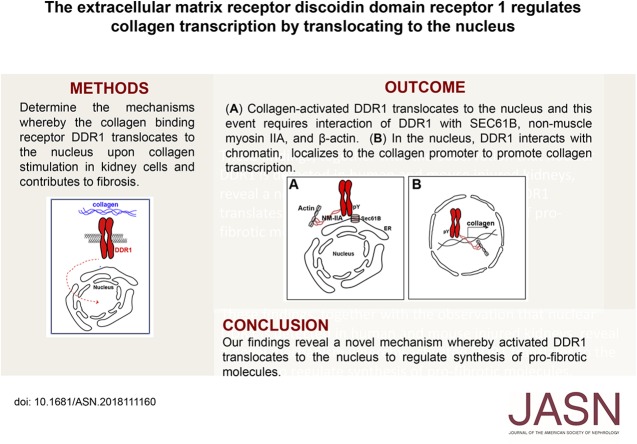
Significance Statement
The receptor discoidin domain receptor 1 (DDR1) is activated by collagen, upregulated in injured kidneys, and contributes to kidney fibrosis, but how DDR1 controls fibrosis is poorly understood. The authors show that upon collagen stimulation, DDR1 translocates to the nucleus. To do this, DDR1 must bind with SEC61B, a component of the Sec61 translocon, as well as with nonmuscle myosin IIA and β-actin. In the nucleus, DDR1 binds to chromatin to increase the transcription of collagen IV, a major collagen upregulated in fibrosis. The study reveals a novel mechanism whereby collagen-activated DDR1 moves to the nucleus to increase the production of profibrotic molecules.
Keywords: chromatin, gene transcription, fibrosis, kidney injury, non muscle myosin
Visual Abstract
Abstract
Background
The discoidin domain receptor 1 (DDR1) is activated by collagens, upregulated in injured and fibrotic kidneys, and contributes to fibrosis by regulating extracellular matrix production, but how DDR1 controls fibrosis is poorly understood. DDR1 is a receptor tyrosine kinase (RTK). RTKs can translocate to the nucleus via a nuclear localization sequence (NLS) present on the receptor itself or a ligand it is bound to. In the nucleus, RTKs regulate gene expression by binding chromatin directly or by interacting with transcription factors.
Methods
To determine whether DDR1 translocates to the nucleus and whether this event is mediated by collagen-induced DDR1 activation, we generated renal cells expressing wild-type or mutant forms of DDR1 no longer able to bind collagen. Then, we determined the location of the DDR1 upon collagen stimulation. Using both biochemical assays and immunofluorescence, we analyzed the steps involved in DDR1 nuclear translocation.
Results
We show that although DDR1 and its natural ligand, collagen, lack an NLS, DDR1 is present in the nucleus of injured human and mouse kidney proximal tubules. We show that DDR1 nuclear translocation requires collagen-mediated receptor activation and interaction of DDR1 with SEC61B, a component of the Sec61 translocon, and nonmuscle myosin IIA and β-actin. Once in the nucleus, DDR1 binds to chromatin to increase the transcription of collagen IV, a major collagen upregulated in fibrosis.
Conclusions
These findings reveal a novel mechanism whereby activated DDR1 translates to the nucleus to regulate synthesis of profibrotic molecules.
Discoidin domain receptors (DDRs) are receptor tyrosine kinases (RTKs) that undergo tyrosine autophosphorylation upon interaction with collagens.1,2 The DDR family consists of two members: DDR1 that binds to and is activated by fibrillar and nonfibrillar collagens and DDR2 that binds to and is activated by fibrillar collagens.3
DDR1 regulates multiple physiologic cellular functions including cytokine secretion,4 collective cell migration,5 and extracellular matrix (ECM) homeostasis6 as well as pathologic conditions including cancer,7 inflammation,8 and fibrosis.6,9 DDR1 activates canonical intracellular signaling pathways including ERK and the RhoGTPase Cdc42, thus controlling cell migration.10,11 In diabetes, DDR1 promotes RUNX2 activity and atherosclerotic vascular calcification via Akt activation.12 In addition to canonical signaling, DDR1 mediates activation of PKCα and STAT3 phosphorylation via TM4SF1-mediated DDR1 coupling to syntenin 2.7 This noncanonical DDR1-mediated signaling drives metastatic reactivation of breast cancer stem cells in various organs.7
DDR1 expression is upregulated in fibrosed organs including the kidney and it contributes to disease progression by regulating inflammatory and fibrotic responses.13 We showed that DDR1 promotes collagen IV production, a major ECM upregulated in fibrotic diseases. This effect requires collagen binding to DDR1 and receptor kinase domain activation; however, the molecular mechanisms whereby DDR1 increases ECM synthesis and contributes to fibrosis initiation and/or progression is poorly defined.
RTKs regulate cell function by activating intracellular signaling and by translocating to the nucleus, where they regulate gene expression by binding chromatin or by interacting with transcription factors.14–16 Nuclear localization of cell surface–bound RTKs has been reported for several RTK subclasses, including subfamilies of epithelial growth factor (EGF), insulin, PDGF, and vascular endothelial growth factor receptors.17 RTKs can translocate to the nucleus as cleaved or full-length receptors. In the case of full-length receptors, ligand-activated RTKs are internalized via clathrin-coated vesicles. Upon internalization, they undergo retrograde transport from Golgi to the endoplasmic reticulum and are subsequently transported into the nucleus via interaction with SEC61B, a member of the Sec61 translocon, and importins.18 If RTKs do not contain a classic nuclear localization sequence (NLS), they can still translocate to the nucleus by association with their NLS-containing ligands.19
DDR1 and its ligands lack an NLS motif, questioning the ability of this receptor to translocate to the nucleus. Interestingly, DDR1 associates with the actin-binding protein nonmuscle myosin II (NM II) and this association mediates DDR1-driven cell motility.20,21 In addition to controlling cell motility, NM II and its binding partner G-actin interact with and facilitate the nuclear translocation of plasma membrane–bound receptors. Moreover, they are found in the nucleus where they regulate gene transcription by interacting with transcription factors and/or RNA polymerase.22–27
The goal of this study was to investigate whether DDR1 can translocate to the nucleus, the mechanisms regulating its nuclear translocation, and the function of nuclear DDR1. We show that full-length DDR1 is present in the nuclei of proximal tubules of injured human and murine kidneys. Using mass spectrometry (MS) and biochemical and cellular assays, we show that upon collagen stimulation full-length DDR1 interacts with SEC61B in endoplasmic reticulum–enriched fractions. Moreover, DDR1 forms a complex with NM IIA and β-actin, which is crucial for DDR1 nuclear translocation. Finally, we show that NM IIA, actin, and DDR1 interact with chromatin to promote the transcription of collagen IV. Thus, DDR1 contributes to fibrosis by translocating to the nucleus where it acts as a cotranscription factor. This previously unrecognized nuclear role of DDR1 opens unanticipated therapeutic options for the treatment of fibrotic diseases.
Methods
Cells
Human embryonic kidney (HEK) and human kidney 2 (HK-2) cells were obtained from ATCC and cultured in DMEM with 10% FBS. Cells were tested for mycoplasma upon purchase and were characterized and authenticated by the vendor.
Plasmids
pIRES-DDR1-FLAG, pIRES-DDR1, and pIRES-DDR1-R105A were generated as described.6 For DDR1-EGFP, DDR1 cDNA was amplified using the primers 5′-GATGGAATTCGGAGCTATGGGACCAGAGG-3′ and 5′-GCTGGATCCGCACCGTGTTGAGTGCATC-3′ and pRK5-DDR1 as a template. The PCR product was digested with EcoRI and BamHI and cloned into pEGFP-N2. RLC-TS/DD-GFP was a gift from Dr. Donna Webb.
FACS
To generate HEK cells expressing DDR1, DDR1-flag, DDR1-R105A, and DDR1-GFP, cells were transfected with 0.4–1 µg of plasmid using Lipofectamine 2000 (Life Technologies) and selected with puromycin (Sigma) or G418 (Corning). Cells were incubated with antibody to the extracellular domain of DDR1 (Millipore) followed by PE-conjugated secondary antibody and sorted using a FACS Aria II sorter (BD Biosciences) available through the Research Flow Cytometry Core Laboratory at the Nashville VA Medical Center.
Cell Treatment
Serum-starved cells were incubated with 20 µM blebbistatin (cat. B0560; Sigma), 10 µM Leptomycin B (cat. L2913; Sigma), or 1 µM Latrunculin-B (cat. L5288; Sigma). After 30 minutes, cells were treated with vehicle (20 mM acetic acid) or with collagen I (Corning; 50 μg/ml in 20 mM acetic acid) for 0.5–3 hours. Cells were then processed as indicated below.
Non-Nuclear and Nuclear Cell Fractions
Cells were harvested in 10 mM HEPES pH 7.9, 1.5 mM MgCl2, 10 mM KCl, protease inhibitors (Roche Applied Science), 5 mM NaVO3, and supernatant (non-nuclear), and pellet (nuclear) fractions were separated by centrifugation (400 × g for 4 minutes at 4°C). The nuclear fraction was lysed in the buffer above containing 25% glycerol.
Kidney cortex (10 mg) was homogenized in 250 mM sucrose, 10 mM HEPES pH 7.4, 5 mM KCl, 1.5 mM EDTA pH 8.0, 5 mM Na3VO4, and proteases inhibitors. After 15 minutes on ice, tissue lysates were centrifuged as described above. The nuclear fraction was resuspended in 20 mM HEPES pH 7.4, 0.4 M NaCl, 2.5% glycerol, 1 mM EDTA pH 8.0, 0.5 mM NaF, and protease inhibitors.
Nuclear Soluble and Chromatin Fractions
Cells were collected in 10 mM HEPES pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 0.34 M sucrose, 10% glycerol, 1 mM DTT, proteases inhibitors, and 5 mM NaVO3, as described.28 After addition of Triton X-100 (0.1%), the nuclear pellet (P1) and non-nuclear fractions were separated by centrifugation (700 × g for 5 minutes at 4°C). P1 was incubated in 3 mM EDTA, 0.2 mM EGTA, 1 mM DTT, protease inhibitors, and 5 mM NaVO3. The nuclear soluble fraction and total chromatin were collected by centrifuging P1 at 1200 × g for 5 minutes at 4°C.
Endoplasmic Reticulum Fractionation
Cell pellets were homogenized in 3 ml of 0.25 M sucrose and 0.1 M Tris (pH 7.4, 4°C). After sonication, cells were centrifuged at 10,000 × g for 20 minutes at 4°C. The supernatant underwent further ultracentrifugation at 100,000 × g for 90 minutes at 4°C and the pellets were saved as microsomal fractions.
Western Blotting
Cell or tissue lysates were resolved in 8% or 4%–20% SDS-PAGE and transferred to nitrocellulose membranes. Membranes were incubated with the primary antibodies described in Supplemental Table 1 followed by horseradish peroxidase–conjugated secondary antibodies. Immunoreactive bands were quantified by densitometry analysis using ImageJ and normalized against the appropriate loading controls.
