Abstract
Objectives: Youth with parental substance use disorder (family-history positive [FH+]) are at an elevated risk for substance use problems, but not all FH+ youth experience this outcome. Frontostriatal brain networks involved in inhibitory control and reward responsivity underlie risk-taking behaviors, but the role of these networks in substance use heterogeneity among FH+ youth has not been examined. The present study examined resting state functional connectivity (RSFC) in frontostriatal networks in FH+ youth with and without risky substance use.
Methods: Participants were 36 FH+ adolescents (mean age 14.96 years at the scan date; 36% female) from a longitudinal, community-based functional magnetic resonance imaging study enriched for parental alcohol use disorder. Groups were based on the absence (resilient) or presence (high-risk) of at least one occasion of any substance use by age 14 and also use of at least two different types of substances by the most recent substance use assessment (mean age 16.89 years). Bilateral masks of the dorsolateral prefrontal cortex (DLPFC) and the nucleus accumbens were used for seed-based RSFC due to the importance of these regions in executive control and salience networks, respectively.
Results: Compared with FH+/high-risk youth, FH+/resilient youth displayed greater connectivity between the left DLPFC seed and the left posterior cingulate cortex. No other brain regions showed significantly different RSFC between resilient and high-risk groups.
Conclusion: FH+/resilient youth showed stronger synchrony between brain regions associated with cognitive control, particularly those associated with flexible adaptation of thoughts and behaviors. Although preliminary, the results of this study set the stage for a continued focus on risk-group heterogeneity to better identify neural markers of resilience against substance use problems in vulnerable populations.
Keywords: resting state, functional connectivity, resilience, substance use, adolescence
Introduction
Adolescence is a dynamic time when simultaneous biological, psychological, and social changes lead to an increase in risk-taking behaviors, including substance use. According to data from the 2017 Monitoring the Future Study, 61.5% of 12th graders reported a lifetime history of alcohol use and 48.9% of 12th graders reported a lifetime history of illicit drug use (Miech et al. 2018). Substance use during adolescence confers both immediate and long-term risk; alcohol misuse is cited as a factor in 4358 deaths (Centers for Disease Control and Prevention [CDC] 2016) and 188,000 emergency room visits (Substance Abuse and Mental Health Services Administration [SAMHSA] 2016) for youth under the age of 21 each year, and the majority of adults who meet criteria for having a substance use disorder (SUD) started using substances during adolescence (Substance Abuse and Mental Health Services Administration [SAMHSA] 2017).
Developmental changes in the brain are believed to contribute to risk-taking behaviors that emerge during adolescence, including the initiation and escalation of substance use. Dual system models of brain development describe a functional and maturational mismatch between the top-down cognitive control network (localized in prefrontal cortex) and bottom-up reward network (localized primarily in the striatum, particularly the nucleus accumbens [NAcc]) (Casey et al. 2008; Shulman et al. 2016). The top-down control network matures linearly over development from late childhood through early adulthood (e.g., Galvan et al. 2006; Somerville and Casey 2010), whereas manifestations of the bottom-up reward network follow a curvilinear trajectory, increasing during late childhood, peaking during early adolescence, and then declining through the early twenties (Shulman et al. 2016 for review).
This imbalance between the function and maturity of brain systems underlying cognitive control and reward responsivity is believed to underlie the normative increases in sensation seeking, impulsivity, and reward-driven behavior observed during adolescence. However, not all adolescents engage in risky, reward-driven behavior such as substance use. Rather, individual variability in top-down control and bottom-up reward system connectivity is likely to be informative in determining why certain individuals engage in substance use while others abstain.
Understanding these individual differences may be particularly important for adolescents with a family history of SUDs (family-history positive [FH+]) because they are at an elevated risk for developing alcohol and drug use disorders compared to youth without such familial risk (e.g., Sher et al. 1991; Zimić and Jukić 2012). Various neurobiological markers have been identified that may predispose FH+ youth to problem substance use, including differences in reward and inhibitory control (e.g., Ivanov et al. 2012; Hardee et al. 2014), and resting state functional connectivity (RSFC) (e.g., Cservenka et al. 2014); notably, these risk markers have been found before the onset of any substance use (Heitzeg et al. 2015a; Cservenka 2016; Squeglia and Cservenka 2017, for reviews).
Existing literature has typically viewed FH+ youth as a homogenous group and compared FH+ youth to lower-risk youth who have no family history of SUD (e.g., Stice and Yokum 2014). Yet, not all FH+ youth go on to experience heavy and/or problematic substance use despite having familial risk. Certain FH+ youth are resilient, defined here as the ability to avoid a pathological outcome despite experiences of adversity (e.g., Windle and Zucker 2010; Hurd and Zimmerman 2016).
In particular, subgroups of FH+ adolescents that display low or modest rates of substance use during adolescence through early adulthood have been identified (e.g., Chassin et al. 2002; Park and Schepp 2015; Martz et al. 2018). Because this is a developmental period during which substance use and SUD onset typically begin (Substance Abuse and Mental Health Services Administration [SAMHSA] 2017), these youth can be considered FH+/resilient. Yet, little is known regarding the neural factors underlying heterogeneity in FH+ individuals.
