Abstract
The aim of this study was to understand how high- and low-intensity locomotor training (LT) affects sympathetic-somatomotor (SS) coupling in people with incomplete spinal cord injury (SCI). Proper coupling between sympathetic and somatomotor systems allows controlled regulation of cardiovascular responses to exercise. In people with SCI, altered connectivity between descending pathways and spinal segments impairs sympathetic and somatomotor coordination, which may have deleterious effects during exercise and limit rehabilitation outcomes. We postulated that high-intensity LT, which repeatedly engages SS systems, would alter SS coupling. Thirteen individuals (50 ± 7.2 years) with motor incomplete spinal cord injuries (American Spinal Injury Association Impairment Scale C or D; injury level >T6) participated in a locomotor treadmill training program. Patients were randomized into either a high-intensity (high-LT; 70–85% of maximum predicted heart rate; n = 6) group or a low-intensity (low-LT; 50–65% of maximum predicted heart rate; n = 7) group and completed up to 20 LT training sessions over 4–6 weeks, 3–5 days/week. Before and after taining, we tested SS coupling by eliciting reflexive sympathetic activity through a cold stimulation, noxious stimulation, and a mental math task while we measured tendon reflexes, blood pressure, and heart rate. Participants who completed high- versus low-LT exhibited significant decreases in reflex torques during triggered sympathetic activity (cold: −83 vs. 13%, p < 0.01; pain: −65 vs. 54%, p < 0.05; mental math: −43 vs. 41%; p < 0.05). Mean arterial pressure responses to sympathetic stimuli were slightly higher following high- versus low-LT (cold: 30 vs. −1.5%; pain: 6 vs. −12%; mental math: 5 vs. 7%), although differences were not statistically significant. These results suggest that high-LT may be advantageous to low-LT to improve SS coupling in people with incomplete SCI.
Keywords: locomotor training, rehabilitation, spinal cord injury, sympathetic nervous system
Introduction
Numerous responses to physiological stressors, including exercise and cold exposure, require coordinated responses of both somatomotor and sympathetic systems for appropriate volitional activity and autonomic regulation. After an incomplete (iSCI) spinal cord injury (SCI), however, partial loss of descending control over spinal networks can disrupt the coordination between these systems. In addition, injury above the mid-thoracic spinal segments (>T6) not only disrupts communication between descending motor commands and spinal networks impairing voluntary movements, but can also interrupt communication of central autonomic pathways for cardiovascular regulation.1 Physical interventions using therapeutic exercises require a combination of somatomotor and sympathetic control for appropriate neuromuscular activity and cardiovascular responses, respectively, although this coordination is typically not a focus for therapy.
Although greater understanding of the coordination of sympathetic and somatic systems in iSCI may have important implications during exercise interventions, alterations in these systems typically have been investigated separately. For example, in the somatomotor (i.e., somatic) system, motoneurons and selected interneuronal circuits often become hyperexcitable and react to a wider variety of sensory inputs after iSCI.2–4 This phenomenon is likely a homeostatic mechanism to restore neuronal excitability in the absence of descending supraspinal inputs and can facilitate volitional motor function in animals and human iSCI.5–8 As a consequence of these changes, seemingly mild sensory provocations often trigger exaggerated reflex behaviors (i.e., spastic motor behaviors) in individuals with iSCI,9 which can interfere with volitional movement.10,11 Sympathetic circuits are affected similarly, such that a variety of stimuli from afferent sources below the injury (e.g., bladder, bowel, cutaneous, and muscle receptors) can reflexively elicit sympathetic activity.12–14 Without descending inputs to regulate spinal sympathetic outflow, reflexive sympathetic activity can result in an uncontrolled increase in blood pressure, resulting in acute hypertension (i.e., autonomic dysreflexia [AD]). The effects of iSCI on the control over both systems can therefore result in devastating problems that interfere with voluntary movements and cardiovascular regulation.
Although altered sympathetic-somatomotor (SS) coupling has largely been reported anecdotally in SCI patients, a few studies do provide quantitative descriptions of this relationship. In the clinic, increased spinal coupling of sympathetic and somatomotor control is often observed as exaggerated reflex behaviors during bouts of AD,15,16 and these symptoms tend to emerge at approximately the same time after injury.17 A few studies have also demonstrated changes in reflex behaviors during increased reflexive sympathetic activity.13,14,18 In response to cold exposure,17 long-standing clinical observations suggest increased spastic responses in patients with SCI, although more recent data indicate variations among patients.18 In addition, Garrison and Schmit14 demonstrated decreased stretch reflex excitability with noxious sympathetic inputs, further suggesting the link between somatic and sympathetic activity.
Improvements in SS coordination through physical exercise may be an important factor for locomotor function in iSCI. Repeated cardiopulmonary exercise, such as locomotor training (LT), could alter the coupling of somatomotor and sympathetic systems, particularly if the intensity of the exercise is sufficiently high.19,20 Recently, physical activity guidelines in adults with SCI are being re-evaluated for their efficacy, with recommendation for training at or above 70% of maximum predicted heart rate (HR)21,22 (see also, more recent recommendations23). Further, recent evidence in SCI patients has shown that goal-directed LT may promote recovery24 and influence plasticity of both somatomotor25–27 and sympathetic28,29 nervous systems. High-intensity exercises may also be a necessary component to improve locomotor function in patients with neurological injury.19,20,30,31 One potential issue in training individuals with iSCI during high-intensity therapeutic exercise is the long-standing belief32–34 that high-intensity exercise may exacerbate spastic motor behaviors and AD. For example, in post-stroke individuals, increasing locomotor intensity (i.e., walking speed) increases the magnitude of spastic motor behaviors, altering movement patterns of the arm.35 Given the importance of appropriate SS coupling during intense physical activity, altered coupling between the systems might influence tolerance to physical activity and muscle function. Yet, no studies demonstrate SS coupling changes after exercise training in people with iSCI.
The aim of this study was to assess changes in SS coupling after locomotor training in iSCI. To examine this coupling, we assessed patellar tendon reflexes, strength, blood pressure, and HR during known sympathetic nervous system triggers before and after high- and low-intensity locomotor training. We hypothesized that training intensity alters SS coordination in iSCI participants. A change in the somatomotor response to sympathetic stressors would indicate changes in coupling of these systems that could underlie improvements in metabolic function associated with LT at high intensity.36
Methods
Participants
Thirteen people (50 ± 7.2 years) with motor iSCI (classified as American Spinal Injury Association Impairment Scale C or D, administered by registered physical therapists) with neurological injury T6 or above and injury duration >1 year were recruited for this study. Participants were recruited by therapist referral from local outpatient rehabilitation centers. Additional characteristics are listed in Table 1. Participants were not asked to alter their medication dosage or schedule during participation. Inclusion criteria consisted of: 18–75 years of age; overground self-selected walking speed <1.0 m/s without physical assistance, but with assistive devices and bracing below the knee as needed; and intact quadriceps and plantarflexor tendon reflexes. Exclusion criteria consisted of: severe lower extremity contractures that limited walking performance; history of osteoporosis; cardiovascular, or metabolic instability; severe medical illness, including unhealed decubiti or existing infection; active heterotrophic ossification in lower extremities; known history of peripheral nerve injury in lower legs; previous history of other central nervous system injury; and inability to adhere to study requirements. All procedures were approved by the Northwestern University Institutional Review Board with written informed consent and medical clearance required to participate.