Immunoprecipitation
Cell fractions (200–500 µg proteins) were precleared using 40 µl Protein G Sepharose (cat. SE-75184; GE healthcare). The lysates were then incubated with 20 µl Protein G Sepharose together with 1 µg anti–NMHC-IIA, 1 µg anti–β-actin, or 20 µl anti-FLAG M2 affinity gel agarose beads (Sigma); or 1 µg anti-SEC61B or 1 µg of IgG control antibody. After 18 hours, the immunoprecipitates were washed with 50 mM Tris pH 7.2, 150 mM NaCl, and 1% Triton X-100; eluted in sample buffer; and analyzed by western blot and as indicated above.
Immunohistochemistry and Immunofluorescence
Human paraffin kidney sections were stained with rabbit anti-DDR1 antibody (cat. 5583; Cell Signaling) followed by horseradish peroxidase–conjugated anti-rabbit secondary antibody (cat. 711035152; Jackson Immunoresearch) and Sigma Fast DAB chromogenic tablets (Sigma). For double immunostaining, paraffin sections were stained with anti-DDR1 antibody, anti-collagen IV antibody (600–401–106–0.5; Rockland), collagen I antibody (ab34710; Abcam), and anti–NMHC-IIA antibody (ab89837; Abcam), together with biotinylated Lotus tetragonolobus agglutinin (cat. B-1325; Vector Laboratories), followed by secondary antibodies conjugated to AlexaFluor 555 and Fluorescein-Streptavidin (cat. SA-5001; Vector Laboratories), and mounted using ProLong Gold Antifade Mountant with DAPI (cat. P36931; Thermo Scientific).
Serum-starved HEK cells expressing DDR1-GFP or GFP, plated in multiwell chamber slides (cat. PEZG S0416; Millipore), were treated with vehicle or 50 µg/ml collagen I with or without 10 µM Leptomyocin B or 20 µM blebbistatin. After 1–3 hours, cells were fixed in 4% PFA and slides were mounted using ProLong Gold Antifade Mountant with DAPI. For double staining, cells were stained with anti-histone H3 or anti–NMHC-IIA antibodies followed by secondary antibodies, AlexaFluor 555 conjugated, and mounted as indicated above. Serum-starved HEK cells expressing GFP or the constitutively active RLC-GFP, plated in multiwell chamber slides, were fixed in 4% PFA, permeabilized with 0.5% Triton-X in PBS, and stained with rhodamine phalloidin (cat. Ab235138; Abcam) to visualize the cytoskeleton. Slides were mounted as indicated above. Confocal images were collected using an LSM710 META inverted confocal microscope. Confocal z-stacks consisting of up to 35 images were reconstructed into 3D animations using the ZEN lite microscope image software.
Image Quantification
To quantify the degree of nuclear colocalization of DDR1 and histone H3 or NMHC-IIA, we used Manders’ coefficient through the software ImageJ (30–40 images analyzed). To quantify the area of nuclear DDR1-GFP–positive structures, we used the software ImageJ (90–129 cells analyzed).
Ischemia-Reperfusion AKI
Surgery was performed as described.29 Anesthetized 10–12-week-old male 129Sv mice were kept on a water bath–heated platform at 38°C. To induce ischemia-reperfusion AKI, mice underwent left renal pedicle clamping for 31 minutes. Three days later, mice were euthanized and the left kidney removed and used for analysis of nuclear and non-nuclear DDR1 levels by western blot. Uninjured mice served as control. Protocols were approved by the Vanderbilt Institutional Animal Care and Use Committee.
LC-MS/MS Analyses
Lysates of HEK cells expressing DDR1-GFP treated with collagen I (50 µg/ml) or acetic acid for 1 hour were immunoprecipitated with GFP-binding protein coupled magnetic beads (ChromoTek). Proteins were eluted by heating magnetic beads in gel loading buffer and subjected to short-stack SDS-PAGE and in-gel digestion with trypsin. Tryptic peptides were analyzed by LC-MS/MS and the resulting Thermo .raw files were searched with MyriMatch algorithm against a human database (20,232 entries; Uniprot). Identified peptide-spectrum matches were imported into IDPicker (version 3.1) for the parsimonious grouping of identified proteins at a 0% protein global FDR.30 Proteins identified in vehicle- and collagen I–treated samples were manually curated to identify proteins only present in cells stimulated with collagen I. To discriminate between potential bona fide interactors or background contaminants (e.g., proteins that interact with magnetic beads or GFR protein), the resulting protein subset was further curated by querying against the Contaminant Repository for Affinity Purification (CRAPome).31 Proteins showing a high frequency of occurrence in control experiments were eliminated as they were likely to be background contaminants. Spectral counting was used to assess relative abundance of proteins in both vehicle- and collagen I–treated samples. To identify phosphorylation of tyrosine residues on DDR1, Thermo .raw files were subjected to a second search using the subset of identified proteins and setting phosphotyrosine [Tyr+79.9663] as variable modification as described.32,33 The raw data can be accessed at https://massive.ucsd.edu/ProteoSAFe/static/massive.jsp?redirect=auth bearing a massive ID “MSV000082595” (username- “basakt,” password–“vanderbilt2015”).
RT-rqPCR
RNA was isolated from cells with Agilent Total RNA Isolation Kit (Agilent Technologies). cDNA synthesis was performed using 0.5 µg RNA with iScript cDNA Synthesis Kit (Bio-Rad) and rqPCR was performed with the SYBR green method using iQ Real-Time Sybr Green PCR Supermix Kit (Bio-Rad). Fluorescence was acquired at each cycle on a CFX96 system (Bio-Rad) using the following cycling conditions: 94°C/2 min, 94°C/30 s and 60°C/30 s for 40 cycles, and 72°C/10 min. The Cq values were analyzed using the CFX96 system and normalized to GAPDH levels. Primers used for human collagen IV α1 chain (COL4A1, NM_001845.5) were: 5′-TGTGCAACTTTGCATCACGAA-3′ (forward) and 5′-TCACACACAGCACACCTACTAA-3′ (reverse); and for human GAPDH 5′-AAGGTGAAGGTCGGAGTCAA-3′ (forward) and 5′-AATGAAGGGGTCATTGATGG-3′ (reverse).34
Chromatin Immunoprecipitation
Serum-starved HEK-DDR1b-GFP or HK-2 cells were treated with acetic acid (20 mM) or collagen I (50 µg/ml in 20 mM acetic acid) for 30 or 60 minutes, respectively. Cells were harvested and the nuclear DDR1-DNA complex was isolated using ChIP-IT Express Enzymatic Magnetic Chromatin Immunoprecipitation Kit (cat. 53009; Active Motif) according to manufacturer instructions. Briefly, proteins and DNA were crosslinked with 1% paraformaldehyde. After 15 minutes, the crosslink reaction was stopped by incubation with Glycine Stop-Solution and, after chromatin shearing, DDR1 was immunoprecipitated using GFP-TRAP beads (Cromteck, for HEK-DDR1-GFP) or rabbit anti-DDR1 (Santa-Cruz) or rabbit IgG (Cell Signaling) antibodies with Protein G agarose beads (for HK-2 cells). DNA was eluted from the beads with Elution Buffer AM2 and, after incubation with Proteinase K for 1 hour, the DNA was further purified using phenol-chloroform extraction. DNA amplification was performed using the following human collagen IV promoter primers: 5′-AAATACGCCCAAAGCTGCTC-3′ (forward) and 5′-GGACCGAGCCTCCTTTGTAT-3′ (reverse).
Biotinylation Assay
Serum-starved HK-2 cells were rinsed with cold PBS and incubated with freshly prepared EZ-Link Sulpho-NHS-SS-Biotin (cat. 21331; ThermoFisher Scientific) at 0.5 mg/ml in PBS pH 8.0. After 20 minutes, unreacted biotinylating reagent was quenched with 100 mM glycine in PBS and rinsed with PBS to remove glycine. After biotinylation, cells were treated with vehicle or collagen I (50 μg/ml) at 37°C (to allow internalization) or at 4°C (to prevent internalization and detect total levels of cell surface biotinylated proteins). Cells treated with vehicle or collagen I at 37°C in the absence of biotinylation served as negative control. After 1 hour, nuclear fractions (200 µg for cells incubated at 37°C) were incubated with streptavidin agarose resin (cat. 20357; ThermoFisher Scientific). After 2 hours, the resin was washed with Tris 50 mM, NaCl 150 mM, and Triton 1% and Igepal 1%, followed by a wash with 2% SDS in Tris 50 mM. Biotinylated proteins were eluted with reducing SDS-PAGE sample buffer and analyzed by western blot analysis for levels of DDR1 or total biotinylated proteins.
Statistical Analyses
Data are shown as mean±SD. Unpaired two-tailed t test was used to evaluate statistically significance differences (P<0.05) between two groups. GraphPad Prism software (GraphPad) ANOVA followed by Bonferroni’s multiple comparison test when appropriate was used to evaluate statistically significant differences (P<0.05) among multiple groups.
Results
Nuclear DDR1 Expression in Proximal Tubules of Injured Human Kidneys
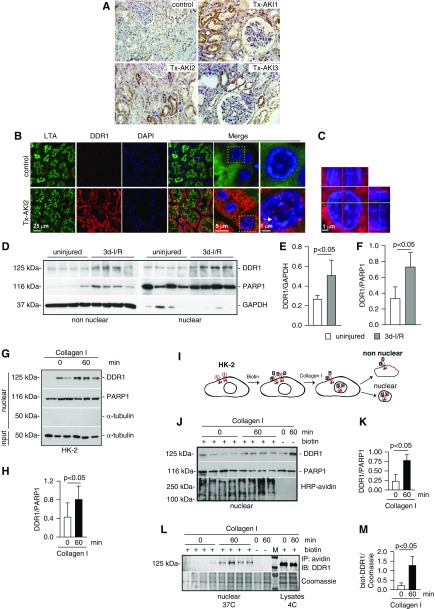
DDR1 levels are upregulated in humans with kidney disease35,36 and in several mouse models of kidney injury.4,35,37,38 To determine where DDR1 is expressed, we stained transplant kidney biopsy specimens with AKI with anti-DDR1 antibody. Tubular DDR1 was increased in AKI compared with control healthy subjects (Figure 1A). Double staining with anti-DDR1 antibody and Lotus tetragonolobus agglutinin (a marker of proximal tubules) showed that DDR1 localized mainly in proximal tubules (Figure 1B). Surprisingly, we detected cytoplasmic and nuclear DDR1 staining in injured proximal tubule cells (Figure 1, B and C). To confirm DDR1 nuclear localization, we evaluated DDR1 expression in non-nuclear and nuclear fractions of kidney cortices of mice uninjured or 3 days after proximal tubule injury induced by ischemia-reperfusion. Full-length DDR1 expression was significantly increased in non-nuclear and nuclear fractions of kidney cortices from injured mice (Figure 1, D–F). Thus, DDR1 expression is upregulated in the injured kidney and the full-length receptor can translocate to the nucleus.
Figure 1.
