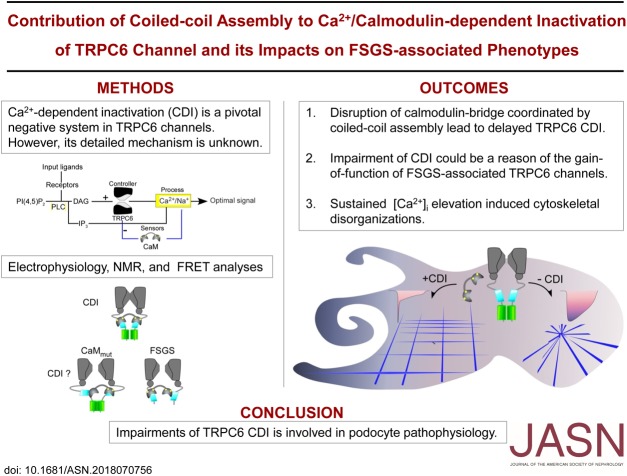
Significance Statement
TRPC6 is a receptor-activated nonselective cation channel. Naturally occurring mutations in this channel are associated with FSGS. FSGS-associated TRPC6 channel mutations appear to have a “gain-of-function” mechanism, but the exact mechanism remains unclear. The authors show that negative feedback regulation induced by cellular calcium levels, called Ca2+-dependent inactivation (CDI), is impaired in FSGS-associated TRPC6 channel mutations. Dysfunctional coiled-coil assembly in the mutated TRPC6 disrupts calmodulin bridging which is essential for CDI. The authors reveal how disruption in a Ca2+-dependent regulatory mechanism may play a role in FSGS.
Keywords: electrophysiology, chronic kidney disease, focal segmental glomerulosclerosis, TRPC channel, Calcium signal, podocyte
Visual Abstract
Abstract
Background
TRPC6 is a nonselective cation channel, and mutations of this gene are associated with FSGS. These mutations are associated with TRPC6 current amplitude amplification and/or delay of the channel inactivation (gain-of-function phenotype). However, the mechanism of the gain-of-function in TRPC6 activity has not yet been clearly solved.
Methods
We performed electrophysiologic, biochemical, and biophysical experiments to elucidate the molecular mechanism underlying calmodulin (CaM)-mediated Ca2+-dependent inactivation (CDI) of TRPC6. To address the pathophysiologic contribution of CDI, we assessed the actin filament organization in cultured mouse podocytes.
Results
Both lobes of CaM helped induce CDI. Moreover, CaM binding to the TRPC6 CaM-binding domain (CBD) was Ca2+-dependent and exhibited a 1:2 (CaM/CBD) stoichiometry. The TRPC6 coiled-coil assembly, which brought two CBDs into adequate proximity, was essential for CDI. Deletion of the coiled-coil slowed CDI of TRPC6, indicating that the coiled-coil assembly configures both lobes of CaM binding on two CBDs to induce normal CDI. The FSGS-associated TRPC6 mutations within the coiled-coil severely delayed CDI and often increased TRPC6 current amplitudes. In cultured mouse podocytes, FSGS-associated channels and CaM mutations led to sustained Ca2+ elevations and a disorganized cytoskeleton.
Conclusions
The gain-of-function mechanism found in FSGS-causing mutations in TRPC6 can be explained by impairments of the CDI, caused by disruptions of TRPC’s coiled-coil assembly which is essential for CaM binding. The resulting excess Ca2+ may contribute to structural damage in the podocytes.
Influxes of calcium ion (Ca2+) through calcium channels initiate crucial chains of events that lead to numerous cellular biologic activities, including morphologic dynamics.1 However, excessive Ca2+ influx can trigger aberrant Ca2+-mediated signaling and occasionally lead to diseases. In renal podocytes, alterations in Ca2+ signaling have been shown to cause proteinuria and progressive disease, wherein Transient Receptor Potential Canonical 6 (TRPC6) channel is considered a key molecule.2–4 TRPC6 is a tetrameric Ca2+-permeable nonselective cation channel, and is widely expressed in human tissues including kidney.5,6 Mutations of this gene are known to be associated with a severe form of kidney disease, FSGS.2–4,7 To date, >20 FSGS-associated mutations have been reported in TRPC6 channels. These mutations often exhibit TRPC6 current amplitude amplification and/or delay of the channel inactivation (i.e., gain-of-function phenotype).8,9 This phenotype may occur because FSGS-associated TRPC6 channel mutations are located on the cytosolic side, where a regulatory domain affected by intracellular events may be present. In previous reports, couplings between TRPC6 and nephrotic proteins have been shown to regulate surface expression of TRPC6.10,11 Although enhanced channel expression could explain the current amplification, it is not fully accountable for the delayed inactivation observed with some FSGS mutations, such as in TRPC6K874×.9 Therefore, the mechanism of the gain-of-function in TRPC6 activity has not yet been clearly solved.
TRPC channels are activated by the stimulation from phospholipase C (PLC)–coupled receptors.12–14 The activated PLC hydrolyzes phosphatidylinositol-(4,5)-bisphosphate [PI(4,5)P2] into diacylglycerol (DAG), the physiologic agonist of TRPC3/6/7 channels,12 and inositol-triphosphate (IP3), a second messenger triggering Ca2+ release from intracellular stores. Consequently, TRPC channels are exposed to substantial elevations in the intracellular Ca2+ concentration ([Ca2+]i) via Ca2+ influx through the channels as well as Ca2+ release from internal stores. Ca2+-dependent regulation is the most common regulatory mechanism fine-tuning Ca2+-influx, including TRPC channels. In the case of TRPC4/5 channels, elevation of [Ca2+]i leads to potentiation of channel activity,15,16 whereas it generally inhibits other TRPC channels.17–20 This negative regulation is termed Ca2+-dependent inactivation (CDI) and is crucial for the intrinsic feedback system to prevent Ca2+ overload.
Theoretic studies of CDI or inactivation of an open-state in the voltage-gated ion channels have proposed a clear correlation between current amplitudes and inactivation kinetics.21,22 These reports suggest that the involvement of CDI could explain the phenotypes reported in FSGS mutations.3,4,9,11,23,24 Nevertheless, both the precise mechanism of CDI in TRPC6 channels and its physiologic effect remain largely unknown.
We initially expected that CDI in TRPC6 channels might be caused by depletion of PI(4,5)P2, because PI(4,5)P2-mediated regulation has been proposed for many types of TRP channels.25,26 However, our preliminary data suggest that under a low Ca2+-buffering condition, the reduction of PI(4,5)P2 is not fast enough to cause the TRPC6 current inactivation (Supplemental Figure 1E). Alternatively, the Ca2+-sensing protein calmodulin (CaM) could be considered in CDI. CaM is a ubiquitous calcium sensor expressed in all eukaryotic cells and is a bilobed (N-lobe and C-lobe) molecule which regulates various targets including ion channels. CaM binding to TRPC6 has been shown to occur within the channel’s CaM-binding domain (CBD: residues 853−874).17,27–29 Therefore, to understand the mechanism underlying CDI of TRPC6, we considered how CaM regulates TRPC6 by coexpressing the channel with the Ca2+-insensitive CaM mutants. Through electrophysiologic and biophysical experiments, we showed that both lobes of CaM are important for CDI and are bound to the CBDs with 1:2 (CaM/CBD) stoichiometry. A supporting element for this stoichiometry may be the association of coiled-coil segments (residues 877−920) which are located downstream of the CBD, deletion of which abolishes CDI. We, therefore, propose that CDI can be explained by a CaM-bridge mechanism where CaM binds to two CBDs in neighboring TRPC6 subunits, and that respective coiled-coil interaction coordinates the position of CBD.
Coiled-coil assembly of TRPC6 is a costructural element for CaM binding and CDI, and FSGS-associated mutations within the coiled-coil region would be expected to have an effect on CDI. Impairment of CDI by FSGS-associated mutations was clearly observed in human embryonic kidney (HEK293) cell expression systems, and was confirmed in cultured podocytes. Podocytes expressing FSGS-associated TRPC6 channels showed a sustained Ca2+ influx after receptor stimulation and often exhibited disorganization of actin stress fibers. Such disruption of cytoskeletal structures may represent the characteristic effacements of podocyte foot processes. Overall, our study reveals the mechanism underlying CDI of TRPC6 channels, and suggests the Ca2+-insensitive channels or the regulation by CaM as effective therapeutic targets for the treatment of FSGS.
Methods
Molecular Biology
All TRPC6 channels were constructed from a plasmid encoding human TRPC6 (GenBank accession no. NM_004621 provided by T. Hofmann) in pIRESn vector that was modified from the vector pIRES2 (Invitrogen) via deletion of the IRES to EGFP coding region. Mutations in the CBD or coiled-coil of TRPC6 (including the deletion of coiled-coil mutation which was terminated at 875 a.a.) were generated by overlap extension PCR utilizing mutagenic primers in which segments were replaced into the SacI and XbaI sites of the TRPC6 expression vector. For GFP-fused TRPC6 channels, AcGFP (Clontech) was inserted into the N-terminal side of TRPC6 channels at NheI and KpnI sites and transfected in podocytes. The sequences of primers are shown in the Supplemental information (Supplemental Table 3). CaM mutations were made by a similar mutagenesis strategy; CaM1234 (four Ca2+ binding sites mutation, D21A, D57A, D90A, D130A), CaM12, and CaM34 from the rat CaMWT in pCI-neo expression vector as a template.30 Coding regions at 1−75 a.a. and 76−145 a.a. of CaMWT obtained using PCR were respectively cloned to generate the CaM N-lobe (CaMΔC) and C-lobe (CaMΔN) in pCI-neo. For FRET measurements, monomeric superenhanced cyan fluorescence protein (CFP)–fused CaM12,31 CaM34, and CaM1234/pIRESn constructs were generated by PCR and substituted for CFP-CaMWT as in a previous report.27 The cDNAs encoding the CBD segment of a human TRPC6 CBD segment spanning residues 853−874 a.a. and the CBD with the coiled-coil (CBD-CC) segment spanning residues 853−927 a.a. were generated by PCR and digested at the NotI and XbaI sites, after which YFP or CFP was fused to the N-terminal side of the CBD or CBD-CC at the NheI and NotI sites in the pIRESn expression vector. A pEF-BOS expression vector encoding human muscarinic type 1 receptor (M1R) was provided by T. Haga (Gakushuin University).
