Significance Statement
Patients with ESRD receiving chronic hemodialysis often have persistent predialysis hyperkalemia. In the DIALIZE randomized study, the authors evaluated treatment with the potassium binder sodium zirconium cyclosilicate versus placebo in managing hyperkalemia in such patients. The authors report that compared with placebo, sodium zirconium cyclosilicate (SZC) significantly increased the proportion of patients who maintained predialysis serum potassium 4.0–5.0 mmol/L during ≥3 of 4 HD treatments following the long interdialytic interval and who did not require urgent rescue therapy. Sodium zirconium cyclosilicate was well tolerated: the two groups had comparable proportions of patients experiencing serious adverse events, comparable interdialytic weight gain, and few episodes of hypokalemia. The results indicate that sodium zirconium cyclosilicate is an option for the management of hyperkalemia in this setting.
Keywords: end-stage renal disease, hemodialysis, clinical trial
Visual Abstract
Abstract
Background
Patients with ESRD have minimal renal potassium excretion and, despite hemodialysis, often have persistent predialysis hyperkalemia. The DIALIZE study (NCT03303521) evaluated sodium zirconium cyclosilicate (SZC) in the management of hyperkalemia in hemodialysis patients.
Methods
In the DIALIZE study, a double-blind, placebo-controlled, phase 3b multicenter study, we randomized adults with ESRD who were managed by three-times weekly hemodialysis and had predialysis hyperkalemia to receive placebo or SZC 5 g once daily on non-dialysis days, and titrated towards maintaining normokalemia over 4 weeks, in 5 g increments to a maximum of 15 g. The primary efficacy outcome was proportion of patients during the 4-week stable-dose evaluation period who maintained predialysis serum potassium of 4.0–5.0 mmol/L during at least three of four hemodialysis treatments after the long interdialytic interval and did not require urgent rescue therapy to reduce serum potassium.
Results
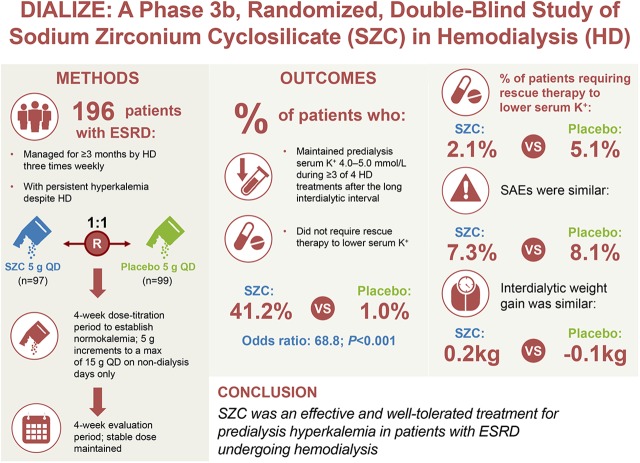
In total, 196 patients (mean [standard deviation (SD)] age =58.1 [13.7] years old) were randomized to sodium zirconium cyclosilicate or placebo. Of 97 patients receiving sodium zirconium cyclosilicate, 41.2% met the primary end point and were deemed treatment responders compared with 1.0% of 99 patients receiving placebo (P<0.001). Rescue therapy to reduce serum potassium during the treatment period was required by 2.1% of patients taking sodium zirconium cyclosilicate versus 5.1% taking placebo. Serious adverse events occurred in 7% and 8% of patients in sodium zirconium cyclosilicate and placebo groups, respectively. The two groups displayed comparable interdialytic weight gain. There were few episodes of hypokalemia.
Conclusions
Sodium zirconium cyclosilicate is an effective and well-tolerated treatment for predialysis hyperkalemia in patients with ESRD undergoing adequate hemodialysis.
Patients with ESRD have severely reduced renal potassium excretion and require hemodialysis (HD) and peritoneal dialysis to maintain normal serum potassium (sK+). Despite dialysis, many patients have persistent predialysis hyperkalemia,1,2 a potentially life-threatening condition that is associated with cardiac arrhythmias and death:3,4 an sK+ concentration of ≥5.6 mmol/L is associated with increased all-cause and cardiac death.1,2 Moreover, the unadjusted hazard ratio between different sK+ categories is U shaped, with the best survival in patients with sK+ concentrations of 4.6–5.6 mmol/L.1 Indeed, in patients with ESRD receiving HD, arrhythmias and sudden cardiac death are leading causes of death.5,6 Of note, rates of hospitalization, all-cause death, and cardiac death are higher after the long interdialytic interval, with hyperkalemia being a potential contributing factor.7,8
Patients with ESRD managed by HD depend on additional strategies to treat hyperkalemia. Therapeutic options for the treatment of hyperkalemia include potassium binding resins, such as sodium polystyrene sulfonate (SPS), patiromer, and sodium zirconium cyclosilicate (SZC; AstraZeneca AB, Södertälje, Sweden).9–11 The efficacy and safety of SPS have been questioned,9,12,13 and SPS and patiromer are limited by a lack of evidence from randomized, controlled trials in patients undergoing HD. Therefore, there is a need for novel treatments that correct hyperkalemia and maintain sK+ within a safe range in patients with ESRD who have persistent hyperkalemia despite HD.
SZC is an orally administered, insoluble, nonabsorbed, inorganic crystalline compound.14 SZC selectively captures potassium ions in exchange for hydrogen and sodium ions in the gastrointestinal lumen, thereby reducing the free concentration of sK+ and increasing potassium fecal excretion to resolve hyperkalemia.14–16 The efficacy and safety of SZC for the treatment of hyperkalemia have been demonstrated in phase 2 and 3 clinical trials of nondialysis populations.15–18 SZC is approved in the United States and the European Union for the treatment of hyperkalemia in adults.19,20
SZC has not been studied in patients receiving HD treatment. Here, we report the findings from a phase 3b study conducted to evaluate the efficacy and safety of SZC in stable patients with ESRD managed by adequate HD.