DDR1 is upregulated in injured kidney proximal tubules. (A) Paraffin kidney sections from control and biopsy specimens from three different patients with transplant AKI (Tx-AKI) were stained with anti-DDR1 antibody. Upregulated DDR1 expression is evident in the tubules of injured kidneys. (B) Paraffin kidney sections from control or one patient with Tx-AKI were stained with anti-DDR1 antibody and Lotus tetragonolobus agglutinin (LTA, a marker of proximal tubule) and analyzed by confocal microscopy. Expression of DDR1 is evident both in the cytoplasm and in the nuclei of injured proximal tubules (arrow). (C) Orthogonal projection of confocal images of kidney sections from the patient shown in (B) was performed using the imaging program Zen (black edition). Red, DDR1; blue, DAPI. (D) Non-nuclear and nuclear fractions (20 µg/lane) from kidney cortices of wild-type mice uninjured or 3 days after ischemia-reperfusion (3d-I/R) were analyzed by western blot for levels of DDR1. (E and F) Non-nuclear DDR1 and GAPDH (E) or nuclear DDR1 and PARP1 (F) bands were quantified by densitometry. Values represent DDR1/GAPDH or DDR1/PARP1 ratio and are the mean±SD of four animals. (G) Serum-starved HK-2 cells were treated with collagen I (50 µg/ml) for the time indicated. Time 0 represents cells incubated with vehicle (20 mM acetic acid) for 60 minutes. Nuclear fractions (20 µg/lane) were analyzed by western blot for levels of DDR1. (H) Nuclear DDR1 and PARP1 bands were quantified by densitometry. Values represent DDR1/PARP1 ratio and are the mean±SD of two experiments performed in triplicate. PARP1 (nuclear marker), GAPDH, or α-tubulin (non-nuclear markers) was used to evaluate fraction purity. (I) Schematic representation of the biotinylation assay performed on HK-2 cells. See text for details. (J) Nuclear fractions of HK-2 cells biotinylated (+ biotin) and treated at 37°C with collagen I for the time indicated were analyzed for levels of DDR1 or total biotinylated proteins using HRP-avidin. Nonbiotinylated (- biotin) cells treated with collagen I for the times indicated served as control. (K) Nuclear DDR1 and PARP1 of biotinylated cells were quantified and expressed as indicated above. (L) Nuclear fractions (200 µg) of biotinylated HK-2 cells treated at 37°C with collagen I for the times indicated were immunoprecipitated using streptavidin beads. Immunoprecipitated biotinylated proteins were analyzed for levels of DDR1. Cells treated at 37°C with collagen I for the time indicated in the absence of biotinylation (- biotin) or biotinylated by kept at 4°C served as negative (background for streptavidin beads) and positive (total biotinylated DDR1) controls, respectively. (M) Nuclear biotinylated DDR1 was quantified to the Coomassie protein band shown. IP, immunoprecipitation; IB, immunoblot.
DDR1 Translocates to the Nucleus upon Collagen I Treatment
RTKs can translocate to the nucleus upon ligand binding.39 Thus, we analyzed nuclear fractions of human proximal tubule HK-2 cells (which express endogenous DDR1 and are the main cell types affected in AKI) treated with vehicle or soluble collagen I (which activates DDR1, but not other collagen-binding receptors such as integrins40–42). Nuclear levels of DDR1 were significantly increased in collagen I–treated compared with vehicle-treated cells (Figure 1, G and H). To further confirm that endogenous DDR1 translocates to the nucleus upon collagen I stimulation, we performed cell-surface protein biotinylation in HK-2 cells followed by treatment with collagen I, nuclei isolation, streptavidin pulldown, and western blot analysis (Figure 1, I–M). Nuclear fractionation of biotinylated NK-2 cells treated with vehicle or collagen I showed that nuclear levels of DDR1 were significantly increased in collagen I–treated compared with vehicle-treated cells, indicating that biotinylation did not compromise DDR1 trafficking (Figure 1, J and K). Streptavidin pulldown of nuclear fractions, followed by western blot analysis of DDR1 levels, revealed the presence of biotinylated DDR1 primarily in the nuclei of cells treated with collagen I, but not vehicle (Figure 1, L and M).
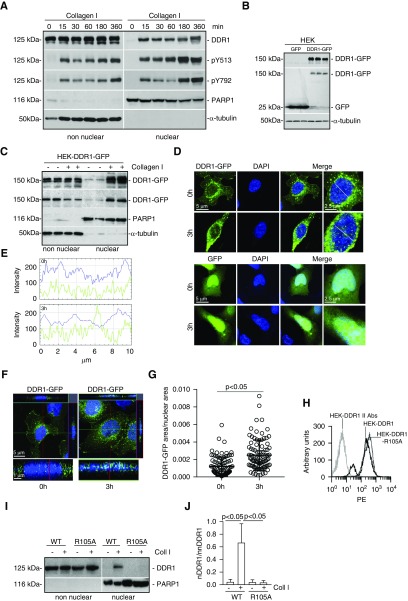
To define how activated DDR1 translocates to the nucleus, we generated HEK cells (which express low levels of endogenous DDR1) that express the human DDR1b isoform (HEK-DDR1). We focused on DDR1b because, among the five DDR1 isoforms, it is well characterized in terms of collagen-mediated activation and downstream signaling.6,43 DDR1 was detected in non-nuclear, but not nuclear, fractions of unstimulated cells (Figure 2A). Upon collagen I treatment, full-length nuclear DDR1 became evident 15 minutes after treatment and it remained in nuclear fractions for at least 360 minutes (Figure 2A). Phosphorylation of the receptor, measured with antibodies to pY513 (specific to DDR1b) and Y792 (in the activation loop of the receptor), was evident only after collagen I treatment and was present in both the nuclear and non-nuclear fractions (Figure 2A).
Figure 2.
Activated DDR1 translocates to the nucleus. (A) Serum-starved HEK-DDR1 cells were treated with collagen I (50 µg/ml) for the times indicated. Time 0 represents cells incubated with 20 mM acetic acid for 360 minutes. Non-nuclear and nuclear fractions were analyzed by western blot for total (DDR1) and phosphorylated (pY513 and pY792) DDR1. PARP1 and α-tubulin were used to verify purity of nuclear versus non-nuclear fractions, respectively. (B) Cell lysates of triplicate samples of HEK cells expressing GFP (HEK-GFP) or the fusion DDR1-GFP (HEK-DDR1-GFP) cDNA were analyzed by western blot using anti-DDR1 (upper panel) or anti-GFP (middle panel) antibody. α-tubulin was used to verify loading. (C) Serum-starved HEK-DDR1-GFP cells were treated with vehicle or collagen I (50 µg/ml) for 3 hours. Nuclear and non-nuclear fractions were analyzed by western blot using anti-DDR1 (upper panel) or anti-GFP (second panel) antibody. PARP1 and α-tubulin were used to verify the fraction purity. (D) Confocal images of HEK-DDR1-GFP cells treated with collagen I (50 µg/ml; 3h) or acetic acid (20 mM; 0h) for 3 hours. (E) Line scanning profile showing intensity of DDR1-GFP (green) and DAPI (blue) pixels, measured along the white line in (D). (F) Orthogonal projection of confocal images of HEK-DDR1-GFP cells was performed using the imaging program Zen (black edition). (G) The area occupied by DDR1-GFP–positive pixels/nuclear area was evaluated in HEK-DDR1-GFP cells. Circles represent single cells (n=90 for 0 hours and n=92 for 3 hours), whereas the bars represent the mean±SD. (H) HEK cells were transfected with DDR1 or DDR1-R105A cDNA and cell populations expressing comparable levels of DDR1 were sorted by FACS. PE, phycoerythrin. (I) Serum-starved HEK cells expressing DDR1 (WT) or DDR1-R105A (R105A) were treated with collagen I (50 µg/ml) for 0 and 3 hours. Time 0 is as indicated above. Non-nuclear and nuclear fractions were analyzed by western blot for total DDR1. PARP1 was used to verify the purity of nuclear fractions. (J) Nuclear DDR1 (nDDR1) and non-nuclear DDR1 (nnDDR1) bands were quantified by densitometry. Values represent nDDR1/nnDDR1 ratio and are the mean±SD of three experiments.
To confirm DDR1 nuclear translocation upon collagen I stimulation, we generated HEK cells expressing human DDR1-GFP cDNA (HEK-DDR1-GFP) (Figure 2B) and analyzed DDR1-GFP localization by western blot and confocal microscopy upon collagen I treatment. Full-length DDR1-GFP was evident in non-nuclear fractions of vehicle- and collagen I–treated cells (Figure 2C, non-nuclear); however, nuclear DDR1-GFP was detected only in collagen I–stimulated cells (Figure 2C, nuclear). In unstimulated cells, DDR1-GFP was present at the cell membrane and in perinuclear regions only (Figure 2, D–F). After collagen I treatment, DDR1-GFP was visualized in aggregates in both cytoplasm and nuclei (Figure 2, D–F) and there was significantly more nuclear DDR1-GFP in collagen I– than vehicle-treated HEK-DDR1-GFP cells (Figure 2G).
To determine whether collagen I binding is required for DDR1 nuclear translocation, we generated HEK cells expressing the collagen I–binding–deficient DDR1-R105A mutant (HEK-DDR1-R105A)44 (Figure 2H). Collagen I treatment failed to promote nuclear translocation of DDR1-R105A (Figure 2, I and J), suggesting that the collagen I/DDR1 interaction is a key step in promoting receptor nuclear translocation.
Identification of Proteins Involved in DDR1 Nuclear Translocation
DDR1 does not contain an NLS, suggesting that its nuclear translocation is mediated by its interaction with chaperones carrying this signal. To identify these interactors, we immunoprecipitated DDR1-GFP in HEK-DDR1-GFP cells treated with vehicle or collagen I and then analyzed the immunoprecipitated proteins by MS.
MS analysis resulted in the identification of 629 and 563 proteins from vehicle- and collagen I–treated DDR1-GFP immune complexes, respectively. DDR1 was the most abundant protein identified in immunoprecipitates of both vehicle- and collagen I–treated samples. DDR1 peptides containing phosphotyrosine resides (Y484, Y513, Y520, Y792) were present only in collagen I–treated samples, confirming that DDR1-GFP undergoes phosphorylation at sites known to activate DDR1 upon collagen stimulation43 (Supplemental Figure 1). We identified 73 proteins in collagen I–treated cells, two of which were protein transport protein Sec61 subunit β (SEC61B) and histone-binding protein RBBP4 (Supplemental Table 2). We observed similar spectral counts for β-actin peptides in both vehicle- and collagen I–treated samples. Moreover, twice as many spectral counts for nonmuscle myosin heavy chain II A (NMHCII-A, encoded by the MYH9 gene) peptides were present in collagen I–treated samples (Supplemental Table 2). This result suggests selective interaction with SEC61B and increased interaction of NMHCII-A with DDR1 upon collagen I treatment. SEC61B regulates the nuclear translocation of full-length growth factor receptors.45 NM IIA associates with DDR1 at the plasma membrane,21 is coexpressed with DDR1 in the inner ear in vivo,20 interacts with DDR1 via its C-terminal kinase domain,46 drives internalization of growth factor receptors,47 and interacts with β-actin and regulates gene expression upon nuclear translocation.22
DDR1 Forms a Complex with SEC61B
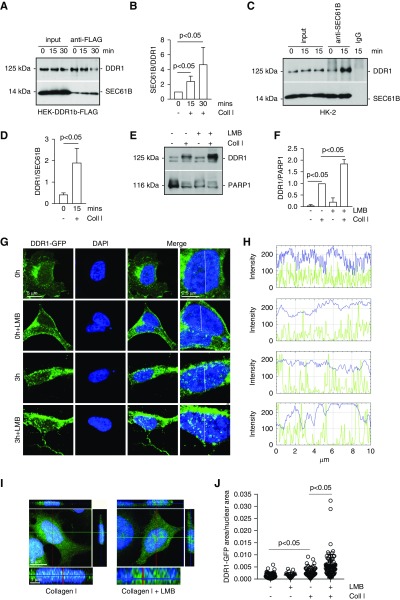
To validate the MS finding, we generated HEK-DDR1-FLAG cells and isolated microsomes from cells treated with vehicle or collagen I. Immunoprecipitation assays using anti-FLAG antibody revealed that collagen I treatment significantly increased the DDR1/SEC61B interaction compared with that detected in vehicle-treated cells (Figure 3, A and B). To validate the finding to endogenously expressed DDR1, we isolated microsomes from HK-2 cells treated with vehicle or collagen I. Immunoprecipitation assays using anti-SEC61B antibody or IgG control antibody followed by western blot for DDR1 showed pronounced DDR1/SEC61B interaction in cells treated with collagen compared with that detected in vehicle-treated cells (Figure 3, C and D). No DDR1/SEC61B interaction was observed in lysates of collagen-treated cells immunoprecipitated with IgG control antibody (Figure 3, C and D).