Table 1.
Patient Characteristics
| Demographics | Medications | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject no. | Group | Age | Level injury | AIS | Weight (kg) | Height (cm) | Antispastics? | Antihypertensives | Antidepressants |
| SCI 1 | High | 63 | C3–C4 | D | 95 | 185 | N | N | Y |
| SCI 2 | High | 53 | C5 | D | 79 | 183 | Y | N | Y |
| SCI 3 | High | 52 | C5–C6 | D | 75 | 179 | N | N | N |
| SCI 10 | High | 47 | T4–T6 | D | 63 | 157 | Y | N | N |
| SCI 14 | High | 55 | T3 | C | 98 | 198 | N | N | Y |
| SCI 19 | High | 42 | C5 | D | 93 | 185 | N | N | N |
| SCI 4 | Low | 53 | T1 | D | 123 | 185 | N | Y | N |
| SCI 5 | Low | 54 | C4 | D | 98 | 191 | N | N | N |
| SCI 7 | Low | 45 | C4 | D | 68 | 178 | N | N | N |
| SCI 9 | Low | 35 | C5–C6 | D | 64 | 185 | Y | Y | N |
| SCI 12 | Low | 56 | C2–C3 | D | 79 | 178 | N | N | N |
| SCI 16 | Low | 45 | C4–C5 | D | 75 | 180 | Y | N | N |
| SCI 17 | Low | 47 | T6 | D | 86 | 160 | N | N | N |
AIS, American Spinal Injury Association Impairment Scale; SCI, spinal cord injury.
Experimental approach
Patients were randomized into groups receiving either high-intensity LT (high-LT; n = 6) or low-intensity LT (low-LT; n = 7), which has been described previously in detail19,36,37 and is detailed briefly here. Patients performed up to 20 training sessions (3–5 × a week) over 4–6 weeks. Each training session consisted of 40 min of stepping activities, with focus on maintaining a target cardiovascular training zone that approximated HR ranges observed during conventional physical therapy19,38; the high-LT group maintained an HR within 70–85% of predicted maximum HR, whereas the low-LT group maintained a target HR 50–65% of predicted maximum (maximum determined by the following: HR = 208–(0.7 × Age)).39 Maximum achievable HRs were reduced by a modest 10 beats/min for those who were taking beta-blockade medications,19 and HRs were monitored continuously using pulse-oximetry with forehead sensors. Not all study participants could tolerate 40-min exercise at 70–85% of their max-predicted heart rate at the beginning of the training period. In these participants, the total amount of training in each session was increased over the training period and the amount of exercise was documented using accelerometers. In these participants, the tolerance improved substantially throughout the training period and all participants tolerated 40 min before the end of the training period. As a secondary measure of intensity, we used the Rating of Perceived Exertion (RPE) scale (6–20),40 if participants could not achieve the target HR zone during training. The target rating for the high-LT group was 15–17 (“hard to “very hard”) and was 11–13 (below “somewhat hard”) for the low-LT group. Measures of HR and RPE were evaluated every 5 min of training. During training sessions, both groups performed four different stepping tasks: speed-dependent treadmill training, skill-dependent treadmill training, over-ground training, and stair climbing.
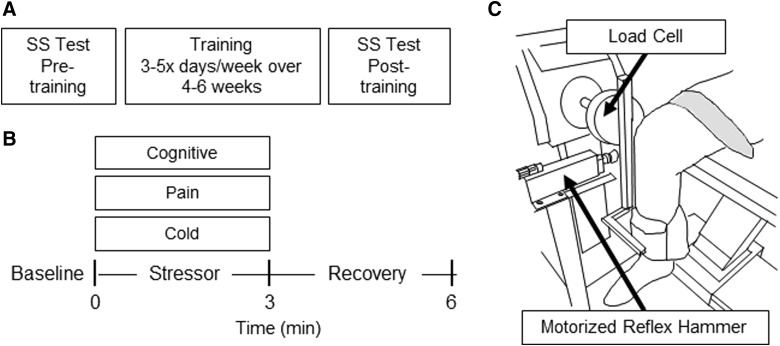
Before and after the training sessions, we assessed sympathetic function (HR and blood pressure) and somatomotor function (stretch reflexes from patellar tendon taps; voluntary strength from maximum voluntary contractions) before, during, and after stimuli that trigger sympathetic reflexes. Specific stressors included a cold stimulus (foot in ice bath for 3 min), noxious electrical stimulation at the stomach (10 stimuli/s for 10 sec over 3 min), and a cognitive mathematical task (counting backward from a random number by 13). Subjects completed this SS experimental session ∼1 week before the start of training and then again ∼1 week after the end of training (Fig. 1A). Figure 1B illustrates the experimental timeline during the SS tests. The sympathetic stimulus (stressor) for each trial was applied during the first 3 min of the trial. After 3 min, the stimulus was removed, and we continued measurements for an additional 3 min (recovery). The procedures for the trials are described below.
FIG. 1.
(A) Experimental timeline. Subjects underwent a pre-training sympathetic-somatomotor (SS) testing session before training and then repeated the post-training SS testing session. The prescribed training comprised 20 sessions, with frequency (3–5 × per week) and duration (4–6 weeks) dependent on scheduling. (B) SS tests experimental timeline. Measurements were recorded during a baseline condition when subjects were relaxed, during 3 min while we applied a known sympathetic stressor, and during a recovery period when the sympathetic stressor was removed. (C) Experimental setup for SS tests. The approximate knee joint center was aligned to a load cell. A motorized reflex hammer was aligned with the patellar tendon.
Test procedures and outcomes
Baseline measurements
At the start of the SS session, we recorded baseline measurements for blood pressure, HR, and patellar tendon reflex responses (torque and electromyogram [EMG]) while subjects were relaxed. We then measured baseline strength from maximal voluntary contractions (MVCs) of the right knee extensors. Participants repeated the MVCs up to five trials during the baseline portion of the test.
Experimental Measurements
We performed six trials to test the coupling between sympathetic reflexes and somatomotor function. For three trials, we elicited patellar tendon reflexes during the sympathetic stressors. We applied five consecutive patellar tendon perturbations (20-ms pulse duration, 1 pulse per second) 20 sec after the application of the stressor, and we repeated these at 1-min intervals (six bouts of five consecutive tendon perturbations). For the other three trials, we repeated each sympathetic stressor while subjects performed MVCs of the right knee extensors. Participants performed one MVC per minute over the 6-min trial. Subjects held the contraction for 5 sec and were verbally encouraged during the contraction.
