Abstract
Objective:
Biebersteinia multifida is one of the native plants of Iran and its root is used in folk medicine. This study aimed to evaluate the gastro-protective effect of the hydro-methanolic extract of this plant's roots against ethanol-induced gastric ulcer in rats.
Materials and Methods:
The following five groups of seven rats were included in this study: control (C), gastric ulcer (GU), control omeprazole (CO) and two treatment groups (the latter 3 groups were rats with gastric ulcer that orally received omeprazole, 20 mg/kg, or the root extract at 150 and 300 mg/kg (BM 150 and BM 300, respectively) 1 hour before ulcer induction). One hour after ulcer induction, blood sampling was performed and after sacrificing animals, the stomachs were immediately removed. Gastric mucosal injury was studied grossly to determine the number and area of gastric ulcers. The level of nitric oxide (NO) and total antioxidant capacity (TAC) in gastric mucosa as well as serum TNF-α were determined.
Results:
In GU group, severe mucosal injuries were observed (p<0.0001 as compared to C group). The lesions in CO and treatment groups were much milder than GU group by regarding ulcer area and number (p<0.001 for all cases). In treated (BM 150 and BM 300) groups, the gastric mucosal TAC and NO level were significantly higher than GU group (p<0.05 for all cases). Serum TNF-α level was not significantly different between GU and other groups.
Conclusion:
B. multifida possesses gastro-protective effects against ethanol-induced ulcer model; this effect is at least partly related to plant’s antioxidant and NO production accelerating properties.
Key Words: Biebersteinia multifida, Peptic ulcer, Rat, Antioxidant, Anti-inflammatory
Introduction
A common gastrointestinal disease is peptic ulcer that primarily affects the stomach and the duodenum (Konturek et al., 2005 ▶). A wide range of factors may cause gastric ulcer including excessive production of gastric acid or pepsin, insufficient mucosal defense, reflux of bile and pancreatic juice into the stomach, Helicobacter pylori, smoking, alcohol consumption, non-steroidal anti-inflammatory drugs use and psychosocial stress (Richardson, 1990 ▶; Everhart et al., 1998 ▶). Although ethanol consumption is a risk factor for gastric ulcer, the magnitude of its effect depends on the level of consumption and is most damaging in very heavy drinkers (Razvodovsky, 2006 ▶), however, there is a weak association between alcohol consumption and ulcers in moderate drinkers (Chou, 1994 ▶).
Different mechanisms are proposed for ethanol induced-stomach ulcers including oxidative stress (Repetto and Llesuy, 2002 ▶), microcirculation disruption (Hernandez-Munoz et al., 2000 ▶), infiltration of neutrophils, secretion of inflammatory mediators (De Souza et al., 2011 ▶), loss of the protective layer of gastric epithelial cells and subsequently increased vulnerability to HCl and pepsin secreted into gastric lumen (Oates et al., 1988 ▶).
Ethanol-induced gastric ulcer model is different from other models of gastric ulcer that majorly rely on gastric acid secretion (Brazozowski et al., 1998 ▶). In fact, this model is commonly recommended for studying factors which have cytoprotective and/or antioxidant properties (Adinorty et al., 2013).
Drugs which are routinely used to treat gastric ulcer cause side effects; moreover, long-term treatment is usually needed and above all, no complete recovery may be achieved. Therefore, alternative and/or adjunct approaches are demanded. Herbal medicines have shown a diverse range of different mechanisms for their effects on peptic ulcers including acceleration of mucous cell proliferation, antioxidant properties, and reduction of gastric acid secretion (Bi et al., 2014 ▶). These agents especially those with a history of use in traditional medicine might be beneficial for treatment of and protection against peptic ulcers usually with proper safety and cost-effectiveness (Bi et al., 2014 ▶).
Biebersteinia is a genus of plants in the flowering plant order Sapindales (Christenhusz and Byng, 2016 ▶). They occur from the East Mediterranean to West Siberia and Central Asia (Muellner, 2011 ▶) with Biebersteinia multifida DC (BM) as the native species of Iran. In folk medicine, the ointment made of the tuberous root of this plant has been used for curing muscle and skeletal disorders and bone fractures (Amin, 1991 ▶; Farsam et al., 2000 ▶; Amirghofran, 2010 ▶). Besides, BM has been used for the treatment of nocturia in children (Aboutorabi, 2001 ▶). Alkaloids, flavonoids, and polysaccharides are the basic constituents of BM (Kurbanov and Zharekeev, 1974 ▶; Omurkamzinova et al., 1991; Arifkhodzhaev and Rakhimov, 1994 ▶). Flavonoids including 7-glucosides of apigenin, luteolin, and tricetin, as well as the 7-rutinoside of apigenin and luteolin, contribute to the antioxidant and anti-hemolytic activities of BM (Greenham et al., 2001; Omurkamzinova et al., 1991; Nabavi et al., 2010 ▶). Other main components of BM include (E)-nerolidol, phytol, 6, 10, 14-trimethyl-2-pentadecanone and hexadecanoic acid (Javidnia et al., 2010). In different studies, diverse beneficial effects of the plant like anti-inflammatory and analgesic effects (Farsam et al., 2002 ▶) as well as anti-bacterial (Godrati et al., 2012 ▶), anti-hemolytic and anti-oxidant activities (Nabavi et al., 2010 ▶), have been confirmed. It has also been found effective against some psychological disorders (Monsef-Esfahani et al., 2013 ▶).