Figure 3.
Inhibition of exportins increases collagen I–induced DDR1 nuclear retention and levels. (A) Serum-starved HEK cells transfected with human DDR1-FLAG cDNA were treated with collagen I (50 µg/ml) for 15 or 30 minutes. Time 0 represents cells incubated with 20 mM acetic acid for 30 minutes. Equal amounts of microsomes (200 µg) were immunoprecipitated with anti-FLAG antibody and the immunoprecipitates were analyzed by western blot for levels of DDR1 and SEC61B. (B) SEC61B and DDR1 bands were quantified by densitometry. Values represent SEC61B/DDR1 ratio and are the mean±SD of three experiments. (C) Serum-starved HK-2 cells were treated with 20 mM acetic acid or collagen I (50 µg/ml) for 15 minutes. Equal amounts of microsomes (200 µg) were immunoprecipitated with anti-SEC61B antibody or IgG control and the immunoprecipitates were analyzed by western blot for levels of DDR1 and SEC61B. (D) SEC61B and DDR1 bands were quantified and expressed as indicated above. Values represent DDR1/SEC61B ratio and are the mean±SD of six experiments. (E) Serum-starved HEK-DDR1 cells were treated with 20 mM acetic acid or collagen I (50 µg/ml) for 3 hours in the presence or absence of Leptomycin B (LMB, 10 ng/ml). Nuclear fractions (20 µg/lane) were analyzed by western blot for levels of DDR1 or PARP1 (to verify equal loading of nuclear fractions). (F) Nuclear DDR1 and PARP1 bands were quantified by densitometry. Values represent DDR1/PARP1 ratio and are the mean±SD of three experiments normalized to samples treated with collagen I only. (G) Confocal images of serum-starved HEK-DDR1-GFP cells treated with collagen I (50 µg/ml) for 3 hours in the presence or absence of LMB (10 ng/ml). Time 0 represents cells incubated with 20 mM acetic acid for 3 hours. (H) Line scanning profile showing intensity of DDR1-GFP (green) and DAPI (blue) pixels (measured along the white line in [G]) in HEK-DDR1-GFP cells treated as described in (E). (I) Orthogonal projection of confocal images of serum-starved HEK-DDR1-GFP cells treated as indicated above was performed using the imaging program Zen (black edition). Green, DDR1-GFP; blue, DAPI. (J) The area occupied by DDR1-GFP–positive pixels/nuclear area was evaluated in HEK-DDR1-GFP cells treated as indicated in (E) using ImageJ. Circles represent single cells (n=90 for 0 hour, n=27 for 0 hour+LMB, n=92 for 3 hour, and n=87 for 3 hour+LMB), whereas the bars represent the mean±SD. Coll I, collagen I.
Inhibition of Nuclear Exports Increases Collagen I–Mediated DDR1 Nuclear Levels
To confirm that activated DDR1 is translocated to the nucleus, we stimulated HEK-DDR1 cells with collagen I with or without the exportin inhibitor Leptomycin B. Western blot of nuclear fractions showed that Leptomycin B significantly increased the nuclear levels of DDR1 in collagen I–stimulated, but not vehicle-treated, cells (Figure 3, E and F). Confocal microscopy confirmed a significant increase in DDR1-GFP–positive nuclear clusters in cells treated with collagen I together with Leptomycin B (Figure 3, G–J).
Collagen I–Mediated DDR1 Nuclear Translocation Is Acto-Myosin Dependent
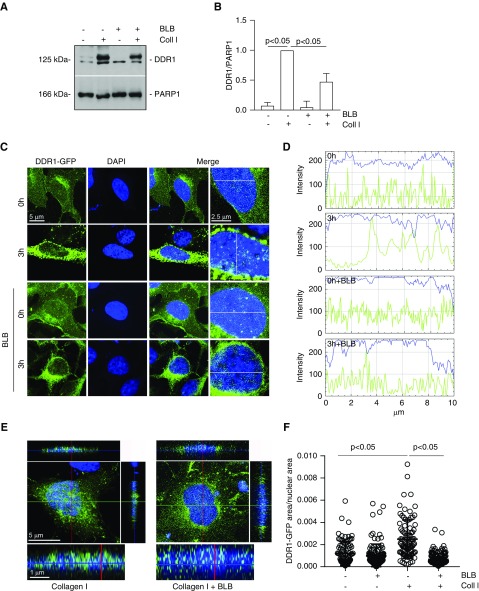
We identified NMHCII-A as a potential DDR1 interactor (Supplemental Table 2). To determine whether NMHCII-A mediates DDR1 nuclear translocation, we stimulated HEK-DDR1 cells with collagen I with or without blebbistatin, which inhibits myosin ATPase activity. Western blot of nuclear fractions and confocal microscopy showed that treatment with blebbistatin significantly decreased collagen I–mediated DDR1 nuclear translocation, but had no effect in vehicle-treated cells (Figure 4, A–F).
Figure 4.
Inhibition of nonmuscle myosin II ATPase activity decreases collagen I–induced DDR1 nuclear translocation. (A) Serum-starved HEK-DDR1 cells were treated with vehicle or collagen I (50 µg/ml) for 3 hours in the presence or absence of blebbistatin (BLB, 20 µM). Nuclear fractions (20 µg/lane) were analyzed by western blot for levels of total DDR1 and PARP1 (to verify equal loading of nuclear fractions). (B) Nuclear DDR1 and PARP1 bands were quantified by densitometry. Values represent DDR1/PARP1 ratio and are the mean±SD of three experiments normalized to samples treated with collagen I only. (C) Confocal images of serum-starved HEK-DDR1-GFP cells treated with collagen I (50 µg/ml) for 0 or 3 hours in the presence or absence of BLB (20 µM). Time 0 represents cells incubated with 20 mM acetic acid for 3 hours. (D) Line scanning profile showing intensity of DDR1-GFP (green) and DAPI (blue) pixels (measured along the white line in [C]) in HEK-DDR1-GFP cells treated as described in (C). (E) Orthogonal projection of confocal images of serum-starved HEK-DDR1-GFP cells treated as indicated above was performed using the imaging program Zen (black edition). Green, DDR1-GFP; blue, DAPI. (F) The area occupied by DDR1-GFP–positive pixels/nuclear area was evaluated in HEK-DDR1-GFP cells treated as indicated above using ImageJ. Circles represent single cells (n=90 for 0 hour, n=107 for 0 hour+BLB, n=92 for 3 hour, and n=129 for 3 hour+BLB), whereas the bars represent the mean±SD. Coll I, collagen I.
Activation of NM II Activity Enhances Collagen I–Mediated DDR1 Nuclear Translocation
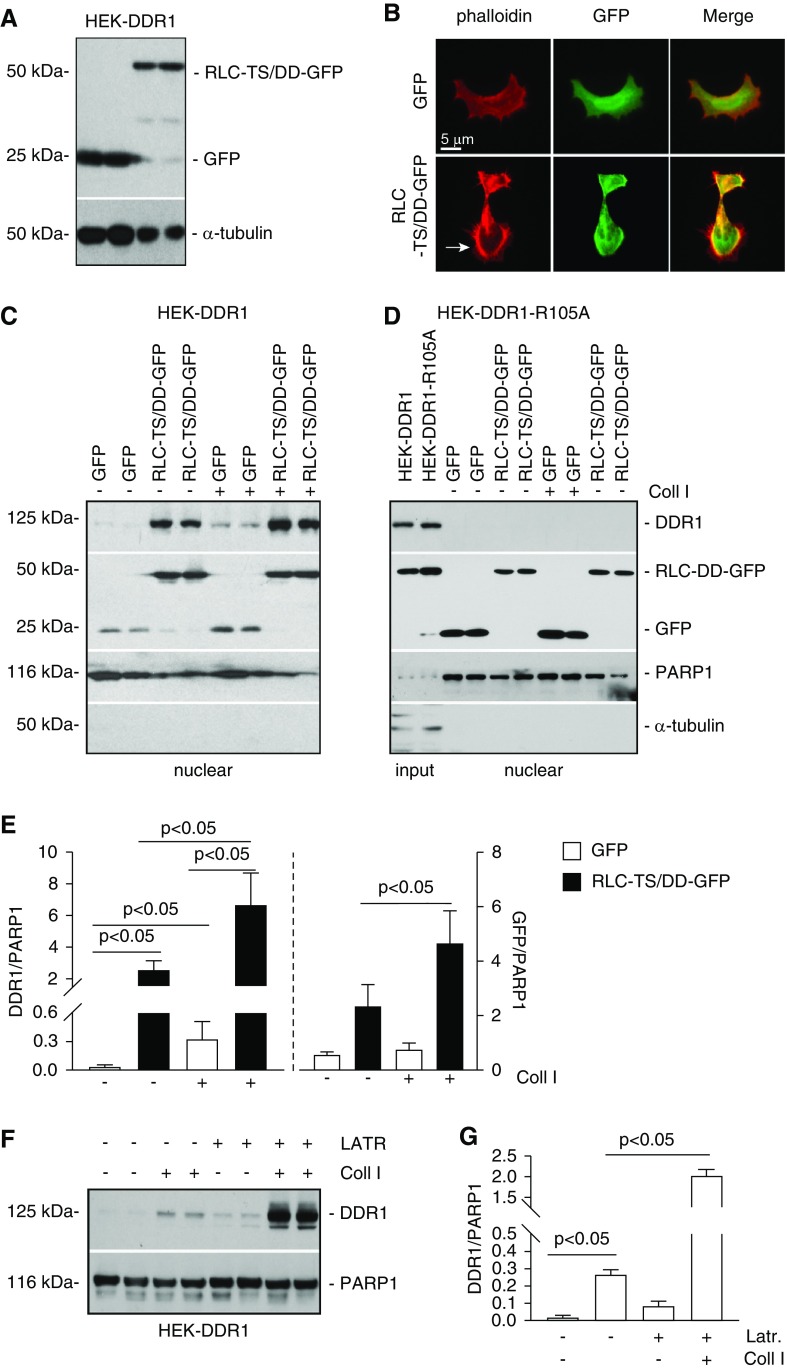
NM II activity is regulated by the regulatory light chains (RLCs).48 RLCs contain two amino acids, threonine 18 (T18) and serine 19 (S19) that, when phosphorylated, relieve the inhibition imposed on the myosin molecule.48 We therefore generated HEK-DDR1 cells expressing GFP or the constitutively active RLC-GFP (T18 and S19 mutated to aspartic acid, RLC-TS/DD-GFP) (Figure 5A). Because myosin motors generate contractile stresses promoting the formation of cortical actin filaments,49 we stained HEK-DDR1-GFP or HEK-DDR1-RLC-GFP cells with rhodamine phalloidin (to visualize the cytoskeleton) and observed presence of cortical actin only in cells expressing constitutively active RLC (Figure 5B). We treated these cells with vehicle or collagen I and analyzed the nuclear level of DDR1. We detected nuclear localization of GFP and RLC-TS/DD-GFP at baseline and after collagen I stimulation (Figure 5C); however, the levels of RLC-TS/DD-GFP were significantly higher in collagen I–treated cells compared with vehicle-treated cells (Figure 5E). We detected nuclear DDR1 in HEK-GFP cells treated with collagen I, whereas no visible signal was evident in vehicle-treated cells (Figure 5, C and E). By contrast, we detected baseline nuclear DDR1 in vehicle-treated HEK-RLC-TS/DD-GFP cells (consistent with the increased baseline of nuclear RLC-TS/DD-GFP levels) that significantly increased upon collagen I treatment (consistent with the further increased levels of RLC-TS/DD-GFP after collagen I treatment) (Figure 5, C and E).
