Abstract
Growing evidence indicates that risk for bipolar disorder is characterized by elevated activation in a fronto-striatal reward neural circuit involving the ventral striatum and orbitofrontal cortex, among other regions. It is proposed that individuals with abnormally elevated reward-related neural activation are at risk for experiencing an excessive increase in approach-related motivation during life events involving rewards or goal striving and attainment. In the extreme, this increase in motivation is reflected in hypomanic/manic symptoms. By contrast, unipolar depression (without a history of hypomania/mania) is characterized by decreased reward responsivity and decreased reward-related neural activation. Collectively, this suggests that risk for bipolar disorder and unipolar depression are characterized by distinct and opposite profiles of reward processing and reward-related neural activation. The objective of the present paper is threefold. First, we review the literature on reward processing and reward-related neural activation in bipolar disorder, and in particular risk for hypomania/mania. Second, we propose that reward-related neural activation reflects a biological marker of differential risk for bipolar disorder versus unipolar depression that may help facilitate psychiatric assessment and differential diagnosis. We also discuss, however, the challenges to using neuroscience techniques and biological markers in a clinical setting for assessment and diagnostic purposes. Lastly, we address the pharmacological and psychosocial treatment implications of research on reward-related neural activation in bipolar disorder.
Keywords: Reward, bipolar disorder, fronto-striatal neural circuitry, depression, assessment, treatment
The Diagnostic and Statistical Manual of Mental Disorders (5th ed.; American Psychiatric Association, 2013) defines the bipolar spectrum disorders as encompassing three diagnoses: cyclothymia, bipolar II disorder, and bipolar I disorder. All three diagnoses involve extreme highs (hypomania or mania) and lows (depression) of mood, motivation, cognition, and behavior, but differ in severity level with bipolar I disorder being the most severe and cyclothymia the least severe. Moreover, having a milder form of bipolar disorder (cyclothymia, bipolar II) increases the risk for developing full-blown bipolar I disorder in both children/adolescents (Birmaher, Axelson, Goldtein et al., 2009; Kochman, Hantouche, Ferrari, et al., 2005) and adults (Alloy, Urosevic, Abramson et al., 2012), supporting the concept that bipolar disorder involves a spectrum of severity.
Bipolar disorder is associated with significant work impairment, high rates of divorce and substance abuse, a 10-year earlier mortality rate, and leads to suicide in nearly one out of every five diagnosed individuals (Angst, Stassen, Clayton, et al., 2002; Chen & Dilsaver, 1996; Goodwin & Jamison, 2007; Isometsa, 1993; Kupfer, Frank, Grochocinski et al., 2002). Indeed, bipolar disorder has been ranked one of the six most debilitating of all non-communicable illnesses in the world (Ayuso-Mateos, 2000). Despite the severity of this illness, bipolar disorder is either frequently misdiagnosed, or there is a lengthy time period from the point of illness onset to correct diagnosis. Delays ranging from 6 to 10 years or longer have been reported before individuals with bipolar disorder receive an accurate diagnosis and appropriate treatment (Ghaemi, Boiman, Goodwin, et al., 2000; Ghaemi, Sachs, Chiou et al., 1999). Hirschfeld et al. (2003) reported data from the National Depressive and Manic Depressive Association survey indicating that close to 70% of respondents with bipolar disorder were initially misdiagnosed, with the most frequent misdiagnosis being unipolar depression (60%). Those who were misdiagnosed consulted a mean of four physicians prior to receiving the correct diagnosis and over one-third waited 10 years or more before receiving an accurate diagnosis. The misdiagnosis of bipolar disorder not only delays administration of appropriate treatment but may have a deleterious effect on the course of bipolar disorder given research suggesting that antidepressant medications typically used for treating unipolar depression may be a risk factor for ‘switching’ into hypomanic/manic episodes among individuals at risk for bipolar disorder (Altshuler, Post, Leverich, et al., 1995; Ghaemi, Boiman, Goodwin, et al. 2000; Ghaemi, Hsu, Soldani, & Goodwin, 2003; Valenti, Pacchiarotti, Bonnin, et al., 2012) [Although see Visser and colleagues (2005) for research indicating no strong evidence that antidepressant use in bipolar disorder increases risk for hypomanic/manic episodes].
Converging factors contribute to the frequent mis- and under-diagnosis of bipolar disorder. For example, the significant impairment associated with bipolar depression (Calabrese, Muzina, Kemp et al., 2006) results in individuals with bipolar disorder being more likely to present for treatment when depressed, increasing the likelihood of an inaccurate diagnosis of unipolar depression (Keck, Kessler, & Ross, 2008). Another contributor of misdiagnosis may involve the absence of biologically relevant diagnostic markers to help differentiate risk for bipolar disorder from other psychiatric disorders, particularly unipolar depression. In considering a biological marker, it is important to assess whether the marker is common to multiple psychiatric disorders, or specific to a particular disorder. As an example, both elevated cortico-amygdala threat-related neural activation and deficits in pre-frontal executive control are observed across multiple psychiatric disorders, including unipolar depression (Hamilton, Etkin, Furman, et al., 2012; Wagner, et al., 2006), bipolar disorder (Phillips & Vieta, 2007; Almeida, Versace, Hassel, et al., 2010), and anxiety disorders (Etkin & Wager, 2007; Pacheco-Unguetti, Acosta, Marques, & Lupianez, 2011). Thus, while threat- and executive control-related neural activation may reflect biological markers for transdiagnostic symptomatology, they may not be particularly helpful in differentiating risk for specific disorders. By contrast, there is growing evidence that bipolar disorder and unipolar depression may be characterized by distinct profiles of fronto-striatal reward-related neural activation. Specifically, bipolar disorder has been associated with abnormally elevated or hyperactive reward-related neural activation (Nusslock, Almeida, Forbes et al., 2012a; Bermpohl, Kahnt, Dalanay et al., 2010), whereas unipolar depression has been associated with reduced or blunted reward-related neural activation (Heller, Johnstone, Shackman, et al., 2009; Forbes, Hariri, Martin, et al., 2009b; McCabe, Cowen, & Harmer, 2009). Thus, the central premise of this paper is that elevated reward-related neural activation may reflect a biological marker for bipolar disorder that can help distinguish risk for bipolar disorder from unipolar depression. The present paper has three objectives. First, we review the behavioral approach system (BAS)/reward hypersensitivity model of bipolar disorder, giving particular attention to neuroimaging research on fronto-striatal reward-related neural activation. As summarized below, reward hypersensitivity appears to be particularly implicated in hypomania/mania, and less so in bipolar depression. Thus, the present paper focuses primarily on reward-related neural activation in the pathophysiology of hypomania/mania. Second, we examine the extent to which reward-related neural activation reflects a biological marker of differential risk for bipolar disorder (particularly hypomania/mania) versus unipolar depression that could potentially be used to facilitate psychiatric assessment and differential diagnosis. Included in this discussion is an overview of the challenges to using neuroscience techniques and biological markers in a clinical setting for assessment and diagnostic purposes. Lastly, we will examine the pharmacological and psychosocial treatment implications of research on reward processing and reward-related neural activation in bipolar disorder.
Behavioral Approach System (BAS)/Reward Hypersensitivity Model of Bipolar Disorder
The BAS/reward hypersensitivity model of bipolar disorder argues that abnormalities in reward processing and approach-related affect play an important role in the pathophysiology of bipolar disorder, and in particular hypomanic/manic symptoms (Alloy & Abramson, 2010; Johnson, 2005; Johnson, Edge, Holmes, & Carver, 2012b; Urosevic, Abramson, & Harmon-Jones, & Alloy, 2008). Reward processing relates to the value an individual places on potential rewards, the perceived probability of reward receipt, and the mechanisms by which an individual processes rewards or goal-relevant cues. These cues can be either external (presence of a desired reward) or internal (expectancies of reward attainment). Approach motivation involves the engagement of cognitive, behavioral, and motoric resources to pursue the potential reward or goal. Considerable evidence implicates a fronto-striatal neural circuit in both reward processing and approach-related affect (Berridge, Robinson, & Aldridge, 2009; Haber, & Knutson, 2010; Kringelbach & Rolls, 2004). Activation of reward and approach-related systems is associated with increased incentive-motivation, positive goal-striving emotions, and increased motor-related behavior (Depue & Collins, 1999), as well as anger when goal striving is frustrated or blocked (Carver, 2004; Harmon-Jones & Sigelman, 2001).
The BAS/reward hypersensitivity model proposes that risk for hypomania and mania is characterized by a hypersensitivity to goal- and reward-relevant cues. This hypersensitivity can lead to an excessive increase in approach-related affect and motivation during life events involving rewards or goal striving and attainment. In the extreme, this excessive increase in approach-related affect is reflected in hypomanic/manic symptoms, such as elevated or irritable mood, decreased need for sleep, increased psychomotor activation, extreme self-confidence, and pursuit of rewarding activities without attention to risks. Thus, from the perspective of the BAS/reward hypersensitivity model, symptoms of hypomania/mania involve extreme expressions along an underlying core brain-behavior dimension of positive valence, reward-processing and approach-related affect.1
In its original conceptualization the BAS/reward hypersensitivity model proposed that reward hypersensitivity can lead to both hypomanic/manic or bipolar depressive symptoms in response to positive and negative reward-relevant events, respectively (Depue, Krauss, & Spoont, 1987; Alloy & Abramson, 2010; Urosevic, Abramson, Harmon-Jones, & Alloy, 2008). The basis for this prediction is that reward hypersensitivity should make individuals hypersensitive to both cues signaling the possible attainment and loss of reward. To date, however, there is not strong support for a linkage between reward hypersensitivity and the course and onset of bipolar depression (Alloy et al., 2008). Instead, research highlights the role that reward hypersensitivity plays in the pathophysiology of hypomania/mania, suggesting that other etiological mechanisms (e.g., threat-related processing) may underlie the depressive phase of bipolar disorder. Accordingly, the present paper primarily focuses on the role that reward hypersensitivity plays in hypomania/mania.
In line with the BAS/reward hypersensitivity model is considerable psychosocial research indicating that individuals with bipolar disorder self-report a hypersensitivity to reward-relevant cues. This research has been aided by the development of the BAS scale of the Behavioral Inhibition System/Behavioral Activation System (BIS/BAS) scales, a self-report measure of reward and approach system sensitivity by Carver and White (1994). According to Carver and White (1994), “greater self-reported BAS sensitivity should be reflected in greater proneness to engage in goal-directed efforts and experience positive feelings when the person is exposed to cues of impending reward” (pp. 319). Compared to relevant control groups, individuals with bipolar I disorder (Meyer, Johnson, & Winters, 2001; Salavert, Caseras, Torrubia, et al., 2007), bipolar II disorder and cyclothymia (Alloy et al., 2008), and people prone to hypomanic symptoms (Meyer, Johnson, & Carver, 1999) self-report elevated BAS sensitivity. Using a retrospective and concurrent behavioral high-risk design, Alloy et al. (2006b) found that individuals selected on the basis of high self-reported BAS sensitivity were six times more likely to obtain a lifetime diagnosis of a bipolar spectrum disorder than were individuals with moderate BAS sensitivity. Individuals with or vulnerable to bipolar spectrum disorders also exhibit ambitious goal-setting relative to controls, particularly for goals involving fame, achievement, and financial success (Gruber & Johnson, 2009; Johnson & Carver, 2006; Johnson, Carver, & Gotlib, 2012a), and display greater behavioral, emotional, and cognitive responses to rewards (Eisner, Johnson, & Carver, 2008; Johnson, Ruggero, & Carver, 2005). Related, bipolar individuals display biased expectations of positive outcomes when considering goals, particularly following initial success (Johnson et al., 2008). The relationship between bipolarity and BAS/reward sensitivity appears to be state-independent in that it is not related to current levels of mania (Lozano & Johnson, 2001; Scott, Stanton, Garland, & Ferrier, 2000), and reward sensitivity continues to be elevated into remission relative to controls (Lam, Wright, & Smith, 2004; Meyer et al., 2001).
