Abstract
Background
The effect of premorbid β-blocker exposure on clinical outcomes in patients with sepsis is not well characterized. We aimed to examine the association between premorbid β-blocker exposure and mortality in sepsis.
Methods
EMBase, MEDLINE, and Cochrane databases were searched for all studies of premorbid β-blocker and sepsis. The search was last updated on 22 June 2019. Two reviewers independently assessed, selected, and abstracted data from studies reporting chronic β-blocker use prior to sepsis and mortality. Main data extracted were premorbid β-blocker exposure, mortality, study design, and patient data. Two reviewers independently assessed the risk of bias and quality of evidence.
Results
In total, nine studies comprising 56,414 patients with sepsis including 6576 patients with premorbid exposure to β-blockers were eligible. For the primary outcome of mortality, two retrospective studies reported adjusted odds ratios showing a reduction in mortality with premorbid β-blocker exposure. One study showed that premorbid β-blocker exposure decreases mortality in patients with septic shock. Another study showed that continued β-blockade during sepsis is associated with decreased mortality.
Conclusion
This systematic review suggests that β-blocker exposure prior to sepsis is associated with reduced mortality. There was insufficient data to conduct a bona fide meta-analysis. Whether the apparent reduction in mortality may be attributed to the mitigation of catecholamine excess is unclear.
Trial registration
PROSPERO, CRD42019130558 registered June 12, 2019.
Electronic supplementary material
The online version of this article (10.1186/s13054-019-2562-y) contains supplementary material, which is available to authorized users.
Keywords: Sepsis, Mortality, Beta blockers, Systematic review
Introduction
The Sepsis-3 consensus defines sepsis as a life-threatening organ dysfunction caused by a dysregulated host response to infection [1]. While our understanding of sepsis pathophysiology is increasing, controversies in haemodynamic management persist [2, 3]. The most recent surviving sepsis guidelines recommend noradrenaline as the first-choice vasopressor because of its vasopressor and positive inotropic properties [strong recommendation, moderate quality of evidence [4]]. In contrast, the concept of ‘decatecholamisation’ emerged in the last decade stemming from the recognized negative effects of catecholamines in sepsis [3, 5, 6]. Interestingly, the β-adrenergic blockade has emerged as a possible treatment option for blunting the adrenergic response in early sepsis with potential effects on the modulation of cardiogenic, metabolic, immunologic, and coagulopathic derangements in sepsis [7].
Early administration of the short-acting β-blocker esmolol in a recent trial showed a reduction in 28-day sepsis mortality [8, 9]. Furthermore, some studies have suggested a benefit of premorbid β-blocker exposure on sepsis outcomes [10, 11]. Multiple systematic reviews have since concluded that there is limited preliminary evidence for the use of β-blockers during sepsis [12–14], while others are skeptical [15]. However, to date, no published systematic review exists on the effects of premorbid β-blocker exposure on sepsis outcomes, including mortality. Therefore, we set out to systematically examine the evidence from all human studies on premorbid β-blocker exposure and sepsis.
Materials and methods
This study follows the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines [16] and was registered with the international prospective register of systematic reviews (PROSPERO; CRD 42019130558). The MOOSE checklist is appended as Additional file 1: Table S1.
Data sources and searches
Three databases, EMBase, MEDLINE, and Cochrane were searched on 30 January 2019 for records dating from database conception to the date of search that was last updated on 22 June 2019. The search was only limited to human research. Duplicates were removed using the Ovid platform and checked for any incorrect removal. Hand searching from reference lists was also performed. The full search strategy is appended as Additional file 4: Figure S1.
Study selection
Inclusion criteria for this review were guided by the ‘Patient, Population, or Problem, Intervention, Comparison, Outcome, Study Design or Setting’ (PICOS) framework [17] (Table 1). Patients exposed to β-blockers prior to an episode of sepsis or septic shock and were cared for in the emergency department (ED) or intensive care unit (ICU) were included in this review. Observational studies were eligible. Excluded were case studies/small series (< 20 patients overall) and review articles. The abstracts were assessed by two investigators (KT, MH) independently, and disagreements were resolved with a third investigator (MN).
Table 1.
‘PICOS’ approach for selecting clinical studies in the systematic search. PICOS Patient, Population, or Problem, Intervention, Comparison, Outcome, Study Design or Setting
| PICOS | Study characteristics |
|---|---|
| 1. Participants | Patients with sepsis and/or septic shock |
| 2. Intervention | Premorbid exposure to beta blockers |
| 3. Comparison | No premorbid exposure to beta blockers |
| 4. Outcomes | Mortality |
| 5. Study design | Prospective observational or retrospective cohort studies |
Data extraction and quality assessment
Data from eligible studies were independently extracted by two investigators (KT, MH). Where required, study authors were contacted directly to kindly provide missing research data. The Risk Of Bias In Non-randomized Studies - of Interventions (ROBINS-I) tool [18] was used to independently assess (KQ, MH) the quality of studies.