Figure 5.
Nonmuscle myosin II A and β-actin promote DDR1 nuclear translocation. (A) HEK-DDR1 cells were transiently transfected with empty vector (GFP) or constitutively active nonmuscle myosin II RLC (RLC-TS/DD-GFP) cDNA. Cell lysates (20 µg/lane) of cells transfected in duplicate were analyzed by western blot using anti-GFP antibody. α-tubulin was used to verify equal loading. (B) Representative image of HEK-DDR1 cells transfected as indicated above stained with rhodamine phalloidin showing the presence of cortical actin in cells transfected with RLC-TS/DD-GFP cDNA. (C and D) Serum-starved HEK-DDR1 or HEK-DDR1-R105A cells, transfected with GFP or RLC-TS/DD-GFP cDNA, were treated with vehicle or collagen I (50 µg/ml) for 3 hours. Nuclear fractions (20 µg/lane) were analyzed by western blot for levels of DDR1 (with anti-DDR1 antibody) or RLC-TS/DD-GFP and GFP (with anti-GFP antibody). PARP1 and α-tubulin were used to verify equal loading and purity of nuclear fractions. (E) DDR1, RLC-TS/DD-GFP, and PARP1 bands of cells in (B) were quantified by densitometry. Values represent DDR1/or RLC-TS/DD-GFP/PARP1 ratio and are the mean±SD of three experiments. (F) Serum-starved HEK-DDR1 cells were treated with vehicle or collagen I (50 µg/ml) for 3 hours in the presence or absence of Latrunculin B (LATR, 10 µM). Nuclear fractions (20 µg/lane) were analyzed by western blot for levels of DDR1 and PARP1 (used to verify equal loading of nuclear fractions). (G) Nuclear DDR1 and PARP1 bands were quantified by densitometry. Values represent DDR1/PARP1 ratio and are the mean±SD of one experiment performed in triplicate. Coll I, collagen I.
To confirm that RLC-TS/DD–mediated DDR1 nuclear translocation is potentiated by collagen I stimulation, we analyzed the nuclear localization of DDR1-R105A in vehicle- or collagen I–treated HEK-DDR1-R105A cells expressing GFP or RLC-TS/DD-GFP. Although we detected both GFP and RLC-TS/DD-GFP in the nuclei, we failed to detect nuclear DDR1-R105A in either vehicle- or collagen I–treated cells (Figure 5, D and E).
G-Actin Regulates DDR1 Nuclear Translocation upon Collagen I Stimulation
NM IIA is an actin-binding protein. Increased actin depolymerization leads to the translocation of actin monomers from cytoplasm to the nucleus 50 and actin can regulate gene expression upon nuclear translocation.22 On the basis of the MS data showing that DDR1 interacts with β-actin (Supplemental Table 2), we investigated the contribution of actin in DDR1 nuclear translocation. HEK-DDR1 cells were treated with collagen I with or without Latrunculin-B, and nuclear levels of DDR1 were significantly increased in cells treated with collagen I in the presence of Latrunculin-B compared with cells treated with collagen I only (Figure 5, F and G). A plausible reason for this result is that Latrunculin-B disassembles F-actin and promotes G-actin formation.
DDR1 Forms a Nuclear Complex with NMHC-IIA and Actin upon Collagen I Stimulation
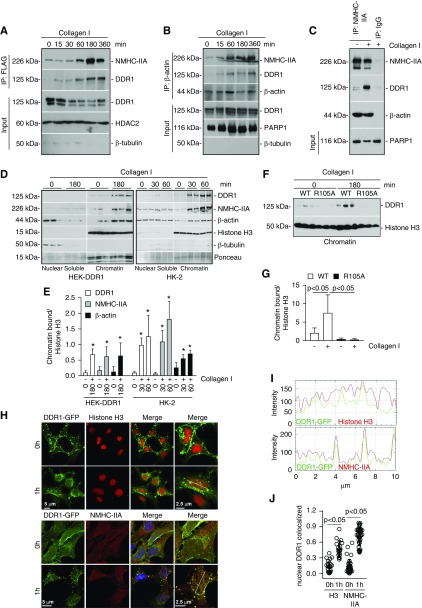
To determine whether DDR1 forms a nuclear complex with NM IIA and actin, we stimulated HEK-DDR1-FLAG cells with collagen I for different time points and then performed immunoprecipitation assays using anti-FLAG antibody (Figure 6A). No immunoprecipitated nuclear DDR1 was evident in cells treated with vehicle; however, immunoprecipitated nuclear DDR1 was evident 15 minutes after collagen I stimulation, which increased at 60 minutes and reached a plateau at 360 minutes (Figure 6A). Anti-FLAG antibody also immunoprecipitated NMHCII-A, which was particularly evident starting 60 minutes after collagen I stimulation (Figure 6A). Immunoprecipitation of HEK-DDR1 nuclear extracts with anti–β-actin antibody revealed β-actin, NMHCII-A, and DDR1 in a complex starting at 15 minutes after collagen I stimulation, which persisted up to 360 minutes (Figure 6B). Finally, immunoprecipitation of nuclear extracts with anti–NMHCII-A antibody confirmed that the three proteins form a complex only in collagen I–stimulated cells (Figure 6C). No proteins were visible in samples incubated with IgG controls (Figure 6C).
Figure 6.
DDR1, NM IIA, and β-actin exist as a nuclear complex in chromatin fractions. (A) Serum-starved HEK cells transfected with DDR1-FLAG cDNA were treated with collagen I (50 µg/ml) for the times indicated. Time 0 represents cells incubated with 20 mM acetic acid for 360 minutes. Nuclear lysates (200 µg) were immunoprecipitated with anti-FLAG antibody and the immunoprecipitates were analyzed by western blot for levels of NMHC-IIA and DDR1. Input represents non-nuclear lysates (20 µg/lane) analyzed for total levels of DDR1, or nuclear lysates analyzed for total levels of HDAC2 (nuclear marker) and β-tubulin (cytoplasmic marker). (B) Serum-starved HEK-DDR1 cells were treated as in (A). Nuclear lysates (200 µg) were immunoprecipitated with anti–β-actin antibody and the immunoprecipitates were analyzed by western blot for levels of NMHC-IIA, DDR1, and β-actin. Input represents non-nuclear lysates (20 µg/lane) analyzed for total levels of DDR1, or nuclear lysates analyzed for total levels of PARP1 and β-tubulin. (C) To verify specificity of the immunoprecipitation, serum-starved HEK-DDR1 cells were treated with vehicle and collagen I for 3 hours. Nuclear lysates (200 µg) were immunoprecipitated with anti–NMHC-IIA or IgG control and the immunoprecipitates were analyzed by western blot for levels of NMHC-IIA, DDR1, and β-actin. Input represents nuclear lysates (20 µg/lane) analyzed for levels of PARP1. (D) Serum-starved HEK-DDR1 cells or HK-2 cells were treated with collagen I (50 µg/ml) for the times indicated. Time 0 represents cells incubated with 20 mM acetic acid for 60 or 180 minutes. Nuclei were separated into nuclear soluble and chromatin fractions which were analyzed by western blot (20 µg/lane) for levels of DDR1, NMHC-IIA, β-actin, and histone H3. (E) Chromatin-bound DDR1, NMHC-IIA, β-actin, and histone H3 bands in HEK-DDR1 or HK-2 cells were quantified by densitometry. Values represent DDR1/, NMHC-IIA/, or β-actin/histone H3 ratio and are the mean±SD of three experiments. (F) Serum-starved HEK-DDR1 and HEK-DDR1-R105A cells were treated with collagen I (50 µg/ml) for the times indicated. Chromatin-bound fractions (20 µg/lane) were analyzed by western blot for level of DDR1 and histone H3. (G) Chromatin bound DDR1 and histone H3 bands were quantified by densitometry. Values represent DDR1/histone H3 ratio and are the mean±SD of two experiments performed in triplicate. (H) Confocal images of serum-starved HEK-DDR1-GFP cells treated with collagen I (50 µg/ml) for 0 or 1 hour and stained with anti-histone H3 (left panel) or anti–NMHC-IIA (right panel) antibodies. Time 0 represents cells incubated with 20 mM acetic acid for 3 hours. (I) Line scanning profile showing intensity of DDR1-GFP and histone-3 or NMHC-IIA pixels, measured along the white line in (E). (J) The degree of nuclear colocalization between DDR1 and histone H3 or NMHC-IIA was performed using Manders’ coefficient. Circles represent single cells, whereas the bars represent the mean±SD.