Methods
Study Design
The DIALIZE study (NCT03303521) is a randomized, double-blind, placebo (PBO)-controlled, phase 3b study conducted at 54 sites across Japan, Russia, the United States, and the United Kingdom between December 14, 2017 and November 7, 2018. The study was performed in accordance with the Declaration of Helsinki, the International Council for Harmonisation, and Good Clinical Practice. The informed consent form, protocol, and amendments were approved by an independent ethics committee or institutional review board for each center before study initiation. All participants provided written informed consent.
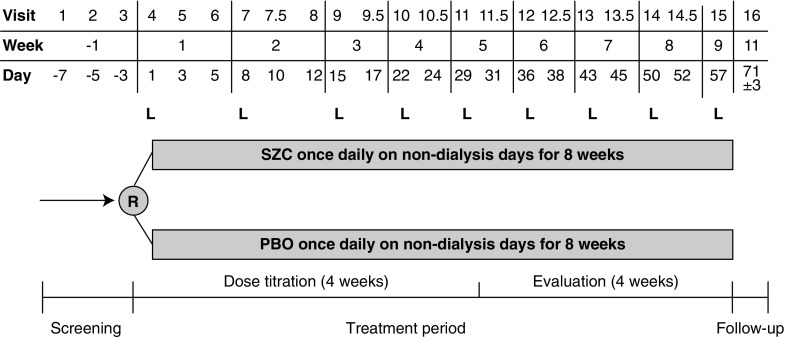
The study included a 1-week screening period, an 8-week treatment period (4 weeks for dose titration and 4 weeks for evaluation on stable dose), and a 2-week (±3 days) follow-up period, ending with a final visit (Figure 1). Screening began on the day of HD after the long interdialytic interval (day –7), included the HD treatments after the two short interdialytic intervals on days –5 and –3, and was completed on the day before randomization. In the treatment period, patients were randomized 1:1 to receive orally a starting dose of SZC 5 g or PBO once daily on nondialysis days. Details of study treatments, randomization, blinding, and dose titration are provided in Supplemental Material. During dose titration, the doses of SZC and PBO were adjusted weekly over a 4-week period to attain normokalemia, defined as achieving and maintaining a predialysis sK+ concentration of 4.0–5.0 mmol/L after the long interdialytic interval. Doses were titrated in 5-g increments to a maximum dose of 15 g once daily on nondialysis days. Treatment was maintained at a stable dose during the 4-week evaluation period.
Figure 1.
The DIALIZE study design. L, visit after the long interdialytic interval; PBO, placebo; R, randomization; SZC, sodium zirconium cyclosilicate.
Patients
Eligible patients were men or women aged ≥18 years old with ESRD managed for ≥3 months before randomization by HD three times weekly. During the 1-week screening period, patients were required to have persistent hyperkalemia despite adequate HD, which was defined as predialysis sK+ >5.4 mmol/L after the long interdialytic interval on day –7, as well as predialysis sK+ >5.0 mmol/L after at least one short interdialytic interval on days –5 and –3. Exclusion criteria included hemoglobin <9 g/dL at screening; noncompliance with HD prescription during the 2 weeks before screening; treatment with SPS, calcium polystyrene sulfonate (Calcium Resonium®), or patiromer within 7 days before screening; myocardial infarction, acute coronary syndrome, stroke, seizure, or a thrombotic/thromboembolic event within 12 weeks before randomization; or pregnancy or breastfeeding. Full inclusion and exclusion criteria are provided in Supplemental Material.
Study Assessments
During screening, consenting patients were assessed to ensure that eligibility criteria were met. sK+ concentrations were measured using central laboratory assessment and a point-of-care i-STAT device (Abbott Point of Care, Inc., Princeton, NJ), which has been shown to produce accurate, reliable, and robust measurements.21,22 Local assessment of sK+ using the i-STAT device was used for dose titration made at weekly intervals predialysis after the long interdialytic interval. Central laboratory sK+ samples were obtained throughout the study (both pre- and postdialysis at visits after the long interdialytic interval and only predialysis for visits after the short interdialytic interval). The dialysate potassium (dK+) prescription was recorded at randomization and weekly intervals. Safety assessments included the recording of adverse events (AEs), physical examination, including assessment of interdialytic weight gain (IDWG), vital signs, laboratory safety measures, and predialysis electrocardiogram (ECG). Full details of the study assessments are provided in Supplemental Material.
Study End Points
The primary efficacy outcome was the proportion of patients classified as “responders,” defined as those who, during the 4-week evaluation period, maintained sK+ of 4.0–5.0 mmol/L during three or more of four HD treatments after the long interdialytic interval and who did not require rescue therapy. Rescue therapy was defined as any urgent therapeutic intervention considered necessary to reduce sK+ in the setting of severe hyperkalemia (defined by protocol as sK+ >6.0 mmol/L). Use of rescue therapy was not strictly protocolized, and it was left to the investigator’s clinical judgment to be given in accordance with local practice patterns. Rescue therapies included but were not restricted to SPS, calcium polystyrene sulfonate, patiromer, β-adrenergic agonists, sodium bicarbonate, insulin/glucose, and any additional dialysis or other forms of RRT when used specifically to treat severe hyperkalemia. Any reduction in the dK+ concentration that was prescribed for the treatment of severe hyperkalemia was also considered rescue therapy. No clinically justified therapy for severe acute hyperkalemia was withheld. Any rescue therapy was followed by SZC dose adjustment if appropriate and documentation of the event. Patients with more than one missing sK+ measurement during the evaluation period or those who received rescue therapy were classified as nonresponders. This composite outcome allowed for the assessment of the proportion of patients who achieved and maintained normokalemia at the majority of study visits without the need for rescue therapy.
The secondary efficacy outcome was the proportion of patients requiring any urgent rescue intervention to reduce sK+ in the setting of severe hyperkalemia (>6.0 mmol/L). The proportion of patients who increased dK+ concentration at end of treatment (EOT) compared with baseline was an explorative efficacy outcome (Supplemental Material). Additional efficacy measurements included the intradialytic potassium shift, defined as the difference between pre- and postdialysis sK+, and the dialysis potassium gradient, defined as the difference between the predialysis sK+ and dK+ concentrations.
Safety outcomes included assessment of AEs, laboratory parameters/vital signs, ECG, and IDWG (Supplemental Material).
