced transcriptional activation of NFAT signaling in the absence of EBF1. These findings demonstrate a new way that direct signals from the mesangium directly control capillary tuft branching during renal development.
Disclosures
None.
Funding
Prof. Fretz is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant R00 DK093711 and the Yale Department of Orthopedics and Rehabilitation. The Yale O’Brien Center for Kidney Research is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant P30 DK079310-11.
Supplementary Material
Acknowledgments
The authors thank the Yale O’Brien Center for Kidney Research for their facilities and assistance in this project. We thank R. Grosschedl for sharing the Ebf1-KO and Ebf1-floxed mice, Shuta Ishibe for sharing the Podocin-cre animals, and Richard Flavell and H. Herschman for sharing the Ptgs2CR and Ptgs2-COE mice.
Prof. Fretz wrote the paper, designed, acquired, and interpreted most of the experiments, and generated the figures. T. Nelson maintained the mouse colony. Dr. Velazquez performed the GFR analysis. N. Troiano prepared the histological sections.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Mesangial Cells: The Tuft Guys of Glomerular Development,” on pages 1551–1553.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018070699/-/DCSupplemental.
Supplemental Figure 1. Proteinuria in Ebf1fl/fl,Podo+ mice.
Supplemental Figure 2. mT/mG reporter and the Foxd1 lineage in kidney and adipose.
References
- 1.Quaggin SE, Kreidberg JA: Development of the renal glomerulus: Good neighbors and good fences. Development 135: 609–620, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Abrahamson DR: Origin of the glomerular basement membrane visualized after in vivo labeling of laminin in newborn rat kidneys. J Cell Biol 100: 1988–2000, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lelongt B, Makino H, Kanwar YS: Maturation of the developing renal glomerulus with respect to basement membrane proteoglycans. Kidney Int 32: 498–506, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Sariola H: Incomplete fusion of the epithelial and endothelial basement membranes in interspecies hybrid glomeruli. Cell Differ 14: 189–195, 1984 [DOI] [PubMed] [Google Scholar]
- 5.Sariola H, Timpl R, von der Mark K, Mayne R, Fitch JM, Linsenmayer TF, et al.: Dual origin of glomerular basement membrane. Dev Biol 101: 86–96, 1984 [DOI] [PubMed] [Google Scholar]
- 6.Kikkawa Y, Virtanen I, Miner JH: Mesangial cells organize the glomerular capillaries by adhering to the G domain of laminin alpha5 in the glomerular basement membrane. J Cell Biol 161: 187–196, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindahl P, Hellström M, Kalén M, Karlsson L, Pekny M, Pekna M, et al.: Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development 125: 3313–3322, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, et al.: Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell 124: 161–173, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Quaggin SE, Schwartz L, Cui S, Igarashi P, Deimling J, Post M, et al.: The basic-helix-loop-helix protein pod1 is critically important for kidney and lung organogenesis. Development 126: 5771–5783, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Imaki J, Onodera H, Tsuchiya K, Imaki T, Mochizuki T, Mishima T, et al.: Developmental expression of maf-1 messenger ribonucleic acids in rat kidney by in situ hybridization histochemistry. Biochem Biophys Res Commun 272: 777–782, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Takemoto M, He L, Norlin J, Patrakka J, Xiao Z, Petrova T, et al.: Large-scale identification of genes implicated in kidney glomerulus development and function. EMBO J 25: 1160–1174, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liberg D, Sigvardsson M, Akerblad P: The EBF/Olf/Collier family of transcription factors: Regulators of differentiation in cells originating from all three embryonal germ layers. Mol Cell Biol 22: 8389–8397, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garel S, Garcia-Dominguez M, Charnay P: Control of the migratory pathway of facial branchiomotor neurones. Development 127: 5297–5307, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Wang MM, Reed RR: Molecular cloning of the olfactory neuronal transcription factor Olf-1 by genetic selection in yeast. Nature 364: 121–126, 1993 [DOI] [PubMed] [Google Scholar]
- 15.Wang SS, Tsai RY, Reed RR: The characterization of the Olf-1/EBF-like HLH transcription factor family: Implications in olfactory gene regulation and neuronal development. J Neurosci 17: 4149–4158, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akerblad P, Lind U, Liberg D, Bamberg K, Sigvardsson M: Early B-cell factor (O/E-1) is a promoter of adipogenesis and involved in control of genes important for terminal adipocyte differentiation. Mol Cell Biol 22: 8015–8025, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hesslein DG, Fretz JA, Xi Y, Nelson T, Zhou S, Lorenzo JA, et al.: Ebf1-dependent control of the osteoblast and adipocyte lineages. Bone 44: 537–546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jimenez MA, Akerblad P, Sigvardsson M, Rosen ED: Critical role for Ebf1 and Ebf2 in the adipogenic transcriptional cascade. Mol Cell Biol 27: 743–757, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagman J, Belanger C, Travis A, Turck CW, Grosschedl R: Cloning and functional characterization of early B-cell factor, a regulator of lymphocyte-specific gene expression. Genes Dev 7: 760–773, 1993 [DOI] [PubMed] [Google Scholar]
- 20.Fretz JA, Nelson T, Velazquez H, Xi Y, Moeckel GW, Horowitz MC: Early B-cell factor 1 is an essential transcription factor for postnatal glomerular maturation. Kidney Int 85: 1091–1102, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin H, Grosschedl R: Failure of B-cell differentiation in mice lacking the transcription factor EBF. Nature 376: 263–267, 1995 [DOI] [PubMed] [Google Scholar]