Sympathetic stressors
We used two methods to elicit spinal sympathetic responses below the level of injury: 1) cold stimulation and 2) noxious electrical stimulation. The cold stimulation was used because it has limited direct impact on motor systems. Electrical stimulation was used over other nociceptive testing methods (e.g., pinprick) in order to accurately control the amplitude with repeated applications. A cognitive stressor was also used because it invokes descending sympathetic drive with little direct impact on motor systems. For each stressor, the stimuli were applied for 3 min, followed by 3 min of recovery. This provided a reliable sympathetic stimulus and produced no autonomic dysreflexia. Detailed methods for each stressor are provided below.
Cold stressor
The left foot was submerged up to the malleolus in a bucket of ice water (∼2–3°C) for 3 min. After 3 min, the foot was removed and we briefly measured the foot's skin temperature using an infrared thermometer to ensure each subject's foot was cooled to the same temperature. The foot was then submerged into a warm (∼27°C) water bath for an additional 3 min.
Noxious pain stressor
Pain was induced using electrical stimulation (100 pulses per second, 20-ms train duration, 1-ms pulse width) to 1–2 cm rostral to the navel using two 40-mm diameter electrodes (Blue Sensor; Medicotest Corporation, Rolling Meadows, IL) and a constant current stimulator (Digitimer North America, LLC, Ft. Lauderdale, FL). The stimulus location was placed at midline of the trunk and not at either limb or side of the body to minimize excitation of specific spinal reflex pathways (e.g., flexor withdrawal or crossed extensor reflex) that may directly interfere with motor output. During the first 3 min of the trial, the electrical stimulation was applied each second for 15 sec with 60 sec between bouts of stimulation. The stimulation output was based on each subject's pain rating using a numerical rating scale (0 is no pain, 10 is worst pain imaginable). When determining the stimulation voltage, we incrementally adjusted the voltage until the subject consistently responded to a 6 on the scale. We did this to ensure that subjects received a similar sensation of pain, which may be different between subjects due to differences in injury severity.
Cognitive stressor
Participants counted aloud backward by 13 beginning from a random three-digit number provided by the experimenter. Participants were encouraged to do their best to subtract as accurately as possible. Backward counting lasted 3 min, followed by 3 min of relaxation.
Experimental arrangement
Figure 1C illustrates the experimental setup used during the SS tests. Participants were seated in a slightly reclined position in a Biodex System 4 Pro chair (Biodex, Shirley, NY) with their knees ∼90 degrees flexed and a strap to secure the right thigh to the seat. The right shank was secured to the Biodex System's dynamometer using an attachment and padded strap and the left leg rested freely on the chair. Participants rested their arms on armrests approximately mid-chest level.
We used a custom-built motorized reflex hammer to deliver controlled patellar tendon perturbations (Fig. 1C). The motorized reflex hammer consisted of a LinMot linear motor powered by a LinMot amplifier and was controlled using LinMot Talk software (LinMot Inc., Delavan, WI). A small force transducer (Kistler, Amherst, NY) was screwed onto the end of the motor shaft. We measured the tendon perturbation force to ensure that consistent perturbations were applied to the tendon. A rubber tip (∼10 cm diameter), attached at the end of the force transducer, was placed 5.0 cm away from the tendon and aligned perpendicular (∼90 degrees) to the tibia to deliver patellar tendon taps with consistent force.
Muscle activity was measured from the rectus femoris (RF), vastus lateralis (VL), medial gastrocnemius, and the tibialis anterior of the right leg (Delsys Inc., Boston, MA). The electrode was placed on the belly of the respective muscle. Before placing the electrodes, the skin was slightly abraded and cleaned with an alcohol swab. EMG signals were pre-amplified ( × 1000–10,000), band-pass filtered (10–500 Hz), and sampled at 1 kHz using a data acquisition card (National Instruments Corp., Austin, TX) and PC.
Knee torque was measured from a load cell (ATI Industrial Automation, Apex, NC) attached to the Biodex motor (Fig. 1C). The approximate knee joint center was aligned with the center of a load cell. Torque signals were sampled at 1 kHz using a data acquisition card (National Instruments) and low-pass filtered (500-Hz analog filter) before acquisition.
To monitor changes in sympathetic activity, we continuously measured blood pressure and HR using the Finometer PRO (Finapres Medical Systems, Amsterdam, The Netherlands). Finger arterial pressure waveforms were continuously recorded from the middle finger of the left hand. The blood pressure recordings were corrected to account for the height difference between the hand and the heart. HR was monitored using a three-lead ECG. Blood pressure and HR signals were recorded with the same data acquisition card used to record EMG, and torque signals and were measured continuously throughout each trial.
Data analysis
All data were analyzed using custom-written Matlab programs (The MathWorks Inc., Natick, MA). The data were first normalized to the value recorded at baseline and then divided into 1-min bins for each trial (i.e., cold, pain, and cognitive). For each of the dependent variables described below, we calculated as the percent change from pre-training to post-training (%Δ = 100 × (post-training – pre-training) / pre-training). In the Results, a positive %Δ indicates the dependent variable increased from pre-training to post-training and vice versa for a negative %Δ.
We used the peak-to-peak (P-P) reflex amplitude to quantify the reflex response from each patellar tendon tap. The P-P reflex amplitude is the difference in volts between the positive and negative peak. We used both the torque and EMG (both VL and RF) signals to calculate the P-P reflex torque and the P-P EMG reflex. Before calculating the P-P reflexes, the torque signal was low-pass filtered at 20 Hz (second-order Butterworth, bidirectionally) to remove noise, and EMG signals were notched filtered (second-order Butterworth, 59–61 Hz, bidirectionally) and detrended. An event detection algorithm was then used to identify the reflex response from each tendon tap, and these were visually inspected to verify a reflex was elicited. Reflexes were differentiated from background noise by their triphasic shape and the latency from the tap (∼20 ms). The P-P reflex amplitudes were then averaged across the five tendon taps for each 1-min interval.
The MVC torque signal was first low-pass filtered (5 Hz, second-order Butterworth filter, bidirectionally). The peak torque was then identified and the MVC was calculated as the median of the torque signal −500 to +500.
HR signals were low-pass filtered at 30 Hz (second-order Butterworth, bidirectionally) and the blood pressure signal was low-pass filtered at 10 Hz (second-order Butterworth, bidirectionally). An event detection algorithm was used to identify systolic and diastolic deflections. The mean arterial pressure (MAP; = ⅔ diastolic + ⅓ systolic blood pressures) and HR were averaged within a 10-sec window during each 1-min interval for each trial. In this study, we are presenting the MAP and HR during the trials with patellar tendon taps.