Statistical Analyses
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC). The target sample size for the primary efficacy end point was 180 patients (90 patients each for the SZC and PBO groups). This would achieve a power of ≥90% assuming that ≤30% of the PBO group would be responders, with a difference in the proportion of PBO and SZC responders of 25% using a two-sided Fisher's exact test at a significance level of 5%.
Efficacy analyses were conducted on the full analysis set (all randomized patients, regardless of whether they received any trial medication or not). For the primary end point analysis, the difference in the likelihood of being a responder between the two treatment groups was analyzed using a two-sided Fisher's exact test at 5% significance level. The odds ratio (OR) between the SZC and PBO groups was estimated and presented with corresponding 95% confidence intervals (95% CIs).
A sensitivity analysis was conducted to determine the effect of being classified as a nonresponder due to missing sK+ data. Missing central laboratory sK+ measurements were replaced using available i-STAT data adjusted for the mean paired difference between the values in patients with both values available at the relevant time point. The last observation carried forward method was used to impute the remaining missing values.
The proportion of patients requiring rescue therapy was reported descriptively. For the proportion of patients who were able to increase dK+ concentration, the difference in the likelihood of being a responder was calculated. Missing dK+ values were imputed according to the last observation carried forward method. Patients with no dK+ concentration registered on any long interdialytic interval visits starting with visit 4 were excluded from the analysis. There were no adjustments for multiple hypothesis testing, and therefore, the P value from this analysis was descriptive. Intradialytic potassium shift and dialysis potassium gradient were summarized by treatment group and visit.
All safety analyses were conducted on the safety analysis set (all randomized patients who received one or more doses), and they were reported descriptively. IDWG was summarized alongside change from baseline and presented by visit and treatment group.
Results
Patient Disposition and Baseline Characteristics
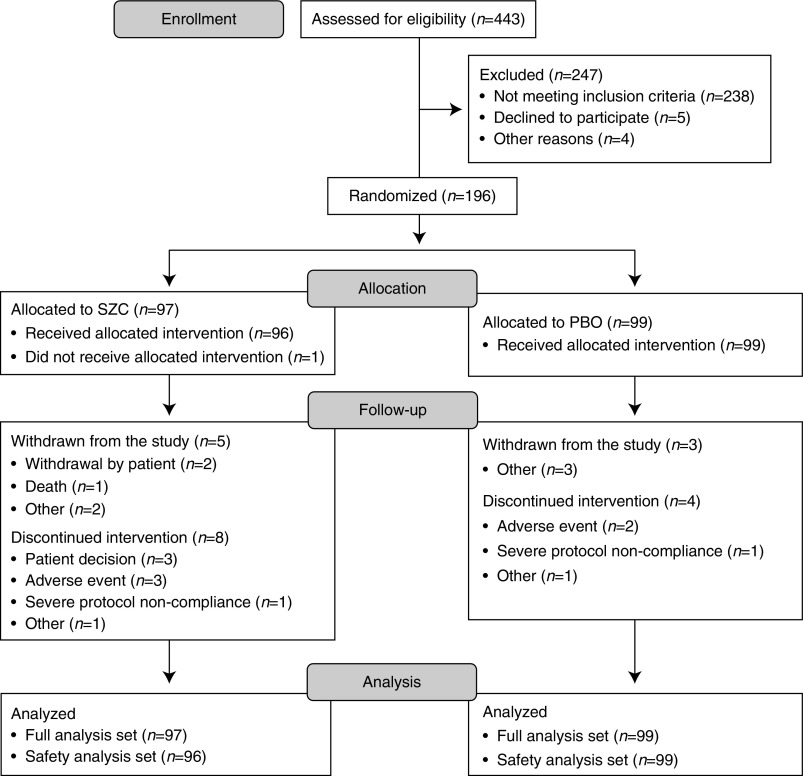
A total of 443 patients were screened, and 247 were excluded (Figure 2); patients were predominantly excluded for not meeting the inclusion criteria (Supplemental Table 1). Overall, 97 patients were randomized to receive SZC, and 99 patients were randomized to receive PBO. All randomized patients except for one patient in the SZC group received treatment (99.5% [n=195 of 196]). In total, 95.9% of patients (n=188 of 196) completed the study. Rates of study completion were balanced between treatment groups (SZC, 94.8% [n=92 of 97]; PBO, 97.0% [n=96 of 99]).
Figure 2.
Flow diagram of patient disposition. “Other” reasons for withdrawal from study for sodium zirconium cyclosilicate (SZC): adverse event (n=1) and transferred to a nonstudy dialysis center (n=1). “Other” reasons for withdrawal from study for placebo (PBO): kidney transplantation (n=1), discontinuation of investigational product (n=1), and noncompliance to study protocol (n=1). “Other” reasons for discontinuation of intervention for SZC: transferred to a nonstudy dialysis center (n=1). “Other” reasons for discontinuation of intervention for PBO: patient hospitalized due to nontreatment-related serious adverse event and was unable to receive treatment during hospitalization and until end of treatment visit (n=1).
Baseline patient characteristics are reported in Table 1. Overall, 58.7% of patients were men, mean (SD) age was 58.1 (13.7) years old, and mean weight was 71.0 (19.1) kg. Most patients were white (52.0%), Asian (33.7%), or black or African American (9.7%). Patient characteristics were generally balanced between treatment groups, except for a small difference in age distribution, such that patients in the SZC group had a younger age compared with the PBO group (mean age [SD] =55.7 [13.8] versus 60.4 [13.2] years old, respectively) (Table 1). Dialysis adequacy parameters were comparable between treatment groups (Table 1). Medical history is provided in Supplemental Material.
Table 1.