RSFC analysis has recently shown great promise for characterizing heterogeneity in brain development between FH+ and FH− youth (e.g., Cservenka et al. 2014). Thus, RSFC may also be useful to examine potential differences in neural connectivity between FH+/resilient and FH+/high-risk groups. This method was developed following the observation that spontaneous fluctuations in functional magnetic resonance imaging (fMRI) signal collected from brain regions when participants were at rest co-varied with signal fluctuations in other regions that have shared functional properties and anatomical connections (Biswal et al. 1997; Xiong et al. 1999; Cordes et al. 2000).
Under the assumption that the degree of covariance between spatially distinct brain regions reflects the strength of functional links between them, RSFC analyses have allowed researchers to make inferences about the organization of large-scale neural networks and the role of these networks in behavior (Fox and Raichle 2007). Relevant to dual system models of brain development, RSFC analyses have identified networks of regions that appear to be involved in top-down control, such as areas within the executive control network, as well as regions within the salience network associated with reward processing (Seeley et al. 2007; Sutherland et al. 2012).
Beyond normative functioning, RSFC analyses have been used to characterize individual differences in neural development that are linked to risk behaviors and psychopathology; altered RSFC of regions linked to both top-down control and limbic/reward system functions has been reported in various externalizing psychopathologies and substance use (Ma et al. 2010; Wetherill et al. 2012; Krmpotich et al. 2013; Sripada et al. 2014a; Cohn et al. 2015; Weissman et al. 2015). Hence, RSFC appears to provide an effective method for characterizing interactions within and between neural systems involved in cognitive control and reward processing and for linking individual differences in these interactions to risk for substance use and related outcomes.
The present study used seed-based RSFC analyses to identify neural indicators of heterogeneity in substance use behaviors between two subgroups of FH+ youth—those who were resilient and those who were high-risk adolescents. “Resilience” was defined as having a vulnerability for substance use due to family history of alcohol use disorder (AUD) and yet not displaying risky substance use behavior. “High-risk” was defined as having the same familial vulnerability as the FH+/resilient group but exhibiting risky substance use, thus suggesting that they were already on the pathway to problem use in adulthood.
Dual criteria used to determine potentially risky substance use were use by age 14, as well as polysubstance use in adolescence. Substance use by the age of 14 is associated with an increased risk for disordered use in adulthood (Grant et al. 2001; Hingson et al. 2006; Swift et al. 2008; Perez et al. 2010; Buchmann et al. 2011; Kendler et al. 2013). Polysubstance use, especially during early adolescence, is also a risk factor for later substance use problems (e.g., Connor et al. 2014; Morley et al. 2015).
RSFC analyses were conducted in resilient and high-risk groups using seeds in the dorsolateral prefrontal cortex (DLPFC) and NAcc, given their importance to executive control and salience networks, respectively (e.g., Li et al. 2009). Furthermore, task-based activation in these regions has been found to differ between resilient and high-risk youth in the few existing studies comparing neural function between these groups (NAcc: Heitzeg et al. 2008; Yau et al. 2012; DLPFC: Martz et al. 2018).
Methods
Participants and study design
Participants were 36 right-handed adolescents (36.1% female; M = 14.96 years old at scan date, standard deviation [SD] = 1.36) from an ongoing fMRI study of youth recruited from the Michigan Longitudinal Study (MLS). The MLS is a longitudinal, community-based study of families with parental AUD and a contrast sample of families without parental AUD living in the same neighborhoods (Zucker et al. 2000).
To focus on resilient and high-risk FH+ youth, participants in the present study were only from families in which at least one parent had an AUD diagnosis. Parental AUD was established by clinical assessments (Diagnostic Interview Schedule—Version 4) (Robins et al. 2000) conducted at the time of recruitment and through multiple in-person diagnostic assessments over the course of the study. Families in which the children displayed signs of fetal alcohol syndrome were excluded from participation. Full details on the prospective assessment and data collection protocol in the MLS can be found elsewhere (Zucker et al. 2000).
Exclusionary criteria for the neuroimaging subsample of the MLS were any neurological or chronic medical illness, any current or recent (within 6 months) treatment with centrally active medications, and a history of psychosis or schizophrenia in first-degree relatives. The presence of an Axis I psychiatric or developmental disorder was exclusionary except for conduct disorder, attention deficit disorder, or SUD, as exclusion of these disorders would preferentially eliminate part of the phenomenon of interest (i.e., heavy substance use).
Participants were instructed to abstain from alcohol and drug use for 48 hours before scanning and then given a multidrug five-panel urine screen just before scanning. Given that THC metabolites are detectable in the urine for a week or even longer, participants who tested positive for marijuana were asked about recent marijuana use. Report of marijuana use in the prior 48 hours was exclusionary. The study protocol was approved by the University of Michigan Institutional Review Board. Participants under the age of 18 gave signed assent, and at least one parent provided written informed consent for their child to participate.
FH+/resilient and FH+/high-risk groups were determined by the absence or presence, respectively, of the following: (1) at least one occasion of alcohol, cigarette, marijuana, or other illegal drug use by age 14 and (2) use of at least two different types of substances (e.g., marijuana and alcohol) by the most recent substance use assessment (M = 16.89 years old, SD = 1.50). To further ensure the absence of problem drinking, the FH+/resilient group must have also reported no drunkenness by age 17. These criteria defined a FH+/resilient group (n = 21) and a FH+/high-risk group (n = 15).