Growing evidence indicates that profiles of BAS/reward sensitivity have predictive validity for the course of bipolar spectrum disorders. Elevated self-reported BAS/reward sensitivity is associated with a greater likelihood of having a lifetime bipolar spectrum diagnosis (Alloy et al., 2006b), a greater likelihood of developing a first onset of a bipolar spectrum disorder (Alloy et al., 2012a), a shorter time to recurrences of hypomanic/manic episodes (Alloy et al., 2008), an increase in manic symptoms among recovered individuals with bipolar I disorder (Meyer, Johnson, & Winters, 2001), and a greater likelihood of progressing to a more severe bipolar diagnosis among those with milder bipolar spectrum diagnoses (Alloy et al., 2012b). Furthermore, both reward-striving (Nusslock, Abramson, Harmon-Jones, et al., 2007) and reward-attainment (Johnson, Sandrow, Meyer, et al., 2000) relevant life events have been shown to trigger hypomanic/manic episodes, and self-reported BAS sensitivity interacts with reward-relevant events to prospectively predict increases in hypomanic symptoms (Alloy et al., 2009b).
Neurophysiological Indices of Reward Hypersensitivity in Bipolar Disorder
Research has recently begun examining the neurophysiological and neurobiological basis of reward hypersensitivity in bipolar disorder. With respect to neurophysiology, much of this work has focused on relative left versus right frontal electroencephalographic (EEG) activity. Close to thirty years of research suggests that relative left frontal EEG activity may reflect a neurophysiological index of approach system sensitivity and reward-related affect (see Coan & Allen, 2004 for review). Increased relative left-frontal EEG activity indicates a propensity to approach or engage a stimulus, whereas decreased relative left-frontal activity indicates a propensity toward reduced approach-related affect or increased withdrawal motivation. In line with this perspective, increased relative left-frontal EEG activity has been associated with elevated self-reported BAS sensitivity (Harmon-Jones & Allen, 1997; Sutton & Davidson, 1997), positive activation (Tomarken, Davidson, Wheeler, & Doss, 1992), and a response bias toward reward-relevant stimuli (Pizzagalli, Sherwood, Henriques, et al., 2005). Increased relative left frontal activity has also been associated with elevated state and trait anger (Harmon-Jones, 2003), an approach-related affect despite its negative valence (Carver & Harmon-Jones, 2009).
Consistent with the BAS/reward hypersensitivity theory, bipolar disorder is characterized by elevated relative left frontal EEG activity. Harmon-Jones and colleagues reported that both individuals prone to hypomanic/manic symptoms (2002) and individuals with a bipolar spectrum diagnosis (2008) display elevated relative left-frontal activity during approach or reward-related laboratory tasks compared to healthy controls. Amongst individuals with a bipolar spectrum diagnosis, elevated relative left-frontal activity is a risk factor for a more severe course. Specifically, Nusslock and colleagues (2012b) reported that elevated left frontal activity was associated with a greater likelihood of converting from cyclothymia or bipolar II disorder to bipolar I disorder over a five-year follow-up period. This is the first study to identify a neurophysiological risk factor for conversion to a more severe bipolar diagnosis and parallels the previously mentioned research indicating that elevated self-reported BAS/reward sensitivity is associated with a more severe bipolar course.
Complimenting neurophysiological research on relative left frontal EEG activity is work employing event-related potentials (ERP). Embedded within EEG data, ERPs are electrical potentials that occur in preparation for, or in response to, discrete events, whether they be internal or external to the participant. An advantage of ERP is that it provides strong temporal resolution, allowing researchers to examine brain activation on the order of milliseconds (Nusslock, Young, Pornpattanananangkul, & Damme, in press). Increasing evidence suggests that reward sensitivity can be measured using the feedback negativity (FN), an ERP component elicited by stimuli that indicate monetary gain versus loss. In gambling tasks, the FN appears as a relative negative deflection in the waveform approximately 300 ms following feedback indicating monetary loss compared to gain (Hajcak, Moser, Holroyd, & Simons, 2007; Yeung, Holroyd, & Cohen, 2005). The reinforcement learning model of the FN (Holroyd & Coles, 2002) proposes that this signal represents rapid evaluation of the motivational impact of behavioral outcomes and the encoding of these outcomes as either better or worse than expected. Thus, the FN encodes reward prediction error that reflects the discrepancy between desired and actual outcomes. The FN is generated when midbrain dopamine neurons encode a reward prediction error and send afferent signals to the anterior cingulate cortex and adjacent motor and prefrontal regions to adapt one’s behavioral performance to pursue gains and/or minimize loss (Cohen & Ranganath, 2007).
Mason and colleagues (2012) recently examined the relationship between the FN to monetary gain/loss stimuli and hypomanic traits, as assessed by the Hypomanic Personality Scale (HPS; Eckblad & Chapman, 1986). In a 10-year prospective study, more than 75% of individuals with high scores on the HPS developed manic or hypomanic episodes (Kwapil et al., 2000), demonstrating that the HPS is a good index of risk for bipolar disorder. In line with the BAS/reward hypersensitivity model, individuals with elevated HPS scores displayed greater reward responsiveness, as indexed by the FN, compared to individuals with both moderate and low HPS scores (Mason et al., 2012). Research examining the FN in individuals with bipolar spectrum diagnoses, including bipolar I disorder, will be an important area for future research.
In summary, both research on relative left frontal EEG activity and the FN ERP component indicate that bipolar spectrum disorders are associated with a neurophysiological hypersensitivity to reward-relevant cues. Next, we examine neuroimaging studies of reward-related neural activation in bipolar disorder.
The Fronto-Striatal Reward-Neural Circuit
Although many regions in the brain respond to reward, the fronto-striatal neural circuit is at the heart of the reward system (Berridge, Robinson, & Aldridge, 2009; Haber & Knutson, 2010; Kringelbach & Berridge, 2009, Schultz, 2000; Schultz, Tremblay, & Hollerman, 2000). This circuit involves dopaminergic projections from midbrain nuclei (e.g., the ventral tegmental area), to subcortical regions that are central to processing the rewarding properties of stimuli (e.g., the ventral striatum, including the nucleus accumbens), to cortical target regions (e.g., the oribitofrontal cortex, medial prefrontal cortex, anterior cingulate cortex). Both animal and human-based research highlight the central role that this circuit plays in reward-responsivity, incentive-based learning, assessing probability of reward receipt, prediction error, and goal directed behavior. For example, functional magnetic resonance imaging (fMRI) research in humans indicates increased midbrain activation in regions such as the substantia nigra and ventral tegmental area during anticipation of pleasant tastes (O’Doherty, Deichmann, Critchley et al., 2002), anticipation of monetary gains (Knutson, Taylor, Kaufman, et al., 2005), reward-predicting cues (Adcock, Thangavel, Whitfield-Gabrieli, et al., 2006), and during exposure to positive or rewarding visual stimuli (Aron, Fisher, Mashek, et al., 2005).
Midbrain dopamine neurons have substantial projections to the ventral striatum, which is a central hub of reward processing. Anatomical definitions of the ventral striatum vary across animal and human based research; however, in human neuroimaging it frequently includes the nucleus accumbens, the ventral medial caudate, and the rostroventral putamen (Haber & Knutson, 2010). Both metabolic positron emission tomography (PET) and fMRI studies indicate that exposure to both primary (e.g., pleasant tastes, sounds and sights) and secondary rewards (e.g., monetary rewards) increase striatal activity in humans (Blood & Zatorre, 2001; Delgado, Nystrom, Fissell, et al., 2000; Haber & Knutson, 2010; Knutson et al., 2005; Small, Zatorre, Dagher, et al., 2001). The observed elevation in striatal activity to both primary and secondary rewards is consistent with the notion that striatal activation does not depend on sensory modality. Many of these studies included unpleasant and neutral stimuli, thus controlling for arousal and other confounds (e.g., perceptual and behavioral demands). A number of factors modulate striatal activity to reward cues, including the magnitude of the reward, the probability of reward receipt, the amount of time until the anticipated reward can be obtained (i.e., delay), and the effort required to pursue the reward (see Haber & Knutson, 2010 for review). Furthermore, elevated ventral striatal activity during reward anticipation is associated with elevated self-reported BAS/reward sensitivity (Caseras, Lawrence, Murphy et al., 2013; Hahn, Dresler, Ehlis, et al., 2009).
The region of the cortex most often associated with reward is the orbitofrontal cortex (OFC; Haber & Knutson, 2010; Kringelbach & Rolls, 2004; Schultz, Tremblay, & Hollerman, 2000). There is variability in how the OFC is anatomically defined, particularly across animal and human based studies. Drawing from research on reward-related neural activation in bipolar disorder (Bermpohl et al., 2010; Nusslock et al., 2012a), we define the OFC as Broadmann Area (BA) 10, 11, and 47 for the present paper. Several neuroimaging studies indicate that sensory and abstract rewards recruit the OFC (Blood & Zatorre, 2001; Knutson, Westdorp, Kaiser, & Hommer, 2000; Small et al., 2001). A meta-analysis of these findings (Kringelbach & Rolls, 2004) suggests a potentially important distinction between medial and lateral regions of the OFC. This analysis indicates that the medial OFC (BA 10, 11) is clearly sensitive to the rewarding properties of stimuli and the generation of positive or approach-related affect, but the lateral OFC (e.g., BA 47) appears to be sensitive to both positive and negative (i.e., punishment cues) cues. Accordingly, activation of the lateral OFC has been interpreted in terms of arousal (Schmidt, Clery-Melin, Lafargue et al., 2009) and salience (Lewis, Critchley, Rothstein, & Dolan, 2007) as opposed to positive hedonic evaluation. Finally, the dorsal anterior cingulate cortex (dACC) is frequently activated in studies of reward-related learning and decision making and likely helps mediate action selection under situations involving conflict between competing responses to maximize reward (Bush, Vogt, Holmes, et al., 2002; Hampton & O’Doherty, 2007).
Both animal and human research highlights the central role of dopamine neurotransmission in the fronto-striatal reward circuit (Haber & Knutson, 2010; Schultz, 2002; Wise, 2002). Relative to placebo injection, ligand-based PET research indicates that amphetamine injection robustly increases striatal dopamine, and these increases correlate with positive and arousing affective experiences (Drevets, Gautier, & Price, et al., 2001; Volkow, Wang, Fowler, et al., 1999). Alcohol, cocaine, and secondary rewards such as gambling all increase dopamine release in the striatum (Boileau, Assaad, Pihl, et al., 2003; Cox, Benkelfat, Dagher, et al., 2009). Dopamine, however, appears to be more involved in reward anticipation and ‘wanting’, and less involved in reward outcome and ‘liking’ (Berridge et al., 2009). For example, suppression of endogenous dopamine neurotransmission reduces ‘wanting’ but not liking (Berridge, 2007), and amplification of ‘wanting’ without ‘liking’ has been produced by the activation of dopamine systems by amphetamine or similar drugs (Robinson & Berridge, 1993). Reward consumption or ‘liking’ has been linked to endogenous opiods and endocannabinoids (see Berridge et al., 2009 for review).