Considering the successful use of BM in traditional medicine, and antioxidant and anti-inflammatory effects of this plant, we were persuaded to evaluate the gastro-protective effect of BM's extract against ethanol-induced gastric ulcer in vivo.
Materials and Methods
Preparing BM root extract
Taxonomical identification of fresh plant was done by the research center of natural products health (NPH), North Khorasan University of Medical Sciences (Iran), after collecting BM in April 2016 from the mountains of Khorasan Province, northeastern Iran. To prepare the hydro-methanolic extract, dried root powder of BM (100 g) was percolated with methanol 70% (1.0 L) in a sterile environment for 5 days. After filtration and removing the solvent under vacuum at 40°C, 10 g of extract was yielded. In order to prepare the appropriate dose of the extract, distilled water was used as diluent. Doses (150 and 300 mg/kg) were chosen based on our pilot tests.
Animals and study design
Thirty-five male Wistar rats (200-250 g) were used in this study, they were maintained on a standard diet, water ad libitum, ambient temperature around 23°C, and 12/12 light/dark cycle and were left for a week for adaptation. The food was withdrawn 24 hours before the experiment but water was freely available. Rats were randomly allocated into 5 groups of 7 animals each as follows.
1: Control group (C), normal rats that received no treatment during the experiment; 2: Gastric ulcer (GU) group, gastric ulcer was induced in this group and the animals were treated with distilled water, 3: Control omeprazole group (CO), gastric ulcer was induced in this group and the animals received omeprazole (Abidi Pharmaceutical Co., Iran), 20 mg/kg (Segawa et al., 1987 ▶) in distilled water, and 4 and 5: BM treatment groups, gastric ulcer was induced in these groups and the animals received extract of BM at 150 and 300 mg/kg, respectively. One hour before induction of peptic ulcer (by oral administration of 4 ml/kg of 75% ethanol (Singh et al., 2007) to groups 2-5), different agents were administered orally.
One hour after ulcer induction, blood sampling was done under diethyl ether anesthesia via cardiocentesis. After that, animals were sacrificed by deepening anesthesia and the stomachs were removed quickly. After opening the stomach from its greater curvature, it was rinsed with normal saline. Photographs were taken and glandular part of the stomachs was assessed for ulcer formation. Mucosal damages in glandular part of the stomach were determined as hemorrhagic streaks or linear breaks (erosions) in the mucosal surface.
All procedures were done in accordance with institutional ethical guidelines for use of experimental animals and were consistent with European convention for the protection of vertebrate animals used for experimental and other scientific purposes.
Determination of number and area of peptic ulcers
The number of ulcers was enumerated and planimetric method was used for calculating the ulcer area (Shomali et al., 2014 ▶) using Axio Vision Software. Then, stomachs were immediately transferred to a -70°C freezer.
Analysis of the gastric tissue nitric oxide (NO) content and total antioxidant capacity (TAC)
After weighing the glandular part of each stomach, samples were homogenized in cold phosphate buffered solution pH 7.4 (100 mg/ml); then, the homogenized samples were centrifuged (at 4°C and 9500 rpm for 5 min) and supernatant was removed and used as the sample for analyzing NO content (Pan et al; 2005) and total antioxidant capacity (TAC) was measured by colorimetric methods (Shomali et al., 2014 ▶). The assays were done according to guidelines of the manufacturer of the kits prepared by Biocore Diagnostik (ZellBio), Germany.
Determination of serum tumor necrosis factor-α (TNF-α) level
For measuring TNF-α, blood samples were used. The samples were centrifuged (3000 rpm for 10 min) and sera were harvested and kept at −70°C until analysis (Du et al., 2013 ▶). TNF-α was measured by an ELISA kit (Biorbyt’s rat TNF-α ELISA kit, UK) based on the sandwich ELISA method and according to the manufacturer instructions. The absorbance of the specimens was determined using an ELISA reader at 450 nm and the levels of TNF-α were expressed as pg/ml.