Measures
Substance use
Quantity and frequency of substance use were assessed through the self-report Drinking and Drug History (DDH) Questionnaire (Zucker et al. 1990), which was administered annually beginning at age 11. Past year drink volume was calculated as counts of drinking days per month that were then multiplied by drinks usually consumed per drinking day. Past year cigarette use was measured by the approximate number of days in the past 12 months that participants reported smoking cigarettes. Past year marijuana use was measured by the approximate number of days in the past 12 months that participants reporting using marijuana. Past year illicit drug use was determined by the number of drugs (other than marijuana) that participants reported using that year.
Cumulative measures of drink volume, cigarette use, marijuana use, and illicit drug use were calculated as sum measures of yearly use reported up to and including age 14 and up to including age 16. First use/drunk measures were determined from the first annual DDH questionnaire in which participants endorsed each substance use behavior (alcohol use, drunkenness, cigarette use, marijuana use, and illicit drug use other than marijuana).
fMRI data acquisition
Whole-brain blood oxygen level-dependent images were acquired on a 3.0 Tesla GE Signa System (Milwaukee, WI) using a standard radio frequency coil. Functional imaging was performed using T2*-weighted single-shot combined spiral in-out acquisition sequence (Glover and Law 2001) with the following parameters: repetition time (TR) = 2000 mseconds; echo time (TE) = 30 mseconds, flip angle = 90°, field-of-view (FOV) = 0 cm, 64 × 64 matrix, slice thickness = 4 mm, 29 slices. High-resolution anatomical T1 scans were acquired for spatial normalization (three-dimensional [3D] spoiled gradient-recalled echo, TR = 25 mseconds; min TE; FOV = 25 cm; 256 × 256 matrix, slice thickness = 1.4 mm).
During the 6-minute resting state scan, participants were instructed to focus on a plus sign shown through a screen at the head of the scanner bore and a mirror attached to the head coil. They were told to remain as still as possible without closing their eyes or falling asleep. In addition to emphasizing the importance of remaining still, participant motion was minimized with foam pads placed around the head and a forehead strap.
Data analysis
RSFC analysis
Connectivity Toolbox (ConnTool), a seed-based functional connectivity analysis approach in SPM developed by the University of Michigan Methods Core, was used to conduct RSFC analyses (e.g., Welsh et al. 2010; Sripada, et al. 2014b; Watanabe et al. 2014). ConnTool has four steps as follows: (1) input data specification; (2) preprocessing specification; (3) region of interest (ROI) specification; and (4) output specification.
Input data specification
Time-series data from the resting state scan were entered as input data. The resting state scan consisted of one run, with data collected from 190 volumes at 2-second intervals. Removal of artifacts arising from head motion was performed on the smoothed functional time series data using ICA-AROMA (Pruim et al. 2015).
Preprocessing specification
Preprocessing steps occurred in the following order: detrending, motion regression, cerebrospinal fluid regression, white matter regression, band-pass filtering, and motion scrubbing of frames that exceed a framewise displacement of 0.5 mm.
ROI specification
In line with the study focus on connectivity within cognitive control and reward circuitry as potential indicators of resilience and high-risk among FH+ youth, a priori ROI masks central to executive control (bilateral DLPFC) and salience (bilateral NAcc) networks were created with Wake Forest University Pickatlas (Maldjian et al. 2003) using coordinates from prior neuroimaging studies that examined brain function associated with executive control and salience networks. A 6 mm diameter sphere was centered at the DLPFC coordinates (left DLPFC: x = −42, y = 34, z = 20; right DLPFC: x = 44, y = 36, z = 20; as per Seeley et al. 2007; Sherman et al. 2014; Woodward et al. 2011); and a 5 mm diameter sphere was centered at the NAcc coordinates (left NAcc: x = −10, y = 13, z = −8; right NAcc: x = 10, y = 13, z = −8; as per Yau et al. 2012; Martz et al. 2016).
Output specification
NIFTI 3D images of z-maps were output to conduct second level analyses of group differences (i.e., FH+/resilient versus FH+/high-risk group contrasts). Type I error was controlled at α = 0.05 by establishing the voxel-wise significance threshold at p < 0.001 uncorrected with a voxel cluster-extent threshold of 162, based on simulation results generated by 3dClustSim in AFNI (Version AFNI_16.3.18).
Results
FH+/resilient versus FH+/high-risk group characteristics
Table 1 shows characteristics for resilient and high-risk FH+ groups. Groups were well matched on scan age (t = 1.30, p = 0.204), sex (t = 0.40, p = 0.692), and age at most recent substance use assessment (t = 1.99, p = 0.060).
Table 1.