Baseline patient characteristics (full analysis set)
| Summary Statistic | SZC, n=97 | PBO, n=99 | Total, n=196 |
|---|---|---|---|
| Demographics/baseline characteristic | |||
| Age, yr | |||
| n | 97 | 99 | 196 |
| Mean | 55.7 | 60.4 | 58.1 |
| SD | 13.8 | 13.2 | 13.7 |
| Age group, yr, n (%) | |||
| 18–50 | 31 (32.0) | 21 (21.2) | 52 (26.5) |
| 51–64 | 34 (35.1) | 32 (32.3) | 66 (33.7) |
| 65–84 | 32 (33.0) | 45 (45.5) | 77 (39.3) |
| ≥85 | 0 (0.0) | 1 (1.0) | 1 (0.5) |
| Total | 97 (100.0) | 99 (100.0) | 196 (100.0) |
| Sex, n (%) | |||
| Men | 57 (58.8) | 58 (58.6) | 115 (58.7) |
| Women | 40 (41.2) | 41 (41.4) | 81 (41.3) |
| Race, n (%) | |||
| White | 50 (51.5) | 52 (52.5) | 102 (52.0) |
| Black or African American | 11 (11.3) | 8 (8.1) | 19 (9.7) |
| Asian | 33 (34.0) | 33 (33.3) | 66 (33.7) |
| American Indian or Alaska Native | 1 (1.0) | 2 (2.0) | 3 (1.5) |
| Other | 2 (2.1) | 4 (4.0) | 6 (3.1) |
| Height, cm | |||
| n | 97 | 99 | 196 |
| Mean | 166.4 | 165.1 | 165.7 |
| SD | 9.9 | 9.2 | 9.5 |
| Weight, kg | |||
| n | 97 | 99 | 196 |
| Mean | 72.0 | 70.0 | 71.0 |
| SD | 22.0 | 15.9 | 19.1 |
| BMI, kg/m2 | |||
| n | 97 | 99 | 196 |
| Mean | 26.9 | 26.7 | 26.8 |
| SD | 7.1 | 5.4 | 6.3 |
| Dialysis history | |||
| Vintage, yr | |||
| n | 92 | 97 | 189 |
| Mean | 8.0 | 7.8 | 7.9 |
| SD | 6.1 | 7.6 | 6.9 |
| Access type, n (%) | |||
| Arteriovenous fistula | 84 (87.5) | 90 (90.9) | 174 (89.2) |
| Arteriovenous graft | 7 (7.3) | 3 (3.0) | 10 (5.1) |
| Tunneled central venous catheter | 4 (4.2) | 6 (6.1) | 10 (5.1) |
| Other | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Total | 96 (100.0) | 99 (100.0) | 195 (100.0) |
| Dialysis parameters | |||
| spKt/V | |||
| n | 74 | 71 | — |
| Mean | 1.7 | 1.7 | — |
| SD | 0.3 | 0.4 | — |
| Urea removal rate, % | |||
| n | 52 | 58 | — |
| Mean | 72.9 | 74.6 | — |
| SD | 6.7 | 5.6 | — |
| Dialysate flow, mL/min | |||
| n | 96 | 99 | — |
| Mean | 512.0 | 538.5 | — |
| SD | 162.8 | 136.0 | — |
| dK+ concentration, mmol/L | |||
| n | 96 | 99 | — |
| Mean | 2.3 | 2.3 | — |
| SD | 0.5 | 0.5 | — |
| Minimum, maximum | 1.0, 3.0 | 1.0, 3.0 | — |
| Blood flow, mL/min | |||
| n | 96 | 99 | — |
| Mean | 322.0 | 318.5 | — |
| SD | 110.7 | 96.3 | — |
SZC, sodium zirconium cyclosilicate; PBO, placebo; BMI, body mass index; spKt/V, single-pool Kt/V; dK+, dialysate potassium.
After the dose titration period, 37%, 43%, and 19% of patients in the SZC group received SZC 5, 10, and 15 g, respectively, and 8%, 8%, and 83% of patients in the PBO group received PBO 5, 10, and 15 g, respectively. During the overall treatment period, the mean (SD) rate of patient compliance with treatment was high (98.7% [5.2]) and balanced between the treatment groups (SZC, 98.9% [4.1]; PBO, 98.4% [6.1]). The mean rate of patient compliance with treatment during the evaluation period was high (99.0% [4.7]) and balanced between treatment groups (SZC, 98.6% [6.0]; PBO, 99.4% [3.1]).
Primary Efficacy Outcome: Proportion of Responders
There was a significantly higher proportion of responders (i.e., those who, during the evaluation period, maintained predialysis sK+ of 4.0–5.0 mmol/L during three or more of four HD treatments after the long interdialytic interval and who did not require urgent rescue therapy) in the SZC group compared with the PBO group: 41.2% (n=40 of 97) versus 1.0% (n=1 of 99; OR, 68.8; 95% CI, 10.9 to 2810.9; P<0.001) (Supplemental Figure 1A). A sensitivity analysis was performed to determine the effect of being deemed a nonresponder due to missing central laboratory assessment by using adjusted i-STAT sK+ data. Findings from the sensitivity analysis were consistent with those from the primary analysis, with there being a higher proportion of responders in the SZC group than the PBO group: 42.3% (n=41 of 97) versus 2.0% (n=2 of 99; OR, 35.5; 95% CI, 8.5 to 309.5; P<0.001) (Supplemental Figure 1B).
All nonresponders had fewer than three sK+ measurements of 4.0–5.0 mmol/L (Supplemental Table 2). Findings from a post hoc analysis that considered the number of occurrences where sK+ was reduced to the 3.5–5.5-mmol/L range (i.e., levels reported to have a lower cardiovascular risk than more severe hyperkalemia) for each patient during the evaluation period showed that the majority of nonresponders receiving SZC achieved clinically meaningful decreases in sK+ during the evaluation period despite not meeting the full responder criteria (Supplemental Table 3).
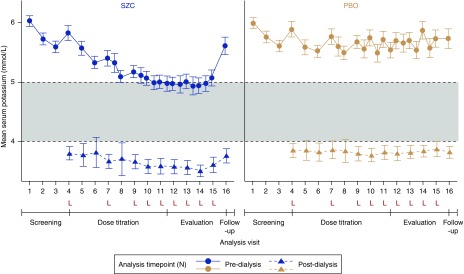
Pre- and postdialysis mean sK+ values were comparable at baseline in the two treatment groups: predialysis mean sK+ value for SZC of 5.8 (0.6) mmol/L compared with PBO of 5.9 (0.6) mmol/L and postdialysis mean sK+ value for SZC of 3.8 (0.6) mmol/L compared with PBO of 3.9 (0.6) mmol/L (visit 4). Mean pre- and postdialysis sK+ values were lower with SZC than with PBO both at the end of the titration period and during the evaluation period (Figure 3, Supplemental Table 4). In a post hoc analysis, there were fewer patients in the SZC group with a maximum sK+ concentration ≥6.0 mmol/L after the long interdialytic intervals during the evaluation period compared with in the PBO group (Supplemental Table 5).