Fronto-Striatal Indices of Reward Hypersensitivity in Bipolar Disorder
Structural and functional neuroimaging studies implicate the fronto-striatal neural circuit in the pathophysiology of bipolar disorder. Structural imaging studies report abnormalities in prefrontal volume (Lopez-Larson, DelBello, Zimmerman, et al., 2002) and increased striatal size (Strakowski, DelBello, Zimmerman, et al., 2002) in individuals with bipolar disorder. Gray matter deficits in the striatum and the ACC are also present in individuals at genetic risk for bipolar disorder, but who have not yet developed the illness (McDonald, Bullmore, Sham et al., 2004). This latter finding suggests that structural abnormalities in the fronto-striatal circuit may reflect a preexisting risk factor for bipolar disorder, as opposed to a consequence of hypomanic/manic and depressive episodes. Positron emission tomography (PET) studies report increased metabolism in the striatum (among other regions) in both currently depressed (Mah, Zarate, Singh, et al., 2007; Drevets, Price, Videen, et al., 1995; Ketter, Kimbrell, George, et al., 2001) and manic (Blumberg, Stern, Martinez, et al., 2000) bipolar individuals, suggesting that elevated metabolic activity in the striatum may be a state-independent marker of bipolar disorder [although see Brooks et al. (2009) for evidence of decreased metabolic activity in the striatum in bipolar depression]. Functional neuroimaging studies also indicate alterations in prefrontal (including orbitofrontal) and ventral striatal neural activation during cognitive and emotional tasks, including emotional-face matching (Altshuler, Bookheimer, Proenza, et al., 2005a), probability-based decision making (Rubinsztein, Fletcher, Rogers, et al. 2001), motor inhibition (Leibenluft, Rich, Vinton, et al., 2007), and both affective (Elliot, Ogilvie, Rubinsztein, et al., 2004) and letter-based (Altshuler, Bookheimer, Townsend, et al., 2005b) Go/NoGo paradigms.
In line with the BAS/reward-hypersensitivity model, bipolar disorder is associated with an excessive increase in fronto-striatal reward-related neural activation to positive or approach-related stimuli. For example, bipolar individuals display elevated striatal (Hassel, Almeida, Kerr, et al., 2008; Lawrence, Williams, Surguladze, et al., 2004), OFC (Elliott, et al., 2004), and amygdala (Bermpohl, Dalanay, Kahnt, et al., 2009) activation to pictures of happy faces or pleasant stimuli compared to healthy controls. There is preliminary evidence that this effect is state-independent, as elevated reward-related neural activation to positive emotional stimuli has been observed in both remitted (Hassel, et al., 2008) and manic (Bermpohl, et al., 2009; Elliott et al., 2004) bipolar individuals [although see Liu, Blond, van Dyck, et al. (2012) for evidence of decreased striatal, OFC, and ACC activation in bipolar individuals to happy versus neutral faces].
Although it is informative to examine reward-related neural activation to happy faces or pleasant stimuli, this approach has its limitations. First, passively viewing pleasant stimuli may be suboptimal for engaging fronto-striatal neural circuitry given this circuits involvement in facilitating approach-related affect and behavior to pursue possible rewards. Second, as typically used, this approach does not allow researchers to separately examine anticipatory (wanting) versus consummatory (liking) reward-related neural activation, which may be important for understanding the pathophysiology of bipolar disorder. Accordingly, researchers in the area of bipolar disorder have begun to employ established fMRI reward paradigms to examine neural activation to the anticipation and receipt of monetary reward and loss. The two most common fMRI reward paradigms used in research on bipolar disorder, and mood disorders more generally, are a card guessing paradigm and the monetary incentive delay task (MID). In the card guessing paradigm [adapted from Forbes et al., (2009b)], participants guess, via button press, whether the value of a visually presented card with a possible value of 1–9 is higher or lower than five. If participants make a correct guess, they can win money during win trials and avoid losing money during loss trials. After a guess is made, participants wait to see whether they guessed correctly (anticipation period) and then receive feedback as to whether they won or lost money for that trial based on their guess (outcome period). The MID task, developed by Knutson and colleagues (2001), involves a comparable trial structure to the card guessing paradigm except that participants’ monetary gain versus loss depends on their performance on a motoric reaction time task.
The handful of studies on bipolar disorder that have employed fMRI reward paradigms provide compelling, albeit nuanced, support for the BAS/reward hypersensitivity model (see Table 1). Nusslock and colleagues (2012a) used the card guessing paradigm to examine reward-related neural activation in euthymic bipolar I disorder versus healthy control participants. In line with prediction, euthymic bipolar I disorder participants displayed greater ventral striatal, medial OFC (BA 10), and left lateral OFC (BA 47) activation during the anticipation, but not the outcome, of monetary reward relative to healthy controls. There were no differences in neural activation between bipolar I and healthy control participants during anticipation or receipt of monetary loss. The fact that reward-related neural activation was abnormally elevated in bipolar I individuals during remission suggests that this profile of fronto-striatal activity may reflect a trait-like risk factor for bipolar disorder. To establish a biological marker of a disorder, however, it is important to examine the marker across multiple phases of the illness. To date, there have been two studies that used an fMRI reward paradigm with bipolar I individuals during a manic episode, and one study that used such a paradigm with bipolar I individuals during a depressive episode. With respect to mania, Bermpohl and colleagues (2010) reported that fifteen bipolar I individuals in a manic episode displayed elevated left lateral OFC (BA 47) activation during reward anticipation in the MID task, while healthy participants showed the inverse effect. In a second study, Abler and colleagues (2007) reported increased activation in the ventral striatum coupled with reward omission in manic versus healthy participants, suggesting that bipolar individuals in a manic episode have a reduced capacity to discriminate between rewards on the basis of their actual value and relevance.
Table 1.
fMRI studies of fronto-striatal neural activation to monetary reward cues in individuals with bipolar disorder.
| Contrast | Ventral Striatum | Medial OFC | Lateral OFC | ACC | Citation |
|---|---|---|---|---|---|
| BD IEuthymic vs. HC | + | + | + | Nusslock et al., 2012a | |
| BD IManic vs. HC | + | Bermpohl et al., 2010 | |||
| BD IManic vs. HC | + ǂ | Abler et al., 2007 | |||
| BD I Depressed vs. HC | + | − | Chase et al., 2013 | ||
| BD II Euthymic vs. HC | + | + | Caseras et al., 2013 | ||
| Hyperthymic vs. Non-Hyperthymic Temperament | + | + | Harada et al., 2013 |
A blank cell indicates there was either no difference between the two groups at the specified neural region or the study did not directly compare the groups at that region. + = BD greater than HC; − = BD less than HC. BD = Bipolar disorder participants; HC = Healthy control participants; OFC = orbitofrontal cortex; ACC = anterior cingulate cortex.
Medial OFC corresponds to Brodmann area (BA) 10 and 11, and lateral OFC corresponds to BA 47.
Increased ventral striatal activation in Abler et al. (2007) was coupled with reward omission.
With respect to bipolar depression, Chase and colleagues (2013) used the card guessing task to compare reward-related neural activation in currently depressed bipolar I individuals, currently depressed individuals with major depressive disorder (MDD), and healthy controls. Analyses examined anticipation and outcome of reward and loss trials separately, as well as the effect of collapsing anticipation of both reward and loss into a single regressor. This regressor reflects anticipation per se, independent of anticipated value. The authors report that both bipolar I and MDD depressed participants displayed decreased ACC activation during reward anticipation relative to healthy controls. This finding highlights the presence of state-dependent effects of depression on reward-related neural activation in the ACC in individuals with bipolar I disorder. The authors further report, however, that bipolar depressed participants displayed elevated left lateral OFC (BA 47) activation during anticipation, collapsing across reward and loss trials. Thus, even during depression, individuals with bipolar I disorder maintain heightened activation in regions of the fronto-striatal neural circuit.
Further evidence for elevated reward-related neural activation in bipolar disorder comes from research on individuals with a bipolar spectrum diagnosis (i.e., bipolar II disorder), and individuals at elevated risk for bipolar disorder who have not yet developed the illness. Using the card-guessing paradigm, Caseras and colleagues (2013) reported that euthymic bipolar II participants displayed greater ventral striatal and left lateral OFC activation during reward anticipation compared to healthy controls (contrary to prediction, this study did not find elevated ventral striatal activity during reward anticipation among bipolar I individuals). Depressed bipolar II participants also displayed elevated metabolism in the ventral striatum, anteroventral putamen, and the left OFC, as indexed by PET (Mah et al., 2007). Lastly, individuals with a hypomanic temperament who have not yet developed bipolar disorder (Harada, Hoaki, Terao, et al., 2013) display elevated ventral striatal activation and left-lateral OFC activation during reward processing. This latter finding suggests that, like structural abnormalities in the fronto-striatal circuit, elevated functional reward-related neural activation may reflect a preexisting risk factor for bipolar disorder, as opposed to a consequence of the illness.
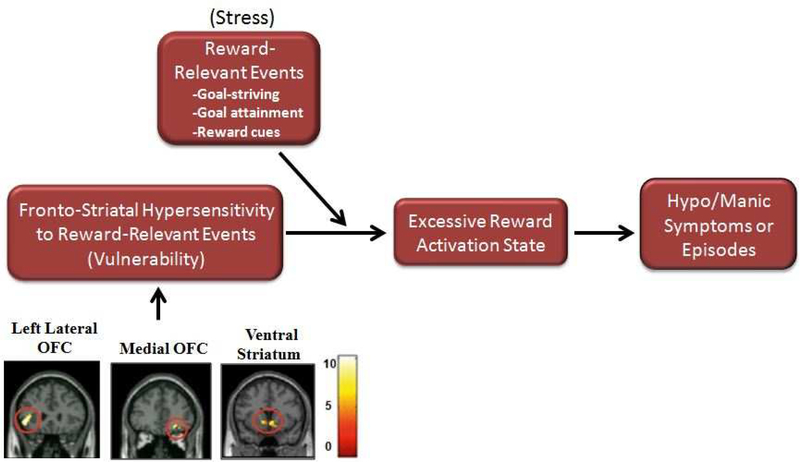
In summary, there is compelling evidence that bipolar disorder is characterized by abnormally elevated fronto-striatal reward-related neural activation. Structural imaging studies report increased ventral striatal volume in individuals with bipolar disorder (Strakowski et al., 2002), and functional imaging studies report elevated reward-related neural activation in individuals with bipolar I disorder (Nusslock et al., 2012a; Bermpohl, et al., 2010; Chase, et al., 2013; Abler, et al., 2008), bipolar II disorder (Caseras, et al., 2013; Mah, et al., 2007), and individuals at elevated risk for bipolar disorder who have not yet developed the illness (Harada, et al., 2013). We propose that elevated reward-related neural activation is likely the biological mechanism underlying elevated self-report, behavioral, and neurophysiological indices of reward sensitivity that have been documented in bipolar disorder research (Alloy & Abramson, 2010; Johnson, 2005; Johnson, et al., 2012b; Urosevic, Abramson, Harmon-Jones, & Alloy, 2008). We and others (Alloy & Abramson, 2010; Depue, Krauss, & Spoont, 1987; Johnson, et al., 2012b; Urosevic, Abramson, Harmon-Jones, & Alloy, 2008) further propose that a hypersensitivity of the fronto-striatal reward circuit is likely a central mechanism through which individuals with bipolar disorder are at risk for developing hypomanic/manic episodes in the presence of goal or reward-relevant life events. Specifically, it is proposed that individuals with bipolar disorder experience an excessive increase in reward-related neural activation to reward-relevant life events which is reflected in an excessive increase in reward or approach-related affect and behavior. In the extreme, this increase in reward-related neural activation and approach-related affect is reflected in hypo/manic symptoms (see Figure 1).
Figure 1:
Fronto-striatal reward hypersensitivity model of bipolar disorder. fMRI images reflect anatomical location of euthymic bipolar I disorder participants displaying greater activation in fronto-striatal circuit (left lateral OFC, right OFC, ventral striatum) during anticipation of monetary gain (reward anticipation) compared to healthy control participants. Color bars reflect beta values. Significant clusters were overlaid on coronal brain slices (adapted from Nusslock et al., 2012a).