Cell Culture
HEK293 cells were obtained from the ATCC, and maintained in DMEM (Gibco) supplemented with 10% FBS and penicillin/streptomycin (Invitrogen), at 37°C and 5% CO2. Transfections for electrophysiology and FRET measurements were achieved with SuperFect (Qiagen) following the manufacturers recommendations. Transfected HEK293 cells were used within 48 hours. Immature mouse podocyte clone 5 (MPC-5) cells were conditionally immortalized and cultured at 33°C in RPMI-1640 medium with 10% FBS, penicillin/streptomycin, and 50 U/ml recombinant mouse IFN-γ (Wako) during the initial culture. This was replaced with 10 U/ml IFN-γ thereafter until the cell number was sufficient for maturation.32 To permit podocyte maturation, cells were switched to the 37°C incubator, and IFN-γ was omitted from the culture medium, followed by cultivation for at least 14 days. Transfection of the mature podocytes was achieved by using Lipofectamine 3000 (Invitrogen) with a standard protocol. pEGFP-N1 (Clontech) was cotransfected as a transfection marker in the electrophysiologic and the Ca2+-imaging experiments (0.3 μg for HEK293, 0.6 μg for podocytes). The transfected cells were used within 24–48 hours.
Electrophysiology
Current signals were recorded with a low noise patch-clamp amplifier (AxoPatch 200B; Axon Instruments). Signals were filtered at 2 kHz and digitized at 1 kHz. Internal solutions contained (in mM): 120 CsOH, 120 aspartate, 20 CsCl, 2 MgCl2, 1 EGTA, 0.3 CaCl2, 2 ATP-Na2, 0.1 GTP, 10 HEPES, and 10 glucose, pH 7.2 (adjusted with Tris-Base), approximately 290 mOSm. Fire-polished patch pipettes had 5−8 MΩ resistance when backfilled with the internal solution. The external solution contained (in mM): 140 NaCl, 5 KCl, 1.8 CaCl2, 1.2 MgCl2, 10 HEPES, and 10 glucose, pH 7.4, 297−300 mOsm (adjusted with glucose). To confirm TRPC6 currents, NaCl was replaced with an equal concentration of N-methyl-D-gluconate (NMDG) in the external solution. Currents were evoked by applying 100 μM carbamylcholine chloride (carbachol, CCh; Sigma). The currents were recorded at a holding potential of −50 mV. For inside-out recordings, the external solution contained (in mM): 120 CsOH, 120 aspartate, 20 CsCl, 2 MgSO4, 0–3 CaCl2, 2 EGTA, 2 HEDTA, 10 HEPES, 10 glucose, 2 ATP-Na2, 0.1 GTP, and 0.001 CaM protein, pH 7.2. Free Ca2+ concentrations in the solutions were calculated using MaxChelator software (https://maxchelator.stanford.edu). The pipette solution contained (in mM): 140 NaCl, 5 KCl, 0.1 CaCl2, 2 MgSO4, 10 HEPES, 10 glucose, and 0.0003 CCh, pH 7.4 (adjusted with Tris-base). Pipettes were coated with Sylgard 184 (Dow Corning) to minimize noise. Data were sampled at 20 kHz and filtered at 5 kHz (−3 dB, 4-pole Bessel). During the inside-out experiment, patch-membrane was clamped at +70 mV. For podocyte whole-cell patch-clamp experiments, the external solution contained in (mM): 130 NaOH, 135 MeSO4, 5 TEA-OH, 1.8 CaCl2, 1.2 MgCl2, 10 HEPES, 10 Glucose, and 0.1 4,4′-Diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS; Cayman Chemical), pH 7.4 (adjusted with NaOH). The composition of the internal solution and the recording conditions were the same as in HEK293 cell experiments. During experiments, HEK293 or podocyte cells were continuously perfused with a gravity-fed external solution at a flow rate of 0.5 ml/min. The perfusion could be turned on and off using electromagnetic solenoid microvalves (Takasago), which enabled us to exchange the external solutions within 1 second.
In-Cell FRET Measurements
The Ca2+-dependent interaction between CaM and CBDs was confirmed using the FRET technique as described previously.27 In brief, transfected cells were incubated on coverslips and loaded with 5 μM Fura-2/AM for 30 minutes. Later, the cells were mounted on an inverted fluorescence microscope (IX83; Olympus) in a glass-bottom dish. Cells were illuminated with five filter cubes, of which two were for Fura-2 and three were for C/Y-FRET. Filter cubes (Olympus) used were as follows (excitation, dichroic, emission): 340 cube (D340/20, 410DCLP, D510/40m) and 360 cube (D360/10, 410DCLP, D510/40m) were used for Fura-2 measurement. CFP cube (D435/20M, 455DCLP, D480/30M), YFP cube (D500/25, 515DCLP, D535/30M), and FRET cube (D435/20M, 455DCLP, D535/30M) were used for the FRET measurement. To minimize crosstalk between fluorescence emissions, bleed-through of Fura-2 in the CFP, YFP, and FRET cubes was calculated by measuring nontransfected cells, and these were subtracted. The background intensity, captured using the corresponding filter setting with untransfected cells, was also subtracted from specimen fluorescence signals. For FRET without Ca2+-imaging, data were acquired and analyzed by the 33-FRET method as previously described.33 By fitting the curve of FR−[D]free to the following equation, the (relative) affinity (Kd,eff) of FRET pairs was evaluated: FR=1+dFRmax(1+Kd,eff/[D]free), where dFRmax represents the maximum differential FRET ratio (FR) and [D]free denotes the equivalent free donor (CFP-tagged) concentration.
Protein Purification
Rat CaM protein encoded by pET24b (Novagen) was expressed in Escherichia coli Tuner (DE3) pLysS (Novagen) upon induction by 1.0 mM IPTG for 4 hours at OD600=0.7. For 13C-15N–labeled CaM, proteins were expressed in the M9 minimal medium containing 13C-labeled glucose and 15N-labeled ammonium chloride. The soluble fraction of CaM was purified by HiTrap Phenyl HP prepacked columns (GE Healthcare). To obtain tryptic fragments of CaM (CaMΔN, CaMΔC), the purified CaM was digested by trypsin in the presence of Ca2+ and then purified by HPLC as described previously.34
Nuclear Magnetic Resonance Experiment
Nuclear magnetic resonance (NMR) measurements were performed at 25°C, 30°C, or 37°C on Bruker AVANCE II and III spectrometers equipped with cryogenic probes (500, 700, and 800 MHz). All NMR data were processed with NMRPipe software,35 and analyzed using Sparky assignment program.36 The NMR samples were prepared in 20 mM Bis-Tris (pH 6.8), 100 mM NaCl, 2–5 mM CaCl2, and 5%–10% D2O. For the titration experiments, nonlabeled CBD peptide (CBDp; TORAY Research Center) was added to 13C-15N–labeled CaM (all 200 μM) up to 4.0 molar equivalents (eqs), and for CaMΔN or CaMΔC up to 2.0 eq. At each titration point, 2D 1H-15N SOFAST-HMQC spectra were recorded. The Δδ was calculated according to the formula for each amino acid residue: Δδ=√([ΔδH]2+[ΔδN/5]2), where ΔδH and ΔδN are the amide proton and nitrogen chemical shift (differences in resonance frequency) differences, respectively. For assigning the chemical shifts of the backbone nuclei of 13C-15N–labeled CaM, 3D HNCA, HNCACB, HN(CO)CA, and CBCA(CO)NH spectra were recorded with or without 2.0 eq of nonlabeled CBDp. Crosspeaks (NMR signal) in the 2D spectra of other titration points were assigned on the basis of these data with tracing of the chemical shift perturbation (CSP).
Isothermal Titration Calorimetry
Isothermal titration calorimetry (ITC) experiments were performed on the iTC200 (Malvern). CaM, CaMΔN, CaMΔC, and CBDp were dissolved in 20 mM Bis-Tris (pH 6.8), 100 mM NaCl, and 2 mM CaCl2. The CBDp concentration was adjusted to 1.0 mM and titrated into the chamber containing CaM at 0.05 mM or the dissolving buffer and held at 25°C. The data were obtained from 25 sequential injections of 0.4-μl (initial point), 1-μl (second to approximately 17th), or 2-μl (18th to approximately 25th) aliquots of CBDp solution. ITC data were processed with Origin (OriginLab) after subtraction of the background heats of peptide mixing to buffer alone from heats obtained from mixing with CaM. Thermodynamic parameters of CaM solutions were obtained by fitting the data to a two–independent binding sites model (Microcal from Malvern), whereas those of CaMΔN and CaMΔC were obtained by fitting the data to a one–binding site model.