Figure 3.
Mean pre- and postdialysis sK+ values were lower with SZC than with PBO both at the end of the titration period and during the evaluation period. Postdialysis measurements after the short interdialytic interval were obtained only for some of the patients, because these were scheduled according to an earlier version of the study protocol. Dashed horizontal lines represent the upper and lower limits of normal predialysis sK+. Error bars indicate 2×SD of the mean. Predialysis mean (SD) sK+ was comparable between treatment groups at randomization (visit 4): SZC, 5.8 (0.6) mmol/L; PBO, 5.9 (0.6) mmol/L. Postdialysis mean (SD) sK+ at randomization was also comparable in each treatment group: SZC, 3.8 (0.6) mmol/L; PBO, 3.9 (0.6) mmol/L. L, visit after the long interdialytic interval.
Secondary Efficacy Outcome: Rescue Therapy
Comparable and low proportions of patients in the SZC group (2.1% [n=2 of 97]) and the PBO group (5.1% [n=5 of 99]) needed rescue therapy to reduce sK+ during the overall treatment period (Table 2).
Table 2.
Patients needing rescue therapy (full analysis set)
| Study Perioda and Patients Requiring Rescue Therapyb | Patients, n (%) | ||
|---|---|---|---|
| SZC, n=97 | PBO, n=99 | Total, n=196 | |
| Treatment (overall) | |||
| Patients requiring rescue therapy | 2 (2.1) | 5 (5.1) | 7 (3.6) |
| Sodium polystyrene sulfonate | 0 (0.0) | 3 (3.0) | 3 (1.5) |
| Calcium polystyrene sulfonate | 1 (1.0) | 1 (1.0) | 2 (1.0) |
| Dialysis | 1 (1.0) | 1 (1.0) | 2 (1.0) |
| Calcium gluconate | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Insulin | 1 (1.0) | 1 (1.0) | 2 (1.0) |
| Furosemide | 0 (0.0) | 1 (1.0) | 1 (0.5) |
| Salbutamol | 0 (0.0) | 1 (1.0) | 1 (0.5) |
| Dose titration | |||
| Patients requiring rescue therapy | 1 (1.0) | 5 (5.1) | 6 (3.1) |
| Sodium polystyrene sulfonate | 0 (0.0) | 3 (3.0) | 3 (1.5) |
| Dialysis | 1 (1.0) | 1 (1.0) | 2 (1.0) |
| Calcium gluconate | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Insulin | 1 (1.0) | 1 (1.0) | 2 (1.0) |
| Calcium polystyrene sulfonate | 0 (0.0) | 1 (1.0) | 1 (0.5) |
| Furosemide | 0 (0.0) | 1 (1.0) | 1 (0.5) |
| Salbutamol | 0 (0.0) | 1 (1.0) | 1 (0.5) |
| Evaluation | |||
| Patients requiring rescue therapy | 1 (1.0) | 1 (1.0) | 2 (1.0) |
| Calcium polystyrene sulfonate | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Sodium polystyrene sulfonate | 0 (0.0) | 1 (1.0) | 1 (0.5) |
| Follow-up | |||
| Patients requiring rescue therapy | 0 (0.0) | 1 (1.0) | 1 (0.5) |
| Calcium gluconate | 0 (0.0) | 1 (1.0) | 1 (0.5) |
| Dialysis | 0 (0.0) | 1 (1.0) | 1 (0.5) |
SZC, sodium zirconium cyclosilicate; PBO, placebo.
Study period is defined using the date of the adverse event requiring rescue therapy.
Rescue therapy was defined as any urgent therapeutic intervention considered necessary to reduce serum potassium (sK+) in the setting of severe hyperkalemia (defined by protocol as sK+>6.0 mmol/L). Use of rescue therapy was not strictly protocolized, and it was left to the investigator’s clinical judgment to be given in accordance with local practice patterns.
Exploratory Efficacy Outcome
At baseline, mean (SD) dK+ concentration was comparable in the two treatment groups: that is, each was 2.3 (0.5) mmol/L (minimum, maximum, 1.0, 3.0). At visit 15 (EOT), mean (SD; minimum, maximum) dK+ concentrations were 2.3 (0.5; 1.2, 3.0) mmol/L with SZC and 2.3 (0.5; 1.0, 3.0) mmol/L with PBO. Of 181 patients, three (1.7%) had an increase in their dK+ concentration at visit 15 (EOT) compared with visit 1, with no significant differences between treatment groups: SZC, 2.3% (n=2 of 87) versus PBO, 1.1% (n=1 of 94; OR, 2.2; 95% CI, 0.1 to 130.5; P=0.61).
Additional Efficacy Analyses
At visit 4 (randomization), mean (SD) intradialytic potassium shift values (defined as the difference between pre- and postdialysis sK+) were comparable in each treatment group (SZC, 2.0 [0.7] mmol/L; PBO, 2.1 [0.7] mmol/L). Between visits 9 and 15 (EOT), mean intradialytic potassium shift values had reduced in the SZC group (range, 1.4–1.5 mmol/L) to a greater extent than in the PBO group (range, 1.9–2.0 mmol/L) (Supplemental Table 6).
Mean (SD) dialysis potassium gradient (defined as the difference between predialysis sK+ and dK+ concentrations) was comparable in each treatment group at visit 4 (randomization): SZC, 3.6 (0.7) mmol/L; PBO, 3.7 (0.6) mmol/L. Between visits 8 and 15 (EOT), mean dialysis potassium gradient values had reduced in the SZC group (range, 2.7–2.9 mmol/L) to a greater extent than in the PBO group (range, 3.3–3.6 mmol/L) (Supplemental Table 7).
Safety
AEs are summarized in Table 3. Overall, 40 patients (41.7%) had an AE in the SZC group, and 46 patients (46.5%) had an AE in the PBO group. Most AEs reported were mild or moderate in intensity. The most common AEs by system organ class were gastrointestinal disorders, with 19 patients (19.8%) in the SZC group and 17 patients (17.2%) in the PBO group. Infections were reported in 12 patients (12.5%) in the SZC group and nine patients (9.1%) in the PBO group.