In studies that have employed an fMRI reward paradigm, one of the most reliable findings is that bipolar individuals display an abnormal increase in left lateral OFC activation during reward anticipation. This has now been observed across the entire bipolar spectrum, including individuals with bipolar I disorder (Nusslock et al., 2012a; Bermpohl, et al., 2010; Chase et al., 2013), bipolar II disorder (Caseras, et al., 2013), and individuals at risk for bipolar disorder (Harada, et al., 2013). Elevated left lateral OFC activation during reward anticipation has also been observed across all phases of bipolar disorder, including mania (Bermpohl et al., 2010), euthymia (Nusslock et al., 2012a), and depression (Chase et al., 2013). Collectively, these findings suggest that there may be a trait-like, and perhaps endophenotypic, abnormality in this region in individuals with bipolar disorder during reward processing. The lateral OFC is a complicated area of neuronal real estate, and the precise function of this region is still debated. Indeed, in many areas of cognitive neuroscience, BA 47 is discussed as being a part of the ventrolateral prefrontal cortex (vlPFC; Badre & Wagner, 2007) or the inferior frontal gyrus (Kensinger & Corkin, 2004), as opposed to the OFC. This region has been implicated in many cognitive and affective processes, including the regulation of affect (Wager, Davidson, Hughes, et al., 2008), the effect of emotion on memory (Murty, Ritchey, Adcock, et al., 2010), and the flexible control of task performance (Hampshire, & Owen, 2006). This region has also been observed to be dysfunctional in individuals with bipolar disorder and first degree relatives across a variety of non-reward-related fMRI paradigms (Pompei, Joiga, Tatarelli, et al., 2011; Robinson, Monkul, Tordesillas-Gutierrez, et al., 2008). As indicated above, the lateral OFC in the context of reward processing is implicated in arousal (Schmidt, et al., 2009) and salience (Lewis, et al., 2007), as opposed to positive hedonic evaluation. Thus, in line with the BAS/reward hypersensitivity model, this suggests that bipolar disorder is likely characterized by abnormalities in regulating arousal and behavioral activation during reward processing and goal pursuit. The fact that this profile of elevated left lateral OFC activation during reward processing is state-independent suggests that even when depressed bipolar individuals maintain an elevated cortical representation of reward. Furthermore, the left lateralization of this effect is consistent with neurophysiological data summarized above indicating that bipolar disorder is characterized by elevated relative left frontal EEG activity (Harmon-Jones et al., 2002, 2008; Nusslock et al., 2012b). Thus, at multiple levels of analysis, the left-lateral prefrontal cortex appears to play an important role in abnormalities in reward processing in bipolar disorder, suggesting this should be an important target for future research.
The fronto-striatal neural circuit operates as an integrated network to facilitate reward-related affect and approach-related behavior (see Haber & Knutson, 2010 for review). It will be important for future research on reward-related neural activation in bipolar disorder to move beyond examining neural regions in the fronto-striatal network in isolation of each other and to instead take a ‘connectivity perspective’. The advent of connectivity analyses allows researchers to examine whether neural regions are functionally related to each other and, using techniques such a dynamic causal modeling (DCM; Friston, Harrison, & Penny, 2003), the extent to which activation in one neural region modulates activity in another. Highlighting the potential value of these analyses, Trost and colleagues (2014) reported that bipolar I individuals had disturbed top-down control of subcortical reward signals by the prefrontal cortex during reward processing. Employing functional connectivity analyses will allow researchers to better determine whether the core abnormality in reward-processing in bipolar disorder involves too much ‘bottom-up’ activation in subcortical regions such as the ventral striatum to reward-relevant stimuli, deficits in top-down’ control of subcortical regions by the prefrontal cortex, or both.
Lastly, all of the studies on bipolar disorder that have employed fMRI reward paradigms have used monetary cues as the rewarding stimulus. This stimulus may be particularly effective for examining reward-related neural activation in bipolar disorder given individuals with bipolar disorder are characterized by elevated achievement motivation (Johnson & Carver, 2006) and achievement oriented cognitive styles (Alloy et al., 2009a). Future research should examine whether this profile of abnormally elevated fronto-striatal activation in bipolar disorder generalizes to primary and social reward cues.
Assessment Implications of Reward-Related Neural Activation in Bipolar Disorder
In the beginning of the present paper we highlighted the fact that close to 70% of individuals with bipolar disorder were initially misdiagnosed (Hirschfeld et al., 2003) and that it can take 6 to 10 years or longer for an individual with bipolar disorder to receive an accurate diagnosis and treatment (Ghaemi et al., 1999; 2000). Certain researchers have argued that biological markers could be used to compliment self-report based diagnostic strategies to facilitate accurate assessment and differential diagnosis (Almeida & Phillips, 2013). In this next section, we provide supporting evidence for the argument that elevated reward-related neural activation may reflect a unique biological marker of risk for bipolar disorder. We focus on the potential utility of reward-related neural activation in differentiating between bipolar disorder and unipolar depression, given that unipolar depression is the most frequent misdiagnosis of bipolar disorder. Indeed, approximately 60% of individuals with bipolar disorder were initially misdiagnosed as having unipolar depression (Hirschfeld et al., 2003. In considering reward-related neural activation as a possible biological marker of bipolar disorder, we also discuss some of the considerable challenges to using neuroscience techniques in real life clinical assessment.
Psychosocial, neurophysiological, and neuroimaging research indicate that unipolar depression is characterized by decreased reward-related affect, behavior, and neural activation. Collectively, this research suggests that risk for bipolar disorder and unipolar depression are characterized by distinct and opposite profiles of reward processing and reward-related neural activation. For example, at the psychosocial level of analysis, individuals with unipolar depression self-report decreased BAS sensitivity (Kasch, Rottenberg, Arnow, & Gotlib, 2002), experience dampened positive affect and reduced motivation (Forbes, 2009a), display a reduced hedonic capacity on behavioral probabilistic reward tasks (Pizzagalli, Iosifescu, Hallett, et al., 2008), and engage less frequently in goal-directed behavior (Forbes, 2009a). This profile is in stark contrast to the literature reviewed in the present paper indicating that bipolar disorder is associated with elevated self-reported BAS/reward sensitivity (Meyer, Johnson, & Winters, 2001), ambitious goal-setting (Gruber & Johnson, 2009; Johnson & Carver, 2006), and biased expectations of positive outcomes when considering goals (Johnson et al., 2008).
At the neurophysiological level, individuals with unipolar depression show decreased relative left frontal EEG activity (see Thibodeau, Jorgensen, & Kim, 2006 for meta-analytic review), reflecting reduced approach system sensitivity and blunted reward-related affect. Individuals with unipolar depression show decreased relative left frontal EEG activity during both depressive (Gotlib, Ranganath, & Rosenfeld, 1998; Henriques & Davidson, 1991) and euthymic states (Henriques & Davidson, 1990), suggesting that reduced left frontal activity may be a state-independent marker of unipolar depression. Decreased left frontal EEG activity has been observed in offspring of depressed individuals who have yet to experience a depressive episode (Dawson, Frey, Panagiotides, et al., 1997), is associated with genetic risk for unipolar depression (Smit, Posthuma, Boomsma, & De Geus, 2007), is predictive of treatment response for unipolar depression (Bruder, Stewart, Tenke, et al., 2001), and prospectively predicts onset of first unipolar depressive episode (Nusslock, Shackman, Coan, et al., 2011). Similar to psychosocial indices, this profile is in stark contrast to individuals with bipolar disorder who display elevated relative left frontal EEG activity (Harmon-Jones, et al., 2002; 2008; Nusslock et al., 2012b), reflecting a propensity towards increased approach-related affect and behavior. Additionally, preliminary research indicates that bipolar disorder and unipolar depression are characterized by distinct profiles of the feedback negativity (FN) ERP component during reward processing. As indicated, individuals with elevated scores on the Hypomanic Personality Scale (HPS; Eckblad & Chapman, 1986) display greater reward responsiveness, as indexed by the FN, compared to individuals with both moderate and low HPS scores (Mason et al., 2012). By contrast, unipolar depression is characterized by blunted reward responsiveness, as indexed by the FN (Foti & Hajcak, 2009), and a blunted FN prospectively predicts onset of a first major depressive episode (Bress, Foti, Kotov, et al., 2013).
Lastly, unipolar depression is associated with decreased ventral striatal activation during reward processing in functional neuroimaging studies [Heller, Johnstone, Shackman, et al., 2009; Forbes, Hariri, Martin, et al., 2009b; McCabe, Cowen, & Harmer, 2009; although see Knutson et al. (2008) for contradictory results]. OFC data are less consistent, with some studies reporting decreased (Osuch, Bluhm, Williamson, et al., 2009), and others increased (Smoski, Felder, Bizzell, et al., 2009) OFC activity to reward cues in depressed individuals. These discrepancies may be driven by developmental considerations (i.e., different profiles of reward processing in adolescents versus adults) and differences in how the OFC is anatomically defined across studies. There is compelling evidence that reduced ventral striatal responsivity to reward-cues in depression reflects anhedonia, or the decreased ability to experience pleasure from activities usually found enjoyable (Stoy, Schlagenhauf, Sterzer, et al., 2012; Wacker, Dillon, & Pizzagalli, 2009).
Thus, across psychosocial, neurophysiological, and neurobiological levels of analysis bipolar disorder and unipolar depression are characterized by distinct and opposite profiles of reward processing, with bipolar disorder being characterized by elevated reward sensitivity, and unipolar depression by blunted reward sensitivity. These findings have important implications for understanding the pathophysiology of bipolar disorder and unipolar depression. Both these disorders are characterized by elevated cortico-amygdala threat-related brain function (Hamilton, et al., 2012; Almeida et al., 2010; Etkin & Wager, 2007) and deficits in pre-frontal executive control (Wagner et al., 2006; Phillips & Vieta, 2007; Pacheco-Unguetti, et al., 2011). We argue that deficits in threat processing and executive control likely relate more to depressive symptoms that are common across bipolar disorder and unipolar depression, as well other transdiagnostic symptoms such as general distress. These mechanisms, however, may not be particularly informative in distinguishing what puts an individual at risk for bipolar disorder versus unipolar depression. We argue, however, that reward processing and fronto-striatal reward-related neural activation is highly relevant for understanding differential risk for bipolar disorder versus unipolar depression. Specifically, it is proposed that what differentiates risk for bipolar disorder versus unipolar depression is risk for mania, and one of the primary risk factors for mania involves abnormally elevated fronto-striatal reward-related neural activation. Thus, the fronto-striatal neural circuit is clearly important for understanding what distinguishes bipolar disorder from unipolar depression, whereas cortico-amygdala threat processing and executive control may be more informative in understanding what is common across these illnesses.
As discussed, profiles of reward-related neural activation may also reflect an important biological marker that could help facilitate the assessment of, and differential diagnosis between bipolar disorder and unipolar depression. Additionally, reward-related neural activation may serve as a useful biological marker for differentiating bipolar disorder from other psychiatric disorders such as schizophrenia and attention deficit hyperactivity disorder (ADHD), both of which have also been associated with blunted fronto-striatal neural activation (Grimm, Heinz, Walter, et al., 2014; Volkow, Wang, Kollins, et al., 2009). For a biological marker to be useful in the assessment and diagnosis of a psychiatric disorder, however, it should be state-independent and measureable during all phases of the illness. This is particularly important for bipolar disorder given that individuals with bipolar disorder typically present for treatment when depressed (Keck, Kessler, & Ross, 2008). As summarized, there is growing evidence that elevated left lateral OFC activation during reward processing is present across all phases of the illness, including bipolar depression (see Table 1). This suggests that even during depression, individuals with bipolar disorder maintain elevated cortical representation of reward. Thus, lateral left OFC activation during reward processing may reflect a particularly important candidate marker of bipolar risk status that has diagnostic value. Future research is needed to test this hypothesis.