Nuclear DDR1 Associates with Chromatin
RTKs can regulate gene transcription by forming a nuclear complex with chromatin, chaperone proteins, and transcription factors.17 MS analysis of proteins associated with DDR1 in collagen I–treated cells revealed the presence of histone-binding protein RBBP4 (Supplemental Table 2). This nuclear protein is present in protein complexes involved in both histone acetylation and chromatin assembly.51 To validate the MS analysis, we stimulated HEK-DDR1-GFP with vehicle or collagen I and then analyzed the association between nuclear DDR1-GFP and RBBP4 by performing immunoprecipitation assays using GFP-TRAP beads. Collagen I treatment significantly increased the RBBP4/DDR1 nuclear interaction compared with that detected in vehicle-treated cells (Supplemental Figure 2). Interestingly, histone H3 (a marker of chromatin) also formed a complex together with DDR1-GFP and RBBP4 in collagen I–treated cells (Supplemental Figure 2), suggesting that nuclear DDR1 forms a complex with chromatin which is mediated by collagen I. To validate these findings, we stimulated HEK-DDR1 or HEK-DDR1-R105A cells with collagen I and then analyzed DDR1 levels in nuclear soluble and/or chromatin fractions. Undetectable and/or low levels of DDR1 were present in nuclear soluble or chromatin fractions of vehicle-treated HEK-DDR1 or HEK-DDR1-R105A cells (Figure 7, D–G). Upon collagen I treatment, DDR1 levels were significantly upregulated primarily in chromatin fractions in HEK-DDR1, but not HEK-DDR1-R105A, cells (Figure 7, D–G). Collagen I–stimulated HEK-DDR1 cells also showed significantly more chromatin-bound NMHC-IIA and β-actin compared with vehicle-treated cells (Figure 7, D and E). Similar results were obtained when analysis was performed on HK-2 cells treated with vehicle or collagen I, suggesting that endogenous DDR1 can form a complex with nuclear chromatin upon activation (Figure 7, D and E). Confocal microscopy of vehicle- and collagen I–treated HEK-DDR1-GFP cells stained with anti-histone H3 or anti–NMHC-IIA antibody further confirmed that upon collagen I treatment nuclear DDR1 is associated with chromatin and colocalizes with NMHC-IIA (Figure 7, H–J). Thus, nuclear DDR1 exists as a complex with these two proteins primarily in chromatin-rich fractions.
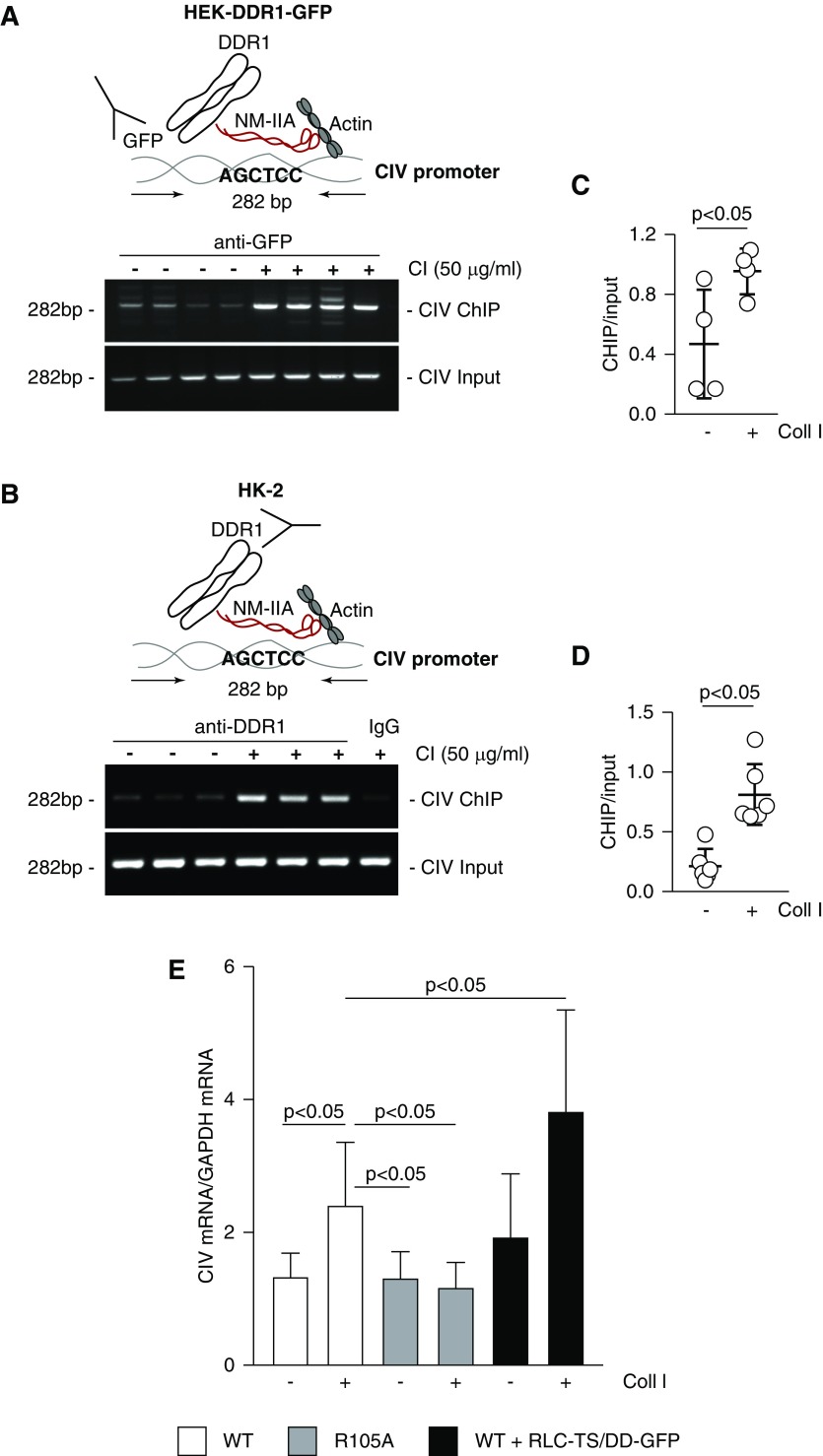
Figure 7.
DDR1 forms a complex with the human collagen IV promoter and promotes transcription. (A–D) Overview of the ChIP assay performed in (A) HEK-DDR1-GFP or (B) HK-2 cells. Crosslinked DNA-protein complexes from cells treated with acetic acid or collagen I for 30 or 60 minutes, respectively, were immunoprecipitated with (A) GFP-TRAP beads or (B) anti-DDR1 or IgG control antibody. After DNA elution and purification, DNA fragments were amplified using collagen IV (CIV) promoter primers spanning the putative NM-IIA binding site. Input represents amplified CIV promoter in total DNA isolated from untreated or collagen I–treated cells before immunoprecipitation. (C and D) CIV ChIP and input CIV bands were quantified by densitometry. Values represent ChIP/input ratio and are the mean±SD of four samples. (E) Serum-starved HEK-DDR1 (WT), HEK-DDR1-R105A, or HEK-DDR1 cells expressing RLC-TS/DD-GFP cDNA were treated with vehicle or collagen I (50 µg/ml). After 24 hours, the levels of collagen IV α1 mRNA were analyzed by RT-rqPCR. Bars and errors are the mean±SD of two to three experiments performed at least in duplicate.
Nuclear DDR1 Associates with Chromatin and Regulates Gene Transcription
Activated DDR1 induces the synthesis of collagens at both the RNA and protein level.6,43 The finding that DDR1, NMHCII-A, and β-actin exist as a complex in nuclear chromatin fractions led to the hypothesis that nuclear DDR1 may function as a cotranscription factor by binding NMHCII-A, which can act as an actin-based nuclear motor to regulate gene transcription. Analysis of the human bidirectional promoter of collagen IV α1 and α2 chains revealed the presence of four AGCTCC sequences, which have been identified as putative NM IIA binding motifs.52,53 To determine whether DDR1 forms a complex with the collagen IV promoter and whether this is dependent on collagen I stimulation, we performed the chromatin immunoprecipitation (ChIP) assay on HEK-DDR1-GFP and HK-2 cells treated with vehicle or collagen I. To do this, nuclei of cells untreated or treated with collagen I were immunoprecipitated with GFP-TRAP beads or anti-DDR1 antibody and the immunoprecipitated DNA was amplified using primers spanning an NM IIA binding motif at positions 1351–1357 (Figure 7, A–D). ChIP analysis showed a significant increase in binding of DDR1 to this collagen IV promoter region in cells stimulated with collagen I compared with cells treated with vehicle only (Figure 7, A–D).
The ChIP analysis suggests that collagen IV might be transcriptionally controlled by DDR1. To determine the functionality of our finding, we analyzed the contribution of DDR1/NM IIA interaction in driving collagen IV mRNA expression. To do this, we measured collagen IV mRNA in HEK-DDR1 and HEK-DDR1-R105A cells treated with vehicle or collagen I. Treatment with collagen I significantly enhanced collagen IV mRNA synthesis only in HEK-DDR1 cells (Figure 7E), consistent with the finding that collagen I promotes nuclear translocation and chromatin association of DDR1, but not the DDR1-R105A receptor (Figures 2, I and J and 5, C–E).
To analyze the contribution of NM IIA in driving DDR1-mediated collagen IV mRNA synthesis, we measured collagen IV mRNA levels in HEK-DDR1 cells expressing RLC-TS/DD-GFP treated with vehicle or treated with collagen I. Collagen I treatment significantly increased collagen IV mRNA levels in HEK-DDR1 cells expressing RLC-TS/DD-GFP compared with collagen I–stimulated HEK-DDR1 cells (Figure 7E). This result is consistent with our finding that collagen I treatment significantly increased DDR1 nuclear levels in HEK-DDR1 cells expressing RLC-TS/DD-GFP compared with collagen I–stimulated HEK-DDR1 cells (Figure 5, C and E).
Finally, to determine the relevance of our finding in vivo, we stained control kidneys and transplant kidney biopsy specimens with AKI with anti-NM IIA, -collagen IV, or -collagen I antibody. NM IIA and collagen expression was evident in both uninjured and injured proximal tubules (Supplemental Figure 3). However, in the tubules of transplant patients with AKI, NM IIA was evident in both cytoplasm and nuclei, reassembling the pattern observed for DDR1 (Figure 1B). In addition, increased expression of the two fibrotic collagens I and IV was observed in the basement membranes and surrounding injured proximal tubules (Supplemental Figure 3).
Discussion
DDR1 is a nonclassic RTK that activates intracellular signaling pathways upon collagen-induced receptor activation. In this study, we show that activated full-length DDR1 is able to form a complex with Sec61B, NM-IIA, and β-actin. This complex mediates DDR1 translocation to the nucleus where it interacts with chromatin and regulates collagen IV transcription (Figure 8). Our study reveals a novel mechanism whereby activated DDR1 regulates gene transcription by directly translocating to the nucleus where it acts as a cotranscription factor.
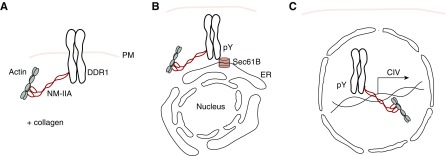
Figure 8.
Schematic representation of the steps and molecules involved in nuclear translocation of activated DDR1. (A) In unstimulated cells, DDR1 interacts with NM-IIA and β-actin at the plasma membrane (PM). (B) Upon collagen stimulation and DDR1 auto-phosphorylation (pY), DDR1/NM-IIA/β-actin complex interacts with Sec61B in the endoplasmic reticulum (ER). (C) This interaction promotes DDR1 nuclear translocation and its association to chromatin where, together with NM-IIA and β-actin, it promotes the transcription of collagen IV (CIV).