Construction and Purification of CBD-CC Protein
The PCR products of the sequence encoding TRPC6 854–923 a.a., which contained the CBD and coiled-coil (CBD-CC) region, were digested with NdeI and BamHI and then ligated into 6xHis-Tag containing pET14b vector (Novagen). Constructs carrying the inserts were confirmed by DNA sequencing. CBD-CC protein was expressed in E. coli Tuner (BL21) grown in 2xYT, after a 4-hour induction with 0.4 mM IPTG at OD600=0.8. CBD-CC protein appeared in the insoluble fractions. Insoluble fractions were then solubilized by adding 6 M guanidine hydrochloride (GdnHCl) and further centrifuged (20,000 × g for 20 minutes). The supernatant was applied onto a Ni-NTA column (Qiagen), and washed with buffer containing 20 mM Tris-HCl, 10 mM imidazole, and 6 M GdnHCl, and eluted by 20 mM Tris-HCl, 150 mM NaCl, 0.5 M imidazole, and 6 M GdnHCl. Fractions containing the CBD-CC were desalted using a PD-10 column (Pierce) before being subjected to HPLC purification.
For HPLC, protein samples were injected into an Inertsil C8 column (GL science). The mobile phase was 5% acetonitrile, 0.05% trifluoroacetic acid, with linear gradient elution using acetonitrile (5% to 60% over 30 minutes). The solvent of eluted fractions was removed with an evaporator. Purity was analyzed in 15% SDS-PAGE. CBD-CC peptides were kept at −80°C until further use.
Chemical Crosslinking of the Coiled-Coil Complex
Oligomerization of the CBD-CC fragment was achieved by mixing 2.5 mg/ml (approximately 200 μM) CBD-CC with amide-amide crosslinking reagent EGS [ethylene glycol bis(succinimidyl succinate), a 12-atom spacer arm, Sigma] with different concentrations (0.1–3 mM) in the presence of coupling buffer (40 mM Na2HCO3 pH 8.3, 100 mM NaCl, and 0.1 mM CaCl2). The mixture was incubated for 30 minutes at room temperature (RT) and was quenched by adding 100 mM Tris-HCl (pH 8.0). These mixtures were loaded on SDS-PAGE (15%) and visualized by Coomassie brilliant blue (CBB) staining. The crosslinking procedure between CaM and CBD-CC was basically performed as recommended by the manufacturer (Thermo Scientific). Briefly, CaM (2.5 mg/ml) was equilibrated in 25 mM MES (pH 7.0), 125 mM NaCl, 1 mM CaCl2, or Ca2+-free condition (1 mM EGTA) for 10 minutes. The CaM solution was then subjected to an amide-carboxy crosslinking reagent EDC (1 mM) together with its stabilizer NHS (1 mM) for 15 minutes at RT. The reactivity of EDC was quenched by addition of β-mercaptoethanol. CBD-CC (2.5 mg/ml) was then mixed together with activated CaM and incubated for an additional 1 hour, followed by quenching with 100 mM Tris-HCl. Samples were then resolved using 12% SDS-PAGE and imaged by CBB staining or immunoblotting for CaM as in the following section.
Immunoblotting for Calmodulin
Samples were separated as described in the previous section. The proteins in the gel were then electro-transferred to a nitrocellulose membrane (Bio-Rad, 15×9.2 cm2, pore size 0.45 μm) with constant current at 0.40 A for 1 hour in a cold room. The membrane was then incubated in blocking buffer (20 mM Tris, 150 mM NaCl, pH 7.5, 0.05% Tween 20 [TBST] with 3% BSA) for 1 hour at RT and was incubated overnight at 4°C with rabbit anti-CaM (cat. no. 4830, 1:1000 dilution with blocking buffer; Cell Signaling Technologies). After treatment with the primary antibody, the membrane was washed thrice with TBST for 5 minutes each time. The secondary antibody (anti-rabbit IgG, HRP conjugated; Amersham, 1:2000 dilution) was applied onto the membrane for 1 hour at RT with gentle shaking. The bands were detected by chemiluminescence (West Pico; Thermo Scientific). An imager equipped with CCD was used for data analysis (LAS-4000 mini; Fujifilm).
Size Exclusion Chromatography
Samples of the purified CBD-CC (0.25 mg/ml) and its complex with CaM (0.5 mg/ml) were injected into a Superdex 75 10/300 (GE Healthcare) column with buffer containing 150 mM KCl, 10 mM Tris-HCl (pH 7.5), and 4 mM CaCl2. The flow rate was set to 0.7 ml/min. Mol wt estimations of the CaM/CBD-CC complex and CBD-CC were obtained by constructing a standard curve using aprotinin (6.5 kDa), cytochrome-C (12.5 kDa), carbonic anhydrase (29 kDa), albumin (66 kDa), and blue dextran (2000 kDa) (analytical grade; Sigma) as mol wt standards.
Calcium Imaging of Podocytes
Transfected podocytes were seeded onto glass coverslips and allowed to attach for 3 hours. The coverslips were then incubated with 5 μM Fura-2/AM for 30 minutes and transferred to a custom-made glass-bottom chamber apparatus on an inverted microscope stage (IX-83). The solution flow rate was set to 1 ml/min using a gravity-fed system. The bath solution had an identical composition to the external solution used in the electrophysiologic experiments for HEK293 cells. Rapid solution application with drugs was done by a custom-made “Y-tube” system. Cells exhibiting GFP fluorescence were selected for measurement. Fluorescence images of the cells were captured with a CCD camera (EXi Blue; QImaging) and recorded with software (cellSens; Olympus). The 340/380-nm Fura-2 ratios from images were obtained on a pixel-by-pixel basis every 5 seconds for a total of 400 seconds. CCh (100 μM) was applied after 60 seconds of recording. Ratiometric 340/380 values were calculated by averaging the ROI intensity and analyzed by a custom-written program in MATLAB (Mathworks). Ca2+ concentrations were estimated from the ratio of the maximal over minimal calcium responses by utilizing the calcium calibration buffer kit (Wako).
Phalloidin Staining
Cultured podocytes spread at a density of 2×105/ml were transfected with plasmid DNA and cultured for 48–72 hours. The transfected podocytes were treated with or without CCh (100 μM) for 10 minutes in a CO2 incubator. The cells were then fixed for 10 minutes with 4% paraformaldehyde at RT. After washing three times with PBS, cells were permeabilized with ice-cold acetone for 5 minutes. F-actin was stained for 30 minutes with Alexa Fluor 546 phalloidin (1:40 dilution; Molecular Probes) in PBS, 1% BSA. The stained fibers were captured under an inverted fluorescence microscope (20× or 40× objective lenses, IX83). The architecture of actin filament (F-actin) was evaluated by the four categories as reported previously.37
Confocal Microscopy
Cultured podocytes were transfected with GFP-TRPC6WT/M1R or with GFP-TRPC6K874×/M1R. After 48 hours, the cells were then seeded to a glass-bottomed culture dish (MatTek) coated with 0.1 mg/ml collagen type I (Wako) and incubated for >3 hours before imaging. Fluorescence and optical images were obtained using an inverted confocal microscope (LSM710; Carl Zeiss) equipped with a 63× oil objective lens (1.25 numerical aperture) at 1024×1024 resolution with a pixel dwell time of 6.4–25.4 µs. We used the 488-nm line of an argon laser to excite GFP-fused channels. During confocal microscopy, the cells were bathed in the same external solution as in electrophysiologic experiments for HEK293 cells.
Statistical Analysis
All data are expressed as the mean±SEM. Statistical significance was evaluated by one-way ANOVA and denoted by the following symbols in the figures: *P<0.05, **P<0.01, ***P<0.001.
Results
Effect of CaM Mutations in CDI and CBD Binding
The Ca2+-dependent negative regulation of receptor-activated TRPC6 currents was verified at several concentrations of intracellular EGTA (Ca2+ chelator) in HEK293 cells (Supplemental Figure 1A). Lower Ca2+-buffering condition (1EG/0.3Ca) in the patch pipettes demonstrated a clearly faster decay compared with the high Ca2+ buffering (20EG/6Ca). The fast current decay at the low Ca2+-buffering condition manifested as a process reflecting CDI.
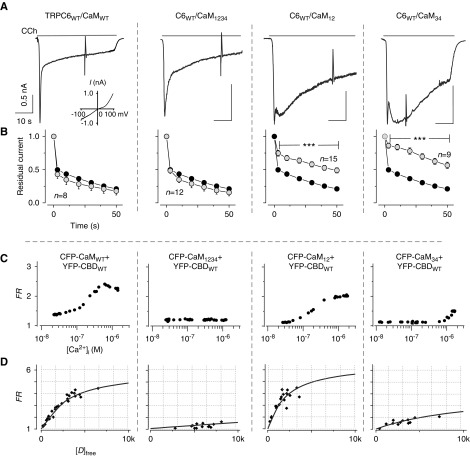
To examine the effect of CaM on CDI, Ca2+-binding EF-hand motif–mutated CaM was coexpressed with TRPC6 in HEK293 cells (Figure 1A). We observed no difference in CDI between cells expressing TRPC6 with endogenous CaM (CaMendo, Supplemental Figure 1A), and those coexpressing TRPC6 with exogenous wild-type CaM (Figure 1A, left). The cells coexpressed with Ca2+-insensitive CaM1234 (all four EF-hands mutated from aspartate to alanine) also exhibited normal inactivation comparable to that seen with CaMendo or CaMWT. However, cells coexpressing with lobe-specific Ca2+-insensitive mutations, CaM12 and CaM34, showed delayed inactivation of TRPC6 currents compared with TRPC6 with CaMWT or CaMendo. The effect of CaM mutations differed from that reported from the L-type Ca channels (CaV1.2), wherein CaM1234 has often exerted a dominant negative effect through the Ca2+-independent association.33 It appears, therefore, that CaM binding properties need to be understood to obtain the mechanistic insight into CDI.
Figure 1.