Statistical Analysis
Data are expressed as mean±SD and analyzed statistically by the analysis of variance (one way ANOVA) method. Differences among groups were investigated using Tukey's multiple comparison tests and p<0.05 was considered the level of significance.
Results
The number and area of ulcers
Severe hyperemia and hemorrhage were observed in stomachs of GU group. The severity of lesions was much lower in groups treated with omeprazole and BM extract at both doses (Figure 1).
Figure 1.
Effect of hydro-methanolic extract of Biebersteinia multifida root on gastric ulcers in the glandular part of the stomach from rats in different groups. The severity of lesions was most prominent in gastric ulcer group, while rats that received omeprazole or B. multifida showed milder changes
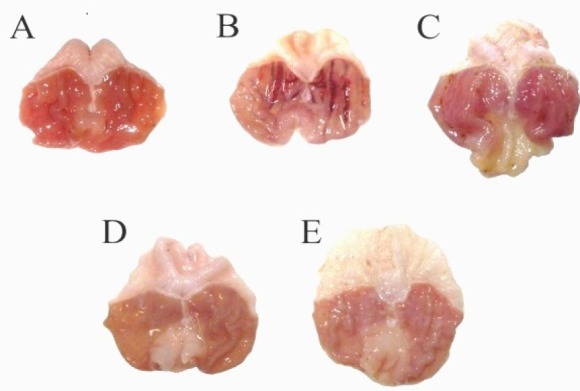
A: Control; B: Gastric ulcer; C: Control omeprazole (omeprazole 20 mg/kg); D and E: Biebersteinia multifida 150 and 300 mg/kg, respectively.
Data on total ulcer area and number in stomachs of rats in different groups are presented in Figures 2A and 2B; a significant difference was found in this regard between GU group and other groups (p<0.001 for all cases). Ulcer area and number in treatment groups and CO group were statistically similar to that of the C group (p>0.05) (Figure 2 A and 2 B).
Figure 2.
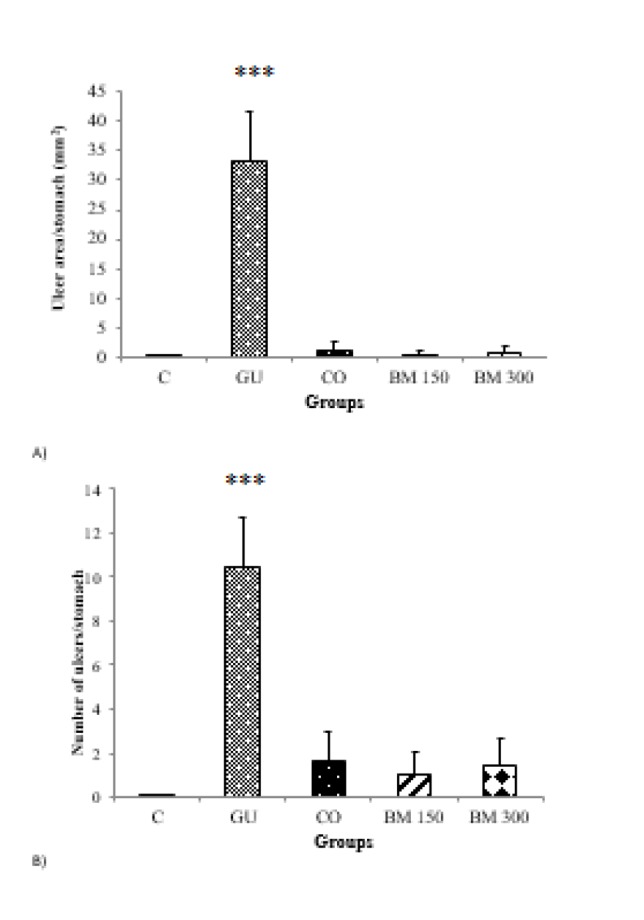
Effect of hydro-methanolic extract of Biebersteinia multifida root on the ulcer area (A) and number (B) /stomach in rats. Data are presented as mean±SD. ***p<0.001 as compared to other groups. C: Control; GU: Gastric ulcer; CO: Control omeprazole (omeprazole 20 mg/kg, orally); BM 150 and BM 300 mg/kg: B. multifida 150 and 300 mg/kg by oral administration
No significant difference was observed between BM 150 and BM 300 groups for both parameters (p>0.05).
Mucosal TAC and NO content and serum TNF-α level
Data showed that mucosal TAC in rats which were treated by BM 150 and BM 300 mg/kg was significantly higher than that of GU (p<0.001 and p<0.05, respectively) and C groups (p<0.001 and p<0.05, respectively). Mucosal TAC in BM 150 group was also significantly higher than BM 300 and CO groups (p<0.001 for both cases). No significant change was observed in mucosal TAC of GU and CO groups as compared to C group (p>0.05) (Figure 3).