Data synthesis and analysis
Adjusted outcome data were combined using the inverse variance method [19]. Heterogeneity between studies was measured by Higgin’s and Thomson’s I2 [20]. Statistical analyses were performed using Review Manager version 5.3 (Copenhagen: The Cochrane Collaboration, 2014)
Results
Study selection
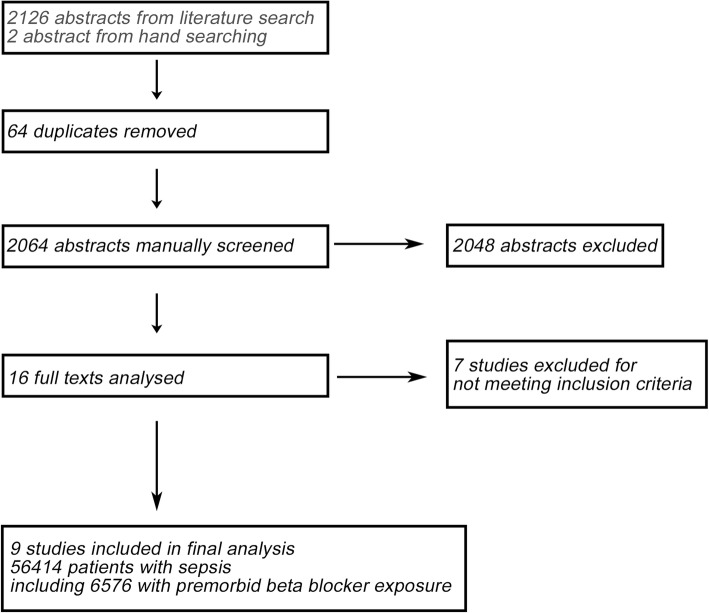
The initial search returned 2128 abstracts, all in English. Two thousand sixty-four abstracts were manually screened after removal of 64 duplicates. After screening, 16 studies were initially selected for data extraction. Where required, the corresponding authors were contacted to obtain necessary data for statistical analysis. Seven studies were excluded for not meeting all inclusion criteria. The list of studies excluded is appended (Additional file 2: Table S2). Overall, a total of nine studies were found to be eligible, comprising 56,414 patients with sepsis, including 6576 patients with premorbid exposure to β-blockers (Fig. 1).
Fig. 1.
Flow diagram of the study selection process
Characteristics and type of studies
All studies were retrospective cohort studies, and the data were collected between 1999 and 2017.
The study populations described patients with sepsis, severe sepsis, or septic shock in an ED [21] or ICU [10, 11, 21–26] setting. The definitions of sepsis, severe sepsis, septic shock, and premorbid β-blocker exposure varied slightly across the studies, but were reasonable and comparable to current definitions [1]. Two studies [10, 27] included patients with sepsis, severe sepsis, and septic shock, while seven studies [11, 21–26] included patients with severe sepsis and/or septic shock. One study (Alsolamy et al.) included patients ≥ 14 years of age; all other studies included adult (18 years and above) patients. Four studies by Sharma et al., Charles et al., Alsolamy et al., and Al-Qadi et al. were reported as conference abstracts [22–25]. The characteristics of the studies are appended (Table 2).
Table 2.
Characteristics of included studies
| First author | Year of publication | Type of study | Study period (month/year) | Country | Number of centres | Diagnosis | Setting (ED/ICU) | ICU type (medical/surgical) | Outcome | Premorbid beta blocker exposure | Inclusions | Exclusions | Select cohort | No. of patients with premorbid beta blocker use |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Singer et al. [11] | 2017 | Retrospective matched cohort study | 2009–2011 | USA | Medicare Provider and Analysis Review data | Severe sepsis, septic shock | ICU | Mixed | Primary: mortality | Filled prescription within 30 days of admission, including date of admission. Data obtained from Medicare Part D claims data. | Patients admitted with an urgent/emergent hospital admission code, requiring intensive care upon admission, and carrying a primary diagnosis of sepsis or systemic inflammatory response syndrome (SIRS) by ICD9 diagnosis codes, > 65 years, asthma, heart block, 1 year of continuous Part A and Part B coverage, with Part D enrolment. | In hospital claims without ICU admission, Part C enrolment (coverage through healthcare maintenance organizations), beta blocker prescriptions extending into 30 days prior to admission but not through the admission date. | 6839 | 2838 |
| Macchia et al. [10] | 2012 | Retrospective matched cohort study | 2003–2008 | Italy | 22 | Sepsis | ICU | Mixed | Primary: mortality | 3 or more filled prescriptions within 4 months of admission. Data obtained from local health authority drug claims database. | Sepsis with codes 038 [septicemia], 020.0 [septicemic], 790.7 [bacteremia], 117.9 [disseminated fungal infection], 112.5 [disseminated candida infection], and 112.81 [disseminated fungal endocarditis]. Admission direct to ICU or transferred to ICU from other hospital departments within 48 h of admission. | < 40 years old, transfer to ICU from other departments 48 h after admission. | 9465 | 1061 |
| Hsieh et al. [27] | 2019 | Retrospective matched observational study | 1999–2013 | Taiwan | National Health Insurance Research Database (NHIRD) of Taiwan data | Sepsis, septic shock | – | – | Primary: mortality | Patients were classified as using certain drugs if they took them for more than 1 week within a 3-month period prior to the index date. | First episode of severe sepsis or septic shock. ICD-9-CM coding was used. | Second episode of sepsis. | 33,213 | 1040 |
| Fuchs et al. [26] | 2017 | Secondary analysis of prospective observational single-centre trial | 2010–2013 | Germany | 1 | Sepsis, severe sepsis, septic shock | ICU | Surgical |
Primary: mortality; survival analysis Secondary: length of stay |
Pre-existing oral beta blocker therapy was defined as a treatment started at least 7 days before sepsis onset. | First episode of severe sepsis or septic shock. | < 18 years old, no infection, no chronic beta blocker therapy, no sepsis or septic shock, second episode of sepsis. | 296 | 296 |
| Contenti et al. [21] | 2015 | Retrospective cohort study | 2012–2014 | France | 1 | Severe sepsis, septic shock | ED/ICU | – |