Functional analysis of CaM mutants and Ca2+-dependent binding. (A) Representative currents recorded from HEK293 cells coexpressing with TRPC6 and CaMWT or a CaM mutant (CaM12, CaM34, or CaM1234). Muscarinic receptor (M1R) is also coexpressed for the electrophysiologic experiments in HEK293 cells. The inset shows typical I–V curve of TRPC6. (B) Fractions of residual currents plotted as a function of time after the peak. Black circles indicate control data from TRPC6 with M1R-transfected cells only (n=14, CaMendo), here and throughout. (C) Representative profiles of Ca2+-dependent binding of CaMs to the TRPC6 CBD. FRET measurement is explained in the Methods section. (D) Quantification of the affinity of binding of CBDWT to CaM mutants by plotting donor free ([D]free) concentrations against FRET signal (FR). Data from various cells were collected at their maximum FR values in (C). Lines represent fitting of CBDWT versus CaMMUT. FRET data are summarized in Supplemental Table 1.
We next confirmed the Ca2+-dependent interaction between CaM and CBD of TRPC6 by using FRET measurement with fluorescence fusion proteins CFP and YFP, respectively. This enabled us to evaluate the binding of two molecules by bringing fused-CFP (donor) and YFP (acceptor) together within a distance of 100 Å, leading to an energy transfer from CFP to YFP. Using FRET analysis applied to individual cells, the Ca2+-dependent binding profiles of the interactions between CaMWT and a CBD fragment were examined, as reported previously (also shown in Figure 1C, left).27 However, Ca2+-insensitive CaM1234 molecule and CBD showed a weak FRET, suggesting that only a small amount of apoCaM binds to the TRPC6 CBD (Figure 1C, middle left). In contrast to CaMWT and CBD, which produced a bell-shaped Ca2+-dependent profile, the lobe-specific mutants CaM12 and CaM34 both demonstrated FRET enhancements at distinctive [Ca2+]i ranges (Figure 1C, middle right and right). Their Ca2+-dependent bindings were altered to a sigmoidal shape. The functional effects of TRPC6 CBD mutations on CDI and CaM binding are shown in Supplemental Figure 2, where CDI was also delayed, as reported previously, with a decreased affinity to CaM.17 These mutational studies resulted in the following findings: (1) apoCaM binding to TRPC6-CBD is almost negligible, and thus CDI would be produced by endogenous CaM; and (2) both lobes of CaM contribute to produce normal CDI in TRPC6. These findings suggest a unique and more complex mechanism by which CaM binding to CBD induces CDI in TRPC6 channels.
Nonclassic Stoichiometry of Ca2+CaM Binding to TRPC6-CBD
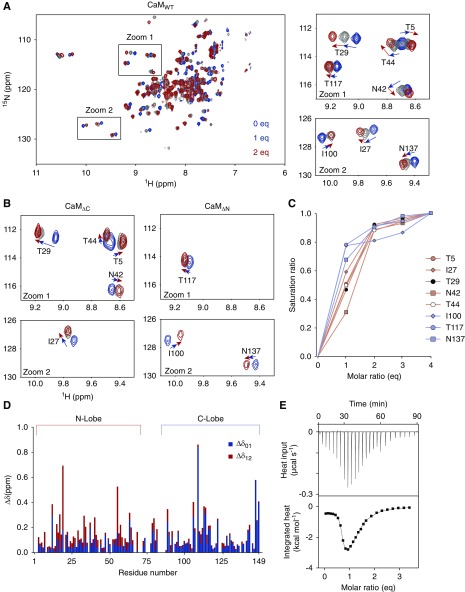
Determining the status of the CaM/TRPC6 CBD complex in the presence of Ca2+ is key to solving the mechanism underlying CDI. To address this point, we performed NMR titration, which is a spectroscopic method to acquire structural information on proteins. Proteins to be measured with NMR are prepared with 13C and/or 15N and 1H, which generate NMR signals and are extremely sensitive to the environment. Because 15N nuclei will exist in every amino acid residue, signals arising from them can be used to determine residue-specific structural changes. Upon titrating 15N-CaM with one eq of CBDp, the NMR signals shifted linearly in one direction (Figure 2A). However, for some signals assigned to residues within the N-lobe (1–75 a.a.) of CaM, additional CBDp (up to 2 eq) induced signal shifts with an altered direction (Figure 2A). With >2 eq, however, little additional signal shift was observed (Figure 2C). On the basis of these observations, the stoichiometry of Ca2+CaM to TRPC6-CBD complex is assumed to be 1:2 (most of the signal shifts in the C-lobe residues were saturated between 0 and 1 eq [Δδ01], whereas those of N-lobe residues were saturated between 1 and 2 eq [Δδ12]) (Figure 2D). Hence, the shifts Δδ01 and Δδ12 could be due to an independent binding of CBDp to the C- and N-lobes, respectively. The 1:1 binding of CBDp to each lobe was confirmed by additional NMR experiments, wherein CBDp was added to labeled CaMΔC or CaMΔN, CaM derivatives lacking C- or N-lobes, respectively (Figure 2B). These results support the notion of 1:2 binding stoichiometry between CaM and TRPC6 CBD.
Figure 2.
NMR and ITC titrations of CBDp binding to Ca2+CaM. (A) Overlay of 2D 1H-15N SOFAST-HMQC spectra of 13C, 15N–labeled CaM with increasing amounts of nonlabeled CBDp. The right panels show zoomed views of crosspeaks (NMR signals). T5, I27, T29, N42, and T44, which are residues within the N-lobe of CaM, showed nonlinear direction changes during the titration. (B) Zoomed view of 2D 1H-15N SOFAST-HMQC spectra of 13C, 15N–labeled CaMΔC and CaMΔN with the increasing amounts of nonlabeled CBDp. The color code is the same as in (A). In contrast to CaMWT, signals of all residues shifted in a linear manner. (C) Saturation of NMR signal shift changes as a function of the equivalent molar ratio of added CBDp. Each value (Δδij) was divided by Δδtotal (=Δδ01+Δδ12+Δδ23+Δδ34) to yield the saturation ratio. All residues reached 80%–90% of saturation at 2 eq. (D) Combined CSPs upon addition of CBD were plotted as a function of the residue number of CaM. In stacked bar graph, Δδ01 (blue) and Δδ12 (red) represent CSPs from 0 to 1 eq and from 1 to 2 eq, respectively. (E) ITC thermograms for CBD binding of CaMWT in the presence of Ca2+. Upper panel represents the raw data for sequential injections of CBDp into CaM. In the lower panel, the data points are obtained by integration of the peaks in the upper panel and plotted against the molar ratios. Fitting was performed with two-site binding model to determine thermodynamic parameters and binding affinity. ITC data for CaMΔC, CaMΔN are shown in Supplemental Figure 3 and summarized in Supplemental Table 2.
This stoichiometry was further validated with ITC, which is a method to measure heat released or absorbed when two proteins are bound. From the titration curve, it is possible to evaluate the binding constant as well as the stoichiometry of the system. The thermogram of Ca2+CaMWT-CBDp demonstrated a biphasic exothermic response that was gradually maximized near 1 eq of CBDp, and then diminished at 2 eq (Figure 2E). This thermogram was fitted with an independent two–binding sites model. The independent two-sites association was also confirmed by using the lobe-separated CaM. These results show that C-lobe of CaM to CBD exhibited a high affinity (30 nM), whereas N-lobe to CBD exhibited a lower affinity (4.8 μM) than the C-lobe, and binds to short α-helical CBD structures (Supplemental Figures 3 and 4, and summarized in Supplemental Table 2). Accordingly, the 1:2 stoichiometry and independent CBD binding to each CaM are verified by NMR and ITC experiments.
The Contribution of the Coiled-Coil Segment
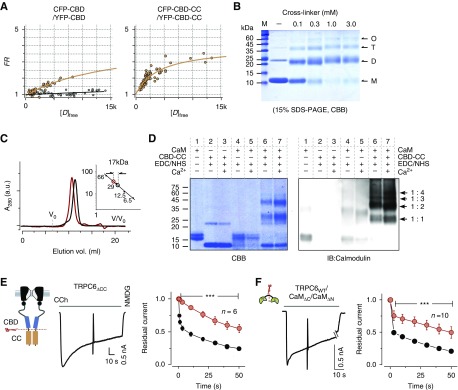
We then considered how the binding stoichiometry of Ca2+CaM to TRPC6 CBD is established in the channel regime. TRPC6 and homologous TRPC3/7 channels possess a highly conserved “coiled-coil” segment, which is a common structural motif to induce dimerization or oligomerization. Within TRPC6, the coiled-coil segment is located at ten amino acid residues on the C-terminal side of the CBD. It is therefore conceivable that coordinated coiled-coil interactions are required to enclose CBDs for CaM binding. To test this idea, FRET values were compared between donor- and acceptor-fused CBD pairs with and without the coiled-coil segment. FRET strength was plotted against the free donor (CFP) concentration in a cell-by-cell manner (Figure 3A). These FRET strength curves indicate that CBDs retaining the coiled-coil (CBD-CC) segments are more tightly associated than those lacking these segments.
Figure 3.
Assembly of coiled-coil segments and their functional role. (A) FRET measurement of binding strength comparison between CBDs (left panel) and CBD-CC segments (right panel) in live cells. The binding affinity was evaluated by the same analysis style as in Figure 1D. A negative control (CFP and YFP only) for FRET measurements is shown in the panel for CBD only (gray circles). (B) Crosslinking of CBD-CC fragments. Purified CBD-CC (200 μM) was treated with 0–3 mM EGS crosslinker. Arrows indicate monomer (M, 11.6 kDa), dimer (D), tetramer (T), and oligomer (O) formations of CBD-CC. (C) Size exclusion chromatography results for CBD-CC with and without CaM are shown as red and black traces, respectively. The inset shows the standard curve estimation of the complex sizes of CBD-CC with and without CaM. (D) Crosslinkings of CaM to CBD-CC by EDC/NHS (1 mM) in the presence (1 mM) or absence of Ca2+ were separated in SDS-PAGE and were imaged by CBB stain (left), or immunoblotting for CaM (right). The positions of the expected mol wt for single CaM (16.7 kDa) and single or multiple CBD-CC complexes (1:1, 1:2, 1:3, 1:4) are indicated by arrows. (E) Currents from TRPC6 with a truncated coiled-coil (TRPC6ΔCC), evoked by CCh. Inactivation plots constructed by plotting residual currents against time for TRPC6WT and TRPC6ΔCC are shown as black and red circles, respectively. (F) Representative TRPC6 currents trace from the cell cotransfected with the N-lobe (CaMΔC) and C-lobe (CaMΔN). Residual current plots of CaMΔC/CaMΔN (red circles) versus TRPC6WT without overexpression of CaM (CaMendo) (black circles). Data from CaMΔC or CaMΔN only are shown in Supplemental Figure 5.