Figure 3.
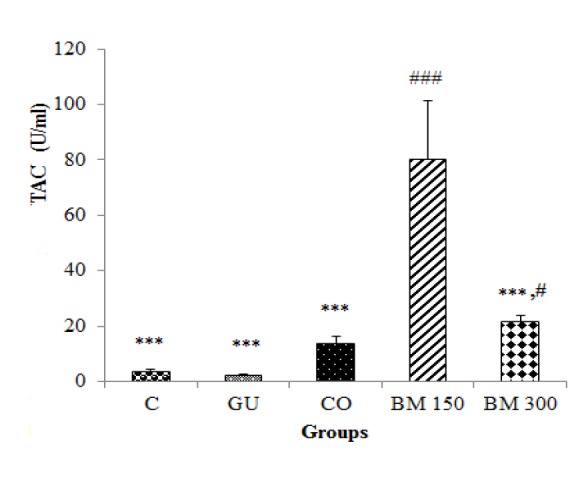
Effect of hydro-methanolic extract of Biebersteinia multifida root on total antioxidant capacity (TAC) of gastric mucosa in gastric ulcer induced by ethanol in rats. Data are presented as mean±SD. #p<0.05, ### p<0.001 as compared to C and GU groups; ***p<0.001 as compared to BM 150 group. C: Control; GU: Gastric ulcer; CO: Control Omeprazole (omeprazole 20 mg/kg, orally); BM 150 and BM 300 mg/kg: B. multifida 150 and 300 mg/kg by oral administration
In relation to mucosal NO content, in rats which were treated by BM (150 and 300 mg/kg) and in CO group, NO content was significantly higher as compared to GU and C groups (p<0.01 for all cases). There was no significant difference between BM 300 and BM 150 group (p>0.05). This parameter was significantly higher in BM 300 as compared to CO group (p<0.05) (Figure 4).
Figure 4.
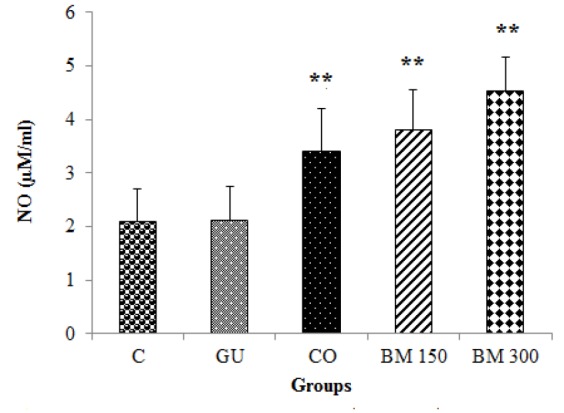
Effects of hydro-methanolic extract of Biebersteinia multifida root on nitric oxide (NO) content of gastric mucosa in gastric ulcer induced by ethanol in rats. Data are presented as mean±SD. **p<0.01 as compared to C and GU groups. C: Control; GU: Gastric ulcer; CO: Control Omeprazole (omeprazole 20 mg/kg, orally); BM 150 and BM 300 mg/kg: B. multifida 150 and 300 mg/kg by oral administration
Serum level of TNF-α was statistically higher in rats treated with BM 150 mg/kg as compared to CO group (p<0.05). Other groups showed statistically similar levels of serum TNF-α in a way that, induction of ulcer in GU group was not associated with a significant change in serum TNF-α levels as compared to the C group (Figure 5).
Figure 5.
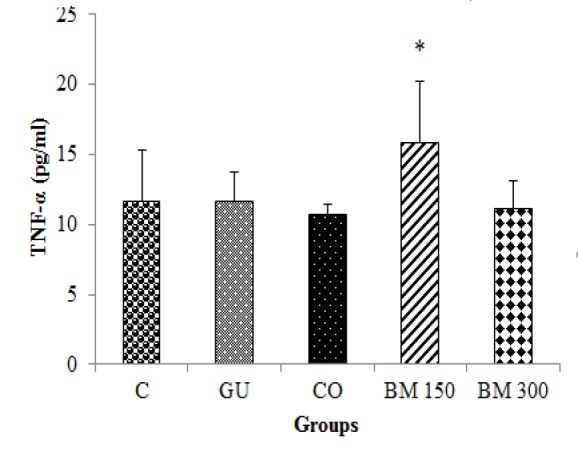
Effect of hydro-methanolic extract of Biebersteinia multifida root on serum TNF-α level in gastric ulcer induced by ethanol in rats. Data are presented as mean±SD. * p<0.05 as compared to CO group C: Control; GU: Gastric ulcer; CO: Control Omeprazole (omeprazole 20 mg/kg, orally); BM 150 and BM 300 mg/kg: B. multifida 150 and 300 mg/kg by oral administration
Discussion
We examined the protective effect of BM extract against ethanol-induced gastric ulcer in rats. Since the ethanol-induced gastric ulcer is more dependent on disturbances in cytoprotective mechanisms, secretion of inflammatory mediators and antioxidant properties (De Souza et al., 2011 ▶; Repetto and Llesuy, 2002 ▶; Hernandez-Munoz et al., 2000 ▶), for studying the protective effect of BM on this gastric ulcer model, we examined some relative parameters which included total antioxidant capacity and mucosal NO content as well as serum TNF-α level.