Activated DDR1 undergoes aggregation followed by cytoplasmic internalization and incorporation into early endosomes.42 DDR1 is internalized alone or together with other RTKs, because IGF-I induces DDR1 tyrosine phosphorylation, cointernalization with the IGF-I receptor, and incorporation into early endosomes.54 Internalized RTKs can recycle back to the plasma membranes, be degraded, or undergo an endosome/Golgi/endoplasmic reticulum retrograde pathway. Once in the endoplasmic reticulum, RTKs can be transported to the nucleus via an interaction with Sec61B. We provide MS and biochemical evidence that activated DDR1 interacts with Sec61B in endoplasmic reticulum–enriched fractions. Sec61B regulates the retrograde pathway and nuclear translocation of full-length growth factor receptors via two distinct retrograde pathways.45 Both pathways require Sec61B and a cytosolic or an endoplasmic reticulum–associated importin. Our MS analysis revealed the presence of importin-β in vehicle- and collagen I–treated DDR1-expressing cells, and importin-5 only in collagen I–stimulated cells (Supplemental Table 2). Importin-5 is a member of the importin β family,55 which acts as a nuclear transport receptor for nuclear myosin 1,56 transcription factors such as Jun and SMADS,57,58 and G-protein–coupled receptors.59 Thus, it is conceivable that importin-β or importin-5, together with Sec61B, regulates collagen I–induced DDR1 nuclear translocation.
DDR1, NMHC-IIA, and β-actin exist as a nuclear complex associated with chromatin. DDR1, NMHC-IIA, and β-actin do not contain a putative NLS domain, yet NMHC-IIA and β-actin are known to translocate to the nucleus. β-actin interacts with cofilin which has an NLS and promotes the nuclear translocation of G-actin.60 We identified cofilin 1 peptides by MS in vehicle- and collagen I–treated cells (Supplemental Table 2), suggesting that an interaction between β-actin and cofilin occurs and promotes translocation of a DDR1/NMHC-IIA/β-actin complex into the nucleus.
Nuclear NMHC-IIA and β-actin act as nuclear chaperones and cotranscription factors. NMHC-IIA regulates annexin I nuclear translocation 61 and, together with α and β actin, regulates ICAM transcription via interaction with its promoter.52 Nuclear β-actin promotes eNOS transcription by binding repeats that have a cis-acting role in eNOS promoter function.62 On the basis of our finding that upon collagen stimulation DDR1 interacts with the collagen IV promoter and with RBBP4, we propose that DDR1 association with chromatin enhances NMHC-IIA and β-actin transcription activity. Consistent with this statement, the transcription of collagen IV, whose promoter contains putative NM II responsive elements, is enhanced in cells expressing DDR1 able to translocate to the nucleus after collagen I stimulation, and is further stimulated in cells expressing both DDR1 and a constitutively active myosin RLC.
RTKs translocate to the nucleus in a ligand-dependent and ligand-independent manner. Our MS analysis suggests that β-actin and NMHC-IIA associate with DDR1 in the absence of collagen; however, nuclear DDR1 is only evident after collagen I stimulation. This observation, together with the fact that the mutant DDR1-R105A fails to translocate to the nucleus after collagen I treatment, suggests that both a collagen I/DDR1 interaction and DDR1 activation are required for DDR1 nuclear translocation. Our finding that DDR1 interacts with NM-IIA agrees with the finding that these two proteins colocalize in the inner ear of mice.20 Furthermore, DDR1 and NMHC-IIA interaction, which requires the kinase domain of DDR1, regulates the assembly of NMHC-IIA into filaments and promotes cell migration.21 Our study reveals a novel function for NMHC-IIA, which is to localize DDR1 to the nucleus. The finding that blocking NM-IIA function by blebbistatin reduces nuclear DDR1 localization further supports a role of NMHC-IIA in regulating DDR1 nuclear translocation. Interestingly, in cells expressing artificially activated NM IIA, DDR1 but not DDR1-R105A translocates the nucleus also in the absence of collagen stimulation. A plausible reason is that low basal (yet undetectable at western blot level) activation of DDR1 by endogenously produced collagen can promote nuclear translocation of DDR1 in the absence of exogenously administered collagen and in the presence of activated NM IIA. On the contrary, DDR1-R105A cannot be activated by endogenous collagen and it cannot be translocated to the nucleus also in the presence of activated NM IIA.
Upon collagen I stimulation, DDR1 regulates the expression of several genes by promoting the activation of transcription factors. In breast cancer cells, DDR1 increases NFkB-DNA binding thus promoting the expression of cyclooxygenase 2.63 In colon cancer cells, DDR1 promotes Notch1 cleavage by γ-secretase, thus enhancing nuclear translocation of cleaved Notch1 and transcription of prosurvival genes such as Hes1 and Hes2.64 In this study, we provide evidence that nuclear DDR1 can regulate gene transcription by interacting with chromatin and acting as cotranscription factors together with NMHCII-A and β-actin.
We show that DDR1 is upregulated by injured tubular cells and that nuclear translocation of DDR1 regulates the synthesis of collagen IV, a DDR1 ligand found in basement membranes.65 Collagen IV, together with collagen I, is a major ECM component upregulated in fibrosis.66 Collagen IV is produced primarily by epithelial cells and, in models of kidney tubule–mediated injury, is deposited primarily along injured tubules (see also Supplemental Figure 3). Collagen I is also upregulated in kidney fibrosis and is considered the classic hallmark of tubule-interstitial fibrosis. A key question is why DDR1 is also upregulated in injured tubules. A plausible hypothesis is that increased DDR1 could strengthen the adhesion of injured proximal tubule cells to collagen IV, thus protecting them from injury or promoting repair. This event might paradoxically contribute to the development of fibrosis by stimulating excessive production of collagen upon DDR1 activation by its two major ligands.
In conclusion, we provide evidence that activated DDR1 translocates to the nucleus, localizes to chromatin, and regulates the transcription of profibrotic genes, including collagen IV. These findings, together with the observation that increased nuclear levels of DDR1 are evident in injured human and injured murine kidneys, reveal a previously unrecognized mechanism whereby DDR1 promotes the synthesis of profibrotic molecules.
Disclosures
None.
Funding
This work was in part supported by Veterans Affairs Merit Reviews 1I01BX002025 (Dr. Pozzi) and 1I01BX002196 (Dr. Zent); National Institutes of Health grants R01-DK119212 (Dr. Pozzi, Dr. Borza), R01-DK069921 (Dr. Zent), R01-DK112688 (Dr. de Caestecker), R01-DK099467 (Dr. Vanacore), R01-DK56942 (Dr. Fogo), and P30-DK114809 (Dr. Pozzi, Dr. Zhang, Dr. Zent, Dr. de Caestecker, Dr. Fogo); and the Department of Defense grant DOD-PR161028 (Dr. de Caestecker).
Supplementary Material
Acknowledgments
We are thankful to Ellen Donnert for her technical help with immunohistochemistry and double immunofluorescence.
Dr. Pozzi is the recipient of a Veterans Affairs Senior Research Career Scientist Award.
Dr. Chiusa, Dr. Hu, Dr. Liao, and Dr. Su conducted plasmid preparations, immunohistochemistry, cell biology, cell fractionations, immunoprecipitations, quantification, and western blots. Dr. Chiusa wrote the first draft of the manuscript. Dr. Borza generated cells expressing wild-type and mutated DDR1. Dr. de Caestecker and Dr. Skrypnyk helped with the establishment of the ischemia/reperfusion injury in mice, organ isolation, and preparation. Dr. Fogo provided samples of human kidneys and performed immunohistochemistry. Dr. Pedchenko helped with the design of primers selective for human collagen IV promoter. Dr. Li generated cells expressing DDR1-GFP fusion protein. Dr. Zhang performed immunofluorescence. Dr. Basak and Dr. Vanacore performed mass spectrometry analysis and analyzed the resultant data. Dr. Pozzi designed and supervised the execution of the experiments in this manuscript. Dr. Hudson, Dr. Zent, and Dr. Pozzi wrote the final version of the manuscript. All authors contributed to and reviewed the final manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018111160/-/DCSupplemental.
Supplemental Table 1. List of primary antibodies used for western blot.
Supplemental Table 2. Mass spectrometry identification of some of the immunoprecipitated DDR1-interacting proteins from HEK-DDR1-GFP expressing cells treated with either vehicle (acetic acid) or collagen I.
Supplemental Figure 1. Mass spectrometry identification of DDR1 phosphotyrosine peptides in collagen I–treated HEK-DDR1-GFP cells.
Supplemental Figure 2. Nuclear DDR1 forms a complex with RBBP4.
Supplemental Figure 3. Analysis of collagens and NMHC-IIA localization in injured human kidneys.