- 22.O’Riordan M, Grosschedl R: Coordinate regulation of B cell differentiation by the transcription factors EBF and E2A. Immunity 11: 21–31, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, et al.: Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35: 39–42, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ: Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis 33: 77–80, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Györy I, Boller S, Nechanitzky R, Mandel E, Pott S, Liu E, et al.: Transcription factor Ebf1 regulates differentiation stage-specific signaling, proliferation, and survival of B cells. Genes Dev 26: 668–682, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L: A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Kamei K, Ishikawa TO, Herschman HR: Transgenic mouse for conditional, tissue-specific Cox-2 overexpression. Genesis 44: 177–182, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Menè P, Stoppacciaro A: Isolation and propagation of glomerular mesangial cells. Methods Mol Biol 466: 3–17, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Clipstone NA, Crabtree GR: Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature 357: 695–697, 1992 [DOI] [PubMed] [Google Scholar]
- 31.Fretz JA, Shevde NK, Singh S, Darnay BG, Pike JW: Receptor activator of nuclear factor-kappaB ligand-induced nuclear factor of activated T cells (C1) autoregulates its own expression in osteoclasts and mediates the up-regulation of tartrate-resistant acid phosphatase. Mol Endocrinol 22: 737–750, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao H, Mejhert N, Fretz JA, Arner E, Lorente-Cebrián S, Ehrlund A, et al.: Early B cell factor 1 regulates adipocyte morphology and lipolysis in white adipose tissue. Cell Metab 19: 981–992, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Treiber T, Mandel EM, Pott S, Györy I, Firner S, Liu ET, et al.: Early B cell factor 1 regulates B cell gene networks by activation, repression, and transcription- independent poising of chromatin. Immunity 32: 714–725, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Singer MA, Morton AR: Mouse to elephant: Biological scaling and Kt/V. Am J Kidney Dis 35: 306–309, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Fretz JA, Nelson T, Xi Y, Adams DJ, Rosen CJ, Horowitz MC: Altered metabolism and lipodystrophy in the early B-cell factor 1-deficient mouse. Endocrinology 151: 1611–1621, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, et al.: Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, et al.: MMKD Study Group : Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: The Mild to Moderate Kidney Disease (MMKD) study. J Am Soc Nephrol 18: 2600–2608, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, et al.: Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature 378: 406–409, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Kömhoff M, Wang JL, Cheng HF, Langenbach R, McKanna JA, Harris RC, et al.: Cyclooxygenase-2-selective inhibitors impair glomerulogenesis and renal cortical development. Kidney Int 57: 414–422, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Norwood VF, Morham SG, Smithies O: Postnatal development and progression of renal dysplasia in cyclooxygenase-2 null mice. Kidney Int 58: 2291–2300, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Seta F, Chung AD, Turner PV, Mewburn JD, Yu Y, Funk CD: Renal and cardiovascular characterization of COX-2 knockdown mice. Am J Physiol Regul Integr Comp Physiol 296: R1751–R1760, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Iñiguez MA, Martinez-Martinez S, Punzón C, Redondo JM, Fresno M: An essential role of the nuclear factor of activated T cells in the regulation of the expression of the cyclooxygenase-2 gene in human T lymphocytes. J Biol Chem 275: 23627–23635, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Liu H, Ye W, Guan G, Dong Z, Jia Z, Yang T: Developmental regulation of calcineurin isoforms in the rodent kidney: Association with COX-2. Am J Physiol Renal Physiol 293: F1898–F1904, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Imai M, Yoshitomi K: Heterogeneity of the descending thin limb of Henle’s loop. Kidney Int 38: 687–694, 1990 [DOI] [PubMed] [Google Scholar]
- 45.Samuel T, Hoy WE, Douglas-Denton R, Hughson MD, Bertram JF: Determinants of glomerular volume in different cortical zones of the human kidney. J Am Soc Nephrol 16: 3102–3109, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Kim JJ, Li JJ, Jung DS, Kwak SJ, Ryu DR, Yoo TH, et al.: Differential expression of nephrin according to glomerular size in early diabetic kidney disease. J Am Soc Nephrol 18: 2303–2310, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Olliges A, Wimmer S, Nüsing RM: Defects in mouse nephrogenesis induced by selective and non-selective cyclooxygenase-2 inhibitors. Br J Pharmacol 163: 927–936, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki H, Tokuriki T, Saito K, Hishida A, Suzuki K: Glomerular hyperfiltration and hypertrophy in the rat hypoplastic kidney as a model of oligomeganephronic disease. Nephrol Dial Transplant 20: 1362–1369, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Boyle SC, Liu Z, Kopan R: Notch signaling is required for the formation of mesangial cells from a stromal mesenchyme precursor during kidney development. Development 141: 346–354, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu J, Valerius MT, Duah M, Staser K, Hansard JK, Guo JJ, et al.: Identification of molecular compartments and genetic circuitry in the developing mammalian kidney. Development 139: 1863–1873, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCright B, Gao X, Shen L, Lozier J, Lan Y, Maguire M, et al.: Defects in development of the kidney, heart and eye vasculature in mice homozygous for a hypomorphic Notch2 mutation. Development 128: 491–502, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Na J, Sweetwyne MT, Park AS, Susztak K, Cagan RL: Diet-induced podocyte dysfunction in drosophila and mammals. Cell Reports 12: 636–647, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.