Statistical analysis
A mixed, repeated-measures analysis of variance (ANOVA) was used to determine differences between training type (main factor: high-LT vs. low-LT) and time (repeated factor: third minute of the stressor vs. third minute of the recovery) on the primary dependent variables: %ΔP-P EMG reflex, %ΔP-P reflex torque, and %ΔHR and %ΔMAP. Statistical tests were performed independently for each stressor condition (e.g., cold stressor during patellar tendon taps, cold stressor during MVCs, etc). As secondary measures, we also performed statistical tests to determine whether training type affected RPE, HR, baseline MVCs, and stimulation amplitude (for the pain sympathetic stressor only). For each of these dependent measures, a mixed, repeated-measures ANOVA was used to test differences between training type (main factor: high-LT vs. low-LT) and time (repeated factor: pre- vs. post-training). The Greenhouse-Geisser correction factor was used when Mauchly's test of sphericity was violated. We tested our data for normality using Shapiro-Wilks' test, and we visually inspected normality using Q-Q plots. Each dependent variable approximated a normal distribution (Shapiro-Wilks, p > 0.05 for all dependent variables). All statistics were performed using IBM SPSS Statistics software (version 21.0; IBM Corp, New York, NY). The α level was set at 0.05. Data are reported as means ± standard deviation in the text and means ± standard error in figures. Only the significant main effects are presented, unless otherwise noted.
Results
All iSCI participants completed the LT protocol. The high-LT group completed an average of 19 ± 3 sessions (1 subject could only complete 11 sessions), with a mean maximum HR 72 ± 12% of age-predicted maximum per training session, with 1 subject presenting with a blunted HR response (mean peak HR was 48% of predicted maximum; see Table 2 for summary). However, the mean RPE for this group was 17 ± 1. For the low-LT group, all participants completed 20 sessions, with mean maximum HR per session of 56 ± 11% of age-predicted maximum, with 1 subject who was unable to walk with reduced HR <65% (i.e., 76%). Nonetheless, the mean RPE for this group was 13 ± 1, with low RPEs reported for that subject. Differences in RPEs (p < 0.01), but not HR (p = 0.07), were significant between training groups.
Table 2.
Peak Performance between Groups
| Training parameters | |||
|---|---|---|---|
| Subject no. | Group | Mean peak RPE | Mean peak % HR max |
| SCI 1 | High | 16 | 0.77 |
| SCI 2 | High | 15 | 0.74 |
| SCI 3 | High | 16 | 0.48 |
| SCI 10 | High | 17 | 0.79 |
| SCI 14 | High | 19 | 0.81 |
| SCI 19 | High | 16 | 0.73 |
| SCI 4 | Low | 14 | 0.52 |
| SCI 5 | Low | 11 | 0.48 |
| SCI 7 | Low | 13 | 0.49 |
| SCI 9 | Low | 12 | 0.62 |
| SCI 12 | Low | 12 | 0.43 |
| SCI 16 | Low | 11 | 0.62 |
| SCI 17 | Low | 15 | 0.76 |
HR, heart rate; RPE, Rating of Perceived Exertion scale; SCI, spinal cord injury.
Baseline measurements were similar between groups. We observed similar pre-training measurements from all subjects (pre high-LT and pre low-LT) with no significant differences between pre-training baseline measurements (p > 0.05: MVC, tendon reflex amplitude, HR, and MAP). For the noxious pain stimulus, there were no differences in stimulation amplitude between groups (p = 0.23) or between pre- and post-training (p = 0.89). Additionally, we tested whether training intensity affected knee extensor strength by comparing baseline MVCs between pre- and post-training. Subjects in the high-LT and low-LT groups did not demonstrate differences in MVCs before and after training (high-LT pre vs. post: p = 0.25; low-LT pre vs. post: p = 0.35).
Patellar tendon reflex and sympathetic stimuli
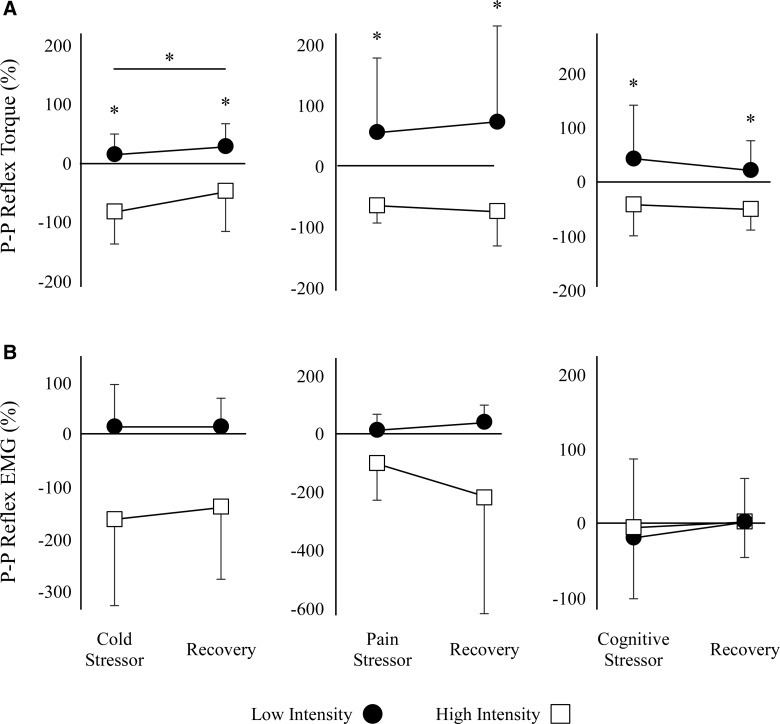
We observed differences in changes the patellar tendon tap torque responses to sympathetic before and after training for the low-LT and high-LT groups (Fig. 2). In Figure 2, the %ΔP-P is a percent change from pre-training to post-training, with the changes shown for both the sympathetic stressor period and recovery period. Subjects in the high-LT group demonstrated a significant decrease in the %ΔP-P reflex torque compared to the low-to group during the cold (p < 0.01), pain (p < 0.05), and cognitive (p < 0.05) trials during both the stressor and the recovery period (Fig. 2A). We also observed a main effect of time (stressor vs. recovery) across both groups during the cold trial (p < 0.01), in which we observed an increase in %ΔP-P reflex torque for both high- and low-LT groups after the cold stimulus was removed. We did not observe this effect for the pain (p = 0.73) or cognitive (p = 0.32) trials. Although we did not find significant differences in the %ΔP-P EMG reflex responses, we did observe similar trends as in the %ΔP-P torque reflex responses (Fig. 2B). During the cold and pain sympathetic stimuli, subjects in the high-LT group exhibited decreased %ΔP-P EMG reflex response, whereas the subjects in the low-LT group exhibited an increase in %ΔP-P EMG reflex response.
FIG. 2.
Training effects on the tendon tap reflex responses to sympathetic stimuli are shown. The percent changes are expressed relative to the pre-training measurements of the effects of sympathetic stimuli on the tendon tap reflex. %ΔP-P reflex torque (A) and %ΔP-P EMG (B) during the third minute of the cold, pain, and cognitive stressor and during the third minute recovery. Asterisks represent significant differences (p < 0.05). EMG, electromyogram; P-P, peak-to-peak.