Table 3.
Summary of adverse events (safety analysis set)
| AE | No. (%) of Patients | |
|---|---|---|
| SZC, n=97 | PBO, n=99 | |
| Any AE | 40 (41.7) | 46 (46.5) |
| Any serious AEa | 7 (7.3) | 8 (8.1) |
| AE leading to discontinuation of treatment | 4 (4.2) | 2 (2.0) |
| Death | 1 (1.0) | 0 (0.0) |
| AEs in >2% patients | ||
| Constipation | 4 (4.2) | 3 (3.0) |
| Diarrhea | 4 (4.2) | 6 (6.1) |
| Headache | 3 (3.1) | 2 (2.0) |
| Nasopharyngitis | 3 (3.1) | 5 (5.1) |
| Hyperkalemia | 2 (2.1) | 6 (6.1) |
| Hordeolum (stye) | 2 (2.1) | 0 (0.0) |
| Muscle spasms | 2 (2.1) | 2 (2.0) |
| Dizziness | 1 (1.0) | 4 (4.0) |
| Dyspnea | 1 (1.0) | 3 (3.0) |
| Pruritus | 1 (1.0) | 3 (3.0) |
| Shunt stenosis | 1 (1.0) | 3 (3.0) |
AE, adverse event; SZC, sodium zirconium cyclosilicate; PBO, placebo.
Including events with death as the outcome.
Serious adverse events (SAEs) occurred in 7.3% of patients in the SZC group and 8.1% of patients in the PBO group. The most common SAE in the SZC group was angina pectoris (n=2 [2.1%]). In the PBO group, the most common SAEs were hyperkalemia requiring rescue therapy (n=3 [3.0%]) and fluid overload (n=2 [2.0%]). All SAEs were considered not related to the study drug by the investigator. One patient who received SZC had an SAE of peripheral arterial occlusive disease that led to death during the study. The SAE started 69 days after the first dose of SZC and 22 days after the last dose. This patient had a concomitant SAE of gangrene of the leg and feet starting 53 days after the first dose of SZC and 6 days after the last dose. The SAE was judged by the investigator as not related to SZC.
Four patients (4.2%) in the SZC group and two patients (2.0%) in the PBO group discontinued the study due to AEs. One event of hyperkalemia in the SZC group led to treatment discontinuation; the AE was considered unlikely related to study drug by the investigator.
There were few notable imbalances in treatment-emergent changes in laboratory values. A higher proportion of patients with low aspartate aminotransferase values was observed in the SZC group compared with the PBO group (40.3% versus 24.6%), and numerically higher bicarbonate values were observed at EOT in the SZC group compared with the PBO group (mean [SD] =20.4 [2.7] versus 19.6 [2.8] mmol/L); however, mean changes from baseline were small in both groups, and variability was high. There were no clinically meaningful changes in heart rate or BP as well as no clinically significantly abnormal ECG results. Change from baseline in IDWG at visit 15 (EOT) was comparable between the SZC and PBO groups (Supplemental Table 8).
There were ten patients with predialysis hypokalemia (defined as sK+<3.5 mmol/L), with five in each treatment group.
Discussion
SZC is a new potassium binder that has been shown to effectively reduce sK+ for up to 1 year in patients with hyperkalemia, including those with CKD.15–20 However, there has been no study specifically in patients on HD to assess the efficacy of SZC on maintaining normal sK+. The DIALIZE study is the first randomized, double-blind, PBO-controlled, phase 3b study to determine the efficacy and safety of a potassium binder in this population (i.e., patients who had persistent hyperkalemia [predialysis sK+ >5.4 mmol/L after the long interdialytic interval] while receiving adequate HD for the management of ESRD). This study demonstrated that SZC is effective in reducing sK+ in patients on HD.
Our study met its primary efficacy end point as demonstrated by a significantly higher proportion of patients in the SZC group who maintained normokalemia (defined as sK+=4.0–5.0 mmol/L) during three or more of four HD treatments after the long interdialytic interval and who did not require rescue therapy to reduce sK+. Rates of nonresponders according to the prespecified criteria were driven by patients having fewer than three predialysis sK+ measurements of 4.0–5.0 mmol/L. However, results of a post hoc analysis of the primary efficacy outcome suggested that use of less stringent responder criteria may have led to even higher response rates that remained clinically meaningful (i.e., the majority of patients had sK+ values below the threshold of ≥5.6 mmol/L associated with increased risk of cardiovascular death1,2). Findings on the basis of mean predialysis sK+ showed that SZC effectively normalizes hyperkalemia in this population. After completing dose titration, the sK+ concentrations of responders, on average, remained controlled throughout the evaluation period on a fixed dose of SZC.
Few patients required rescue therapy during the study. The overall low use of rescue therapy may be attributable to hyperkalemia being managed via HD, the perception that patients undergoing HD have a higher tolerance to high sK+, and the role of rescue medication as an intervention primarily confined to the emergency department.
Few patients in either treatment group had an increase in their dK+ concentration. This finding may be a consequence of changes in dK+ concentration being on the basis of physician’s choice and local practice instead of being mandated in the study protocol and the infrequent (monthly) review of HD prescription. Studies have identified low dK+ (<2 and <3 mmol/L) as being associated with death and sudden cardiac arrest.1,23–27 Very low dK+ is associated with a larger removal of potassium during HD, causing intradialytic and postdialysis hypokalemia. Therefore, avoiding dK+ concentrations <2 mmol/L is supported to prevent a rapid decrease in sK+, particularly in patients with high predialysis sK+.27,28
Reductions were observed in the dialysis potassium gradient (defined as sK+ compared with dK+) with SZC compared with PBO. High dialysis potassium gradient is associated with cardiac arrhythmia, all-cause hospitalization and emergency department visit, and as a result, minimizing the dialysis potassium gradient is recommended.1,29–31 The correction and maintenance of sK+ in a safe range as well as the reduction in dialysis potassium gradient observed with SZC in this study may allow higher dK+ concentration, lower the risk of cardiac arrhythmia, and allow easing of restrictions in quality of life, such as dietary restriction of potassium-containing foods.