Primary: initial blood lactate concentration. Secondary: mortality |
‘Premorbid’ not defined. Data obtained from ED observation data or inpatient records | > 18 years old, severe sepsis, septic shock. | – | 260 | 65 |
| Sharma et al. [25] | 2016 | Retrospective study | 2013–2014 | ICU | Not specified | Septic shock | ICU | Medical | Peak lactate, cumulative norepinephrine dose and duration, mortality | ‘Premorbid’ not defined. Data obtained from ICU medical records. | Medical ICU, septic shock, vasopressors required. | – | 123 | 48 |
| Charles et al. [23] | 2018 | Retrospective study | 2008–2016 | France | Not specified | Septic shock | ICU | – | Heart rate, arterial lactate levels, arterial oxygen content, fluid requirements, norepinephrine requirements, duration of mechanical ventilation, mortality | ‘Premorbid’ not defined. | Adult patients diagnosed with septic shock within 48 h. | – | 938 | 230 |
| Alsolamy et al. [22] | 2016 | Retrospective cohort study | 1/1/2003–31/12/2013 | Saudi Arabia | 1 | Severe sepsis, septic shock | ICU | – | Primary: mortality | Active prescription 3 months prior to admission. | > 14 years old, severe sepsis and septic shock, previous prescription of beta blockers active for 3 months prior to hospital admission. | – | 4629 | 623 |
| Al-Qadi et al. [24] | 2014 | Retrospective study | 2007–2009 | USA | 1 | Severe sepsis, septic shock | ICU | Medical | Primary: mortality | 3 or more months of beta blocker usage prior to admission. Data obtained from electronic records. | Severe sepsis and septic shock, 3 or more months on beta blocker prior to ICU admission. | Patients with comfort care. | 651 | 375 |
Risk of bias assessment
All observational studies of premorbid medication use are at risk of bias because of confounding. Five studies included in this review [10, 11, 24, 26, 27] were judged to be of moderate risk of bias for the primary outcome of mortality as they reported adjustment of confounding variables via statistical analysis. Four studies [21–23, 25] were judged as having serious risk of bias due to confounding as the authors did not perform statistical analysis to correct for confounders. The risk of bias assessment using ROBINS-1 tool for each trial is appended (Additional file 5: Figure S2) with reasonings attached (Additional file 3: Table S3).
Primary outcome: mortality
The smallest study by Contenti et al. included 260 sepsis patients. Results from that study showed a non-significant decrease in 28-day mortality (35% vs 49%, p = 0.08; Table 3). Using multivariate logistic regression, three studies by Singer et al., Macchia et al., and Hsieh et al. reported mortality data as adjusted odds ratios [10, 11, 27]. Singer et al. reported a decrease in hospital mortality in patients with severe sepsis and septic shock (aOR = 0.69; 95% CI [0.62, 0.77]; Table 3). Subgroup analysis between cardioselective β-blockers and non-selective β-blockers showed that non-selective β-blockers were associated with lower hospital mortality, adjusted OR for non-selective β-blockers (aOR = 0.59; 95% CI [0.49, 0.71]) compared to cardioselective β-blockers (aOR = 0.73; 95% CI [0.65, 0.82]). Overall mortality rate for cardioselective β-blocker users was higher, cardioselective β-blocker users vs. non-selective β-blocker users (aOR = 1.23; 95% CI [1.11–1.36]). Hospital mortality was also reduced across all age groups: between ages 65 and 74 (aOR = 0.64; 95% CI [0.52, 0.80]), between ages 75 and 84 (aOR = 0.69; 95% CI [0.58, 0.83]), and above 85 (aOR = 0.73; 95% CI [0.60, 0.90]).
Table 3.
Mortality data for included studies. Premorbid beta blocker exposure vs no premorbid beta blocker exposure
| First author | Select cohort | No. of patients with no premorbid beta blocker use | No. of patients with premorbid beta blocker use | Mortality census day | Mortality | 90-day mortality | 28-day mortality | ICU mortality | Hospital mortality | Survival analysis | Outcome | Adjustment method | Adjusted variables |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Singer et al. [11] | 6839 | 4001 | 2838 | Hospital mortality | – | – | – | – | aOR = 0.69 (CI 0.62–0.77) | – | Premorbid beta blocker usage is significantly associated with decreased mortality | Multivariate logistic regression | Age, class of beta blocker, congestive heart failure, cancer, surgical procedures |
| Macchia et al. [10] | 9465 | 8404 | 1061 | 28-day mortality | – | – | aOR = 0.81 (CI 0.68–0.97), p = 0.025 | – | – | – | Premorbid beta blocker usage is significantly associated with decreased mortality | Multivariate logistic regression | Age, sex, history of hypertension, dyslipidaemia, diabetes mellitus, myocardial infarction, congestive heart failure, atrial fibrillation, chronic obstructive pulmonary disease, depression, and malignancy |
| Hsieh et al. [27] | 33,213 | 32,173 | 1040 | Hospital mortality | – | – | – | – | aOR = 0.89 (CI 0.76–1.04), p = 0.1484 | – | Premorbid beta blocker usage is not significantly associated with decreased mortality | Multivariate logistic regression | Age, sex, insurance premium, urbanization level, and comorbidities |