To verify that the coiled-coil segment is involved in the formation of dimers or oligomers, chemical crosslinking experiments were performed with purified CBD-CC proteins. By the addition of crosslinker (EGS), 2–4-fold increases in the mol wt of CBD-CC protein (11.6 kDa) were observed on the SDS-PAGE (Figure 3B). This confirms that coiled-coil is mainly stabilized as dimer and oligomer (Figure 3B). On the basis of the stoichiometry obtained from the NMR titration experiments, it is feasible that CaM could bind to dimerized or oligomerized CBD-CC proteins. The binding of CaM and CBD-CC protein was then examined with size exclusion chromatography. Peak volume of CBD-CC alone eluted at around 11.5 ml (Figure 3C). With the addition of CaM, peak volume eluted faster (10.5 ml), and the difference corresponded to the mol wt of CaM (16.7 kDa). This result suggests that a single CaM molecule binds to dimerized CBD-CC in the presence of Ca2+. This arrangement was verified with crosslinking experiments, then subjected to SDS-PAGE and immunoblot (IB) analysis with anti-CaM antibody in the presence or absence of Ca2+ (Figure 3D). As expected in CaM-IB, bands appeared at around 39.9, 51.5, and 63.1 kDa, which corresponded to 1:2, 1:3, and 1:4 CaM/CBD-CC complexes (Figure 3D, IB panel, lanes 6 and 7). CaM and CBD-CC complexes were detected even in the absence of Ca2+; however, they appeared stronger in the presence of Ca2+. Collectively, these results indicate that Ca2+ enhances single CaM binding to multiple CBD-CC segments.
The functional role of the coiled-coil assembly on CDI could be tested by expression of its entire deletion mutant (TRPC6ΔCC). Strikingly, the cells expressing TRPC6ΔCC showed an abolished CDI (Figure 3E). This coiled-coil deletion result led us to hypothesize that the binding of one CaM molecule to two CBDs arranged by coiled-coil assembly may be essential for evoking CDI through an intersubunit bridging. With this arrangement, the linker domain in CaM between the N-lobe and C-lobe should contribute to CDI. To test this possibility, CaM was separated into two molecules at the central linker domain (CaMΔC, CaMΔN). In cells coexpressing both CaMΔC and CaMΔN with TRPC6, CDI was delayed (Figure 3F), to an extent comparable to that observed with CaM12 or CaM34 (Figure 1), as well as CaMΔC or CaMΔN alone (Supplemental Figure 5). These results suggest that the coiled-coil assembly and the linker connecting N- and C-lobes of CaM are required to hold two CBDs in neighboring subunits to inactivate the channel. On the basis of this fundamental mechanistic insight into CDI, we explored the possibility that the pathogenesis of renal glomerular disease could be explained by the disruption of TRPC6 CDI.
FSGS-Associated Mutations in TRPC6 Manifest Disrupted CDI
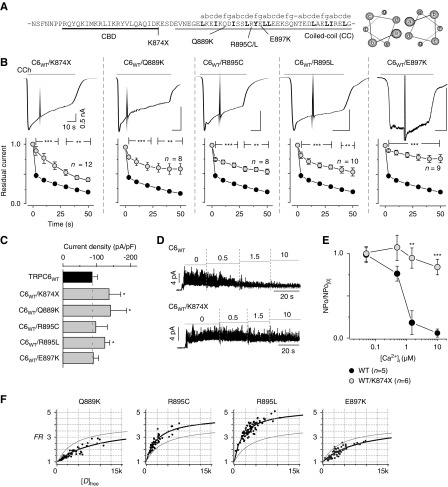
FSGS occurs either sporadically or as an autosomal dominant trait, where both wild-type and mutant TRPC6 channel subunits are expected to be expressed in patients. To conduct functional experiments under such heterogeneity, equal amounts of plasmid DNAs for wild-type TRPC6 channel and its FSGS-associated mutations lacking the entire coiled-coil segment or point mutations within the coiled-coil (Figure 4A) were cotransfected in HEK293 cells (Figure 4B). In previous reports, the delay of inactivation and current amplitude amplification were described for the K874× mutant9; however, only the current amplitude amplification was shown for the remaining coiled-coil mutations.24 Surprisingly, a significant delay of inactivation was observed in all coiled-coil FSGS mutants (Figure 4B), whereas the current amplitudes of TRPC6K874×, TRPC6Q889K, and TRPC6R895L, were significantly increased, but not in TRPC6R895C and TRPC6E897K (Figure 4C). Insensitivity to cellular Ca2+ of the TRPC6WT/TRPC6K874× was confirmed with inside-out mode recording, which demonstrated almost no sign of inactivation by increasing [Ca2+]i (Figure 4, D and E). Accordingly, the disruption of CDI appears to be linked to the gain-of-function seen with the above FSGS-associated mutations.
Figure 4.
FSGS mutations in the coiled-coil segment lead to impairment of CDI. (A) The sequence of the locus of the CBD (black) and coiled-coil segment (gray), and FSGS mutations within the TRPC6 channel. Positions of the coiled-coil heptad repeat (abcdefg) are indicated above the sequence. Helical-wheel diagrams showing the location of residues in the heptad repeat (right). (B) Receptor-activated whole-cell currents from FSGS mutations (TRPC6K874×, TRPC6Q889K, TRPC6R895C, TRPC6R895L, TRPC6E897K) coexpressed with wild-type TRPC6 in HEK293 cells (upper panels). Scale bars depict the same time course and current amplitude throughout. The coexpression of wild-type and K874× mutation showed no difference compared with K874× alone (Supplemental Figure 6). Residual current plots are plotted against time after the peak (data from FSGS-associated TRPC6 channels are shown in gray circles, lower panels). (C) Summary of the peak current densities (pA/pF) induced by the receptor stimulation. (D) Inside-out recordings of TRPC6WT (upper), TRPC6K874× (lower) with step-wise changing of intracellular Ca2+ concentrations (μM). (E) Summary of the inside-out recordings. TRPC6WT (black circles) and TRPC6K874× (gray circles) are represented as a plot of normalized NPo versus [Ca2+]i. Normalized NPo was obtained by dividing NPo at nearly zero [Ca2+]i. (F) Binding affinities between CBD-CC mutants in cells were measured by FRET, where individual data points represent FRET strength from single cells (black circles), and fitted by using 1:1 binding isotherm equations (black curve). The strength of interaction of CBD-CCWT is shown as a gray line (control). FRET data are summarized in Supplemental Table 1.
The interaction strength between the mutated coiled-coil segments was then quantified by FRET measurement (coexpressing CFP/YFP-tagged CBD-CCMUT and plotting FRET strengths against donor-free concentrations). Fitting the curve within CBD-CCQ889K or CBD-CCE897K indicated a weaker affinity (Kd=4.3 or 4.1 μM) compared with wild-type CBD-CC (Kd=1.5 μM), whereas CBD-CCR895C and CBD-CCR895L had a higher affinity (Kd=0.78 and 0.37 μM) (Figure 4F, Supplemental Table 1). These results suggest that the abnormal coiled-coil configuration in FSGS mutants contributes to the dysfunctionality of CDI.
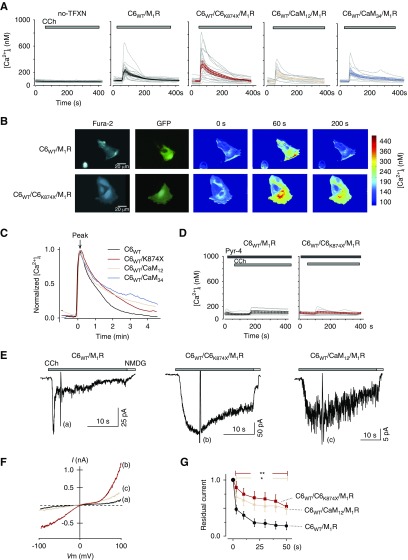
To more directly investigate whether CDI contributes to the dysregulation of renal Ca2+ homeostasis, FSGS-associated mutant (TRPC6K874×) was again coexpressed with TRPC6WT in cultured podocytes. Figure 5A shows the individual and averaged [Ca2+]i responses induced by receptor stimulation. Nontransfected cells showed no clear [Ca2+]i response, but cells expressing TRPC6WT, TRPC6WT/TRPC6K874×, TRPC6WT/CaM12, or TRPC6WT/CaM34 with muscarinic receptor showed a marked increase in [Ca2+]i (Figure 5, A and B). In Figure 5C, the [Ca2+]i response was normalized to the peak and plotted against time. The increased [Ca2+]i returned to the basal level (approximately 100 nM) within several minutes in TRPC6WT, but was clearly sustained in cells expressing TRPC6K874×, CaM12, or CaM34.
Figure 5.