It is important to emphasize, however, that there are significant challenges to using biological markers in a clinical setting for assessment and diagnostic purposes. The first is pragmatic. Neuroimaging is expensive and technically demanding. Thus, there are a limited number of settings in which it would be feasible to use neuroimaging in psychiatric assessment and most of these settings are larger metropolitan cities. One possible way to address this challenge would be to validate behavioral tasks or psyhophysiological indices as proxies of neuroimaging data. These more cost effective and user- friendly measures could then be disseminated into the clinical field. While this approach has its advantages, it also has its disadvantages, which center on whether behavioral or psychophysiological indices are reliable and precise indicators of neural activation. This is an important topic for future research.
The second challenge to using biological markers in a clinical setting relates to the precision with which neuroimaging data are able to classify individual patients and predict individual behavior. The majority of studies reviewed in the present paper involve mean level differences between groups in reward-related neural activation and do not characterize individual patients. Furthermore, if we were to plot the individual data points from these studies there would likely be considerable overlap in the distribution of reward-related neural activation across bipolar disorder, unipolar depressed, and healthy control participants. To add additional complexity, there is extraordinary variability in the symptom profile and course of bipolar disorder across individuals (Nusslock & Frank, 2011), which likely corresponds to individual variability in neural activation. Thus, it is unclear at this point in time what appreciable effect biological markers would have in improving clinical assessment and differential diagnosis. It is our view that for biological makers to be employed in clinical assessment it will be important to develop analytic strategies to characterize neural data at the level of the individual patient and classify individuals into clinically meaningful groups. An important step in this direction is the use of machine-learning-based pattern classification methods on MRI data. Machine learning is a data driven technique to find optimal biomarkers to classify individuals into clinically meaningful groups (Pereira, Mitchell, & Botvinick, 2009). This method has recently been applied to both structural and functional MRI data, successfully classifying between 81% and 92% of individuals with either schizophrenia or bipolar disorder into their appropriate diagnostic category (Davatzikos, et al., 2005; Costafreda et al., 2011). Preliminary research indicates that these accuracy levels may be even higher when incorporating genetic data (Yang, et al., 2010).This will be an important topic for future research to consider in more detail.
Treatment Implications of Reward-Related Neural Activation in Bipolar Disorder
Research on reward-related neural activation in bipolar disorder has important pharmacological and psychosocial treatment implications. With respect to pharmacology, dopamine neurotransmission is at the heart of the fronto-striatal reward circuit (Haber & Knutson, 2010; Schultz, 2002; Wise, 2002), as summarized above. Critically, there is converging data highlighting the central role of dopamine in bipolar disorder, and pharmacological models suggest a role of increased dopaminergic drive in mania and the converse in bipolar depression (see Berk, Dodd, Kauer-Sant’Anna, et al., 2007 for review). For example, administration of the dopamine precursor levdopa (L-DOPA) to patients with bipolar disorder produces hypomanic/manic episodes (van Praag & Korf, 1975). Similarly, amphetamine, which increases levels of synaptic dopamine, can produce a hypomanic-like state in otherwise healthy individuals (Leyton et al., 2002) and hyperactivity in an animal model of mania (Frey, Andreazza, Cereser, et al., 2006). Direct acting dopamine agonists such as bromocriptine and piribedil may also induce mania in some bipolar patients (Berk et al., 2007). By contrast, dopamine deficits play an important role in both bipolar depression and unipolar depression, and symptoms of depression can arise in the context of lowered dopamine transmission (Berk et al., 2007).
The central role of dopamine in reward-related neural activation and bipolar disorder suggests that dopamine neurotransmission may be an important pharmacological target for managing both manic and depressive symptoms. In line with this perspective, selective dopamine antagonists are effective in controlling the symptoms of mania (Tohen, Goldberg, Gonzalez-Pinto, et al., 2003a; Vieta, Ros, Goikolea, et al., 2005). For example, conventional neuroleptics such as haloperidol and chlorpromazine, which block dopamine receptors, are effective antimanic agents (Cipriani, Rendell, & Geddes, 2006) and strategies that deplete dopamine can reduce hypomanic/manic symptoms (Scarna, Gijsman, Mctavish, et al., 2003). There is preliminary evidence that lithium, perhaps the most effective mood stabilizer, decreases the amount of dopamine and its metabolites in unipolar and bipolar women (Tohen, Vieta, Calabrese, et al., 2003b). Furthermore, dopamine-blocking antipsychotics reduce reward-related neural activation in regions such as the ventral striatum (Abler, Erk, & Walter, 2007). By contrast, several agents that target dopaminergic activity have been demonstrated to be effective antidepressants. Buproprion, which inhibits catecholamine transporters including dopamine, is an effective treatment for bipolar depression (Post, et al., 2006; Sachs et al., 2007). Non-dopaminergic antidepressants of diverse mechanisms have also been shown to have effects on increasing dopamine release in many brain areas in animal models, and potentiate dopamine reward-related behaviors (Tanda, Carboni, Frau et al., 1994; D’Aquila, Collu, Gessa, et al., 2000). Lastly, in humans, electroconvulsive therapy, a highly effective treatment for depression, may involve an increase in dopamine receptor responsiveness (Ciapparelli et al., 2001).
Despite progress, the neurochemistry of dopamine is not completely understood. The dopaminergic system is complex and there is differential functioning depending on the neural region and which receptor is expressed. Future research is needed to more precisely modulate dopamine neurotransmission and develop more individualized pharmacological treatments. Importantly, recent evidence suggests that there are also differences in transcriptional neuroanatomy across different groups of dopamine neurons (Berk et al., 2007). Examining these differences in dopamine transcriptional neuroanatomy may be important for producing targeted, selective, and more effective therapeutic agents.
The BAS/reward hypersensitivity model and research on reward-related neural activation also have important implications for psychosocial interventions for bipolar disorder (Nusslock, Abramson, Harmon-Jones, et al., 2009). There are currently three psychosocial interventions for bipolar disorder that have shown promise as adjuncts to pharmacotherapy – Cognitive-Behavioral Therapy (CBT), Psychoeducational Interventions, and Interpersonal and Social Rhythm Therapy (IPSRT). Growing evidence highlights the efficacy of these interventions, as summarized in a meta-analysis that reported a significant reduction in relapse rates (~40%) for individuals with bipolar disorder engaged in these treatments (Scott, Colom, & Vieta, 2007). We next briefly review some of the possible implications of research on reward processing in bipolar disorder for each of these interventions.
CBT has been applied to bipolar disorder given research indicating that individuals with bipolar disorder exhibit underlying cognitive patterns as negative as those of individuals with unipolar depression and that cognitive styles predict the course of bipolar disorder, alone or in combination with both negative and positive life events (Alloy et al., 2006a; Alloy, Reilly-Harrington, Fresco, et al., 1999; Francis-Raniere, Alloy, & Abramson, 2006; Scott and Pope, 2003). The small number of studies conducted to date provide reasonable evidence that CBT has a positive prophylactic effect on bipolar disorder and improves psychosocial functioning and medication adherence (e.g., Scott, Garland, and Moorhead, 2001; Lam et al., 2000; Miklowitz et al., 2007). There is, however, research indicating that CBT is more effective in reducing days of depression over time than days manic (Zaretsky et al., 2008).
In line with the BAS/reward hypersensitivity model’s emphasis on drive and incentive motivation, there is growing evidence that the cognitive style of individuals with bipolar disorder is marked by ambitious goal-setting, autonomy, and perfectionism in the achievement domain (Alloy, Abramson, Walshaw et al., 2009a; Johnson, 2005; Lam, Wright, & Smith, 2004). Moreover, Alloy and colleagues (2009a) reported that only achievement oriented cognitive dimensions predicted the likelihood of onset of depressive and hypomanic/manic episodes among individuals with bipolar disorder over a 3 year follow-up period. This is in contrast to the dependency and attachment attitudes frequently observed among unipolar depressed individuals (for alternative findings, see Hammen, Ellicot, & Gitlin, 1992). Accordingly, we propose that an important contribution of research on reward processing in bipolar disorder for CBT is highlighting the potential value of targeting achievement and reward-oriented cognitive profiles in managing both the depressive and manic phases of bipolar disorder. Preliminary support for this claim comes from a study by Lam and colleagues (2003) that modified CBT for bipolar disorder to specifically target extreme goal striving attitudes (Lam, Watkins, Hayward, et al., 2003). The authors report that this variant of CBT not only reduced bipolar episodes and hospitalizations relative to a treatment as usual group, but also reduced highly driven and extreme goal-attainment beliefs that were viewed as a risk factor for future episodes. Adding nuance to these data, however, is a follow-up study by Lam and colleagues (2005) in which it was found that bipolar patients who perceive themselves to possess hypomanic personal attributes (e.g., dynamism, persuasiveness and productiveness) did less well in CBT (i.e., more relapses sooner) than a treatment as usual group. This suggests that CBT may need to be modified to be effective for subpopulations prone to particularly high levels of reward sensitivity. Future research is needed to more fully examine this issue.
Psychoeducation for bipolar disorder has been informed by the literature on psychoeducational treatments for schizophrenia (Miklowitz & Goldstein, 1997), and there is considerable evidence that psychoeducational interventions for bipolar disorder have a positive prophylactic effect for a number of clinically relevant variables (e.g., Miklowitz, et al., 2003; Miklowitz et al., 2000; Rea et al., 2003). There are several models of psychoeducational interventions for bipolar disorder, many of which are beyond the scope of the present paper. The two components of psychoeducation that we focus on involve helping individuals identify and manage both hypomanic/manic prodromal symptoms and life events that may trigger bipolar episodes. In medicine, prodromes are defined as early signs and symptoms that herald a full episode (Molnar, Feeney, & Fava, 1988). Two advantages in targeting prodromes in the treatment of bipolar disorder are (a) full-blown bipolar episodes may overwhelm the coping strategies of patients, where these strategies may be highly effective in managing prodromes of bipolar episodes; and (b) the prodromal period precedes the full bipolar syndrome (Smith & Tarrier, 1992), representing a window in which intervention might protect patients from relapse. Support for this claim are data indicating that teaching patients to recognize early symptoms of manic relapse and seek early treatment is associated with clinical improvements in time to first manic relapse, social functioning, and employment (Perry et al., 1999).
In line with the BAS/reward hypersensitivity model, growing evidence suggests that prodromes of manic episodes are characterized by extreme goal-setting and heightened expectations of success in the achievement domain. Indeed, increased goal-directed activity is one of the two most common behavioral prodromes of mania and has been independently associated with increased rates of manic episodes (Lam et al., 2001). Accordingly, certain clinicians have begun to take a BAS/reward perspective and are giving particular attention to the importance of helping patients understand the relationship between ambitious goal-setting and the onset of manic episodes (Lam et al., 2003). These clinicians argue that the therapist and patient can develop a treatment plan in which surges of reward sensitivity, ambitious goal-setting, and confidence are identified and challenged during the prodromal period, and behavioral deactivation strategies can be employed to manage mood and motivation (Lam and Wong, 1997). We argue that psychoeducation for bipolar disorder may benefit from the perspective taken by Lam and colleagues of giving particularly close attention to elevated reward sensitivity, ambitious goal-setting, and increased goal-directed activity during the prodromal period.