Maximum voluntary strength and sympathetic stimuli
Training intensity did not have a significant effect between voluntary strength and sympathetic reflexes. Participants in both training groups demonstrated similar knee extensor strength (%ΔMVC) for the cold (p = 0.23), pain (p = 0.98), and cognitive (p = 0.79) trials.
Cardiovascular responses to sympathetic stimuli
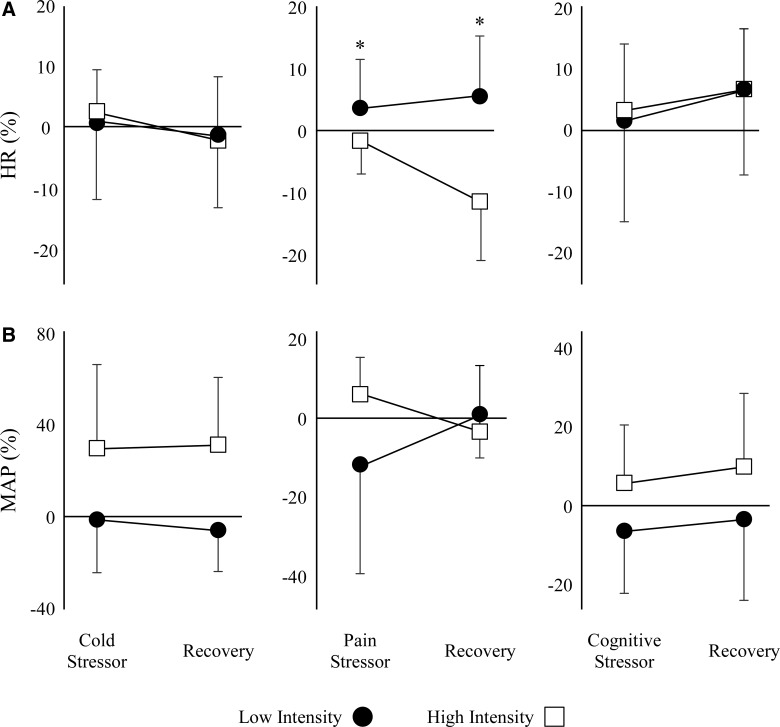
Training intensity affected the %ΔHR during the pain trial (Fig. 3A). We observed a significant difference in %ΔHR between the high- and low-LT groups (p = 0.01). The high-LT group demonstrated a decrease in %ΔHR during the pain trial, whereas the low-LT group demonstrated an increase in %ΔHR during the pain stressor. Further, during the recovery period (when the pain stimulus was removed), the high-LT group had further decreased the %ΔHR, whereas the low-LT group showed increased %ΔHR after the cessation of the pain stressor. The %ΔHR between high-LT and low-LT groups did not differ during the cold (p = 0.93) and cognitive (p = 0.91) trials.
FIG. 3.
Cardiovascular responses to sympathetic stimuli. The %ΔHR (A) and %ΔMAP (B) during the third minute of the cold, pain, and cognitive stressor and the third minute of recovery. HR, heart rate; MAP, mean arterial pressure.
We observed no statistically significant differences in %ΔMAP between training intensities for the cold (p = 0.07), pain (p = 0.41), and cognitive (p = 0.13) trials (Fig. 3B). However, the high-LT group demonstrated a trend of increased %ΔMAP during the cold and cognitive stressors compared with the low-LT group.
Discussion
The aim of this study was to assess SS coordination after high- or low-intensity LT in people with iSCI. We found that the intensity of the exercise training differentially altered the coordination between stretch reflexes and sympathetic activity. After high-intensity LT, stretch reflexes were decreased during sympathetic reflex responses induced by cold, pain, and a mental math task. In contrast, the low-LT group demonstrated either no change or increased reflex response during sympathetic stimuli. These results suggest that LT at a higher intensity may alter SS coordination in people with iSCI.
Sympathetic-somatomotor interactions
In neurologically intact individuals, the sympathetic and somatomotor systems are tightly coupled, and both systems are recruited in a coordinated manner.41 Increasing sympathetic outflow through exercise, mental arithmetic, or cold stimulation, for example, augments stretch reflex activity in the soleus muscle in able-bodied individuals.18,42 After neurological injury, loss or diminished central control over spinal networks leads to dysregulation of both sympathetic1 and motor systems.43 Previous studies have shown there is altered coupling between the two systems after SCI,14,18,44 but there is limited research on whether their relation is modifiable through exercise. In the current study, the most important observation is that high-intensity locomotor training resulted in decreased stretch reflex responses during sympathetic stimuli. These changes observed in the high-LT group may be a result of altered coupling of motor and sympathetic systems at the spinal cord or possibly at the brainstem level.
Recent research on locomotor training in iSCI is showing that improvements in walking function are associated with increased descending activity over spinal networks.26,45 It has been suggested that a central SS circuit controls the simultaneous modulations of spinal interneurons common to both systems.41 In regard to the motor system, treadmill training can increase facilitation of descending excitatory and spinal inhibitory pathways resulting in better voluntary control45 and decreased reflex excitability,27,46,47 respectively. Because stretch reflex excitability can be modulated through sympathetic nervous system activity,14,18,42 changes in descending connectivity to motor systems could also mediate spinal sympathetic networks after high-intensity LT. Results from the current study suggest that intensive exercises are critical in producing neural modifications that are important for motor recovery and SS coupling after SCI, possibly through spinal interneuron pathways.
An alternative explanation for the results observed in this study is high-intensity exercise modifies plasma catecholamine (norepinephrine and epinephrine) levels differently than low-intensity training. In able-bodied individuals, peripheral catecholamine concentration increases with greater workload48,49 and persists after a workout.50 In rats, increased systemic catecholamine levels can cause monosynaptic reflex depression.51,52 Conclusions on plasma norepinephrine levels have been mixed in exercising paraplegics,53 but a few studies have shown that individuals with high thoracic (T1–T6) spinal injuries who perform arm cycle exercises have increased levels of norepinephrine.54,55 Although we did not measure catecholamine levels in this study, it is possible the higher-intensity training produced greater increases in catecholamine levels, both peripherally and centrally, compared to the low-intensity training, resulting in decreased reflex responses in the high-LT group. Moreover, because low plasma catecholamine levels are typical in individuals with SCI,54 increases in their levels may indicate better sympathetic activation56 and thus an improved physiological response to stress.
During the pain stimulus, we observed a decrease in HR for the high-LT group, whereas the low-LT demonstrated increased %ΔHR (Fig. 3A). During exercise, the exercise pressor reflex plays a role in increasing blood pressure, HR, and ventilation attributed to contracting muscle,57 which is mediated through group III and IV muscle afferents. Group III and IV muscle afferents are also responsible for evoking the sensation of pain and overlap areas of the dorsal horn in the spinal cord.58 In chronic SCI, muscle group III/IV actions appear to be altered, producing enhanced flexor reflexes59 and augmented force-feedback inhibition of extensor muscles.60 In the high-LT group of the current study, strengthened descending control may have improved the regulation of group III-IV afferent activity, affecting HR during noxious stimulation.