Impairment of CDI in cultured podocytes. (A) Gray lines represent individual [Ca2+]i of nontransfected cells (left) and cells transfected with TRPC6WT/M1R (middle left) (n=23), TRPC6WT/TRPC6K874×/M1R (center) (n=17), TRPC6 WT/CaM12/M1R (middle right) (n=10), and TRPC6WT/CaM34/M1R (right) (n=10). Cells are evoked by CCh (100 μM) at approximately 60 seconds. Average traces are indicated on the panel in black, red, pink, and light-blue lines, respectively. Note that the scales for [Ca2+]i are identical. (B) Representative Ca2+-imaging of CCh-evoked TRPC6WT and TRPC6WT/TRPC6K874×. In the TRPC6K874× panels, a sustained [Ca2+]i elevation is observed (200 seconds). (C) Decay time course of normalized to peak [Ca2+]i from TRPC6WT (black line) and TRPC6K874× (red), CaM12 (pink) and CaM34 (light blue). The horizontal axis represents time after transient Ca2+ peak. (D) TRPC6 antagonist (Pyr-4, 10 μM) suppresses the [Ca2+]i responses in podocytes expressing TRPC6WT/M1R (left) and TRPC6WT/TRPC6K874×/M1R (right). (E) Whole-cell recordings from podocytes overexpressing TRPC6WT, TRPC6WT/TRPC6K874×, and TRPC6WT/CaM12 with M1R (Vh=−50 mV). (F) I–V relationship curve recorded from the representative traces (a–c). (G) Residual current plots of TRPC6WT, TRPC6WT/TRPC6K874×, and TRPC6WT/CaM12 are shown as black circles, red squares, and pink triangles, respectively (n=5).
To confirm that the [Ca2+]i response was derived from TRPC6 channels, cultured podocytes overexpressing TRPC6 channels were pretreated with Pyrazole-4 (Pyr-4, 10 μM), a TRPC6 channel blocker.38 Application of the blocker reduced the [Ca2+]i response via TRPC6WT and TRPC6K874× to 44% and 25% of the original responses, respectively (Figure 5D). Whole-cell patch-clamp recording in cultured podocytes confirmed the delayed inactivation of TRPC6-like currents for cells expressing TRPC6WT/TRPC6K874× and TRPC6WT/CaM12, compared with TRPC6WT (Figure 5E). These currents demonstrated inward and outward doubly rectifying I–V curves characteristic to TRPC6 currents (Figure 5F). We have also tested flufenamic acid, known to stimulate TRPC6 channels.13,39 Potentiation by flufenamic acid and inhibition by Pyr-4 strongly support that the expressed TRPC6 channel is involved in delayed TRPC6-like currents in the cultured podocytes (Supplemental Figure 8). Residual current plots showed a significant delay in the CDI of TRPC6K874× or CaM12–coexpressing cells, as seen in HEK293 expression system (Figure 5G). However, there was no statistical significance for the whole-cell current densities from cultured podocytes, which were 3.34±1.53 pA/pF (n=5), 9.76±4.96 pA/pF (n=5), and 1.69±0.27 pA/pF (n=5) for C6WT, C6WT/C6K874×, and C6WT/CaM12, respectively.
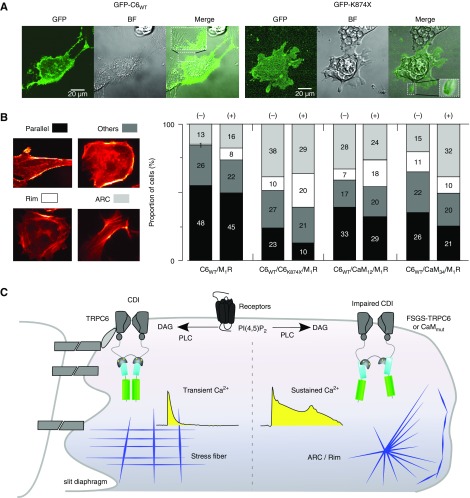
Impairment of CDI Contributes to F-Actin Disorganization
Patients with FSGS show characteristic abnormality in the maintenance of the foot process structure.40,41 Therefore, we have aimed to show that impairments of TRPC6 CDI could lead to disorganization of the cellular structure. The distributions of the TRPC6WT and TRPC6K874× in podocytes were tested using TRPC6 with N-terminally fused GFP. As shown in Figure 6A, both GFP-TRPC6WT and GFP-TRPCK874× were distributed in the cytosol, and more on the plasma membrane, with no apparent irregularity related to the TRPCK874× mutation. However, disorganized F-actin structures (actin-rich center [ARC] or rim) were more apparent in the podocytes transfected with TRPCK874× compared with TRPC6WT (Figure 6B). CaM mutations CaM12 and CaM34 also exerted pathophysiologic actin structures. These tendencies were retained even with CCh treatment. These results suggest that the FSGS-associated TRPC6 channels or lobe-specific CaM mutations lead to podocyte damage via impaired CDI (Figure 6C).
Figure 6.
Distribution of TRPC6 channels and pathologic F-actin organization in podocytes. (A) Confocal images of podocytes overexpressing GFP-TRPC6WT/M1R (left panels; BF, bright field) and GFP-TRPC6K874×/M1R (right panels). Expanded boxes represent GFP fluorescence in foot process–like structure. (B) The frequency of occurrence of the four types of F-actin manifestations in cultured podocytes stained by Alexa Fluor 546 phalloidin (parallel fiber: shown in black bar; rim: white bar; ARC: gray bar; and others: dark gray bar). No stimulation (−) and CCh (100 μM) stimulation for 10 minutes (+). Numbers of cells displaying each F-actin pattern are labeled on each stacked bar. (C) Summary figure. Disruption of the CaM-bridge due to the abnormal assembly between coiled-coil segments disrupts CDI of TRPC6, thereby prolonging channel opening and enhancing ion influx. Sustained Ca2+ elevation stimulates downstream signaling cascades and F-actin rearrangements in podocytes. DAG, diacylglycerol; PLC, phospholipase C.
Discussion
In this study, we uncovered the regulation mechanism of TRPC6 channels in renal disease, involving the impairment of CDI. We found that CDI is triggered by Ca2+-dependent CaM binding to the TRPC6 CBD with a 1:2 stoichiometry. Moreover, coiled-coil segment located immediately C-terminal to the CBD is critical for assembly of the CBD in close proximity to mediate bilobed CaM binding. With FSGS-associated mutations of TRPC6, the lack of the coiled-coil segment or its dysfunctionality impair the affinity of coiled-coil interaction or proximity of CBDs necessary for CaM binding and CDI, leading to gain-of-function phenotype as reported in FSGS.
The Mechanism for CaM Binding and Coiled-Coil Assembly in CDI
In this study, we first confirmed the inhibitory effect of intracellular Ca2+ on TRPC6. This Ca2+-dependent inhibition, or CDI, is weakened by high Ca2+ buffering, resulting in amplified currents and exhibiting delayed inactivation. Mutations in the TRPC6 CBD produced similar results, indicating the indispensable role of CaM in CDI. Moreover, we found that both lobes of CaM contribute to the inactivation of TRPC6 channels. The binding of CaM to the TRPC6 CBD is Ca2+-dependent, but a bell-shaped response curve. This profile gives a hint for decoding the mechanism of TRPC6 CDI. The binding of CaM to a CBD often shows a 1:1 stoichiometry, with both the N- and C-lobes binding to a long stretch of α-helix within a single CBD.42 By contrast, our biophysical analyses reveal a noncanonical 1:2 stoichiometry for the binding of CaM to the TRPC6 CBD (Figure 2, Supplemental Table 2).
The biophysical analyses also revealed the characteristics of the CBD structure, which contains a short α-helix in complex (Supplemental Figure 4). This short α-helix length of CBD may present a crucial structural constraint for the 1:2 stoichiometry. More specifically, whereas longer α-helix CBDs in classic CaM target proteins allow simultaneous contact of both lobes of CaM, and thus the 1:1 stoichiometry, the shorter α-helical CBD in TRPC6 may allow binding of only a single CaM lobe. Such nonclassic stoichiometry has been reported previously for several proteins in which CBDs fold into a short α-helical structure.43–46
The coiled-coil is a structural motif in proteins and in TRP channels which has multiple functions, including channel tetramerization, surface trafficking, assembly to another molecule, and channel gating regulation.47–52 In this report, we explored the novel contribution made by the coiled-coil configuration to CaM binding and CDI of TRPC6 channels. Our findings indicate that the bundling of the coiled-coil segment contributes to bringing two CBDs into close enough proximity for CaM binding. The recently reported cryo-EM structure of TRPC6 may help this idea.53,54 On the basis of this structure, the distance between proximal CBDs is about 3.5 nm, which is nearly identical to the distance between the hydrophobic pockets in the N- and C-lobes of CaM, as was reported in the case of 1 (CaM):2 (targets) stoichiometry (Supplemental Figure 7).43 Thus, deletion of the coiled-coil segment severely impairs CDI, probably because the CBDs are too distant from one another (Figure 6C). This was also true with some of the coiled-coil point mutations (Q889K, E897K), which demonstrate weaker coiled-coil interaction (Figure 4F). By contrast, R895C and R895L mutations actually led to a stronger coiled-coil interaction, and, on the basis of our FRET measurement analysis, minimal distances between CBDs with the R895C/L coiled-coil could be around 5 Å shorter than in the wild-type protein (Supplemental Figure 7). However, despite having opposite interaction profiles, all of the tested coiled-coil mutations impaired CDI. These results suggest that, for a normal TRPC6 CDI, neither too weak nor too strong interactions in the coiled-coil assembly configuration may be required. The coiled-coil segment of TRPC6 thus plays a fundamental role in the production of CaM-mediated CDI. From a structural point of view, by supporting bilobed CaM binding, the coiled-coil may act as a “bridge” between two subunits within the larger tetrameric complex, thereby acting as a minimal unit for inactivation. However, in the cryo-EM structure of TRPC6, it is curious that the CBD is partially covered by the N-terminal cytoplasmic region, including the ankyrin domain.53,54 A similar observation was also reported in TRPC4, a close family member of TRPC6, where the CBD is partly accessible by CaM/IP3.55 In addition, the cryo-EM structure of a Ca2+-selective TRP channel (TRPV5) and CaM complex has shown that single CaM binds to the CBD in the C-terminal domain, and fits under the ankyrin domains.56 These reports support CaM binding to CBD in TRPC6 channels; however, to answer this question in more detail, study of the whole channel complex would be required.