| Fuchs et al.a [26] | 296 | 0 | 296 | ICU, hospital, 28 days, 90 days | – | 40.7% vs. 52.7%, p = 0.046a | 28.7% vs. 41.1%, p = 0.04a | 27.5% vs. 38%, p = 0.06a | 35.3% vs. 48.1%, p = 0.03a | HR = 0.67 (CI 0.48, 0.95), p = 0.03a | Continuation of beta-blockade is associated with decreased 28-day, 90-day, and hospital mortality. | Multivariate cox regression | Sex, known nosocomial pathogen, chronic diseases, body temperature (< 36.0 °C), APACHE II score first 24 h, lactate first 24 h (> 3 mmol/L) |
| Contenti et al. [21] | 260 | 195 | 65 | 28-day mortality | – | – | – | – | 35% vs 49%, p = 0.08 | – | Premorbid beta blocker usage is not significantly associated with decreased mortality | – | – |
| Sharma et al. [25] | 123 | 75 | 48 | Hospital mortality | – | – | – | – | 35.4% vs 32%, p = 0.70 | – | Premorbid beta blocker usage is not significantly associated with decreased mortality | – | – |
| Charles et al. [23] | 938 | 708 | 230 | ICU mortality | – | – | – | 35.7% vs. 37%, p = 0.75 | – | – | Premorbid beta blocker usage is not significantly associated with decreased mortality | – | – |
| Alsolamy et al. [22] | 4629 | 4006 | 623 | ICU mortality | – | – | – | RR = 0.94 (CI 0.82–1.08), p = 0.39 | – | – | Premorbid beta blocker usage is not significantly associated with decreased mortality | – | – |
| Al-Qadi et al. [24] | 651 | 276 | 375 | Not specified | 21.3% vs 27.2%, p = 0.09; aOR 0.62, p = 0.023 | – | – | – | – | – | Premorbid beta blocker usage is not significantly associated with decreased mortality | – | Age, gender, and severity of illness using SOFA and APACHE III scores |
aContinued beta blocker usage during sepsis vs discontinued beta blocker usage during sepsis
Macchia et al. reported a significant decrease in 28-day mortality in patients with sepsis (aOR = 0.81; 95% CI [0.68–0.97]; p = 0.025; Table 3). Subgroup analysis investigating the effect of age, gender, organ dysfunction, and previous comorbidities did not alter the results. Adjustment for previous medication used including calcium channel blockers, amiodarone, angiotensin-converting-enzyme inhibitors, diuretics, or any nonsteroidal anti-inflammatory drugs also did not alter the results. The authors also conducted a propensity matching analysis, which led to similar results (OR = 0.72; 95% CI [0.57–0.91]; p = 0.04).
The study by Hsieh et al. showed that premorbid β-blocker exposure was not associated with a significant decrease in hospital mortality in patients with sepsis and septic shock (aOR = 0.89; 95% CI [0.76, 1.04]; p = 0.1484; Table 3). However, subgroup analysis of patients with septic shock showed that premorbid β-blocker exposure was significantly associated with decreased hospital mortality (aOR = 0.68; 05% CI [0.56, 0.82]; p = 0.0001). In patients without septic shock, premorbid β-blocker exposure was associated with significantly higher mortality (aOR = 1.16; 95% CI [1.11, 1.21]; p < 0.0001).
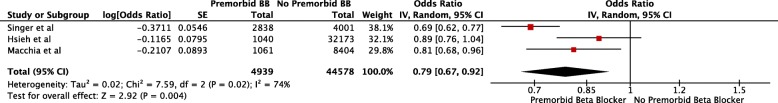
We compared the mortality data from the three studies that adjusted for potential confounders. Pooled analysis of the three studies showed an average odds ratio, aOR = 0.79; 95% CI (0.67, 0.92), p = 0.004; Fig. 2. However, there was substantial heterogeneity (i2 = 74%) between the studies, indicating that a meta-analysis is premature and that further studies and subgroup analyses are needed to validate the results.
Fig. 2.
Adjusted odds ratio analysis via forest plot of sepsis mortality rates in studies comparing populations with premorbid β-blocker (BB) exposure to populations without premorbid β-blocker exposure. Horizontal bars represent 95% confidence intervals
Our systematic search also included grey literature in the form of conference abstracts. Mortality data from three studies reported as conference abstracts showed a trend towards a decrease in mortality with premorbid β-blocker exposure. However, the results were not statistically significant: Charles et al. (ICU mortality; 35.7% vs. 37%, p = 0.75), Alsolamy et al. (ICU mortality; RR = 0.94 (CI: 0.82–1.08), p = 0.39), and Al-Qadi et al. (21.3% vs 27.2%, p = 0.09) (Table 3). Of note, the study by Alsolamy et al. included patients ≥ 14 years old, while all other studies only included adults. Another retrospective study, reported as a conference abstract, involving 123 sepsis patients showed a non-significant increase in mortality with premorbid β-blocker exposure: Sharma et al. (hospital mortality; 35.4% vs 32%, p = 0.70; Table 3).
One interesting study by Fuchs et al. investigated the effect of continuing premorbid β-blocker use in patients with severe sepsis and septic shock. This study included 296 patients on chronic β-blockers, in which β-blockade was continued in 176 patients. Results showed that continuation of β-blockade during sepsis was associated with decreased 28-day (28.7% vs. 41.1%, p = 0.04), 90-day (40.7% vs. 52.7%, p = 0.046), and hospital mortality (35.3% vs. 48.1%, p = 0.03) (Table 3). Survival analysis also indicated that continuation of β-blockade during sepsis is significantly associated with decreased mortality (HR = 0.67; 95% CI [0.48, 0.95]; p = 0.03; Table 3).