Methodological considerations
The equation we used to predict the maximum heart rate (HRmax = 208 – 0.7 × age) for our training groups is based on statistical models from healthy individuals,39 and it may overestimate the attainable HR in patients with SCI. Persons with a high-thoracic (above T6) or cervical spinal injury may have inappropriate responses to exercise because of poorly controlled autonomic function.61 Depending on the integrity of descending autonomic pathways, exercise performance can be altered because of lower blood pressure, lower stroke volume, lower HR, or impaired blood redistribution.61 Although we did not assess the completeness of autonomic function in our iSCI cohort, most of our participants (11 of 13) were able to increase their HR to the predicted maximum target, which is in line with previous results.62 Nonetheless, it is important to keep in mind that the cardiovascular response to exercise depends on the integrity of spinal sympathetic pathways, which may have affected the iSCI training outcomes. Across study participants it was also challenging to maintain a high enough HR in subject 3 in the high-LT group and a low enough HR in subject 17 of the low-LT group (see Table 2). As a result, there may be differences between high-LT and low-LT that were not detected.
Functional implications
Alterations in SS coupling in iSCI through high-intensity LT may have important implications for physical activity after SCI. Common sequelae after SCI include spasticity and loss of voluntary control9,63,64 as well as impaired autonomic function.1,65 Whereas impairments in the respective systems are problematic, impaired interactions between the two systems can be debilitating on several levels. During physical activity, a loss or decrease in sympathetic control results in poor blood redistribution to active muscles.66,67 This results in cardiovascular intolerance during physical activity in individuals with SCI68,69 and impaired exercise performance in SCI athletes.70,71 Equally, impaired muscle activity may not be adequate for increasing spinal sympathetic activity needed in response to exercise. Further, with impaired voluntary control, individuals with iSCI can lead more sedentary lifestyles, leading to muscular deconditioning as well as secondary cardiovascular complications. As such, high-intensity exercise to normalize SS interactions might be critical to therapeutic interventions in individuals with SCI.
Conclusions
In this study, we demonstrate that LT intensity differentially affects SS coupling in individuals with iSCI. After high-intensity LT, we observed decreased reflexes during sympathetic stressors compared to reflexes observed after low-intensity LT. Further, we observed a trend toward increased blood pressure in the high-LT group compared to the low-LT group during sympathetic stimuli. Intensive locomotor treadmill training may have improved descending control over spinal networks common to the sympathetic and motor systems, leading to changes in SS coupling, and they suggest that higher-intensity exercise programs may be more beneficial for recovery in iSCI.
Acknowledgments
This work was funded by NIH R01NS07975. We thank Patrick Hennessy and Jane Woodward for their assistance during locomotor training.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Krassioukov A. (2009). Autonomic function following cervical spinal cord injury. Respir. Physiol. Neurobiol. 169, 157–164 [DOI] [PubMed] [Google Scholar]
- 2. Hyngstrom A., Johnson M., Schuster J. and Heckman C.J. (2008). Movement-related receptive fields of spinal motoneurones with active dendrites. J. Physiol. 586, 1581–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schmit B.D., and Benz E.N. (2002). Extensor reflexes in human spinal cord injury: activation by hip proprioceptors. Exp. Brain Res. 145, 520–527 [DOI] [PubMed] [Google Scholar]
- 4. Schmit B.D., McKenna-Cole A., and Rymer W.Z. (2000). Flexor reflexes in chronic spinal cord injury triggered by imposed ankle rotation. Muscle Nerve 23, 793–803 [DOI] [PubMed] [Google Scholar]
- 5. Onifer S.M., Smith G.M., and Fouad K. (2011). Plasticity after spinal cord injury: relevance to recovery and approaches to facilitate it. Neurotherapeutics 8, 283–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murray K.C., Nakae A., Stephens M.J., Rank M., D'Amico J., Harvey P.J., Li X., Harris R.L., Ballou E.W., Anelli R., Heckman C.J., Mashimo T., Vavrek R., Sanelli L., Gorassini M.A., Bennett D.J., and Fouad K. (2010). Recovery of motoneuron and locomotor function after spinal cord injury depends on constitutive activity in 5-HT2C receptors. Nat. Med. 16, 694–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hornby T.G., Lewek M.D., Thompson C.K., and Heitz R. (2009). Repeated maximal volitional effort contractions in human spinal cord injury: initial torque increases and reduced fatigue. Neurorehabil. Neural Repair 23, 928–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thompson C.K., Lewek M.D., Jayaraman A., and Hornby T.G. (2011). Central excitability contributes to supramaximal volitional contractions in human incomplete spinal cord injury. J. Physiol. 589, 3739–3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benz E.N., Hornby T.G., Bode R.K., Scheidt R.A., and Schmit B.D. (2005). A physiologically based clinical measure for spastic reflexes in spinal cord injury. Arch. Phys. Med. Rehabil. 86, 52–59 [DOI] [PubMed] [Google Scholar]
- 10. Jones C.A., and Yang J.F. (1994). Reflex behavior during walking in incomplete spinal-cord-injured subjects. Exp. Neurol. 128, 239–248 [DOI] [PubMed] [Google Scholar]
- 11. Onushko T., Hyngstrom A,. and Schmit B.D. (2010). Effects of multijoint spastic reflexes of the legs during assisted bilateral hip oscillations in human spinal cord injury. Arch. Phys. Med. Rehabil. 91, 1225–1235 [DOI] [PubMed] [Google Scholar]
- 12. Corbett J.L., Debarge O., Frankel H.L., and Mathias C. (1975). Cardiovascular responses in tetraplegic man to muscle spasm, bladder percussion and head-up tilt. Clin. Exp. Pharmacol. Physiol. Suppl. 2, 189–193 [PubMed] [Google Scholar]
- 13. Corbett J.L., Frankel H.L., and Harris P.J. (1971). Cardiovascular changes associated with skeletal muscle spasm in tetraplegic man. J. Physiol. 215, 381–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garrison M.K., and Schmit B.D. (2009). Flexor reflex decreases during sympathetic stimulation in chronic human spinal cord injury. Exp. Neurol. 219, 507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kewalramani L.S. (1980). Autonomic dysreflexia in traumatic myelopathy. Am. J. Phys. Med. 59, 1–21 [PubMed] [Google Scholar]