As stated above, the results of the total ulcer area and number in stomachs of rats in different groups, revealed that severe mucosal injury was present in GU group but the lesions in CO and treatment groups were very mild as compared to GU group.
Data showed that remission of gastric ulcers by both doses of BM was accompanied by higher mucosal TAC and NO content.
Phytochemical studies have revealed that BM contains neutral polysaccharides described as glucans A, B, and C (Arifkhodzhaev et al., 1985 ▶; Arifkhodzhaev and Rakhimov, 1993 ▶, 1994) and alkaloids (Kurbanov and Zharekeev, 1974 ▶). It is believed that BM's biological activities are related to the presence of phenols, flavonoids and alkaloid components of the plant.
It was suggested that disturbances in the mucus-bicarbonate barrier and cell membrane rupture in the wall of blood vessels in ethanol-induced gastric ulcer are presumably due to lipid peroxidation, formation of free radicals and intracellular oxidative stress. Therefore, oxidative stress, as a major player in this regard, has a pivotal role in the creation of necrotic injuries (Sannomiya et al., 2005 ▶). Consistently, in our study, the appreciable preventive effect and high mucosal TAC were observed in BM extract-treated groups.
In previous studies, Greenham et al. (2001) and Omurkamzinova et al. (1991) characterized some of BM's compounds including polysaccharides, peptides, alkaloids such as vasicinone, and flavonoids like 7-glucosides of apigenin, luteolin, and tricetin, as well as 7-rutinoside of apigenin and luteolin (Greenham et al., 2001; Omurkamzinova et al., 1991). Nabavi et al. (2010) ▶ revealed the anti-hemolytic and antioxidant effects of the BM extract. It is well established that some of the pharmacological properties of BM like antioxidant and antihemolytic activities, are because of the presence of vasicinone (Monsef-Esfahani et al., 2013 ▶; Greenham et al., 2001). Considering our findings about reducing the number and extent of ulcers by BM, it can be concluded that these effects may be related to antioxidant effects of this extract, as shown by higher mucosal TAC.
Nitric oxide is known for regulating gastric blood flow. In the stomach, NO helps to maintain gastric mucosal health and can, therefore, be a protective factor against ethanol damage (TeppermanandSoper, 1994 ▶). Nitric oxide is produced by nitric oxide synthase (NOS) enzymes including gastric endothelial NOS (eNOS) and inducible NOS (iNOS). Among them, eNOS activity is a pivotal factor in the protection of gastric mucosa while iNOS can participate in ulcer formation through the production of peroxide free radicals (Cho, 2001 ▶). In a study done in mice, it was shown that 1 hour after peptic ulcer induction, the expression of eNOS and production of NO in gastric mucosa is high, but after 3 and 6 hours, the expression of iNOS increases. In the present study, for determination of NO content, sampling of gastric tissue was done 1 hour after ulcer induction, hereupon; the increased level of NO due to BM extract is most possibly related to eNOS activity (Pan et al., 2005 ▶).
In our study, omeprazole administration also resulted in significant NO elevation and also showed a tendency to increase TAC.
Although omeprazole is routinely used in gastric ulcer because of its ability in suppressing gastric acid secretion by inhibiting the proton pumps (Shin and Sachs, 2008 ▶), a previous study showed that the acid-reducing effect of omeprazole contributes little to its protective effect in ethanol-induced gastric ulcer (Le et al., 2001 ▶). In fact, a major part of the gastro-protective effect of omeprazole is relevant to its potent antioxidant activity (Biswas et al., 2003 ▶; Shomali et al., 2014 ▶).
Moreover, in a study by Le et al. (2001) ▶ on rats with ethanol-induced gastric ulcer, it was observed that omeprazole can exert important protection against gastric mucosal lesion through NO, which is also in agreement with our results.