References
- 1.Xu H, Raynal N, Stathopoulos S, Myllyharju J, Farndale RW, Leitinger B: Collagen binding specificity of the discoidin domain receptors: Binding sites on collagens II and III and molecular determinants for collagen IV recognition by DDR1. Matrix Biol 30: 16–26, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leitinger B: Molecular analysis of collagen binding by the human discoidin domain receptors, DDR1 and DDR2. Identification of collagen binding sites in DDR2. J Biol Chem 278: 16761–16769, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Leitinger B: Discoidin domain receptor functions in physiological and pathological conditions. Int Rev Cell Mol Biol 310: 39–87, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerrot D, Kerroch M, Placier S, Vandermeersch S, Trivin C, Mael-Ainin M, et al.: Discoidin domain receptor 1 is a major mediator of inflammation and fibrosis in obstructive nephropathy. Am J Pathol 179: 83–91, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hidalgo-Carcedo C, Hooper S, Chaudhry SI, Williamson P, Harrington K, Leitinger B, et al.: Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat Cell Biol 13: 49–58, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borza CM, Su Y, Tran TL, Yu L, Steyns N, Temple KJ, et al.: Discoidin domain receptor 1 kinase activity is required for regulating collagen IV synthesis. Matrix Biol 57–58: 258–271, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao H, Chakraborty G, Zhang Z, Akalay I, Gadiya M, Gao Y, et al.: Multi-organ site metastatic reactivation mediated by non-canonical discoidin domain receptor 1 signaling. Cell 166: 47–62, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hou G, Vogel W, Bendeck MP: The discoidin domain receptor tyrosine kinase DDR1 in arterial wound repair. J Clin Invest 107: 727–735, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avivi-Green C, Singal M, Vogel WF: Discoidin domain receptor 1-deficient mice are resistant to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med 174: 420–427, 2006 [DOI] [PubMed] [Google Scholar]
- 10.El Azreq MA, Kadiri M, Boisvert M, Pagé N, Tessier PA, Aoudjit F: Discoidin domain receptor 1 promotes Th17 cell migration by activating the RhoA/ROCK/MAPK/ERK signaling pathway. Oncotarget 7: 44975–44990, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juin A, Di Martino J, Leitinger B, Henriet E, Gary AS, Paysan L, et al.: Discoidin domain receptor 1 controls linear invadosome formation via a Cdc42-Tuba pathway. J Cell Biol 207: 517–533, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lino M, Wan MH, Rocca AS, Ngai D, Shobeiri N, Hou G, et al.: Diabetic vascular calcification mediated by the collagen receptor discoidin domain receptor 1 via the phosphoinositide 3-kinase/Akt/runt-related transcription factor 2 signaling axis. Arterioscler Thromb Vasc Biol 38: 1878–1889, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorison A, Dussaule JC, Chatziantoniou C: The role of discoidin domain receptor 1 in inflammation, fibrosis and renal disease. Nephron 137: 212–220, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Song S, Rosen KM, Corfas G: Biological function of nuclear receptor tyrosine kinase action. Cold Spring Harb Perspect Biol 5 pii: a009001, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo HW, Hsu SC, Ali-Seyed M, Gunduz M, Xia W, Wei Y, et al.: Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell 7: 575–589, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Maher PA: Nuclear Translocation of fibroblast growth factor (FGF) receptors in response to FGF-2. J Cell Biol 134: 529–536, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HH, Wang YN, Hung MC: Non-canonical signaling mode of the epidermal growth factor receptor family. Am J Cancer Res 5: 2944–2958, 2015 [PMC free article] [PubMed] [Google Scholar]
- 18.Liao HJ, Carpenter G: Role of the Sec61 translocon in EGF receptor trafficking to the nucleus and gene expression. Mol Biol Cell 18: 1064–1072, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stachowiak MK, Fang X, Myers JM, Dunham SM, Berezney R, Maher PA, et al.: Integrative nuclear FGFR1 signaling (INFS) as a part of a universal “feed-forward-and-gate” signaling module that controls cell growth and differentiation. J Cell Biochem 90: 662–691, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Meyer Zum Gottesberge AM, Hansen S: The collagen receptor DDR1 co-localizes with the non-muscle myosin IIA in mice inner ear and contributes to the cytoarchitecture and stability of motile cells. Cell Tissue Res 358: 729–736, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Huang Y, Arora P, McCulloch CA, Vogel WF: The collagen receptor DDR1 regulates cell spreading and motility by associating with myosin IIA. J Cell Sci 122: 1637–1646, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Viita T, Vartiainen MK: From cytoskeleton to gene expression: Actin in the nucleus. Handb Exp Pharmacol 235: 311–329, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Zheng B, Han M, Bernier M, Wen JK: Nuclear actin and actin-binding proteins in the regulation of transcription and gene expression. FEBS J 276: 2669–2685, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falahzadeh K, Banaei-Esfahani A, Shahhoseini M: The potential roles of actin in the nucleus. Cell J 17: 7–14, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu YZ, Thuraisingam T, Morais DA, Rola-Pleszczynski M, Radzioch D: Nuclear translocation of β-actin is involved in transcriptional regulation during macrophage differentiation of HL-60 cells. Mol Biol Cell 21: 811–820, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y, Wang J, Jiang H, Yu Z, Li X, Shi J: Following OGD/R, annexin 1 nuclear translocation and subsequent induction of apoptosis in neurons are assisted by myosin IIA in a TRPM7 kinase-dependent manner. Mol Neurobiol 51: 729–742, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Li LY, Chen H, Hsieh YH, Wang YN, Chu HJ, Chen YH, et al.: Nuclear ErbB2 enhances translation and cell growth by activating transcription of ribosomal RNA genes. Cancer Res 71: 4269–4279, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Méndez J, Stillman B: Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle: Assembly of prereplication complexes in late mitosis. Mol Cell Biol 20: 8602–8612, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skrypnyk NI, Voziyan P, Yang H, de Caestecker CR, Theberge MC, Drouin M, et al.: Pyridoxamine reduces postinjury fibrosis and improves functional recovery after acute kidney injury. Am J Physiol Renal Physiol 311: F268–F277, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma ZQ, Dasari S, Chambers MC, Litton MD, Sobecki SM, Zimmerman LJ, et al.: IDPicker 2.0: Improved protein assembly with high discrimination peptide identification filtering. J Proteome Res 8: 3872–3881, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mellacheruvu D, Wright Z, Couzens AL, Lambert JP, St-Denis NA, Li T, et al.: The CRAPome: A contaminant repository for affinity purification-mass spectrometry data. Nat Methods 10: 730–736, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basak T, Vega-Montoto L, Zimmerman LJ, Tabb DL, Hudson BG, Vanacore RM: Comprehensive characterization of glycosylation and hydroxylation of basement membrane collagen IV by high-resolution mass spectrometry. J Proteome Res 15: 245–258, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabb DL, Fernando CG, Chambers MC: MyriMatch: Highly accurate tandem mass spectral peptide identification by multivariate hypergeometric analysis. J Proteome Res 6: 654–661, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernández-Imaz E, Martín Y, de Conti L, Melean G, Valero A, Baralle M, et al.: Functional analysis of mutations in Exon 9 of NF1 reveals the presence of several elements regulating splicing. PLoS One 10: e0141735, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerroch M, Guerrot D, Vandermeersch S, Placier S, Mesnard L, Jouanneau C, et al.: Genetic inhibition of discoidin domain receptor 1 protects mice against crescentic glomerulonephritis. FASEB J 26: 4079–4091, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Moll S, Yasui Y, Abed A, Murata T, Shimada H, Maeda A, et al.: Selective pharmacological inhibition of DDR1 prevents experimentally-induced glomerulonephritis in prevention and therapeutic regime. J Transl Med 16: 148, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee R, Eidman KE, Kren SM, Hostetter TH, Segal Y: Localization of discoidin domain receptors in rat kidney. Nephron Exp Nephrol 97: e62–e70, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Flamant M, Placier S, Rodenas A, Curat CA, Vogel WF, Chatziantoniou C, et al.: Discoidin domain receptor 1 null mice are protected against hypertension-induced renal disease. J Am Soc Nephrol 17: 3374–3381, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Carpenter G, Liao HJ: Receptor tyrosine kinases in the nucleus. Cold Spring Harb Perspect Biol 5: a008979, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Curat CA, Eck M, Dervillez X, Vogel WF: Mapping of epitopes in discoidin domain receptor 1 critical for collagen binding. J Biol Chem 276: 45952–45958, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Fu HL, Valiathan RR, Payne L, Kumarasiri M, Mahasenan KV, Mobashery S, et al.: Glycosylation at Asn211 regulates the activation state of the discoidin domain receptor 1 (DDR1). J Biol Chem 289: 9275–9287, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mihai C, Chotani M, Elton TS, Agarwal G: Mapping of DDR1 distribution and oligomerization on the cell surface by FRET microscopy. J Mol Biol 385: 432–445, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borza CM, Pozzi A: Discoidin domain receptors in disease. Matrix Biol 34: 185–192, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdulhussein R, McFadden C, Fuentes-Prior P, Vogel WF: Exploring the collagen-binding site of the DDR1 tyrosine kinase receptor. J Biol Chem 279: 31462–31470, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Chen MK, Hung MC: Proteolytic cleavage, trafficking, and functions of nuclear receptor tyrosine kinases. FEBS J 282: 3693–3721, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coelho NM, Arora PD, van Putten S, Boo S, Petrovic P, Lin AX, et al.: Discoidin domain receptor 1 mediates myosin-dependent collagen contraction. Cell Reports 18: 1774–1790, 2017 [DOI] [PubMed] [Google Scholar]
- 47.Kim JH, Wang A, Conti MA, Adelstein RS: Nonmuscle myosin II is required for internalization of the epidermal growth factor receptor and modulation of downstream signaling. J Biol Chem 287: 27345–27358, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR: Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 10: 778–790, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hays T, Ma’ayan A, Clark NR, Tan CM, Teixeira A, Teixeira A, et al.: Proteomics analysis of the non-muscle myosin heavy chain IIa-enriched actin-myosin complex reveals multiple functions within the podocyte. PLoS One 9: e100660, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saha T, Guha D, Manna A, Panda AK, Bhat J, Chatterjee S, et al.: G-actin guides p53 nuclear transport: Potential contribution of monomeric actin in altered localization of mutant p53. Sci Rep 6: 32626, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Millard CJ, Varma N, Saleh A, Morris K, Watson PJ, Bottrill AR, et al.: The structure of the core NuRD repression complex provides insights into its interaction with chromatin. eLife 5: e13941, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Q, Sarna SK,: Nuclear myosin II regulates the assembly of preinitiation complex for ICAM-1 gene transcription. Gastroenterology 137: 1051–1060, 1060.e1–1060.e3, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zerbino DR, Wilder SP, Johnson N, Juettemann T, Flicek PR: The ensembl regulatory build. Genome Biol 16: 56, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malaguarnera R, Nicolosi ML, Sacco A, Morcavallo A, Vella V, Voci C, et al.: Novel cross talk between IGF-IR and DDR1 regulates IGF-IR trafficking, signaling and biological responses. Oncotarget 6: 16084–16105, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flores K, Seger R: Stimulated nuclear import by β-like importins. F1000Prime Rep 5: 41, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dzijak R, Yildirim S, Kahle M, Novák P, Hnilicová J, Venit T, et al.: Specific nuclear localizing sequence directs two myosin isoforms to the cell nucleus in calmodulin-sensitive manner. PLoS One 7: e30529, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baas R, Sijm A, van Teeffelen HA, van Es R, Vos HR, Marc Timmers HT: Quantitative proteomics of the SMAD (Suppressor of Mothers against Decapentaplegic) transcription factor family identifies importin 5 as a bone morphogenic protein receptor SMAD-specific importin. J Biol Chem 291: 24121–24132, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waldmann I, Wälde S, Kehlenbach RH: Nuclear import of c-Jun is mediated by multiple transport receptors. J Biol Chem 282: 27685–27692, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Bhosle VK, Rivera JC, Zhou TE, Omri S, Sanchez M, Hamel D, et al.: Nuclear localization of platelet-activating factor receptor controls retinal neovascularization. Cell Discov 2: 16017, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pendleton A, Pope B, Weeds A, Koffer A: Latrunculin B or ATP depletion induces cofilin-dependent translocation of actin into nuclei of mast cells. J Biol Chem 278: 14394–14400, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Li X, Zhao Y, Xia Q, Zheng L, Liu L, Zhao B, et al.: Nuclear translocation of annexin 1 following oxygen-glucose deprivation-reperfusion induces apoptosis by regulating Bid expression via p53 binding. Cell Death Dis 7: e2356, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ou H, Shen YH, Utama B, Wang J, Wang X, Coselli J, et al.: Effect of nuclear actin on endothelial nitric oxide synthase expression. Arterioscler Thromb Vasc Biol 25: 2509–2514, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Das S, Ongusaha PP, Yang YS, Park JM, Aaronson SA, Lee SW: Discoidin domain receptor 1 receptor tyrosine kinase induces cyclooxygenase-2 and promotes chemoresistance through nuclear factor-κB pathway activation. Cancer Res 66: 8123–8130, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Ross DA, Kadesch T: The notch intracellular domain can function as a coactivator for LEF-1. Mol Cell Biol 21: 7537–7544, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pozzi A, Yurchenco PD, Iozzo RV: The nature and biology of basement membranes. Matrix Biol 57–58: 1–11, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pozzi A, Voziyan PA, Hudson BG, Zent R: Regulation of matrix synthesis, remodeling and accumulation in glomerulosclerosis. Curr Pharm Des 15: 1318–1333, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.