Another component of certain psychoeducational models is to educate clients and their family members on the important role that life events and stressors play in the course of bipolar disorder and to identify triggers of bipolar episodes (Morris, Miklowitz, & Waxmonsky, 2007). To date, however, much of the research on life events and bipolar disorder has been atheoretical, with many studies relying on global classifications of events such as negative events, stressful events, and severe events. An advantage of the BAS/reward hypersensitivity model is that it makes specific predictions about the types of life events relevant to the onset of bipolar episodes, particularly hypo/manic episodes. In line with this model, research indicates that both goal-striving (Nusslock, Abramson, Harmon-Jones, et al., 2007) and goal-attainment (Johnson, et al., 2000) life events precipitate hypomanic/manic episodes among individuals with bipolar disorder. By contrast, positive events that do not involve the pursuit or attainment of a valued reward are not associated with an increase in manic symptoms (Johnson, et al., 2000). Thus, research on reward processing in bipolar disorder may be helpful to both psychoeducation clinicians and clients in generating a better understanding of the role that reward-relevant life events play in the course of bipolar disorder. We of course are not suggesting that individuals with bipolar disorder refrain from pursuing or attaining rewards or goals. We are suggesting, however, that it may be beneficial for individuals with bipolar disorder to enter into these positive life events with an appreciation of the importance of monitoring their affect and behavior to minimize the risk of hypomanic/manic symptoms.
The Social Rhythm or Zeitgeber Theory (Ehlers, Kupfer, Frank, & Monk, 1993; Ehlers, Frank, & Kupfer, 1988) postulates that life events that disrupt daily rhythms or schedules will be especially likely to precipitate bipolar symptoms and episodes. This theory suggests that individuals with bipolar disorder have a predisposition to circadian rhythm and sleep-wake cycle abnormalities that may be responsible, in part, for the onset of hypomanic/manic and depressive symptoms. Based on this work, Frank et al. (1997; 1999) developed IPSRT. This therapy integrates interpersonal psychotherapy for unipolar depression (Klerman, Weissman, Rounsaville, & Chevron, 1984) with behavioral and environmental strategies to help stabilize irregularities of the sleep-wake cycle among bipolar individuals and manage bipolar symptoms. We and others (Grandin, Alloy, & Abramson, 2006; Levenson, Nusslock, Frank, 2013) propose that research on reward processing in bipolar individuals is highly relevant to both the Social Rhythm Theory and IPSRT. As discussed in the present paper, individuals with elevated reward-sensitivity and reward-related neural activation are at risk for experiencing an excessive increase in approach-related affect and motivation in the presence of rewarding life events. This excessive increase in approach-related affect is frequently reflected in excessive goal-striving behaviors which are incongruent with maintaining regular schedules/social rhythms. Thus, individuals with elevated reward sensitivity may work excessively long hours and neglect normal social routines, which, in turn may disrupt circadian rhythms and trigger mood symptoms (Nusslock et al., 2009). Consistent with this perspective, Boland and colleagues (2014) reported that individuals with elevated reward sensitivity experienced more social rhythm disruption following reward-relevant life events. Furthermore, greater social rhythm disruption during these rewarding events prospectively predicted increases in depressive and hypomanic symptoms, as well as first onset of a bipolar spectrum diagnosis. Accordingly, we propose that it may be helpful for the IPSRT therapist to be particularly sensitive to the disruptive effects that elevated reward sensitivity and goal-pursuit have on social and circadian rhythms and to help their clients develop behavioral and environmental strategies to maintain social/circadian regularity in the midst of reward-relevant life events. Many of the existing IPSRT strategies designed to address the effect of interpersonal events on circadian/social rhythms could be applied to reward or goal-relevant events. For example, the interpersonal inventory used in IPSRT is designed to identify and assess the major relationships in an individual’s life. This logic could be extended to the development of a reward or goal-relevant inventory in which the therapist and client identify the meaning and value of relevant goals. Using the Social Rhythm Metric (SRM; Monk, Kupfer, Frank, & Ritenour, 1991), a self-report instrument designed to quantify daily rhythms, the therapist and client could then examine the extent to which these reward-relevant events are likely to induce social/circadian rhythm disruption. Finally, goal-pursuit strategies could be developed to maximize that chances of reward attainment while minimizing social and circadian rhythm disruption.
Concluding Remarks
A considerable challenge in the management and treatment of bipolar disorder is the fact that it can take upwards of ten years for an individual with bipolar disorder to receive an accurate diagnosis (Ghaemi, et al., 2000; Ghaemi, et al., 1999), and over 60% of bipolar individuals report being initially misdiagnosed as having unipolar depression (Hirschfeld et al., 2003). Certain researchers have argued that biological markers could be used to compliment self-report based diagnostic strategies to facilitate accurate assessment and differential diagnosis (Almeida & Phillips, 2013). As outlined in the present paper, bipolar disorder and unipolar depression are characterized by distinct and opposite profiles of reward-related neural activation in the fronto-striatal circuit, with bipolar disorder being characterized by abnormally elevated reward-related neural activation, and unipolar depression by reduced or blunted activation. Accordingly, we propose that profiles of reward-related neural activation may reflect an important biological marker of differential risk for bipolar disorder versus unipolar depression. However, as also discussed, there are considerable challenges to using biological markers in a clinical setting for assessment and diagnostic purposes. These challenges involve the expense and technical demands of neuroimaging, as well as the poor precision with which neuroimaging data are able to classify and predict individual behavior. It will be important for researchers and clinicians to directly address these challenges if neuroscience techniques are to eventually facilitate diagnostic assessment. Lastly, research on reward-related neural activation in bipolar disorder has important pharmacological and psychosocial treatment implications.
Highlights.
We review research on reward-related neural activation in bipolar disorder
We suggest reward-related neural activation is a biological marker of bipolar
We discuss challenges to using neuroscience techniques in assessment and diagnosis
We discuss treatment implications of reward-related neural activation in bipolar
Acknowledgements
Robin Nusslock’s contribution to this work was supported by National Institute of Mental Health (NIMH) grant R01 MH100117-01 and R01 MH077908-01A1, as well as a Young Investigator Grant from the Ryan Licht Sang Bipolar Foundation and the Chauncey and Marion D. McCormick Family Foundation. Christina Young’s contribution to this work was supported by a National Science Foundation (NSF) Graduate Research Fellowship.
Footnotes
Future work is needed to examine the extent to which the BAS/reward hypersensitivity model and research on the fronto-striatal neural circuit in bipolar disorder can account for mixed episodes. Many individuals with bipolar disorder (up to 40% in some clinical samples; Swann et al., 2013) present with mixed symptoms, and these are even more common in individuals with early-onset bipolar disorder (Perlis et al., 2009). Understanding the pathophysiology of mixed states has important scientific, diagnostic, and treatment implications. We propose that mixed states may involve the co-activation of both the fronto-striatal neural circuit, as reflected in excessive approach-related affect, and the cortico-amygdala circuit, as reflected in excessive negative affect. Future research is needed to test this hypothesis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abler B, Erk S, & Walter H (2007). Human reward system activation is modulated by a single dose of olanzapine in healthy subjects in an event-related, double-blind, placebo-controlled fMRI study. Psychopharmacology, 191, 823–833. [DOI] [PubMed] [Google Scholar]
- Abler B, Greenhouse I, Ongur D, Walter H, & Heckers S (2008). Abnormal reward system activation in mania. Neuropsychopharmacology, 33, 2217–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JDE (2006). Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron, 50, 507–517. [DOI] [PubMed] [Google Scholar]
- Alloy LB, & Abramson LY (2010). The role of the Behavioral Approach System (BAS) in bipolar spectrum disorders. Current Directions in Psychological Science, 19, 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Grandin LD, Hughes ME, Iacoviello BM, Whitehouse WG, Urosevic S, Nusslock R, & Hogan ME (2008). Behavioral approach system (BAS) and behavioral Inhibition system (BIS) sensitivities and bipolar spectrum disorders: Prospective prediction of bipolar mood episodes. Bipolar Disorders, 10, 310–322. [DOI] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Neeren AM, Walshaw PD, Urosevic S, & Nusslock R (2006a). Psychosocial risk factors for bipolar disorder: Current and early environment and cognitive styles In Jones S & Bentall R (Eds), The psychology of bipolar disorder –New developments and research strategies (pp. 11–46). Oxford: Oxford University Press. [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Smith J, Hughes M, Iacoviello BM, Gerstein RK, Keyser J, Urosevic S, & Nusslock R (2006b). Behavioral Approach System (BAS) sensitivity and bipolar spectrum disorders: A retrospective and concurrent behavioral high-risk design. Motivation and Emotion, 30, 143–155. [Google Scholar]
- Alloy LB, Abramson LY, Walshaw PD, Gerstein RK, Keyser JD, Whitehouse WG, Urosevic S, Nusslock R, Hogan ME, & Harmon-Jones E (2009a). Behavioral approach system (BAS) – relevant cognitive styles and bipolar spectrum disorders: Concurrent and prospective associations. Journal of Abnormal Psychology, 118, 459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Abramson LY, Whitehouse WG, Sylvia LG, Hafner J, Bender RE, et al. (2009b). Prospective prediction of hypomanic and depressive symptoms by Behavioral Approach System (BAS) activation-relevant and deactivation relevant life events. Unpublished manuscript. [Google Scholar]
- Alloy LB, Urosevic S, Abramson LY, Jager-Hyman S, Nusslock R, Whitehouse WG, & Hogan ME (2012a). Progression along the bipolar spectrum: A longitudinal study of predictors of conversion from bipolar spectrum conditions to bipolar I and II disorders. Journal of Abnormal Psychology, 121, 16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Bender RE, Whitehouse WG, Wagner CA, Liu RT, Grant DA, Jager-Hyman S, Molz A, Choi JY, Harmon-Jones E, & Abramson LY (2012b). High Behavioral Approach System (BAS) sensitivity, reward responsiveness, and goal-striving predict first onset of bipolar spectrum disorders: A prospective behavioral high-risk design. Journal of Abnormal Psychology, 121, 339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Reilly-Harrington NA, Fresco DM, Whitehouse WG, & Zechmeister JS (1999). Cognitive styles and life events in subsyndromal unipolar and bipolar mood disorders: Stability and prospective prediction of depressive and hypomanic mood swings. Journal of Cognitive Psychotherapy: An International Quarterly, 13, 235–240. [Google Scholar]
- Almeida JRC & Phillips ML (2013). Distinguishing between unipolar depression and bipolar depression: Current and future clinical and neuroimaging perspectives. Biological Psychiatry, 73, 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida JRC, Versace A, Hassel S, Kupfer DJC, & Phillips ML (2010). Elevated amygdala activity sad facial expressions: A state marker of bipolar but not unipolar depression. Biological Psychiatry, 67, 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler L, Bookheimer S, Proenza MA, Townsend J, Sabb F, Firestine A, Bartzokis G, Mintz J, Mazziotta J, Cohen MS (2005a). Increased amygdala activation during mania: A functional magnetic resonance imaging study. American Journal of Psychiatry, 162, 1211–1213. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Bookheimer SY, Townsend J, Proenza MA, Eisenberger N, Sabb F, Mintz J, Cohen MS (2005b). Blunted activation in orbitofrontal cortex during mania: A functional magnetic resonance imaging study. Biological Psychiatry, 58,763–769. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Post RM, Leverich GS, Mikalauskas K, Rosoff A, Ackerman L. (1995). Antidepressant-induced mania and cycle acceleration: a controversy revisited. American Journal of Psychiatry, 152, 1130–1138. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Association Press. [Google Scholar]
- Angst F, Stassen HH, Clayton PJ, & Angst J (2002). Mortality of patients with mood disorders: Follow-up over 34–38 years. Journal of Affective Disorders, 68, 167–181. [DOI] [PubMed] [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL (2005). Reward, motivation,and emotion systems associated with early-stage intense romantic love. Journal of Neurophysiology, 94, 327–337. [DOI] [PubMed] [Google Scholar]
- Ayuso-Mateos JL. Global Burden of Bipolar Disorder in the Year 2000: Draft 21–06-06. http://wwwwhoint/healthinfo/statistics/bod_bipolarpdf. 2006.