TNF-α is a pro-inflammatory cytokine that has an important role in the maintenance and regulation of the severity of peptic ulcers (Choi et al., 2010 ▶) as well as gastric mucosal apoptosis (Nakashita et al., 2013 ▶). Du et al. (2013) ▶ reported a significant increase in TNF-α concentration of gastric mucosa of rats with ethanol-induced gastric ulcer after 1 hour which was reduced in rats treated with Veronica strumaxillare (Du et al., 2013 ▶). In 2014, Li et al showed that 4 hours after ethanol administration in mice, TNF-α level in gastric tissue and serum was significantly increased which was attenuated by chelerythrine alkaloid (Li et al., 2014 ▶). As stated above, we evaluated serum levels of TNF-α 1 hour after ulcer induction. This can explain why there was no significant change in this parameter in rats of GU group as compared to C group. Concerning the effect of BM extract on TNF-α, the only significant change was the appreciable increase of this parameter in the BM 150 mg/kg-treated rats as compared to CO group. A previous investigation reported that administration of BM aqueous extract at 500 mg/kg for 8 days to rats with acetic acid-induced ulcerative colitis, results in a significant decrease in intestinal TNF-α levels (Keshavarzi et al., 2018 ▶). These controversies may be related to different factors including the type of the extract, dose, duration of administration, site of evaluation, etc.
Proton pump inhibitors like omeprazole were shown to affect the production of pro-inflammatory cytokines (nuclear factor-κB (NF-κB) and interleukin-8 (IL-8) (Kedika et al., 2009 ▶). We did not observe a significant difference in TNF-α level between the CO group and C or GU groups. In 2006, Handa et al. showed that the effect of proton pump inhibitors on pro-inflammatory cytokines is related to gastric ulcer model, e.g. induction of peptic ulcer by Helicobacter pylori, which induces a significant increase in cytokines (Handa et al., 2006 ▶). Therefore, one of the most important reasons for the inconsistency between these results and the results of our study, can be related to the model of gastric ulcer induction. Consistently, in a similar study by Shomali et al. (2016) ▶, similar results were obtained regarding the effect of omeprazole on TNF-α.
B. multifida root hydro-methanolic possesses gastro-protective properties against ethanol-induced peptic ulcer model that is, at least partly, related to its antioxidant properties and NO accelerating effects. Further studies are necessary to clarify other possible mechanisms.
Acknowledgment
Authors are thankful to Dr. Y. Kamali for his generous donation of the extract.
Conflicts of interest
The authors declare that there is no conflict of interest.
References
- Abdallah IZ, Khattab HA, Heeba GH. Gastroprotective effect of Cordia Myxa L fruit extract against indomethacin-induced gastric ulceration in rats. Life Sci. 2011;8:433–445. [Google Scholar]
- Aboutorabi H. Ethnobotanic and phytochemical study of plants in Rouin region. Pharma Thesis.Tehran University of Medical Sciences. Tehran, Iran: Tehran University of Medical Sciences; 2001. [Google Scholar]
- Arifkhodzhaev AO, Arifkhodzhaev KA, Kondratenko ES. Polysaccharides of saponin-bearing plants Isolation and characterization of the polysaccharides of Biebersteinia multifidi. Chem Nat Compd. 1985;21:714–716. [Google Scholar]
- Arifkhodzhaev AO, Rakhimov D. Polysaccharides of saponin-bearing plantsIV Structure of glucans A, B, and C of Biebersteinia multifidi. Chem Nat Compd. 1993;29:151–153. [Google Scholar]
- Arifkhodzhaev AO, Rakhimov DA. Polysaccharides of saponin-bearing plantsV Structural investigation of glucans A B and C and their oligosaccharides from Biebersteinia multifida plants. Chem Nat Compd. 1994;30:655–660. [Google Scholar]
- Amin G. Popular medicinal plants of Iran. 1st edition. Tehran, Iran: Research Deputy, Ministry of Health, Treatment and Medical Education; 1991. [Google Scholar]
- Amirghofran Z. Medicinal plants as immunosuppressive agents in traditional Iranian medicine. Iran J Immunol. 2010;7:65–73. [PubMed] [Google Scholar]