- 16. Mathias C., and Bannister R. (1999). Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System, 4th ed. Oxford University Press: Oxford, UK [Google Scholar]
- 17. Teasell R.W., Arnold J.M., Krassioukov A., and Delaney G.A. (2000). Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch. Phys. Med. Rehabil. 81, 506–516 [DOI] [PubMed] [Google Scholar]
- 18. Ogata H., Sayenko D.G., Yamamoto E., Kitamura T., Yamamoto S., Miyoshi T., Kamibayashi K., and Nakazawa K. (2011). Effect of spinal cord injury and its lesion level on stretch reflex modulation by cold stimulation in humans. Clin. Neurophysiol. 122, 163–170 [DOI] [PubMed] [Google Scholar]
- 19. Hornby T.G., Holleran C.L., Hennessy P.W., Leddy A.L., Connolly M., Camardo J., Woodward J., Mahtani G., Lovell L., and Roth E.J. (2016). Variable Intensive Early Walking Poststroke (VIEWS): a randomized controlled trial. Neurorehabil. Neural Repair 30, 440–450 [DOI] [PubMed] [Google Scholar]
- 20. Leech K.A., Kinnaird C.R., Holleran C.L., Kahn J., and Hornby T.G. (2016). Effects of locomotor exercise intensity on gait performance in individuals with incomplete spinal cord injury. Phys. Ther. 96, 1919–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Devillard X., Rimaud D., Roche F., and Calmels P. (2007). Effects of training programs for spinal cord injury. [Article in English, French]. Ann. Readapt. Med. Phys. 50, 490–498, 480–489 [DOI] [PubMed] [Google Scholar]
- 22. Nightingale T.E., Metcalfe R.S., Vollaard N.B., and Bilzon J.L. (2017). Exercise guidelines to promote cardiometabolic health in spinal cord injured humans: time to raise the intensity? Arch. Phys. Med. Rehabil. 98, 1693–1704 [DOI] [PubMed] [Google Scholar]
- 23. Billinger S.A., Arena R., Bernhardt J., Eng J.J., Franklin B.A., Johnson C.M., MacKay-Lyons M., Macko R.F., Mead G.E., Roth E.J., Shaughnessy M., and Tang A.; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Clinical Cardiology. (2014). Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 45, 2532–2553 [DOI] [PubMed] [Google Scholar]
- 24. Dobkin B., Apple D., Barbeau H., Basso M., Behrman A., Deforge D., Ditunno J., Dudley G., Elashoff R., Fugate L., Harkema S., Saulino M., andScott M.; Spinal Cord Injury Locomotor Trial (SCILT) Group. (2006). Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology 66, 484–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gorassini M.A., Norton J.A., Nevett-Duchcherer J., Roy F.D., and Yang J.F. (2009). Changes in locomotor muscle activity after treadmill training in subjects with incomplete spinal cord injury. J. Neurophysiol. 101, 969–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thomas S.L., and Gorassini M.A. (2005). Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. J. Neurophysiol. 94, 2844–2855 [DOI] [PubMed] [Google Scholar]
- 27. Smith A.C., Mummidisetty C.K., Rymer W.Z., and Knikou M. (2014). Locomotor training alters the behavior of flexor reflexes during walking in human spinal cord injury. J. Neurophysiol. 112, 2164–2175 [DOI] [PubMed] [Google Scholar]
- 28. Ditor D.S., Kamath M.V., MacDonald M.J., Bugaresti J., McCartney N., and Hicks A.L. (2005). Effects of body weight-supported treadmill training on heart rate variability and blood pressure variability in individuals with spinal cord injury. J. Appl. Physiol. (1985) 98, 1519–1525 [DOI] [PubMed] [Google Scholar]
- 29. Hoekstra F., van Nunen M.P., Gerrits K.H., Stolwijk-Swuste J.M., Crins M.H., and Janssen T.W. (2013). Effect of robotic gait training on cardiorespiratory system in incomplete spinal cord injury. J. Rehabil. Res. Dev. 50, 1411–1422 [DOI] [PubMed] [Google Scholar]
- 30. Holleran C.L., Rodriguez K.S., Echauz A., Leech K.A., and Hornby T.G. (2015). Potential contributions of training intensity on locomotor performance in individuals with chronic stroke. J. Neurol. Phys. Ther. 39, 95–102 [DOI] [PubMed] [Google Scholar]
- 31. Leddy A.L., Connolly M., Holleran C.L., Hennessy P.W., Woodward J., Arena R.A., Roth E.J., and Hornby T.G. (2016). Alterations in aerobic exercise performance and gait economy following high-intensity dynamic stepping training in persons with subacute stroke. J. Neurol. Phys. Ther. 40, 239–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bobath B. (1990). Adult Hemiplegia: Evaluation and Treatment. Butterworth-Heinemann: Oxford, United Kingdom [Google Scholar]
- 33. O'Sullivan S., Schmitz T., and Fulk G. (2014). Physical Rehabilitation, 6th ed. FA Davis Co: Philadelphia, PA [Google Scholar]
- 34. Umphred D., Lazaro R., Roller M., and Burton G. (2012). Neurological Rehabilitation, 6th ed. Mosby: St. Louis, MO [Google Scholar]
- 35. Kline T.L., Schmit B.D., and Kamper D.G. (2007). Exaggerated interlimb neural coupling following stroke. Brain 130, 159–169 [DOI] [PubMed] [Google Scholar]
- 36. Brazg G., Fahey M., Holleran C.L., Connolly M., Woodward J., Hennessy P.W., Schmit B.D., and Hornby T.G. (2017). Effects of training intensity on locomotor performance in individuals with chronic spinal cord injury: a randomized crossover study. Neurorehabil. Neural Repair 31, 944–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Holleran C.L., Straube D.D., Kinnaird C.R., Leddy A.L., and Hornby T.G. (2014). Feasibility and potential efficacy of high-intensity stepping training in variable contexts in subacute and chronic stroke. Neurorehabil. Neural Repair 28, 643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. MacKay-Lyons M.J., and Makrides L. (2002). Cardiovascular stress during a contemporary stroke rehabilitation program: is the intensity adequate to induce a training effect? Arch. Phys. Med. Rehabil. 83, 1378–1383 [DOI] [PubMed] [Google Scholar]
- 39. Tanaka H., Monahan K.D., and Seals D.R. (2001). Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 37, 153–156 [DOI] [PubMed] [Google Scholar]
- 40. Borg G.A. (1982). Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 14, 377–381 [PubMed] [Google Scholar]
- 41. Kerman I.A. (2008). Organization of brain somatomotor-sympathetic circuits. Exp. Brain Res 187, 1–16 [DOI] [PubMed] [Google Scholar]
- 42. Hjortskov N., Skotte J., Hye-Knudsen C., and Fallentin N. (2005). Sympathetic outflow enhances the stretch reflex response in the relaxed soleus muscle in humans. J. Appl. Physiol. (1985) 98, 1366–1370 [DOI] [PubMed] [Google Scholar]