Contribution of TRPC6 CDI to Podocyte Pathophysiology
We then explored the effect of defective CDI on podocyte physiology. Autosomal dominant FSGS-associated mutations in TRPC6 have been established as the source of podocyte damage and foot process effacement (i.e., channelopathy or podocytopathy). These FSGS mutations induce various functional phenotypes in exogenous expression systems, and often demonstrate an enhanced channel activity.3,4,9,23,24,57 It was previously suggested that this gain-of-function reflected an aberrant surface expression of TRPC6 induced by disruption of inhibitory binding of nephrin or synaptopodin.10,58 However, delayed current inactivation was also reported after FSGS-associated deletion of the entire coiled-coil domain (K874×). We think that this delayed inactivation cannot be accounted for by upregulated surface expression.9
Furthermore, it is not clear yet whether FSGS-associated coiled-coil mutations can cause slowing of CDI. In that regard, our electrophysiologic findings demonstrated that all coiled-coil mutations slow inactivation under low Ca2+-buffering conditions. These slower kinetics are similar to those seen with wild-type TRPC6 currents recorded under high Ca2+-buffering conditions (Supplemental Figure 1, A–C), where diminished CDI of TRPC6 contributes to slowed current decay and increased current amplitudes. Similarly, the increments in peak current and slowed inactivation seen with FSGS-associated mutants are also due to a lack of CDI under our experimental conditions. Thus, the observed gain-of-function, which is often used to describe the increased activity of TRPC6 channels carrying FSGS-associated mutations, can be caused by disrupting CDI. Although all five tested FSGS-associated TRPC6 channels impaired CDI, determining whether CDI is also disrupted by the other FSGS-associated mutations will require further study, which we anticipate will provide a comprehensive view of the disease-based CDI mechanism and pathogenesis of FSGS.
In FSGS disorders, the effacement of podocyte foot processes is an invariable feature that involves morphologic changes in the cellular periphery, including retraction and shortening of the foot processes.40,41 Along with the sustained elevation in [Ca2+]i due to CDI disruption, ‘‘ARC” or “rim (ring-like)” formations were often observed in podocytes expressing FSGS-associated mutation. Previous work has shown that short, transient Ca2+ responses in podocytes are not sufficient to functionally alter the renal filtration barrier.59 Thus, a transient [Ca2+]i elevation may not be sufficient to induce podocyte structural disorder. On the other hand, the sustained increases in [Ca2+]i due to disruption of TRPC6 CDI could have adverse, aberrant effects on Ca2+ and CaM signaling pathways.60–63 In addition, along with disruption of TRPC6 CDI, potentiation of TRPC5 channel activity due to their intrinsic Ca2+-dependent facilitation mechanism may also contribute to a sustained elevation in [Ca2+]i during FSGS progression.64 Therefore, excluding or limiting the sustained [Ca2+]i elevation would be critical to an effective therapeutic strategy for FSGS.
In summary, we have demonstrated the Ca2+/CaM-mediated negative regulation of TRPC6 channel gating and how dysregulation of TRPC6 gating due to gain-of-function mutations found in patients with FSGS may lead to podocyte damage. Structural and functional analyses in a heterologous expression system and in cultured podocytes reveal the pathophysiologic mechanism of FSGS-associated mutations that disrupt Ca2+/CaM-mediated inhibition of TRPC6 channel gating. These findings provide novel molecular insight into the importance of Ca2+-regulation in the context of kidney disease.
Disclosures
Dr. Reiser is a co-founder and shareholder of TRISAQ, a biotech company that develops novel therapies for kidney diseases. All other remaining authors have nothing to disclose.
Funding
Mr. Polat was supported by a scholarship from MEXT of Japan. This research was supported by grants from Oxygen Biology (Dr. Y. Mori) and JSPS (Dr. M.X. Mori).
Supplementary Material
Acknowledgments
The authors are grateful to Dr. Masaomi Nangaku and Dr. Tuck W. Soong for critical comments on our manuscript. We thank Dr. K. Akagi and Dr. H. Yamada for help with Nuclear Magnetic Resonance (NMR) measurements at National Institutes of Biomedical Innovation, Health and Nutrition and podocyte culture. Thanks to Hideharu Hase and Tomorou Amatani for preparation of molecular biological tools.
Dr. Uno, Dr. Tochio, Dr. Ariyoshi, and Dr. Imamura performed the NMR and Isothermal Titration Calorimetry (ITC) experimental work and data analysis. Mr. Polat, Mr. Maruyama, Mr. Tran, and Dr. M.X. Mori performed the Electro-Physiology (EP) experiments and data analysis. Mr. Polat and Dr. M.X. Mori contributed to the Förster or Fluorescence Resonance Energy Transfer (FRET) measurements. Dr. Wong, Dr. Itsuki, and Dr. Uno prepared the protein/peptide samples. Dr. Asanuma, Dr. Inoue, and Dr. Ichikawa performed the experimental design in podocytes. Mr. Polat performed the crosslink and podocyte experiments. Dr. Sakaguchi guided confocal microscopy experiments. Dr. Uno and Mr. Tran wrote the sections for NMR and ITC. Mr. Polat and Dr. M.X. Mori wrote the EP, FRET, and podocyte sections. Dr. Shirakawa and Dr. Y. Mori supported and criticized the project. All authors commented on the manuscript. Dr. Tochio and Dr. M.X. Mori interpreted the data.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018070756/-/DCSupplemental.
Simultaneous detection of TRPC currents and intracellular Ca2+.
Simultaneous detection of TRPC currents and PI(4,5)P2.
Preparation for 13C, 15N–doubly labeled CBDp.
Supplemental Figure 1. Ca2+-buffering effects on TRPC6 current.
Supplemental Figure 2. Functional and biophysical analysis of CBD mutants.
Supplemental Figure 3. ITC thermograms for CBD binding of lobe-separated CaM molecules.
Supplemental Figure 4. NMR analysis of the binding of 13C,15N–CBDp to CaM.
Supplemental Figure 5. Single-lobe CaM effect on CDI.
Supplemental Figure 6. Currents from TRPC6K874X (Homo) and TRPC6WT/TRPC6K874X (Hetero).
Supplemental Figure 7. Effective distance between CaM and CBD binding.
Supplemental Figure 8. Potentiating and inhibiting reagent effects on TRPC6-like currents in C6WT/C6K874X channels expressing in cultured podocytes.
Supplemental Table 1. Binding parameters measured by FRET methods.
Supplemental Table 2. Thermodynamic parameters of the binding of CaM to CBD.
Supplemental Table 3. Primer sequences for plasmid constructions.