Clinical parameters
Only four studies by Contenti et al. [21], Charles et al. [23], Sharma et al. [25], and Fuchs et al. [26] provided clinical parameter data. However, reporting of parameters was inconsistent. There was no significant difference in the requirements for vasopressor infusion across all four studies. Contenti et al. and Charles et al. found that premorbid β-blocker exposure was associated with decreased heart rate; Sharma et al., did not report heart rate data. Continuation of β-blockade during sepsis was not associated with a decrease in heart rate in the first 24 h [26]. Premorbid β-blocker use was found to be associated with lower initial plasma lactate levels by Contenti et al., but not by Charles et al.. The continuation of β-blockade during sepsis was associated with lower plasma lactate levels in the first 24 h [26].
There were no significant differences in all other relevant parameters including mean arterial pressure, Sequential Organ Failure Assessment (SOFA) score, Acute Physiology and Chronic Health Evaluation (APACHE)-II or III score, and incidence of mechanical ventilation. The clinical parameter data are presented in Table 4.
Table 4.
Reported clinical parameters
| First author | Heart rate | Initial blood lactate levels | Peak blood lactate levels | Creatinine levels | Arterial pH | Mean arterial pressure | SOFA score | APACHE II score | APACHE III score | Mechanical ventilation | Vasopressor infusion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Contenti et al.a [21] | 100 ± 25 vs 109 ± 25 bpm; p = 0.02 | 3.9 ± 2.3 mmol/L vs 5.6 ± 3.6 mmol/L; p = 0.0006 | – | – | – | 72 mmHg ± 22 vs 70 mmHg ± 21; p = 0.48 | 5.0 ± 2.8 vs 5.3 ± 2.8; p = 0.44 | 21.0 ± 6.0 vs 21.7 ± 6.9; p = 0.41 | – | 15% vs 19%; p = 0.58 | 31% vs 32%; p = 0.94 |
| Sharma et al.a [25] | – | – | 3.2 vs 3.6 mmol/L; p = 0.54 | – | – | – | – | – | 94 vs 84; p = 0.14 | – |
Cumulative dose 11.4 vs 12.6 mg; p = 0.43 Duration of infusion 1563 vs 1730 min; p = 0.37 |
| Charles et al.a [23] | 81 (IQR 82–111) vs. 107 (IQR 89–122) bpm; p < 0.01 | 1.75 (IQR 0.9–3.4) vs. 1.8(IQR 0.8–4) mmol/L; p = 0.97 | – | 165.5 (IQR 108–245) vs 135.5 (IQR 82–108); p < 0.00 | 7.35 (IQR 7.25–7.42) vs 7.34 (IQR 7.23–7.42); p = 0.354 | – | 9 (IQR 6–12) vs 9 (IQR 6–13); p = 0.242 | – | – |
81.7% vs 84.9%; p = 0.112 Days on ventilation 4 (IQR 2–9) vs 5.5 (IQR 2–11); p = 0.055 |
23.2 mg (IQR 5.1–57.0) vs 22.4 mg (IQR 5.2–60.5); p = 0.95 |
| Fuchs et al.b [26] | 111 (IQR 97.0–132.8) vs 118 (IQR 97.0–135.5); p = 0.2 | 2.3 (IQR 1.5–3.8) vs 3.5 (IQR 2.0–6.5); p < 0.01 | – | – | – | – | – | 20.0 (IQR 15.0–24.5) vs 21.0 (IQR 16.2–26.0); p = 0.25 | – | – | Norepinephrine 91% vs 92.2%; p = 0.83 |
aPremorbid beta blocker exposure vs no premorbid beta blocker exposure
bContinued beta blocker usage during sepsis vs discontinued beta blocker usage during sepsis
Discussion
This is the first systematic review examining the role of premorbid β-blocker exposure on mortality outcomes in patients with sepsis. While there was not enough data to conduct a meta-analysis, pooled adjusted odds ratio from three studies indicated a potential decrease in mortality associated with premorbid β-blocker use, albeit with substantial heterogeneity. Our results provide preliminary evidence of a potential association between premorbid β-blocker use and mortality in sepsis and add to the emerging evidence suggesting harmful effects of adrenergic stress on mortality in sepsis. We discuss the effects of premorbid β-blocker exposure on the adrenergic response in early sepsis.
Cardiac dysfunction in sepsis is common and has both systolic and diastolic components [5]. However, only diastolic dysfunction seems to be associated with mortality [28, 29]. While being on premorbid β-blockers may reduce systolic function, the reduction of adrenergic response in sepsis (decreasing heart rate, prolongation of diastolic time, and improved coronary perfusion) can lead to mitigation of diastolic dysfunction [28, 29]. Further, the risks of myocardial ischemia may be decreased due to reduced myocardial oxygen consumption [14].
Patients with septic shock are often treated with large doses of exogenous catecholamines for haemodynamic stabilization. The most recent Surviving Sepsis Campaign guidelines recommend using noradrenaline as the first-line agent for vasopressor therapy, with adrenaline or low-dose vasopressin as second-line agents [4]. Increased dosage and duration of noradrenaline administration has been associated with higher incidence of new onset atrial fibrillation [3]. Excessive catecholamine levels may also play an important role in sepsis-related cardiac dysfunction by causing cardiomyopathy and cardiomyocyte necrosis [5, 7]. β-adrenergic blockade could reduce the amount of exogenous catecholamines used by restoring sepsis-induced downregulation of β-adrenergic receptors [12, 30]. Four of the included studies in this systematic review, however, found that premorbid β-blocker exposure was not associated with a significant difference in vasopressor requirements during sepsis. Similarly, Fuchs et al. found that continuing chronic beta blockers during acute phase of sepsis is not associated with increased use of catecholamines.