Sample Characteristics for Family-History Positive/Resilient and Family-History Positive/High-Risk Groups
| Characteristic | FH+/resilient (n = 21) | FH+/high-risk (n = 15) |
|---|---|---|
| Scan age | 14.71 (1.24) | 15.30 (1.49) |
| Male | 66.7% | 60.0% |
| Age at most recent substance use assessment | 16.45 (1.08) | 17.50 (1.82) |
| ADHD diagnosisa | n = 4 | n = 1 |
| Conduct disorder diagnosisa | n = 0 | n = 1 |
| Cumulative drink volume by age 14 | 0 | 121.13 (451.98) |
| Cumulative marijuana use days by age 14 | 0 | 17.95 (64.10) |
| Cumulative cigarette use days by age 14 | 0 | 0.20 (0.53) |
| Cumulative number of illicit drugsb used by age 14 | 0 | 1.07 (2.12) |
| Cumulative drink volume by age 16 | 0 | 616.00 (1321.40) |
| Cumulative marijuana use days by age 16 | 0 | 123.43 (225.35) |
| Cumulative cigarette use days by age 16c | 0.13 (0.43) | 56.91 (115.60) |
| Cumulative number of illicit drugsb used by age 16 | 0 | 2.60 (2.38) |
| Age at first drink | — | 13.40 (1.84) |
| Age at first drunk | — | 14.15 (1.82) |
| Age at first marijuana use | — | 13.57 (2.10) |
| Age at first cigarette use | 15.52 (0.05) | 15.59 (1.35) |
| Age at first illicit drug useb | — | 14.17 (2.64) |
Any diagnosis reported from ages 9 to 17.
Illicit drugs other than marijuana.
Two FH+/resilient youth reported cigarette use, one at age 15.48 and the other at age 15.55; dashes indicate that the behavior was not endorsed.
ADHD, attention-deficit/hyperactivity disorder; FH+, family-history positive.
Group differences in resting-state functional connectivity
As shown in Figure 1, FH+/resilient youth showed significantly greater RSFC between the left DLPFC seed and the left posterior cingulate cortex (PCC; MNI coordinates: x = −8, y = −42, z = 24, k = 182, peak z-value = 4.17) compared with FH+/high-risk youth. There were no significant differences in right DLPFC RSFC between FH+/resilient and FH+/high-risk groups. There were also no significant group differences in right or left NAcc RSFC.
FIG. 1.
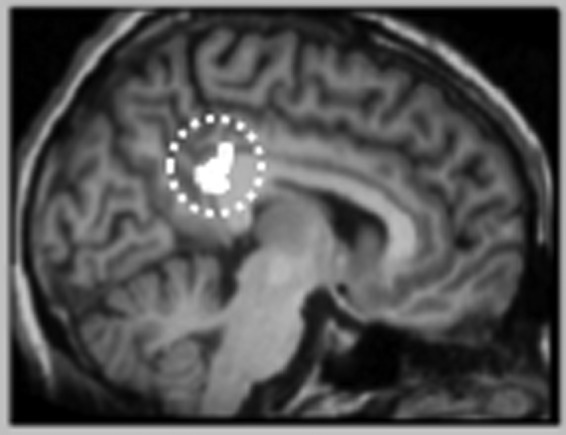
Greater resting state functional connectivity between the left PCC (designated by dashed circle) and the left dorsolateral prefrontal cortex seed for FH+/resilient versus FH+/high-risk youth. Whole-brain contrast map of the PCC is significant at p < 0.05 (cluster-defining threshold of p < 0.001 uncorrected, cluster size threshold of 162; MNI coordinates x = −8, y = −42, z = 24). FH+, family-history positive; PCC, posterior cingulate cortex.
Post hoc analyses with substance use
Due to the high-risk group displaying greater levels of substance use before the scan, the potential impacts of cumulative substance use by age 14 and age 16 on between-group differences in functional connectivity were tested. First, mean z-values for the left PCC cluster showing significantly stronger connectivity with the left DLPFC in the resilient versus high-risk group were extracted using the MarsBaR toolbox (Brett et al. 2002). These values were then imported into SPSS for further analysis. We conducted two analysis of covariance (ANCOVA) analyses with DLPFC-PCC z-values as the dependent variable, group membership as the between-subjects factor, and substance use variables as covariates (two models: cumulative use by age 14; cumulative use by age 16). Group membership remained significant in each model (ps < 0.004), and all substance use variables were nonsignificant (ps > 0.26). These findings suggest that levels of prior substance use were not driving differences in strength of left PCC and left DLPFC connectivity between resilient and high-risk groups.
Discussion
Findings from the present study support recent evidence showing that certain neural markers may differentiate FH+/resilient and FH+/high-risk youth (Heitzeg et al. 2008; Yau et al. 2012; Martz et al. 2018). The present study is the first to use RSFC to identify neural circuitry differentiating these two FH+ groups. Although preliminary, results suggest that stronger synchrony between the left DLPFC and left PCC may be a neural indicator of resilience against early and potentially problematic substance use among FH+ youth.
DLPFC connectivity
In the resilient group, the left DLPFC showed greater coupling with the left PCC. The left PCC, which is a parietal region within the brain's limbic system and a central node within the default mode network (Raichle 2015), is involved in an array of cognitive functions related to memory, attention, and decision-making (Pearson et al. 2011). One function of the PCC that is perhaps most relevant to resilience against heavy substance use is flexible adaptation of thoughts and behaviors in response to changing environmental contexts (Pearson et al. 2011; Leech et al. 2012). This form of context-based self-regulation aligns closely with the personality construct of ego resiliency described by Block and Block (1980) and more recently by Eisenberg et al. (2003).
Associations between ego resiliency and substance use vulnerability have been documented in our own group, as well as the external research literature. Wong et al. (2006) found that children with higher levels of ego resiliency in preschool were less likely to begin alcohol use by age 14 than children with lower resiliency. Weiland et al. (2012) found that greater ego resiliency in adolescence was associated with better performance in an executive functioning task and lower levels of substance use in young adulthood. Heitzeg et al. (2015b) found that adolescent marijuana use was related to lower ego resiliency in young adulthood and that this relationship was mediated by brain activation in the DLPFC in response to negative emotional stimuli.