References
- 1.Berridge MJ: Calcium signalling remodelling and disease. Biochem Soc Trans 40: 297–309, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Greka A, Mundel P: Cell biology and pathology of podocytes. Annu Rev Physiol 74: 299–323, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reiser J, Polu KR, Möller CC, Kenlan P, Altintas MM, Wei C, et al.: TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet 37: 739–744, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, et al.: A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308: 1801–1804, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Clapham DE: TRP channels as cellular sensors. Nature 426: 517–524, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Nilius B, Owsianik G, Voets T, Peters JA: Transient receptor potential cation channels in disease. Physiol Rev 87: 165–217, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Dryer SE, Reiser J: TRPC6 channels and their binding partners in podocytes: Role in glomerular filtration and pathophysiology. Am J Physiol Renal Physiol 299: F689–F701, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietrich A, Chubanov V, Gudermann T: Renal TRPathies. J Am Soc Nephrol 21: 736–744, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Heeringa SF, Möller CC, Du J, Yue L, Hinkes B, Chernin G, et al.: A novel TRPC6 mutation that causes childhood FSGS. PLoS One 4: e7771, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanda S, Harita Y, Shibagaki Y, Sekine T, Igarashi T, Inoue T, et al.: Tyrosine phosphorylation-dependent activation of TRPC6 regulated by PLC-γ1 and nephrin: Effect of mutations associated with focal segmental glomerulosclerosis. Mol Biol Cell 22: 1824–1835, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun ZJ, Ng KH, Liao P, Zhang Y, Ng JL, Liu ID, et al.: Genetic interactions between TRPC6 and NPHS1 variants affect posttransplant risk of recurrent focal segmental glomerulosclerosis. Am J Transplant 15: 3229–3238, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Hofmann T, Obukhov AG, Schaefer M, Harteneck C, Gudermann T, Schultz G: Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397: 259–263, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, et al.: The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca(2+)-permeable cation channel. Circ Res 88: 325–332, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Thakur DP, Tian JB, Jeon J, Xiong J, Huang Y, Flockerzi V, et al.: Critical roles of Gi/o proteins and phospholipase C-δ1 in the activation of receptor-operated TRPC4 channels. Proc Natl Acad Sci U S A 113: 1092–1097, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blair NT, Kaczmarek JS, Clapham DE: Intracellular calcium strongly potentiates agonist-activated TRPC5 channels. J Gen Physiol 133: 525–546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okada T, Shimizu S, Wakamori M, Maeda A, Kurosaki T, Takada N, et al.: Molecular cloning and functional characterization of a novel receptor-activated TRP Ca2+ channel from mouse brain. J Biol Chem 273: 10279–10287, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Kwon Y, Hofmann T, Montell C: Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Mol Cell 25: 491–503, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lintschinger B, Balzer-Geldsetzer M, Baskaran T, Graier WF, Romanin C, Zhu MX, et al.: Coassembly of Trp1 and Trp3 proteins generates diacylglycerol- and Ca2+-sensitive cation channels. J Biol Chem 275: 27799–27805, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Shi J, Mori E, Mori Y, Mori M, Li J, Ito Y, et al.: Multiple regulation by calcium of murine homologues of transient receptor potential proteins TRPC6 and TRPC7 expressed in HEK293 cells. J Physiol 561: 415–432, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh BB, Liu X, Tang J, Zhu MX, Ambudkar IS: Calmodulin regulates Ca(2+)-dependent feedback inhibition of store-operated Ca(2+) influx by interaction with a site in the C terminus of TrpC1. Mol Cell 9: 739–750, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Armstrong CM: Inactivation of the potassium conductance and related phenomena caused by quaternary ammonium ion injection in squid axons. J Gen Physiol 54: 553–575, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chad J, Eckert R, Ewald D: Kinetics of calcium-dependent inactivation of calcium current in voltage-clamped neurones of Aplysia californica. J Physiol 347: 279–300, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gigante M, Caridi G, Montemurno E, Soccio M, d’Apolito M, Cerullo G, et al.: TRPC6 mutations in children with steroid-resistant nephrotic syndrome and atypical phenotype. Clin J Am Soc Nephrol 6: 1626–1634, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Riehle M, Büscher AK, Gohlke BO, Kaßmann M, Kolatsi-Joannou M, Bräsen JH, et al.: TRPC6 G757D loss-of-function mutation associates with FSGS. J Am Soc Nephrol 27: 2771–2783, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imai Y, Itsuki K, Okamura Y, Inoue R, Mori MX: A self-limiting regulation of vasoconstrictor-activated TRPC3/C6/C7 channels coupled to PI(4,5)P2-diacylglycerol signalling. J Physiol 590: 1101–1119, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mercado J, Gordon-Shaag A, Zagotta WN, Gordon SE: Ca2+-dependent desensitization of TRPV2 channels is mediated by hydrolysis of phosphatidylinositol 4,5-bisphosphate. J Neurosci 30: 13338–13347, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mori MX, Imai Y, Itsuki K, Inoue R: Quantitative measurement of Ca(2+)-dependent calmodulin-target binding by Fura-2 and CFP and YFP FRET imaging in living cells. Biochemistry 50: 4685–4696, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z, Tang J, Tikunova S, Johnson JD, Chen Z, Qin N, et al.: Activation of Trp3 by inositol 1,4,5-trisphosphate receptors through displacement of inhibitory calmodulin from a common binding domain. Proc Natl Acad Sci U S A 98: 3168–3173, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saimi Y, Kung C: Calmodulin as an ion channel subunit. Annu Rev Physiol 64: 289–311, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Mori M, Konno T, Ozawa T, Murata M, Imoto K, Nagayama K: Novel interaction of the voltage-dependent sodium channel (VDSC) with calmodulin: Does VDSC acquire calmodulin-mediated Ca2+-sensitivity? Biochemistry 39: 1316–1323, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Itoh RE, Kurokawa K, Ohba Y, Yoshizaki H, Mochizuki N, Matsuda M: Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol Cell Biol 22: 6582–6591, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mundel P, Reiser J, Zúñiga Mejía Borja A, Pavenstädt H, Davidson GR, Kriz W, et al.: Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Erickson MG, Alseikhan BA, Peterson BZ, Yue DT: Preassociation of calmodulin with voltage-gated Ca(2+) channels revealed by FRET in single living cells. Neuron 31: 973–985, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Mori M, Konno T, Morii T, Nagayama K, Imoto K: Regulatory interaction of sodium channel IQ-motif with calmodulin C-terminal lobe. Biochem Biophys Res Commun 307: 290–296, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A: NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6: 277–293, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Goddard TD, Kneller DG. 1999. SPARKY 3. University of California, San Francisco. [Google Scholar]
- 37.Forst AL, Olteanu VS, Mollet G, Wlodkowski T, Schaefer F, Dietrich A, et al.: Podocyte purinergic P2X4 channels are mechanotransducers that mediate cytoskeletal disorganization. J Am Soc Nephrol 27: 848–862, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiyonaka S, Kato K, Nishida M, Mio K, Numaga T, Sawaguchi Y, et al.: Selective and direct inhibition of TRPC3 channels underlies biological activities of a pyrazole compound. Proc Natl Acad Sci U S A 106: 5400–5405, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ilatovskaya DV, Palygin O, Chubinskiy-Nadezhdin V, Negulyaev YA, Ma R, Birnbaumer L, et al.: Angiotensin II has acute effects on TRPC6 channels in podocytes of freshly isolated glomeruli. Kidney Int 86: 506–514, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andrews P: Morphological alterations of the glomerular (visceral) epithelium in response to pathological and experimental situations. J Electron Microsc Tech 9: 115–144, 1988 [DOI] [PubMed] [Google Scholar]
- 41.D’Agati VD, Kaskel FJ, Falk RJ: Focal segmental glomerulosclerosis. N Engl J Med 365: 2398–2411, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Hoeflich KP, Ikura M: Calmodulin in action: Diversity in target recognition and activation mechanisms. Cell 108: 739–742, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Drum CL, Yan SZ, Bard J, Shen YQ, Lu D, Soelaiman S, et al.: Structural basis for the activation of anthrax adenylyl cyclase exotoxin by calmodulin. Nature 415: 396–402, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Zheng X, Mueller GA, Sobhany M, DeRose EF, Zhang Y, et al.: Crystal structure of calmodulin binding domain of orai1 in complex with Ca2+ calmodulin displays a unique binding mode. J Biol Chem 287: 43030–43041, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reichow SL, Clemens DM, Freites JA, Németh-Cahalan KL, Heyden M, Tobias DJ, et al.: Allosteric mechanism of water-channel gating by Ca2+-calmodulin. Nat Struct Mol Biol 20: 1085–1092, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yap KL, Yuan T, Mal TK, Vogel HJ, Ikura M: Structural basis for simultaneous binding of two carboxy-terminal peptides of plant glutamate decarboxylase to calmodulin. J Mol Biol 328: 193–204, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Lee KP, Choi S, Hong JH, Ahuja M, Graham S, Ma R, et al.: Molecular determinants mediating gating of Transient Receptor Potential Canonical (TRPC) channels by stromal interaction molecule 1 (STIM1). J Biol Chem 289: 6372–6382, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li M, Yu Y, Yang J: Structural biology of TRP channels. Adv Exp Med Biol 704: 1–23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paulsen CE, Armache JP, Gao Y, Cheng Y, Julius D: Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 520: 511–517, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsuruda PR, Julius D, Minor DL Jr: Coiled coils direct assembly of a cold-activated TRP channel. Neuron 51: 201–212, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phelps CB, Gaudet R: The role of the N terminus and transmembrane domain of TRPM8 in channel localization and tetramerization. J Biol Chem 282: 36474–36480, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Jansen C, Sahni J, Suzuki S, Horgen FD, Penner R, Fleig A: The coiled-coil domain of zebrafish TRPM7 regulates Mg·nucleotide sensitivity. Sci Rep 6: 33459, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Azumaya CM, Sierra-Valdez F, Cordero-Morales JF, Nakagawa T: Cryo-EM structure of the cytoplasmic domain of murine transient receptor potential cation channel subfamily C member 6 (TRPC6). J Biol Chem 293: 10381–10391, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang Q, Guo W, Zheng L, Wu JX, Liu M, Zhou X, et al.: Structure of the receptor-activated human TRPC6 and TRPC3 ion channels. Cell Res 28: 746–755, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vinayagam D, Mager T, Apelbaum A, Bothe A, Merino F, Hofnagel O, et al.: Electron cryo-microscopy structure of the canonical TRPC4 ion channel. eLife 7: e36615, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hughes TET, Pumroy RA, Yazici AT, Kasimova MA, Fluck EC, Huynh KW, et al.: Structural insights on TRPV5 gating by endogenous modulators. Nat Commun 9: 4198, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu B, Chen N, Wang ZH, Pan XX, Ren H, Zhang W, et al.: Identification and functional analysis of a novel TRPC6 mutation associated with late onset familial focal segmental glomerulosclerosis in Chinese patients. Mutat Res 664: 84–90, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Yu H, Kistler A, Faridi MH, Meyer JO, Tryniszewska B, Mehta D, et al.: Synaptopodin limits TRPC6 podocyte surface expression and attenuates proteinuria. J Am Soc Nephrol 27: 3308–3319, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koehler S, Brähler S, Kuczkowski A, Binz J, Hackl MJ, Hagmann H, et al.: Single and transient Ca2+ peaks in podocytes do not induce changes in glomerular filtration and perfusion. Sci Rep 6: 35400, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schlöndorff J, Del Camino D, Carrasquillo R, Lacey V, Pollak MR: TRPC6 mutations associated with focal segmental glomerulosclerosis cause constitutive activation of NFAT-dependent transcription. Am J Physiol Cell Physiol 296: C558–C569, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, et al.: The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14: 931–938, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chiluiza D, Krishna S, Schumacher VA, Schlöndorff J: Gain-of-function mutations in transient receptor potential C6 (TRPC6) activate extracellular signal-regulated kinases 1/2 (ERK1/2). J Biol Chem 288: 18407–18420, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, et al.: Modification of mineralocorticoid receptor function by Rac1 GTPase: Implication in proteinuric kidney disease. Nat Med 14: 1370–1376, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Zhou Y, Castonguay P, Sidhom EH, Clark AR, Dvela-Levitt M, Kim S, et al.: A small-molecule inhibitor of TRPC5 ion channels suppresses progressive kidney disease in animal models. Science 358: 1332–1336, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.