- Bi WP, Man HB, Man MQ. Efficacy and safety of herbal medicines in treating gastric ulcer: a review. World J Gastroenterol. 2014;20:17020–17028. doi: 10.3748/wjg.v20.i45.17020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas K, Bandyopadhyay U, Chattopadhyay I, Varadaraj A, Ali E, Banerjee RK. A novel antioxidant and antiapoptotic role of omeprazole to block gastric ulcer through scavenging of hydroxyl radical. J Biol Chem. 2003;278:10993–1001. doi: 10.1074/jbc.M210328200. [DOI] [PubMed] [Google Scholar]
- Brzozowski T, Konturek PC, Konturek SJ, Kwiecién S, Pajdo R, Brzozowska I, Hahn EG. Involvement of endogenous cholecystokinin and somatostatin in gastroprotection induced by intraduodenal fat. J Clin Gastroenterol. 1998;27:125–137. doi: 10.1097/00004836-199800001-00020. [DOI] [PubMed] [Google Scholar]
- Cadirci E, Suleyman H, Aksoy H, Halici Z, Ozgen U, Koc A, Ozturk N. Effects of Onosma armeniacum root extract on ethanol-induced oxidative stress in stomach tissue of rats. ChemBiol Interact. 2007;170:40–48. doi: 10.1016/j.cbi.2007.06.040. [DOI] [PubMed] [Google Scholar]
- Cho CH. Current roles of nitric oxide in gastrointestinal disorders. J Physiol Paris. 2001;95:253–256. doi: 10.1016/s0928-4257(01)00034-1. [DOI] [PubMed] [Google Scholar]
- Choi JI, Raghavendran HR, Sung NY, Kim JH, Chun BS, Ahn DH, Choi HS, Kang KW, Lee JW. Effect of fucoidan on aspirin-induced stomach ulceration in rats. Chem Biol Interact. 2010;183:249–254. doi: 10.1016/j.cbi.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Chou SP. An examination of the alcohol consumption and peptic ulcer association: Results of a national survey. Alcohol Clin Exp Res. 1994;18:149–153. doi: 10.1111/j.1530-0277.1994.tb00895.x. [DOI] [PubMed] [Google Scholar]
- Christenhusz MJM, Byng JW. The number of known plants species in the world and its annual increase. Phytotaxa. 2016;261:201–217. [Google Scholar]
- De Souza AES, Filho VC, Niero R, Clasen BK, Balogun SO, Oliveira MDT. Pharmacological mechanisms underlying the anti-ulcer activity of methanol extract and canthin-6-one of Simaba ferruginea A St-Hil In animal models. J Ethnopharmacol. 2011;134:630–636. doi: 10.1016/j.jep.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Du Y, Zhao W, Lu L, Zheng J, Hu X, Yu Z, Zhu L. Study on the anti-ulcer effects of Veronica strumaxillare on gastric ulcer in rats induced by ethanol based on tumor necrosis factor-α (TNF-α) and endothelin-1 (ET-1) Asian Pac J Trop Biomed. 2013;3:925–930. doi: 10.1016/S2221-1691(13)60180-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everhart JE, Byrdholt D, Sonnenberg A. Incidence and risk factors for self-reported peptic ulcer disease in the United States. Am J Epidemiol. 1998;147:529–536. doi: 10.1093/oxfordjournals.aje.a009484. [DOI] [PubMed] [Google Scholar]
- Farsam H, Amanlou M, Dehpour AR, Jahaniani F. Anti-inflammatory and analgesic activity of Biebersteinia multifida DC. root extract. J Ethnopharmacol. 2000;71:443–447. doi: 10.1016/s0378-8741(00)00174-4. [DOI] [PubMed] [Google Scholar]
- Ghodrati N, Asili J, Mohammadi Sani A, Fazli Bazzaz BS. Evaluation of Antibacterial activities of different roots extracts of Biebersteinia multifida DC. Journal of North Khorasan University of Medical Sciences (Natural Products & Medicinal Plants Supplementary) 2012;4:149–154. [Google Scholar]
- Handa O, Yoshida N, Fujita N, Tanaka Y, Ueda M, Takagi T, Kokura S, Naito Y, Okanoue T, Yoshikawa T. Molecular mechanisms involved in anti-inflammatory effects of proton pump inhibitors. Inflamm Res. 2006;55:476. doi: 10.1007/s00011-006-6056-4. [DOI] [PubMed] [Google Scholar]
- Hernandez-Munoz R, Montiel-Ruiz C, Vazquez-Martinez O. Gastric mucosal cell proliferation in ethanol-induced chronic mucosal injury is related to oxidative stress and lipid peroxidation in rats. Lab Invest. 2000;80:1161–1169. doi: 10.1038/labinvest.3780124. [DOI] [PubMed] [Google Scholar]
- Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci. 2009;54:2312–2317. doi: 10.1007/s10620-009-0951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzi Z, Nazari M, Razmi Z, Behnamfar M. The Modulatory effects of aqueous extract of the plant biebersteinia multifida on the gastric acid level and intestinal cytokines in ulcerative colitis model. JBUMS. 2018;20:7–13. [Google Scholar]