In addition, the PCC is associated with the processing of emotionally salient stimuli, such as substance use cues (e.g., Tapert et al. 2003; Feldstein Ewing et al. 2012; Filbey et al. 2016). Thus, stronger coupling between the DLPFC and PCC in the resilient compared to high-risk group may indicate greater synchrony between regions involved in executive functions that are also linked to substance use outcomes.
In the present study, connectivity between the DLPFC and PCC was lateralized to the left hemisphere, and there were no significant differences in connectivity between resilient and high-risk youth in the right DLPFC seed. The literature is mixed in terms of whether left, right, or bilateral DLPFC activation is associated with attention, working memory, inhibitory control, and planning. For example, a sample of adults who were abstinent from alcohol showed reduced activation in the left but not right DLPFC during an impulse control task compared to healthy controls (Li et al. 2009). Studies using transcranial direct current stimulation to reduce cravings for alcohol and marijuana have focused on the left DLPFC (e.g., Boggio et al. 2010; da Silva et al. 2013).
Yet, other studies support the role of the right DLPFC in response inhibition (e.g., Aron 2011; Stramaccia et al. 2015), specifically inhibition of risky decision-making relevant to substance use (Yamamoto et al. 2015). Additional research is needed to test differing hemispheric connectivity in the DLPFC, especially during the developmental time period in which these regions mature.
NAcc connectivity
There were also no differences in left or right NAcc RSFC between resilient and high-risk youth. Due to the centrality of the NAcc in relation to the brain's salience network (e.g., Baler and Volkow 2006) and the role of the prefrontal regions in the executive control network and addiction vulnerability (Goldstein and Volkow 2011), it was expected that resilient youth would show greater frontostriatal connectivity in comparison with high-risk youth.
However, differences in NAcc connectivity in FH+ youth may differ from non-FH+ populations of youth. For example, Cservenka et al. (2014) examined RSFC of the NAcc in FH+ compared with FH− adolescents. Their findings indicated that in FH+ adolescents, the NAcc was more weakly integrated with other brain regions associated with reward responsivity and less differentiated with regions involved in cognitive control. Both FH+ and FH− groups had low levels of prior substance use, suggesting that atypical neural circuitry between reward and cognitive control systems may be a preexisting risk factor in FH+ youth.
Prior work focused on differences in brain function between resilient and high-risk FH+ youth, with resilience and risk based on trajectories of binge drinking and marijuana use through young adulthood, found that these groups differed in relation to inhibitory control (i.e., DLPFC activation during a go/no-go task) but not reward responsivity (i.e., NAcc activation during a monetary incentive delay task; Martz et al. 2018). In line with findings from Martz et al. (2018), it is possible that both resilient and high-risk FH+ youth may share similar proclivities toward heightened reward responsivity but that the differences in FH+ youth who do versus do not show substance use problems are contingent upon neural function in prefrontal regions associated with cognitive control.
Strengths and limitations
An important strength of the present study is its investigation of differences in RSFC between FH+/resilient and FH+/high-risk youth, which has not been done in previous work. Groups were well matched on scan age, sex, and age at most recent substance use assessment; they differed only in levels of substance use and left DLPFC connectivity. Thus, the present study sheds light on the need to study FH+ youth as a heterogeneous rather than homogenous group in terms of substance use behaviors and brain connectivity. This has generally been overlooked in studies interested in inferring risk based on family history, yet the present study suggests that there are significant neural differences, as well as behavioral differences, within these two subsets of youth, and consideration of the two as equally high-risk is inappropriate.
Another key strength of the present study was its use of resting state rather than task-based imaging. Advantages of resting state analyses include having a lower likelihood of confounds inherent to task-based imaging, thus allowing for the study of connectivity between brain regions involved in both cognitive control and reward processing without having to use different task-based paradigms (Sutherland et al. 2012).
In addition to its strengths, some limitations of the present study should be noted. First, the small sample size limits the generalizability of findings. Generalizability is also limited by the sample consisting predominately of white males. Additional studies that are both larger and more diverse are needed to test the replicability and validity of findings across different populations of youth. Second, although we found no significant associations between substance use and DLPFC-PCC RSFC in our ANCOVA models, it remains possible that higher levels of use in the high-risk group may have impacted findings. Additional studies are needed, such as work currently being done by the Adolescent Brain Cognitive Development study (Feldstein Ewing et al. 2018), which conducts neuroimaging assessments before substance use onset to avoid potential confounding effects of substance use on the brain.
Conclusion
In summary, resilient youth showed greater connectivity between brain regions associated with self-control. Results of the present study set the stage for a continued focus on risk-group heterogeneity to better identify protective mechanisms against substance use problems in vulnerable populations. The neural mechanisms underlying resilience remain unknown, but linkages between the DLPFC and PCC may be a starting point for future research.
Future studies can build upon this work in several ways. One avenue for future work is to use longitudinal imaging data to examine potential developmental shifts in frontostriatal connectivity in resilient and high-risk groups from adolescence through early adulthood, coinciding with the protracted maturation of brain networks involved in cognitive control (Casey et al. 2008). Another direction for longitudinal work is to test if RSFC in the high-risk group is a predictor of later SUD diagnosis compared to developmentally limited rates of heavy use. The resilient group should also be assessed longitudinally to determine the extent to which their resilience against problematic substance use is sustained into adulthood.