Patient baseline demographics were generally balanced between the two treatment groups. A small difference in age was observed between treatment groups; however, this was not expected to have any important clinical implications that could influence interpretation of the findings.
SZC had an acceptable safety profile in patients with HD-managed ESRD. The incidence of gastrointestinal AEs was comparable between treatment groups and is typical of this patient population. Furthermore, the safety profile of SZC in this study was similar to that seen in the nondialysis population described previously,15–18 and treatment with SZC raised no safety concerns. IDWG is predominantly the result of salt and water intake between dialysis sessions.32 In our study, IDWG was comparable between treatment groups, suggesting that there was no difference in fluid retention with SZC.
This study was of relatively short duration. Additional studies are required to determine the long-term efficacy and safety of SZC in patients receiving chronic HD, although previous studies in the nondialysis population have demonstrated that SZC effectively reduces sK+ for up to 1 year in patients with hyperkalemia, including those with CKD.15–20 Treatment compliance in our study was high; however, in a real world setting, patients with the highest sK+ are often the least compliant to treatment,33 and therefore, our findings may not be generalizable to all patients. This study also has a number of strengths. The 4-week dose titration period enabled the SZC dose to be adjusted weekly on the basis of measured sK+. After the dose titration period, treatment was maintained at a stable dose during the 4-week evaluation period, which is consistent with clinical practice in which once monthly monitoring is the routine standard of care. Finally, the primary efficacy outcome was designed to measure predialysis sK+ after the long interdialytic interval (i.e., the point in which patients have been shown to have a heightened risk).7,8
Potential areas of future research in this setting include assessment of the effect of SZC on clinical outcomes in patients on HD. This study demonstrates the ability of SZC to correct a biochemical end point (i.e., hyperkalemia), but new studies could further determine the effect on patient-level outcomes, such as hospitalization, cardiac arrhythmia, and/or other cardiovascular events. In addition, such studies could also further elucidate the ability of SZC to increase dK+ concentration and if this will possibly reduce variation in dK+ supplementation used in clinical practice. Finally, strategies could also be assessed toward liberalizing diet to include healthy and enjoyable foods that are also potassium rich.
In conclusion, in the phase 3b DIALIZE study, SZC was effective in reducing sK+ in patients on HD. The safety profile of SZC observed in patients managed by HD was similar to that known in the nondialysis population and raised no new concerns. The results indicate that SZC is an option for the management of hyperkalemia in this setting.
Disclosures
Dr. Fishbane received research support and consulting fees from AstraZeneca. Dr. Ford received travel support from Amgen and AstraZeneca. Dr. Fukagawa received consulting fees from AstraZeneca Japan. Dr. McCafferty is an academic grant holder and advisory board member for AstraZeneca. Dr. Rastogi received research or travel support from and/or is a speaker, consultant, or advisory board member for AstraZeneca, Relypsa, Fresenius Medical Care, Sanofi, Kadmon, AMAG, Otsuka, Genzyme, GSK, Omerus, Janssen, Reata Pharmaceuticals, Ironwood, and Amgen. Dr. Spinowitz received research grants, lecture fees, and/or consulting fees from AstraZeneca, Akebia, Reata Pharmaceuticals, and Fresenius Medical Care. Dr. Staroselskiy received research support from AstraZeneca. Dr. Vishnevskiy received research support from AstraZeneca. Dr. Lisovskaja is an employee of AstraZeneca. Dr. Al-Shurbaji is an employee of AstraZeneca. Dr. Guzman is an employee of AstraZeneca. Dr. Bhandari has given lectures and participated on an advisory board for AstraZeneca and has given lectures sponsored by Vifor Pharma.
Funding
This study was supported by AstraZeneca.
Supplementary Material
Acknowledgments
The authors thank the patients, their families, and all investigators involved in this study. Medical writing support, including assisting authors with the development of the outline and initial draft and incorporation of comments, was provided by Shaun W. Foley, BSc (Hons) CMPP and editorial support, including figure preparation, formatting, proofreading, and submission, was provided by Bethany King, BSc (Hons) both of Core Medica (London, United Kingdom) supported by AstraZeneca according to Good Publication Practice guidelines. Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure (see http://www.icmje.org/recommendations/browse/publishing-and-editorial-issues/clinical-trial-registration.html).
The sponsor was involved in the study design, collection, analysis, and interpretation of data as well as data checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019050450/-/DCSupplemental.
Supplemental Figure 1. Proportion of patients who achieved target sK+ during three or more of four measurements and did not require rescue therapy in the SZC and PBO groups in the (A) full analysis set and (B) sensitivity analysis.
Supplemental Material. Methods.
Supplemental Material. Results.
Supplemental Table 1. Failed inclusion and exclusion criteria for nonrandomized patients (all patients).
Supplemental Table 2. sK+ responder groupings (full analysis set).
Supplemental Table 3. Patients with predialysis sK+ measurements between 4.0 and 5.0 mmol/L or between 3.5 and 5.5 mmol/L during the evaluation period (full analysis set).
Supplemental Table 4. Mean pre- and postdialysis sK+ at each visit for patients treated with SZC (n=97) and PBO (n=99; full analysis set).
Supplemental Table 5. Proportions of patients with maximum sK+ concentrations ≥6.0 mmol/L after the long interdialytic intervals during the evaluation period (full analysis set).
Supplemental Table 6. Mean intradialytic potassium shift by study visit (full analysis set).
Supplemental Table 7. Mean dialysis potassium gradient by study visit (full analysis set).
Supplemental Table 8. IDWG over time (safety analysis set).