Interestingly, Singer et al. reported that patients with premorbid exposure to non-selective β-blockers had lower mortality rates compared to patients with premorbid cardioselective β-blocker exposure [11]. This suggests that β-blocker modulation of non-cardiac adrenergic responses to sepsis may also have an important role. Furthermore, β-blockers may potentially positively modulate the disturbed autonomic (sympathetic-parasympathetic) balance in sepsis [31].
Adrenergic response to sepsis induces a hypermetabolic state characterized by increased energy expenditure, hyperglycaemia, lipolysis and proteolysis, supressed ketogenesis, and negative nitrogen balance resulting in eventual loss of lean body mass [32]. β2-adrenergic blockade appears to have the potential to reverse hyperglycaemia and reduce proteolysis [7]. For example, the use of propranolol in children with severe burns appears to attenuate hypermetabolism and reverse muscle catabolism [33].
The immune system is also modulated by the adrenergic responses to sepsis [34]. The β-adrenergic system regulates apoptosis, mitochondrial function, and inflammatory cytokine production. β-blockers influence the pattern of cytokine synthesis with β1 blockers downregulating a proinflammatory response, whereas β2-antagonization seems to have an opposite effect, at least in chronic heart failure [35].
In sepsis, β2-adrenergic stimulation selectively inhibits CD4+ lymphocyte Th1 function and favours the Th2 responses that inhibit macrophage activation, T cell proliferation, and proinflammatory cytokine production [7]. CD8+ lymphocyte function may also be suppressed by β2-adrenergic stimulation [36]. The derangement in lymphocytic function induced by catecholamines is thus reminiscent of sepsis-induced immune suppression and could even be considered as one of the mechanisms. However, to date, the evidence for any beneficial use of β-adrenergic blockade on immune function in sepsis has been conflicting [7].
Sepsis results in a pro-thrombotic state with increases in plasma tissue factor and von Willebrand factor levels [37]. Platelets also express adrenergic receptors on their surface [38]. However, there are conflicting effects of β1 and β2 pathways on platelet function [7]. The use of β-adrenergic blockade led to decreased endothelial cell damage in a murine model of shock coagulopathy [39]. This suggests that premorbid β-blocker therapy might mitigate shock-induced endotheliopathy (SHINE), attenuating sepsis-associated coagulopathy [40].
Nonetheless, multiple questions on the role of β-adrenergic blockade in sepsis remain unanswered. On top of safety and efficacy concerns, the duration and dosage at which β-blockade should be performed remain to be elucidated. Furthermore, the timing of therapeutic β-adrenergic blockade initiation is also controversial. The results of our systematic review suggest that we should not discount β-blockers during sepsis. Instead, we may consider continuing chronic β-blockers and perhaps introduce β-blocking drugs early in the sepsis management, especially the non-cardioselective ones.
Strengths and limitations
This study analysed data from nine observational studies, four of which were reported as conference abstracts. There was not enough data to conduct a meta-analysis. By nature of observational studies, systematic confounding and risk of bias cannot be ruled out. The risk of bias can be reduced by adjusted analysis. Analysis of pooled adjusted odds ratio revealed a significant decrease in sepsis mortality with premorbid β-blocker exposure, but adjusted data were available only from three studies. Despite the three studies providing data on the majority of patients included in this review, substantial heterogeneity is present and residual confounding is likely. Potential sources of confounding include the variable definitions of premorbid β-blocker exposure used by the included studies, the appropriate prescription of β-blockers to all included patients, and patient compliance to treatment.
The conclusions that can be drawn from this study are also hampered by the lack of clinical parameter data, limiting our ability to decipher the likely mechanism/s by which premorbid β-blocker exposure may lower sepsis mortality.
Conclusion
This systematic review suggests that β-blocker exposure prior to an episode of sepsis could have a role in reducing sepsis mortality. More evidence, however, is needed to elucidate whether premorbid β-blocker treatment is able to mitigate, and by what mechanism, the potentially detrimental effects of endogenous or exogenous catecholamines in early sepsis. Further appropriately powered and ideally prospective observational studies on premorbid β-blocker exposure will be necessary to generate the required evidence.
Additional files
Table S1 MOOSE Checklist. (DOCX 16 kb)
Table S2 List of studies excluded from systematic review. (DOCX 14 kb)
Table S3 Reasoning for Bias Assessment for Mortality Outcome using ROBINS-1 Tool (DOCX 18 kb)
Figure S1 Detailed search strategy. (DOCX 13 kb)
Figure S2 Risk of bias assessment for mortality in individual studies using ROBINS-I assessment tool. (TIF 1123 kb)
Acknowledgements
Not applicable.
Abbreviations
- aOR
Adjusted odds ratio
- APACHE
Acute Physiology and Chronic Health Evaluation
- CI
Confidence interval
- ED
Emergency department
- HR
Hazard ratio
- ICU
Intensive care unit
- MOOSE
Meta-analysis Of Observational Studies in Epidemiology
- PICOS
Patient, Population, or Problem, Intervention, Comparison, Outcome, Study Design or Setting
- PROSPERO
International prospective register of systematic reviews
- ROBINS-I
Risk Of Bias In Non-randomized Studies - of Interventions
- RR
Relative risk
- SHINE
Shock-induced endotheliopathy
- SOFA score
Sequential Organ Failure Assessment
Authors’ contributions
KQ and MN designed the study. KQ, MH, and MN conducted the literature search and data analysis. KQ drafted the manuscript. MH, BT, AM, and MN revised the manuscript. All authors read and approved the final manuscript.