Clinical Significance
Identifying neural markers of resilience against substance use problems using RSFC in FH+/resilient and FH+/high-risk youth provides important targets for intervention. Strengthening executive functions has been a focus of substance use intervention and prevention programs aimed at adolescents (e.g., Pentz et al. 2016). Prevention and intervention efforts specifically tailored to FH+ youth that increase self-control may help to prevent or reduce heavy substance use. These interventions may be particularly important for adolescents, whose brains are undergoing developmental changes that may make them more vulnerable to risk-taking behavior and sensitive to the neurotoxic effects of substances (Spear 2014).
Disclosures
The authors report no conflicts of interest.
References
- Aron AR: From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses. Biol Psychiatry 69:e55–e68, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baler RD, Volkow ND: Drug addiction: The neurobiology of disrupted self-control. Trends Mol Med 12:559–566, 2006 [DOI] [PubMed] [Google Scholar]
- Biswal BB, Kylen JV, Hyde JS: Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed 10:165–170, 1997 [DOI] [PubMed] [Google Scholar]
- Block JH, Block J: The role of ego-control and ego-resiliency in the organization of behavior. In: Minnesota Symposia on Child Psychology, Vol. 13 Edited by Collins WA. Hillsdale, NJ, Erlbaum, 1980, pp. 39–101 [Google Scholar]
- Boggio PS, Zaghi S, Villani AB, Fecteau S, Pascual-Leone A, Fregni F: Modulation of risk-taking in marijuana users by transcranial direct current stimulation (tDCS) of the dorsolateral prefrontal cortex (DLPFC). Drug Alcohol Depend 112:220–225, 2010 [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB: Region of interest analysis using an SPM toolbox. Paper presented at the 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan, June2–6, 2002 Available on CD-ROM in Neuroimage, Vol. 16, No. 2 [Google Scholar]
- Buchmann AF, Blomeyer D, Jennen-Steinmetz C, Schmidt MH, Esser G, Banaschewski T, Laucht M: Early smoking onset may promise initial pleasurable sensations and later addiction. Addict Biol 18:947–954, 2011 [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A: The adolescent brain. Dev Rev 28:62–77, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC): Fact Sheets: Underage Drinking. Atlanta (GA), CDC, 2016 [Google Scholar]
- Chassin L, Pitts SC, Prost J: Binge drinking trajectories from adolescence to emerging adulthood in a high-risk sample: Predictors and substance abuse outcomes. J Consult Clin Psychol 70:67, 2002 [PubMed] [Google Scholar]
- Cohn MD, Pape LE, Schmaal L, van den Brink W, van Wingen G, Vermeiren RR, Doreleijers TA, Veltman DJ, Popma A: Differential relations between juvenile psychopathic traits and resting state network connectivity. Hum Brain Mapp 36:2396–2405, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JP, Gullo MJ, White A, Kelly AB: Polysubstance use: Diagnostic challenges, patterns of use and health. Curr Opin Psychiatry 27:269–275, 2014 [DOI] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski PA, Moritz CH, Quigley MA, Meyerand ME: Mapping functionally related regions of brain with functional connectivity MR imaging. Am J Neuroradiol 21:1636–1644, 2000 [PMC free article] [PubMed] [Google Scholar]
- Cservenka A: Neurbiological phenotypes associated with a family history of alcoholism. Drug Alcohol Depend 158:8–21, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Casimo K, Fair DA, Nagel BJ: Resting state functional connectivity of the nucleus accumbens in youth with a family history of alcoholism. Psychiatry Res Neuroimaging 221:210–219, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva MC, Conti CL, Klauss J, Alves LG, do Nascimento Cavalcante HM, Fregni F, Nitsche MA, Nakamura-Palacios EM: Behavioral effects of transcranial direct current stimulation (tDCS) induced dorsolateral prefrontal cortex plasticity in alcohol dependence. J Physiol 107:493–502, 2013 [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Valient C, Fabes RA, Smith CL, Reiser M, Shepart SA, Losoya SH, Guthrie IK, Murphy BC, Cumberland AJ: The relations of effortful control and ego control to children's resiliency and social functioning. Dev Psychol 39:761–776, 2003 [DOI] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Bjork JM, Luciana M: Implications of the ABCD study for developmental neuroscience. Dev Cogn Neurosci 32:161–164, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Mead HK, Yezhuvath U, DeWitt S, Hutchison KE, Filbey FM: A preliminary examination of how serotonergic polymorphisms influence brain response following an adolescent cannabis intervention. Psychiatry Res 204:112–116, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Dunlop J, Ketcherside A, Baine J, Rhinehardt T, Kuhn B, DeWitt S, Alvi T: fMRI study of neural sensitization to hedonic stimuli in long-term, daily cannabis users: Reward cue-reactivity in marijuana users. Hum Brain Mapp 37:3431–3443, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME: Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711, 2007 [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ: Earlier development of the accumbens relative to the orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci 26:6885–6892, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Law CS: Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med 46:515–522, 2001 [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND: Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nat Rev Neurosci 12:652–669, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Harford TC: Age at onset of alcohol use and DSM-IV alcohol abuse and dependence: A 12-year follow-up. J Subst Abuse 13:493–504, 2001 [DOI] [PubMed] [Google Scholar]