- 43. Field-Fote E.C. (2009). Spinal Cord Injury Rehabilitation. F. A. Davis: Philadelphia, PA [Google Scholar]
- 44. Hung C.Y., Tseng S.H., Chen S.C., Chiu H.C., Lai C.H., and Kang J.H. (2014). Cardiac autonomic status is associated with spasticity in post-stroke patients. NeuroRehabilitation 34, 227–233 [DOI] [PubMed] [Google Scholar]
- 45. Zewdie E.T., Roy F.D., Yang J.F., and Gorassini M.A. (2015). Facilitation of descending excitatory and spinal inhibitory networks from training of endurance and precision walking in participants with incomplete spinal cord injury. Prog. Brain Res. 218, 127–155 [DOI] [PubMed] [Google Scholar]
- 46. Khan A.S., Patrick S.K., Roy F.D., Gorassini M.A., and Yang J.F. (2016). Training-specific neural plasticity in spinal reflexes after incomplete spinal cord injury. Neural Plast. 2016, 6718763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mirbagheri M.M., Kindig M.W., and Niu X. (2015). Effects of robotic-locomotor training on stretch reflex function and muscular properties in individuals with spinal cord injury. Clin. Neurophysiol. 126, 997–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mazzeo R.S., and Marshall P. (1989). Influence of plasma catecholamines on the lactate threshold during graded exercise. J. Appl. Physiol. (1985) 67, 1319–1322 [DOI] [PubMed] [Google Scholar]
- 49. Naveri H., Kuoppasalmi K., and Harkonen M. (1985). Metabolic and hormonal changes in moderate and intense long-term running exercises. Int. J. Sports Med. 6, 276–281 [DOI] [PubMed] [Google Scholar]
- 50. Sagnol M., Claustre J., Pequignot J.M., Fellmann N., Coudert J., and Peyrin L. (1989). Catecholamines and fuels after an ultralong run: persistent changes after 24-h recovery. Int. J. Sports Med. 10, 202–206 [DOI] [PubMed] [Google Scholar]
- 51. McLennan H. (1961). The effect of some catecholamines upon a monosynaptic reflex pathway in the spinal cord. J. Physiol. 158, 411–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schweitzer A., and Wright S. (1937). The action of adrenaline on the knee jerk. J. Physiol. 88, 476–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Frey G.C., McCubbin J.A., Dunn J.M., and Mazzeo R.S. (1997). Plasma catecholamine and lactate relationship during graded exercise in men with spinal cord injury. Med. Sci. Sports Exerc. 29, 451–456 [DOI] [PubMed] [Google Scholar]
- 54. Schmid A., Huonker M., Barturen J.M., Stahl F., Schmidt-Trucksass A., Konig D., Grathwohl D., Lehmann M., and Keul J. (1998). Catecholamines, heart rate, and oxygen uptake during exercise in persons with spinal cord injury. J. Appl. Physiol. (1985) 85, 635–641 [DOI] [PubMed] [Google Scholar]
- 55. Steinberg L.L., Lauro F.A., Sposito M.M., Tufik S., Mello M.T., Naffah-Mazzacoratti M.G., Cavalheiro E.A., and Silva A.C. (2000). Catecholamine response to exercise in individuals with different levels of paraplegia. Braz. J. Med. Biol. Res. 33, 913–918 [DOI] [PubMed] [Google Scholar]
- 56. Claydon V.E., Hol A.T., Eng J.J., and Krassioukov A.V. (2006). Cardiovascular responses and postexercise hypotension after arm cycling exercise in subjects with spinal cord injury. Arch. Phys. Med. Rehabil. 87, 1106–1114 [DOI] [PubMed] [Google Scholar]
- 57. Mitchell J.H., Kaufman M.P., and Iwamoto G.A. (1983). The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu. Rev. Physiol. 45, 229–242 [DOI] [PubMed] [Google Scholar]
- 58. Smith S.A., Mitchell J.H., and Garry M.G. (2006). The mammalian exercise pressor reflex in health and disease. Exp. Physiol. 91, 89–102 [DOI] [PubMed] [Google Scholar]
- 59. Schmit B.D., Benz E.N., and Rymer W.Z. (2002). Afferent mechanisms for the reflex response to imposed ankle movement in chronic spinal cord injury. Exp. Brain Res. 145, 40–49 [DOI] [PubMed] [Google Scholar]
- 60. Hornby T.G., Tysseling-Mattiace V.M., Benz E.N., and Schmit B.D. (2004). Contribution of muscle afferents to prolonged flexion withdrawal reflexes in human spinal cord injury. J. Neurophysiol. 92, 3375–3384 [DOI] [PubMed] [Google Scholar]
- 61. Krassioukov A., and West C. (2014). The role of autonomic function on sport performance in athletes with spinal cord injury. PM R 6, 8 Suppl., S58–S65 [DOI] [PubMed] [Google Scholar]
- 62. West C.R., Romer L.M., and Krassioukov A. (2013). Autonomic function and exercise performance in elite athletes with cervical spinal cord injury. Med. Sci. Sports Exerc. 45, 261–267 [DOI] [PubMed] [Google Scholar]
- 63. Dietz V., and Sinkjaer T. (2007). Spastic movement disorder: impaired reflex function and altered muscle mechanics. Lancet Neurol. 6, 725–733 [DOI] [PubMed] [Google Scholar]
- 64. Onushko T., Hyngstrom A., and Schmit B.D. (2011). Bilateral oscillatory hip movements induce windup of multijoint lower extremity spastic reflexes in chronic spinal cord injury. J. Neurophysiol. 106, 1652–1661 [DOI] [PubMed] [Google Scholar]
- 65. Popa C., Popa F., Grigorean V.T., Onose G., Sandu A.M., Popescu M., Burnei G., Strambu V., and Sinescu C. (2010). Vascular dysfunctions following spinal cord injury. J. Med. Life 3, 275–285 [PMC free article] [PubMed] [Google Scholar]
- 66. Dela F., Mohr T., Jensen C.M., Haahr H.L., Secher N.H., Biering-Sorensen F., and Kjaer M. (2003). Cardiovascular control during exercise: insights from spinal cord-injured humans. Circulation 107, 2127–2133 [DOI] [PubMed] [Google Scholar]
- 67. Thijssen D.H., Steendijk S., and Hopman M.T. (2009). Blood redistribution during exercise in subjects with spinal cord injury and controls. Med. Sci. Sports Exerc. 41, 1249–1254 [DOI] [PubMed] [Google Scholar]
- 68. Davis G.M., Servedio F.J., Glaser R.M., Gupta S.C., and Suryaprasad A.G. (1990). Cardiovascular-responses to arm cranking and FNS-induced leg exercise in paraplegics. J. Appl. Physiol. 69, 671–677 [DOI] [PubMed] [Google Scholar]
- 69. Hoffman M.D., Sheldahl L.M., Wenzler R.B., Plato C., Levandoski S., Kalbfleisch J.H., Dunnick J.S., and Tristani F.E. (1987). Cardiovascular-responses in paraplegics during exercise with lower-extremity compression. Clin. Res. 35, A287–A287 [Google Scholar]
- 70. Hopman M.T., Kamerbeek I.C., Pistorius M., and Binkhorst R.A. (1993). The effect of an anti-G suit on the maximal performance of individuals with paraplegia. Int. J. Sports Med. 14, 357–361 [DOI] [PubMed] [Google Scholar]
- 71. Pitetti K.H., Barrett P.J., Campbell K.D., and Malzahn D.E. (1994). The effect of lower body positive pressure on the exercise capacity of individuals with spinal cord injury. Med. Sci. Sports Exerc. 26, 463–468 [PubMed] [Google Scholar]