Funding
Nepean Institute of Critical Care Education and Research (NICCER). Dr. Nalos and Dr. Harazim were was supported by the Charles University Research Fund (project number Q39) and by project number CZ.02.1.01/0.0/.0/16_019/0000787 ‘Fighting Infectious Diseases,’ awarded by the Ministry of Youth and Education Services of the Czech Republic.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Ethics approval for systematic review is not required.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ [Internet. 2016:i1585 [cited 2019 Mar 24]. Available from: http://www.bmj.com/lookup/doi/10.1136/bmj.i1585. [DOI] [PubMed]
- 3.Lesur O, Delile E, Asfar P, Radermacher P. Hemodynamic support in the early phase of septic shock: a review of challenges and unanswered questions. Ann Intensive Care. 2018;8(1) [cited 2019 Mar 24]. Available from: https://annalsofintensivecare.springeropen.com/articles/10.1186/s13613-018-0449-8. [DOI] [PMC free article] [PubMed]
- 4.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki T, Suzuki Y, Okuda J, Kurazumi T, Suhara T, Ueda T, et al. Sepsis-induced cardiac dysfunction and β-adrenergic blockade therapy for sepsis. J Intensive Care. 2017;5(1) [cited 2019 Mar 24]. Available from: http://jintensivecare.biomedcentral.com/articles/10.1186/s40560-017-0215-2. [DOI] [PMC free article] [PubMed]
- 6.Singer M. Catecholamine treatment for shock—equally good or bad? Lancet. 2007;370(9588):636–637. doi: 10.1016/S0140-6736(07)61317-8. [DOI] [PubMed] [Google Scholar]
- 7.de Montmollin E, Aboab J, Mansart A, Annane D. Bench-to-bedside review: β-adrenergic modulation in sepsis. Crit Care. 2009;13(5):230. doi: 10.1186/cc8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morelli A, Ertmer C, Westphal M, Rehberg S, Kampmeier T, Ligges S, et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: a randomized clinical trial. JAMA. 2013;310(16):1683. doi: 10.1001/jama.2013.278477. [DOI] [PubMed] [Google Scholar]
- 9.Morelli A, Donati A, Ertmer C, Rehberg S, Kampmeier T, Orecchioni A, et al. Microvascular effects of heart rate control with esmolol in patients with septic shock: a pilot study*. Crit Care Med. 2013;41(9):2162–2168. doi: 10.1097/CCM.0b013e31828a678d. [DOI] [PubMed] [Google Scholar]
- 10.Macchia A, Romero M, Comignani PD, Mariani J, D’Ettorre A, Prini N, et al. Previous prescription of β-blockers is associated with reduced mortality among patients hospitalized in intensive care units for sepsis*. Crit Care Med. 2012;40(10):2768–2772. doi: 10.1097/CCM.0b013e31825b9509. [DOI] [PubMed] [Google Scholar]
- 11.Singer KE, Collins CE, Flahive JM, Wyman AS, Ayturk MD, Santry HP. Outpatient beta-blockers and survival from sepsis: results from a national cohort of Medicare beneficiaries. Am J Surg. 2017;214(4):577–582. doi: 10.1016/j.amjsurg.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Loon LM, van der Hoeven JG, Lemson J. Hemodynamic response to β-blockers in severe sepsis and septic shock: a review of current literature. J Crit Care. 2019;50:138–143. doi: 10.1016/j.jcrc.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Chacko C, Gopal S. Systematic review of use of β-blockers in sepsis. J Anaesthesiol Clin Pharmacol. 2015;31(4):460. doi: 10.4103/0970-9185.169063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanfilippo F, Santonocito C, Morelli A, Foex P. Beta-blocker use in severe sepsis and septic shock: a systematic review. Curr Med Res Opin. 2015;31(10):1817–1825. doi: 10.1185/03007995.2015.1062357. [DOI] [PubMed] [Google Scholar]
- 15.McLean AS, Taccone FS, Vieillard-Baron A. Beta-blockers in septic shock to optimize hemodynamics? No. Intensive Care Med. 2016;42(10):1610–1612. doi: 10.1007/s00134-016-4407-3. [DOI] [PubMed] [Google Scholar]
- 16.Stroup DF. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016:i4919 [cited 2018 Jul 30]. Available from: http://www.bmj.com/lookup/doi/10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed]
- 19.Park S, Beretvas SN. Using total sample size weights in meta-analysis of log-odds ratios. J Exp Educ. 2018;1–15. [cited 2019 Mar 25]. Available from: https://www.tandfonline.com/doi/full/10.1080/00220973.2018.1451295
- 20.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 21.Contenti J, Occelli C, Corraze H, Lemoël F, Levraut J. Long-term β-blocker therapy decreases blood lactate concentration in severely septic patients*. Crit Care Med. 2015;43(12):2616–2622. doi: 10.1097/CCM.0000000000001308. [DOI] [PubMed] [Google Scholar]
- 22.Alsolamy S, Ghamdi G, Alswaidan L, Alharbi S, Alenezi F, López-Rodríguez M, et al. 36th International Symposium on Intensive Care and Emergency Medicine: Brussels, Belgium. 15–18 March 2016. Crit Care. 2016;20(S2) [cited 2019 Mar 17]. Available from: http://ccforum.biomedcentral.com/articles/10.1186/s13054-016-1208-6.
- 23.Charles DR, Jean-Francois L, Matthieu J, Charpentier J, Cariou A, Chiche J-D, et al. Proceedings of Réanimation 2018, the French Intensive Care Society International Congress. Ann Intensive Care. 2018;8(S1) [cited 2019 Mar 17]. Available from: https://annalsofintensivecare.springeropen.com/articles/10.1186/s13613-017-0345-7.