- Hardee JE, Weiland BJ, Nichols TE, Welsh RC, Soules ME, Steinberg DB, Zubieta JK, Zucker RA, Heitzeg MM: Development of impulse control circuitry in children of alcoholics. Biol Psychiatry 76:708–716, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Cope LM, Martz ME, Hardee JE: Neuroimaging risk markers for substance abuse: Recent findings on inhibitory control and reward system functioning. Curr Addict Rep 2:91–103, 2015a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Cope LM, Martz ME, Hardee JE, Zucker RA: Brain activation to negative stimuli mediates a relationship between adolescent marijuana use and later emotional functioning. Dev Cogn Neurosci 16:71–83, 2015b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Yau WY, Zubieta JK, Zucker RA: Affective circuitry and risk for alcoholism in late adolescence: Differences in frontostriatal responses between vulnerable and resilient children of alcoholic parents. Alcohol Clin Exp Res 32:414–426, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR: Age at drinking onset and alcohol dependence: Age at onset, duration, and severity. Arch Pediatr Adolesc Med 160:739–746, 2006 [DOI] [PubMed] [Google Scholar]
- Hurd N, Zimmerman M: Adolescent resilience: Promoting more positive outcomes among youth at risk of using and abusing substances. In: The Oxford Handbook of Adolescent Substance Abuse. Edited by Zucker R, Brown S. Oxford, UK, Oxford University Press, 2016, pp. 1–44 [Google Scholar]
- Ivanov I, Liu X, Shulz K, Fan J, London E, Friston K, Halperin JM, Newcorn JH: Parental substance abuse and function of the motivation and behavioral inhibition systems in drug-naïve youth. Psychiatry Res 201:128–135, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Damaj MI, Chen X: Early smoking onset and risk for subsequent nicotine dependence: A monozygotic co-twin control study. Am J Psychiatry 170:408–413, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krmpotich TD, Tregellas JR, Thompson LL, Banich MT, Klenk AM, Tanabe JL: Resting-state activity in the left executive control network is associated with behavioral approach and is increased in substance dependence. Drug Alcohol Depend 129:1–7, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Braga R, Sharp DJ: Echoes of the4 brain within the posterior cingulate cortex. J Neurosci 32:215–222, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CSR, Luo X, Yan P, Bergquist K, Sinha R: Altered impulse control in alcohol dependence: Neural measures of stop signal performance. Alcohol Clin Exp Res 33:740–750, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, Xu HS, Fu XM, Hu X, Zhang DR: Addiction related alteration in resting-state brain connectivity. Neuroimage 49:738–744, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH: An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19:1233–1239, 2003 [DOI] [PubMed] [Google Scholar]
- Martz ME, Trucco EM, Cope LM, Hardee JE, Jester JM, Zucker RA, Heitzeg MM: Association of marijuana use with blunted nucleus accumbens response to reward anticipation. JAMA Psychiatry 73:838–844, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martz ME, Zucker RA, Schulenberg JE, Heitzeg MM: Psychosocial and neural indicators of resilience among youth with a family history of substance use disorder. Drug Alcohol Depend 185:198–206, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech RA, Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE, Patrick ME: Monitoring the Future National Survey Results on Drug Use, 1975–2017: Volume I, Secondary school students. Ann Arbor (MI), Institute for Social Research, The University of Michigan, 2018 [Google Scholar]
- Morley KI, Lynskey MT, Moran P, Borschmann R, Winstock AR: Polysubstance use, mental health and high-risk behaviours: Results from the 2012 Global Drug Survey. Drug Alcohol Rev 34:427–437, 2015 [DOI] [PubMed] [Google Scholar]
- Park S, Schepp KG: A systematic review of research on children of alcoholics: Their inherent resilience and vulnerability. J Child Fam Stud 24:1222–1231, 2015 [Google Scholar]
- Pearson JM, Heilbronner SR, Barack DL, Hayden BY, Platt ML: Posterior cingulate cortex: Adapting behavior to a changing world. Trends Cogn Sci 15:143–151, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentz MA, Riggs NR, Warren CM: Improving substance use prevention efforts with executive function training. Drug Alcohol Depend 163:S54–S59, 2016 [DOI] [PubMed] [Google Scholar]
- Perez A, Ariza C, Sanchez-Martinez F, Nebot M: Cannabis consumption initiation among adolescents: A longitudinal study. Addict Behav 35:129–134, 2010 [DOI] [PubMed] [Google Scholar]
- Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF: ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage 112:267–277, 2015 [DOI] [PubMed] [Google Scholar]
- Raichle ME: The brain's default model network. Annu Rev Neurosci 38:433–447, 2015 [DOI] [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Bucholz KK, Compton WM, North CS, Rourke KM: Diagnostic Interview Schedule for the DSM-IV (DIS-IV). St. Louis (MO), Washington University School of Medicine, 2000 [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD: Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher KJ, Walitzer KS, Wood PK, Brent EE: Characteristics of children of alcoholics: Putative risk factors, substance use and abuse, and psychopathology. J Abnorm Psychol 100:427–448, 1991 [DOI] [PubMed] [Google Scholar]