- Konturek SJ, KonturekPC , Brzozowski T. Prostaglandins and ulcer healing. J Physiol Pharmacol. 2005;56:5–31. [PubMed] [Google Scholar]
- Kurbanov D, Zharekeev B. Alkaloid of Biebersteinia multifida and Peganumharmala from the karakalpak ASSR Russian [Abstract] Him Prir Soedin. 1974;5:685–686. [Google Scholar]
- Le QL, Zhang J, Xu QZ, Gao HY. Role of nitric oxide in omeprazole protection of the gastric mucosa in rats. Di Yi Jun Yi Da Xue Xue Bao. 2001;21:926–928. [PubMed] [Google Scholar]
- Li WF, Hao DJ, Fan T, Huang HM, Yao H, Niu XF. Protective effect of chelerythrine against ethanol-induced gastric ulcer in mice. Chem Biol Interact. 2014;208:18–27. doi: 10.1016/j.cbi.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Monsef-Esfahani HR, Amini M, Goodarzi N, Saiedmohammadi F, Hajiaghaee R, Faramarzi MA, Tofighi Z, Ghahremani MH. Coumarin compounds of Biebersteinia multifida roots show potential anxiolytic effects in mice. Daru. 2013;21:51. doi: 10.1186/2008-2231-21-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota KSDL, Pita JCLR, Estevam EC, Medirose VM, Tavares JF, Agra MF, Diniz MFFM, Silva MS, Batista LM. Evaluation of the toxicity and antiulcerogenic activity of the ethanol extract of Maytenus obtusifolia Mart. leaves. Rev Bras Farmacogn. 2008;18:441–446. [Google Scholar]
- Muellner AN. Biebersteiniaceae. In: ubitzki K, editor. The Families, and Genera of Vascular Plants, Flowering Plants Eudicots. Verlag Berlin: Springer; 2011. p. 72. [Google Scholar]
- Nabavi SF, Ebrahimzadeh MA, Nabavi SM, Eslami B, Dehpour A. Anti-hemolytic and antioxidant activities of Biebersteinia multifida. Eur Rev Med Pharmacol Sci. 2010;14:823–830. [PubMed] [Google Scholar]
- Nakashita M, Suzuki H, Miura S, Taki T, Uehara K, Mizushima T, Nagata H, Hibi T. Attenuation of acetic acid-induced gastric ulcer formation in rats by glucosylceramide synthase inhibitors. Dig Dis Sci. 2013;58:354–362. doi: 10.1007/s10620-012-2350-x. [DOI] [PubMed] [Google Scholar]
- Oates PJ, Hakkinen JP. Studies on the mechanism of ethanol-induced gastric damage in rats. Gastroenterology. 1988;94:10–21. doi: 10.1016/0016-5085(88)90604-x. [DOI] [PubMed] [Google Scholar]
- Pan LR, Tang Q, Fu Q, Hu BR, Xiang JZ, Qian JQ. Roles of nitric oxide in the protective effect of berberine in ethanol-induced gastric ulcer mice. Acta Pharmacol Sin. 2005;26:1334–1338. doi: 10.1111/j.1745-7254.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- Razvodovsky YE. Aggregate level association between alcohol and the peptic ulcer mortality rate. Alcoholism. 2006;42:61–68. [Google Scholar]
- Repetto MG, Llesuy SF. Antioxidant properties of natural compounds used in popular medicine for gastric ulcer. Braz J Med Biol Res. 2002;35:523–534. doi: 10.1590/s0100-879x2002000500003. [DOI] [PubMed] [Google Scholar]
- Richardson CT. Role of aggressive factors in the pathogenesis of peptic ulcer disease. Scand J Gastroenterol. 1990;174:37–43. doi: 10.3109/00365529009091928. [DOI] [PubMed] [Google Scholar]
- Sannomiya M, Fonseca VB, Da Silva MA, Rocha LR, Dos Santos LC, Hiruma-Lima CA, Souza Brito AR, Vilegas W. Flavonoids and anti-ulcerogenic activity from Byrsonimacrassa leaves extracts. J Ethnopharmacol. 2005;97:1–6. doi: 10.1016/j.jep.2004.09.053. [DOI] [PubMed] [Google Scholar]
- Segawa K, Nakazawa S, Tsukamoto Y, Chujoh C, Yamao K, Hase S. Effect of omeprazole on gastric acid secretion in the rat: evaluation of dose, duration of effect, and route of administration. Gastroenterol Jpn. 1987;22:413–418. doi: 10.1007/BF02773807. [DOI] [PubMed] [Google Scholar]
- Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10:528–534. doi: 10.1007/s11894-008-0098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomali T, Raeesi M, Eskandari-Roozbahani N. Zataria multiflora Boiss essential oil against ethanol-induced gastric ulcer in rats by antioxidant properties and an increase in nitric oxide production. J Herb Med Pharmacol. 2016;5:143–148. [Google Scholar]
- Tepperman BL, Soper BD. Nitric oxide synthase induction and cytoprotection of rat gastric mucosa from injury by ethanol. Can J PhysiolPharmacol. 1994;72:1308–1312. doi: 10.1139/y94-188. [DOI] [PubMed] [Google Scholar]