- Badre D, & Wagner AD (2007). Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia, 45, 2883–2901. [DOI] [PubMed] [Google Scholar]
- Balldin J, Granerus AK, Lindstedt G, Modigh K, Walinder J. (1982). Neuroendocrine evidence for increased responsiveness of dopamine receptors in humans following electroconvulsive therapy. Psychopharmacology, 76, 371–376. [DOI] [PubMed] [Google Scholar]
- Berk M, Dodd S, Kauer-Saint’Anna M, Malhi GS, Bourin M, Kapczinski F, & Norman T (2007). Dopamine dysregulation syndrome: Implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatrica Scandinavica, 116, 41–49. [DOI] [PubMed] [Google Scholar]
- Bermpohl. F, Dalaney U, Kahnt T, Sajonz B, Heimann H, Ricken R, Stoy M, Hagele C, Schlagenhauf F, Adli M, Wrase J, Strohle A, Heinz A, & Bauer M (2009). A preliminary study of increased amygdala activation to positive affective stimuli in mania. Bipolar Disorders, 11, 70–75. [DOI] [PubMed] [Google Scholar]
- Bermpohl F, Kahnt T, Dalanay U et al. (2010). Altered representation of expected value in the orbitofrontal cortex in mania. Human Brain Mapping, 31, 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC (2007). The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology, 191, 391–431. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, & Aldridge JW (2009). Dissecting components of reward: ‘Liking’, ‘wanting’, and learning. Current Opinion in Pharmacology, 9, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Goldstein B, Strober M, Gil MK, Hunt J, et al. (2009). Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: The Course and Outcome of Bipolar Youth (COBY) study. American Journal of Psychiatry, 166, 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AJ & Zatorre RJ (2001). Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proceedings of the National Academy of Science, 98, 11818–11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg HP, Stern E, Martinez D, Ricketts S, Asis J, White T, Epstein J, McBride PA, Eidelberg D, Kocsis JH, & Silbersweig DA (2000). Increased anterior cingulate and caudate activity in bipolar mania. Biological Psychiatry, 48, 1045–1052. [DOI] [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M et al. (2003). Alcohol promotes dopamine release in the human nucleus accumbens. Synapse, 49, 226–231. [DOI] [PubMed] [Google Scholar]
- Boland EM, Bender RE, LaBelle DR, Abramson LY, & Alloy LB (2014). Event-specific social rhythm disruption from BAS-relevant life events predicts depressive and hypomanic symptoms and episodes in a biobehavioral high-risk sample. Manuscript under editorial review.
- Bress JN, Foti D, Kotov R, Klein DN, & Hajcak G (2013). Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology, 50, 74–81. [DOI] [PubMed] [Google Scholar]
- Brooks JO, Wang PW, Bonner JC, Rosen AC, Hoblyn JC, Hill SJ, & Ketter. TA (2009). Decreased prefrontal, anterior cingulate, insula, and ventral striatal metabolism in medication-free depressed outpatients with bipolar disorder. Journal of Psychiatric Research, 43, 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Stewart JW, Tenke CE, McGrath PJ, Leite P, et al. (2001) Electroencephalographic and perceptual asymmetry differences between responders and nonresponders to an SSRI antidepressant. Biological Psychiatry, 49, 416–425. [DOI] [PubMed] [Google Scholar]
- Bush G, Voght BA, Holmes J, Dale. AM, Greve D, Jenike MA, & Rosen BR (2002). Dorsal anterior cingulate cortex: A role in reward-based decision making. Proceedings of the National Academy of Sciences, 99, 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese JR, Muzina DJ, Kemp DE et al. (2006). Predictors of bipolar disorder risk among patients currently treated for major depression. Medscape General Medicine, 8, 38. [PMC free article] [PubMed] [Google Scholar]
- Carver C (2004). Negative affect deriving from the Behavioral Approach System. Emotion, 4, 3–22. [DOI] [PubMed] [Google Scholar]
- Carver CS, & Harmon-Jones E (2009). Anger is an approach-related affect: Evidence and implications. Psychological Bulletin, 135, 183–204. [DOI] [PubMed] [Google Scholar]
- Carver CS, & White TL (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology, 67, 319–333. [Google Scholar]
- Caseras X, Lawrence NS, Murphy K, Wise RG, & Phillips ML (2013). Ventral striatum activity in response to reward: Differences between bipolar I and bipolar II disorders. American Journal of Psychiatry, 170, 533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase H, Nusslock R, Almeida JRC, Forbes EE, LaBarbara EJ, Phillips ML (2013). Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar Disorders, 15, 839–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Dilsaver SC. (1996). Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other Axis I disorders. Biological Psychiatry, 39, 896–899. [DOI] [PubMed] [Google Scholar]
- Ciapparelli A, Dell’Osso L, Tundo A, Pini S, Chiavacci MC, Di Sacco I, & Cassano GB (2001). Electroconvulsive therapy in medication-nonresponsive patients with mixed mania and bipolar depression. Journal of Clinical Psychiatry, 62, 552–555. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Rendell JM, Geddes JR. (2006). Haloperidol alone or in combination for acute mania. Cochrane Database of Systematic Reviews, 3, CD004362. [DOI] [PubMed] [Google Scholar]
- Coan JA, & Allen JJB (2004). Frontal EEG asymmetry as amoderator and mediator of emotion. Biological Psychology, 67, 7–49. [DOI] [PubMed] [Google Scholar]
- Cohen MX and Ranganath C (2007). Reinforcement learning signals predict future decisions. The Journal of Neuroscience, 27, 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda SG, Fu CH, Picchioni M, Toulopoulou T, McDonald C, Kravariti E, Walshe M, Prata D, Murray RM, & McGuire PK (2011). Pattern of neural responses to verbal fluency shows diagnostic specificity for schizophrenia and bipolar disorder. BMC Psychiatry, 11, 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox SM, Benkelfat C, Dagher A, Delaney JS, Durand F, McKenzie SA. et al. (2009). Striatal dopamine responses to intranasal cocaine self-administration in humans. Biological Psychiatry, 65, 846–850. [DOI] [PubMed] [Google Scholar]
- D’Aquila PS, Collu M, Gessa GL, & Serra G. (2000). The role of dopamine in the mechanism of action of antidepressant drugs. European Journal of Pharmacology, 405, 65–373. [DOI] [PubMed] [Google Scholar]
- Davatzikos C, Shen D, Gur RC, Wu X, Liu D, Fan Y, et al. (2005). Wholebrain morphometric study of schizophrenia revealing a spatially complex set of focal abnormalities. Archives of General Psychiatry, 62, 1218–1227. [DOI] [PubMed] [Google Scholar]
- Dawson G, Frey K, Panagiotides H, Osterling J, & Hessl D (1997). Infants of depressed mothers exhibit atypical frontal brain activity: A replication and extension of previous findings. Journal of Child Psychology and Psychiatry, 38, 179–186. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA (2000). Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology, 84, 3072–3077. [DOI] [PubMed] [Google Scholar]
- Depue RA, & Collins PF (1999). Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences, 22, 491–517. [DOI] [PubMed] [Google Scholar]
- Depue RA, Krauss SP, & Spoont MR (1987). A two-dimensional threshold model of seasonal bipolar affective disorder In Maggnusson D & Ohman A (Eds.), Psychopathology: An interactional perspective (pp. 95–123). New York: Academic Press. [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA. et al. (2001). Amphetamine-induced dopamine release in human ventral striatum correlateswith euphoria. Biological Psychiatry, 49, 81–96. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Videen TO, Todd RD, Reich T, Vannier ME (1995). Metabolic abnormalities in the subgenual prefrontal cortex and ventral striatum in mood disorders. Astr Soc Neurosci, 21, 260. [Google Scholar]
- Eckblad M, & Chapman L.J. (1986) Development and validation of a scale for hypomanic personality. Journal of Abnormal Psychology, 95, 214–222. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Frank E, & Kupfer DJ (1988). Social zeitgebers and biological rhythms: A unified approach to understanding the etiology of depression. Archives of General Psychiatry, 45, 948–952. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Kupfer DJ, Frank E, & Monk TH (1993). Biological rhythms and depression: The role of zeitgebers and zeitstorers. Depression, 1, 285–293. [Google Scholar]
- Eisner L, Johnson SL, & Carver CS (2008). Cognitive responses to failure and success relate uniquely to bipolar depression versus mania. Journal of Abnormal Psychology, 117, 154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Ogilvie A, Rubinsztein JS, Calderon G, Dolan RJ, Sahakian BJ (2004): Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biological Psychiatry 55, 1163–1170. [DOI] [PubMed] [Google Scholar]
- Etkin A & Wager TD (2007). Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social znxiety disorder, and specific phobia. American Journal of Psychiatry, 164, 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE (2009a). Where’s the fun in that? Broadening the focus on reward function in depression. Biological Psychiatry, 66, 199–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, et al. (2009b). Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. The American Journal of Psychiatry, 166, 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, & Hajcak G (2009). Depression and reduced sensitivity to non-rewards versus rewards: Evidence from event-related potentials. Biological Psychology, 81, 1–8. [DOI] [PubMed] [Google Scholar]
- Francis-Raniere E, Alloy LB, & Abramson LY (2006). Depressive personality styles and bipolar spectrum disorders: Prospective tests of the event congruency hypothesis. Bipolar Disorders, 8, 382–399. [DOI] [PubMed] [Google Scholar]
- Frank E (1999). Interpersonal and social rhythm therapy prevents depressive symptomatology in bipolar I patients. Bipolar Disorders, 1(Suppl. 1), 13.11256649 [Google Scholar]
- Frank E, Hlastala S, Ritenour A, Houck P, Tu XM, Monk TH, et al. (1997). Inducing lifestyle regularity in recovering bipolar disorder patients: Results from the maintenance therapies of bipolar disorder protocol. Biological Psychiatry, 41, 1165–1173. [DOI] [PubMed] [Google Scholar]
- Frey BN, Andreazza AC, Cereser KM et al. (2006). Effects of mood stabilizers on hippocampus BDNF levels in an animal model of mania. Life Science, 79, 281–286. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, & Penny W (2003). Dynamic causal modeling. Neuroimage, 19, 1273–1302. [DOI] [PubMed] [Google Scholar]
- Ghaemi SN, Boiman EE, Goodwin FK. (2000). Diagnosing bipolar disorder and the effect of antidepressants: a naturalistic study. Journal of Clinical Psychiatry, 61, 804–808. [DOI] [PubMed] [Google Scholar]
- Ghaemi SN, Hsu DJ, Soldani F, Goodwin FK. (2003). Antidepressants in bipolar disorder: the case for caution. Bipolar Disorders, 5, 421–433. [DOI] [PubMed] [Google Scholar]
- Ghaemi SN, Sachs GS, Chiou AM, Pandurangi AK, Goodwin K. (1999). Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? Journal of Affective Disorders, 52, 135–144. [DOI] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression, 2nd edn. New York: Oxford University Press, 2007. [Google Scholar]
- Gotlib IH, Ranganath C, & Rosenfeld JP (1998). Frontal EEG asymmetry, depression, and cognitive functioning. Cognition & Emotion, 12, 449–478. [Google Scholar]
- Grandin LD, Alloy LB, & Abramson LY (2006). The social zeitgeber theory, circadian rhythms, and mood disorders: Review and evaluation. Clinical Psychology Review, 26, 679–694. [DOI] [PubMed] [Google Scholar]
- Grimm O, Heinz A, Walter H, Kirschm P, Erk S, Haddad L, et al. (2014). Striatal response to reward anticipation: Evidence for a systems-level intermediate phenotype for schizophrenia. Journal of American Medical Association Psychiatry, Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Gruber J, & Johnson SL (2009). Positive emotional traits and ambitious goals among people at risk for mania: The need for specificity. International Journal of Cognitive Therapy, 2, 176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, & Knutson B. (2010). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology, 35, 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn T, Dresler T, Ehlis AC, Plichta MM, Heinzel S, Polak T, Lesch KP, Breuer F, Jakob PM, Fallgatter AJ. (2009). Neural response to reward anticipation is modulated by Gray’s impulsivity. Neuroimage, 46, 1148–1153. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Holroyd CB, & Simons RF (2007). It’s worse than you thought: The feedback negativity and violations of reward prediction in gambling tasks. Psychophysiology, 44, 905–912. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Etkin A, Furman D, Lemus M, Johnson RF, & Gotlib IH (2012). Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. American Journal of Psychiatry 169, 693–703. [DOI] [PubMed] [Google Scholar]