- Sherman LE, Rudie JD, Pfeifer JH, Masten CL, McNealy K, Dapretto M: Development of the default mode and central executive networks across early adolescence: A longitudinal study. Dev Cogn Neurosci 10:148–159, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman EP, Smith AR, Silva K, Icenogle G, Duell N, Chein J, Steinberg L: The dual systems model: Review, reappraisal, and reaffirmation. Dev Cogn Neurosci 17:103–117, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Casey BJ: Developmental neurobiology of cognitive control and motivational systems. Curr Opin Neurobiol 20:236–241, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP: Adolescents and alcohol: Acute sensitivities, enhanced intake, and later consequences. Neurotoxicol Teratol 41:51–59, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Csesrvenka A: Adolescent and drug use vulnerability: Findings from neuroimaging. Curr Opin Behav Sci 13:164–170, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada CS, Kessler D, Angstadt M: Lag in maturation of the brain's intrinsic functional architecture in attention-deficit/hyperactivity disorder. Proc Natl Acad Sci U S A 111:14259–14264, 2014a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, Swain JE, Evans GW, Welsh RC, Liberzon I: Childhood poverty and stress reactivity are associated with aberrant functional connectivity in default mode network. Neuropsychopharmacology 39:2244–2251, 2014b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S: Brain reward region responsivity of adolescents with and without parental substance use disorders. Psychol Addict Behav 28:805–815, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stramaccia DF, Penolazzi B, Sartori G, Braga M, Mondini S, Galfano G: Assessing the effects of tDCS over a delayed response inhibition task by targeting the right inferior frontal gyrus and right dorsolateral prefrontal cortex. Exp Brain Res 233:2283–2290, 2015 [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA): Key Substance Use and Mental Health Indicators in the United States: Results from the 2015 National Survey on Drug Use and Health. Rockville (MD), SAMHSA, 2016 [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA): Report to congress on the prevention and reduction of underage drinking. Rockville (MD), U.S. Department of Health and Human Services, 2017 [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA: Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage 62:2281–2295, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift W, Coffey C, Carlin JB, Degenhardt L, Patton GC: Adolescent cannabis users at 24 years: Trajectories to regular weekly use and dependence in young adulthood. Addiction 103:1361–1370, 2008 [DOI] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA: Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch Gen Psychiatry 60:727–735, 2003 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Kessler D, Scott C, Angstadt M, Sripada C: Disease prediction based on functional connectomes using a scalable and spatially-informed support vector machine. Neuroimage 96:183–202, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland BJ, Nigg JT, Welsch RC, Yau WYW, Zubieta JK, Zucker RA, Heitzeg MM: Resiliency in adolescents at higher risk for substance abuse: Flexible adaptation via subthalamic nucleus and linkage to drinking and drug use in early adulthood. Alcohol Clin Exp Res 36:1355–1364, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DG, Schriber RA, Fassbender C, Atherton O, Krafft C, Robins RW, Hastings PD, Guyer AE: Earlier adolescent substance use onset predicts stronger connectivity between reward and cognitive control brain networks. Dev Cogn Neurosci 16:121–129, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh RC, Chen AC, Taylor SF: Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull 36:713–722, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Bava S, Thompson WK, Boucquey V, Pulido C, Yang TT, Tapert SF: Frontoparietal connectivity in substance-naive youth with and without a family history of alcoholism. Brain Res 1432:66–73, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle M, Zucker RA: Reducing underage and young adult drinking: How to address critical drinking problems during this developmental period. Alcohol Res Health 33:29–44, 2010 [PMC free article] [PubMed] [Google Scholar]
- Wong MM, Nigg JT, Zucker RA, Puttler LI, Fitzgerald HE, Jester JM, Glass JM, Adams K: Behavioral control and resiliency in the onset of alcohol and illicit drug use: A prospective study from preschool to adolescence. Child Dev 77:1016–1033, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Rogers B, Heckers S: Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res 130:86–93, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Parsons LM, Gao JH, Fox PT: Interregional connectivity to primary motor cortex revealed using MRI resting state images. Hum Brain Mapp 8:151–156, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto DJ, Woo CW, Wager TD, Regner MF, Tanabe J: Influence of dorsolateral prefrontal cortex and ventral striatum on risk avoidance in addiction: A mediation analysis. Drug Alcohol Depend 149:10–17, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau WYW, Zubieta JK, Weiland BJ, Samudra PG, Zucker RA, Heitzeg MM: Nucleus accumbens response to incentive stimuli anticipation in children of alcoholics: Relationships with precursive behavioral risk and lifetime alcohol use. J Neurosci 32:2544–2551, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimić JI, Jukić V: Familial risk factors favoring drug addiction onset. J Psychoactive Drugs 44:173–185, 2012 [DOI] [PubMed] [Google Scholar]
- Zucker RA, Fitzgerald H, Noll R: Drinking and Drug History (Revised edition, Version 4). Ann Arbor (MI), University of Michigan Department of Psychiatry, Addiction Research Center, 1990 [Google Scholar]
- Zucker RA, Fitzgerald HE, Refior SK, Puttler LI, Pallas DM, Ellis DA: The clinical and social ecology of childhood for children of alcoholics: Description of a study and implications for a differentiated social policy. In: Children of Addiction: Research, Health and Policy Issues. Edited by Fitzgerald HE, Lester BM, Zuckerman BS. New York, Routledge Falmer, 2000, pp. 109–141 [Google Scholar]