References
- 1.Kovesdy CP, Regidor DL, Mehrotra R, Jing J, McAllister CJ, Greenland S, et al.: Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol 2: 999–1007, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Yusuf AA, Hu Y, Singh B, Menoyo JA, Wetmore JB: Serum potassium levels and mortality in hemodialysis patients: A retrospective cohort study. Am J Nephrol 44: 179–186, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Jain N, Kotla S, Little BB, Weideman RA, Brilakis ES, Reilly RF, et al.: Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol 109: 1510–1513, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Luo J, Brunelli SM, Jensen DE, Yang A: Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol 11: 90–100, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.USRDS: Annual data report, 2018. Available at: https://www.usrds.org/adr.aspx. Accessed March 25, 2019
- 6.Genovesi S, Valsecchi MG, Rossi E, Pogliani D, Acquistapace I, De Cristofaro V, et al.: Sudden death and associated factors in a historical cohort of chronic haemodialysis patients. Nephrol Dial Transplant 24: 2529–2536, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Foley RN, Gilbertson DT, Murray T, Collins AJ: Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med 365: 1099–1107, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Brunelli SM, Du Mond C, Oestreicher N, Rakov V, Spiegel DM: Serum potassium and short-term clinical outcomes among hemodialysis patients: Impact of the long interdialytic interval. Am J Kidney Dis 70: 21–29, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Beccari MV, Meaney CJ: Clinical utility of patiromer, sodium zirconium cyclosilicate, and sodium polystyrene sulfonate for the treatment of hyperkalemia: An evidence-based review. Core Evid 12: 11–24, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fried L, Kovesdy CP, Palmer BF: New options for the management of chronic hyperkalemia. Kidney Int Suppl 7: 164–170, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovesdy CP, Appel LJ, Grams ME, Gutekunst L, McCullough PA, Palmer BF, et al.: Potassium homeostasis in health and disease: A scientific workshop cosponsored by the National Kidney Foundation and the American Society of Hypertension. Am J Kidney Dis 70: 844–858, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Harel Z, Harel S, Shah PS, Wald R, Perl J, Bell CM: Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: A systematic review. Am J Med 126: 264.e9–e24, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Sterns RH, Rojas M, Bernstein P, Chennupati S: Ion-exchange resins for the treatment of hyperkalemia: Are they safe and effective? J Am Soc Nephrol 21: 733–735, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Stavros F, Yang A, Leon A, Nuttall M, Rasmussen HS: Characterization of structure and function of ZS-9, a K+ selective ion trap. PLoS One 9: e114686, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kosiborod M, Rasmussen HS, Lavin P, Qunibi WY, Spinowitz B, Packham D, et al.: Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: The HARMONIZE randomized clinical trial. JAMA 312: 2223–2233, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Ash SR, Singh B, Lavin PT, Stavros F, Rasmussen HS: A phase 2 study on the treatment of hyperkalemia in patients with chronic kidney disease suggests that the selective potassium trap, ZS-9, is safe and efficient. Kidney Int 88: 404–411, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anker SD, Kosiborod M, Zannad F, Piña IL, McCullough PA, Filippatos G, et al.: Maintenance of serum potassium with sodium zirconium cyclosilicate (ZS-9) in heart failure patients: Results from a phase 3 randomized, double-blind, placebo-controlled trial. Eur J Heart Fail 17: 1050–1056, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Packham DK, Rasmussen HS, Lavin PT, El-Shahawy MA, Roger SD, Block G, et al.: Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med 372: 222–231, 2015 [DOI] [PubMed] [Google Scholar]
- 19.AstraZeneca Pharmaceuticals LP. 2018. LOKELMA—sodium zirconium cyclosilicate powder, for suspension. Available at: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=90bf8e28-748d-4e4b-a19f-9cf483370eff. Accessed March 25, 2019
- 20.European Medicines Agency. 2018. Lokelma. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/lokelma. Accessed March 25, 2019
- 21.Papadea C, Foster J, Grant S, Ballard SA, Cate JC 4th, Southgate WM, et al.: Evaluation of the i-STAT Portable Clinical Analyzer for point-of-care blood testing in the intensive care units of a university children’s hospital. Ann Clin Lab Sci 32: 231–243, 2002 [PubMed] [Google Scholar]
- 22.Gault MH, Harding CE: Evaluation of i-STAT portable clinical analyzer in a hemodialysis unit. Clin Biochem 29: 117–124, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Karnik JA, Young BS, Lew NL, Herget M, Dubinsky C, Lazarus JM, et al.: Cardiac arrest and sudden death in dialysis units. Kidney Int 60: 350–357, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Pun PH, Lehrich RW, Honeycutt EF, Herzog CA, Middleton JP: Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney Int 79: 218–227, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Karaboyas A, Zee J, Brunelli SM, Usvyat LA, Weiner DE, Maddux FW, et al.: Dialysate potassium, serum potassium, mortality, and arrhythmia events in hemodialysis: Results from the Dialysis Outcomes And Practice Patterns Study (DOPPS). Am J Kidney Dis 69: 266–277, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jadoul M, Thumma J, Fuller DS, Tentori F, Li Y, Morgenstern H, et al.: Modifiable practices associated with sudden death among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Clin J Am Soc Nephrol 7: 765–774, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hung AM, Hakim RM: Dialysate and serum potassium in hemodialysis. Am J Kidney Dis 66: 125–132, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Locatelli F, La Milia V, Violo L, Del Vecchio L, Di Filippo S: Optimizing haemodialysate composition. Clin Kidney J 8: 580–589, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Redaelli B, Locatelli F, Limido D, Andrulli S, Signorini MG, Sforzini S, et al.: Effect of a new model of hemodialysis potassium removal on the control of ventricular arrhythmias. Kidney Int 50: 609–617, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Santoro A, Mancini E, London G, Mercadal L, Fessy H, Perrone B, et al.: Patients with complex arrhythmias during and after haemodialysis suffer from different regimens of potassium removal. Nephrol Dial Transplant 23: 1415–1421, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Brunelli SM, Spiegel DM, Du Mond C, Oestreicher N, Winkelmayer WC, Kovesdy CP: Serum-to-dialysate potassium gradient and its association with short-term outcomes in hemodialysis patients. Nephrol Dial Transplant 33: 1207–1214, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.López-Gómez JM, Villaverde M, Jofre R, Rodriguez-Benítez P, Pérez-García R: Interdialytic weight gain as a marker of blood pressure, nutrition, and survival in hemodialysis patients. Kidney Int Suppl 93: S63–S68, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Chaaban A, Abouchacra S, Gebran N, Abayechi F, Hussain Q, Al Nuaimi N, et al.: Potassium binders in hemodialysis patients: A friend or foe? Ren Fail 35: 185–188, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.