- 24.Al-Qadi MO, O’Horo JC, Thakur L, Kaur S, Berrios RAS, Caples SM, et al. American Journal of Respiratory and Critical Care Medicine. San Diego: Conference: Ameriacn Thoracic Society International Conference, ATS 2014; 2014. Long-term use of beta blockers is protective in severe sepsis and septic shock; p. 189. [Google Scholar]
- 25.Sharma A, Vashisht R, Bauer S, Hanane T. Effect of preadmission beta-blocker use on outcomes of patients admitted with septic shock. United States: Critical Care Medicine; 2016. p. 413. [Google Scholar]
- 26.Fuchs C, Wauschkuhn S, Scheer C, Vollmer M, Meissner K, Kuhn S-O, et al. Continuing chronic beta-blockade in the acute phase of severe sepsis and septic shock is associated with decreased mortality rates up to 90 days. Br J Anaesth. 2017;119(4):616–625. doi: 10.1093/bja/aex231. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh M-S, How C-K, Hsieh VC-R, Chen P-C. Preadmission antihypertensive drug use and sepsis outcome: impact of angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs). Shock. 2019;1 [cited 2019 Jun 22]. Available from: http://Insights.ovid.com/crossref?an=00024382-900000000-97629. [DOI] [PubMed]
- 28.Sanfilippo F, Corredor C, Fletcher N, Landesberg G, Benedetto U, Foex P, et al. Diastolic dysfunction and mortality in septic patients: a systematic review and meta-analysis. Intensive Care Med. 2015;41(6):1004–1013. doi: 10.1007/s00134-015-3748-7. [DOI] [PubMed] [Google Scholar]
- 29.Sanfilippo F, Corredor C, Arcadipane A, Landesberg G, Vieillard-Baron A, Cecconi M, et al. Tissue Doppler assessment of diastolic function and relationship with mortality in critically ill septic patients: a systematic review and meta-analysis. Br J Anaesth. 2017;119(4):583–594. doi: 10.1093/bja/aex254. [DOI] [PubMed] [Google Scholar]
- 30.Kimmoun A, Louis H, Al Kattani N, Delemazure J, Dessales N, Wei C, et al. β1-adrenergic inhibition improves cardiac and vascular function in experimental septic shock*. Crit Care Med. 2015;43(9):e332–e340. doi: 10.1097/CCM.0000000000001078. [DOI] [PubMed] [Google Scholar]
- 31.Kohoutova M, Horak J, Jarkovska D, Martinkova V, Tegl V, Nalos L, et al. Vagus nerve stimulation attenuates multiple organ dysfunction in resuscitated porcine progressive sepsis. Crit Care Med. 2019;1 [cited 2019 Apr 19]. Available from: http://Insights.ovid.com/crossref?an=00003246-900000000-95985. [DOI] [PubMed]
- 32.Chioléro R, Revelly JP, Tappy L. Energy metabolism in sepsis and injury. Nutr Burbank Los Angel Cty Calif. 1997;13(9 Suppl):45S–51S. doi: 10.1016/S0899-9007(97)00205-0. [DOI] [PubMed] [Google Scholar]
- 33.Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345(17):1223–1229. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 34.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52(4):595–638. [PubMed] [Google Scholar]
- 35.Shaw SM, Coppinger T, Waywell C, Dunne L, Archer LD, Critchley WR, et al. The effect of beta-blockers on the adaptive immune system in chronic heart failure. Cardiovasc Ther. 2009;27(3):181–186. doi: 10.1111/j.1755-5922.2009.00089.x. [DOI] [PubMed] [Google Scholar]
- 36.Estrada LD, Ağaç D, Farrar JD. Sympathetic neural signaling via the β2-adrenergic receptor suppresses T-cell receptor-mediated human and mouse CD8 + T-cell effector function. Eur J Immunol. 2016;46(8):1948–1958. doi: 10.1002/eji.201646395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schouten M, Wiersinga WJ, Levi M, van der Poll T. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol. 2008;83(3):536–545. doi: 10.1189/jlb.0607373. [DOI] [PubMed] [Google Scholar]
- 38.Hjemdahl P, Larsson PT, Wallén NH. Effects of stress and beta-blockade on platelet function. Circulation. 1991;84(6 Suppl):VI44–VI61. [PubMed] [Google Scholar]
- 39.Xu L, Yu W-K, Lin Z-L, Tan S-J, Bai X-W, Ding K, et al. Chemical sympathectomy attenuates inflammation, glycocalyx shedding and coagulation disorders in rats with acute traumatic coagulopathy. Blood Coagul Fibrinolysis. 2015;26(2):152–160. doi: 10.1097/MBC.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 40.Johansson P, Stensballe J, Ostrowski S. Shock induced endotheliopathy (SHINE) in acute critical illness - a unifying pathophysiologic mechanism. Crit Care. 2017;21(1):25. doi: 10.1186/s13054-017-1605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 MOOSE Checklist. (DOCX 16 kb)
Table S2 List of studies excluded from systematic review. (DOCX 14 kb)
Table S3 Reasoning for Bias Assessment for Mortality Outcome using ROBINS-1 Tool (DOCX 18 kb)
Figure S1 Detailed search strategy. (DOCX 13 kb)
Figure S2 Risk of bias assessment for mortality in individual studies using ROBINS-I assessment tool. (TIF 1123 kb)
Data Availability Statement
Not applicable.