- Hammen C, Ellicott A, & Gitlin M (1992). Stressors and sociotropy / autonomy: A longitudinal study of their relationship to the course of bipolar disorder. Cognitive Therapy and Research, 16, 409–418. [Google Scholar]
- Hampshire A, & Owen AM. (2006). Fractionating attentional control using event-related fMRI. Cerebral Cortex, 16, 1679–1689. [DOI] [PubMed] [Google Scholar]
- Hamptom AN, & O’Doherty JP (2007). Decoding the neural substrates of reward-related decision making with functional MRI. Proceedings of the National Academy of Sciences, 104, 1377–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M, Hoaki N, Tero T, Takeshi T, Hatano K, Kohno K, Araki Y, Mizokami Y, Kodama K, Toyokawa K, Izumi T, Arasaki M, Shimomura T, Fujiki M, & Kochiyama T (2013). Hyperthymic temperament and brightness judgment in healthy subjects: Involvement of left inferior orbitofrontal cortex. Journal of Affective Disorders, 151, 143–148. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E (2003). Clarifying the emotive functions of asymmetrical frontal cortical activity. Psychophysiology, 40, 838–848. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Abramson LY, Nusslock R, Sigelman JD, Urosevic S, Turonie L, Alloy LB, & Fearn M (2008). Effect of bipolar disorder on left frontal cortical responses to goals differing in valence and task difficulty. Biological Psychiatry, 63, 693–698. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Abramson LY, Sigelman J, Bohlig A, Hogan ME, & Harmon-Jones C (2002). Proneness to hypomania/mania symptoms or depression symptoms and asymmetrical frontal cortical responses to an anger-evoking event. Journal of Personality and Social Psychology, 82, 610–618. [PubMed] [Google Scholar]
- Harmon-Jones E, & Allen JJ (1997). Behavioral activation sensitivity and resting frontal EEG asymmetry: Covariation of putative indicators related to risk for mood disorders. Journal of Abnormal Psychology,106, 159–163. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, & Sigelman JD (2001). State anger and prefrontal brain activity: Evidence that insult-related relative left prefrontal activity is associated with experienced anger and aggression. Journal of Personality and Social Psychology, 80, 797–803. [PubMed] [Google Scholar]
- Hassel S, Almeida JR, Kerr N et al. (2008). Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disorders, 10, 916–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller Johnstone, Shackman Light, S.N., Peterson MJ, Kolden GG, Kalin NH, & Davidson RJ (2009). Reduced capacity to sustain positive emotion in major depression reflects dimished maintenance of fronto-striatal brain activation. Proceedings of the National Academy of Sciences, 106, 22445–22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques JB, & Davidson RJ (1990). Regional brain electrical asymmetries discriminate between previously depressed and healthy controls. Journal of Abnormal Psychology, 99, 22–31. [DOI] [PubMed] [Google Scholar]
- Henriques JB, & Davidson RJ (1991). Left frontal hypoactivation in depression. Journal of Abnormal Psychology, 100, 535–545. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RM, Lewis L, Vornik LA. (2003). Perceptions and impact of bipolar disorder: how far have we really come? Results of the national depressive and manic-depressive association 2000 survey of individuals with bipolar disorder. Journal of Clinical Psychiatry, 64, 161–174. [PubMed] [Google Scholar]
- Holroyd CB and Coles MG (2002). The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review, 109, 679–709. [DOI] [PubMed] [Google Scholar]
- Isometsa ET. (1993). Course, outcome, and suicide risk in bipolar disorder: a review. Psychiatric Fennica, 24, 113–124. [Google Scholar]
- Johnson SL (2005). Mania and dysregulation in goal pursuit: A review. Clinical Psychology Review, 25, 241–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, & Carver CS (2006). Extreme goal setting and vulnerability to mania among undiagnosed young adults. Cognitive Therapy and Research, 30, 377–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Edge MD, Holmes MK, & Carver CS (2012a). The behavioral activation system and mania. The Annual Review of Clinical Psychology, 8, 243–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Carver CS, & Gotlib IH (2012b). Elevated ambitions for fame among persons diagnosed with bipolar I disorder. Journal of Abnormal Psychology, 121, 602–609. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Cuellar AK, Ruggero C, Winett-Perlman C, Goodnick P, White R, et al. (2008). Life events as predictors of mania and depression in bipolar 1 disorder. Journal of Abnormal Psychology, 117, 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Ruggero C, & Carver CS (2005). Cognitive, behavioral and affective responses to reward: Links with hypomanic vulnerability. Journal of Social and Clinical Psychology, 24, 894–906. [Google Scholar]
- Johnson SL, Sandrow D, Meyer B, Winters R, Miller I, Solomon D, & Keitner G (2000). Increases in manic symptoms after life events involving goal attainment. Journal of Abnormal Psychology, 109, 721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasch KL, Rottenberg J, Arnow BA, & Gotlib IH (2002). Behavioral activation and inhibition systems and the severity and course of depression. Journal of Abnormal Psychology, 111, 589–597. [DOI] [PubMed] [Google Scholar]
- Keck PE Jr, Kessler RC, Ross R. (2008). Clinical and economic effects of unrecognized or inadequately treated bipolar disorder. Journal of Psychiatric Practice, 14, 31–38. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. (2004). Two routes to emotional memory: distinct neural processes for valence and arousal. Proceedings of the National Academy of Science, 101, 3310–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketter TA, Kimbrell TA, George MS, Dunn RT, Speer AM, Benson BE, et al. (2001). Effects of mood and subtype on cerebral glucose metabolism in treatment-resistant bipolar disorder. Biological Psychiatry, 49, 97–109. [DOI] [PubMed] [Google Scholar]
- Klerman GL, Weissman MM, Rounsaville BJ, & Chevron ES (1984). Interpersonal psychotherapy of depression. New York: Basic Books. [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D (2001). Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience, :RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B Bhanji JP, Cooney RE, Atlas LY, & Gotlib IH (2008). Neural response to monetary incentives in major depression. Biological Psychiatry, 63, 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G (2005). Distributed neural representation of expected value. Journal of Neuroscience, 25, 4806–4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D (2000). FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage, 12, 20–27. [DOI] [PubMed] [Google Scholar]
- Kochman FJ, Hantouche EG, Ferrari P, Lancrenon S, Bayart D, & Akiskal HS (2005). Cyclothymic temperament as a prospective predictor of bipolarity and suicidality in children and adolescents with major depressive disorder. Journal of Affective Disorders, 85, 181–189. [DOI] [PubMed] [Google Scholar]
- Kringelbach M & Berridge KC (2009). Towards a functional neuroanatomy of pleasure and happiness. Trends in Cognitive Science, 13, 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, & Rolls ET (2004). The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology, 72, 341–372. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ, Frank E, Grochocinski VJ, Cluss PA, Houck PR, Stapf DA. (2002). Demographic and clinical characteristics of individuals in a bipolar disorder case registry. Journal of Clinical Psychiatry,63, 120–125. [DOI] [PubMed] [Google Scholar]
- Kwapil TR, Miller MB, Zinser MC, Chapman LJ, Chapman J, & Eckblad M (2000). A longitudinal study of high scorers on the Hypomanic Personality Scale. Journal of Abnormal Psychology, 109, 222–226. [PubMed] [Google Scholar]
- Lam DH, Bright J, Jones S, Hayward P, Schuck N,Chisholm D, et al. (2000). Cognitive therapy for bipolar illness: A pilot study of relapse prevention. Cognitive Therapy and Research, 24, 503–520. [Google Scholar]
- Lam DH, Watkins ER, Hayward P, Bright J, Wright K, Kerr N, et al. (2003). A randomized controlled study of cognitive therapy for relapse prevention for bipolar affective disorder: Outcome of the first year. Archives of General Psychiatry, 60, 145–152. [DOI] [PubMed] [Google Scholar]
- Lam D, & Wong G (1997). Prodromes, coping strategies, insight, and social functioning in bipolar affective disorders. Psychological Medicine, 27, 1091–1100. [DOI] [PubMed] [Google Scholar]
- Lam D, Wong G, & Sham P (2001). Prodromes, coping strategies and course of illness in bipolar affective disorder–A naturalistic study. Psychological Medicine, 31, 1387–1402. [DOI] [PubMed] [Google Scholar]
- Lam D, Wright K, & Sham P (2005). Sense of hyper-positive self and response to cognitive therapy in bipolar disorder. Psychological Medicine, 35, 69–77. [DOI] [PubMed] [Google Scholar]
- Lam D, Wright K, & Smith N (2004). Dysfunctional assumptions in bipolar disorder. Journal of Affective Disorders, 79, 193–199. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S et al. (2004). Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguished patients with bipolar disorder and major depression. Biological Psychiatry, 55, 578–587. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Rich BA, Vinton DT, Nelson EE, Fromm SJ, Berghorst LH, Joshi P, Robb A, Schachar RJ, Dickstein DP, McClure EB, Pine DS (2007): Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. American Journal of Psychiatry, 164, 52–60. [DOI] [PubMed] [Google Scholar]
- Levenson JC, Nusslock R, Frank E (2013). Life events, sleep disturbances, and mania: An integrated model. Clincial Psychology: Science and Practice. 195–210. [Google Scholar]
- Lewis PA, Critchley HD, Rothstein P, Dolan RJ. (2007). Neural correlates of processing valence and arousal in affective words. Cerebral Cortex, 17, 742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, & Dagher A (2002). Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: A PET [11C] raclopride study in healthy men. Neuropsychopharmacology, 27, 1027–1035. [DOI] [PubMed] [Google Scholar]
- Liu J, Blond BN, van Dyck LI, Spencer L, Wang F, & Blumberg HP (2012). Trait and state corticostriatal dysfunction in bipolar disorder during emotional face processing. Bipolar Disorders, 14, 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larson MP, DelBello MP, Zimmerman ME, Schwiers ML, Strakowski SM (2002). Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biological Psychiatry, 52, 93–100. [DOI] [PubMed] [Google Scholar]
- Lozano BE, & Johnson SL (2001). Can personality traits predict increases in manic and depressive symptoms? Journal of Affective Disorders, 63, 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah L, Zarate CA, Singh J, Duan Y, Luckenbaugh DA, Manji HK, & Drevets WC (2007). Regional cerebral glucose metabolic abnormalities in bipolar II depression. Biological Psychiatry, 61, 765–775. [DOI] [PubMed] [Google Scholar]
- Mason L, O’Sullivan N, Bentall. RP, & El-Deredy W. (2012). Better than I thought: Positive evaluation bias in hypomania. PLOS One, 7, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Cowen PJ, Harmer CJ. (2009). Neural representation of reward in recovered depressed patients. Psychopharmacology, 205, 667–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald C, Bullmore ET, Sham PC, Chitnis X, Wickham H, Bramon E et al. (2004). Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Archives of General Psychiatry, 61, 974–984. [DOI] [PubMed] [Google Scholar]
- Meyer B, Johnson SL, & Carver CS (1999). Exploring behavioral activation and inhibition sensitivities among college students at risk for bipolar spectrum symptomatology. Journal of Psychopathology and Behavioral Assessment, 21, 275–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B, Johnson SL, & Winters R (2001). Responsiveness to threat and incentive in bipolar disorder. Relations of the BIS/BAS scales with symptoms. Journal of Psychopathology and Behavioral Assessment, 23, 133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklowitz DJ, George EL, Richards JA, Simoneau TL, & Suddath RL (2003). A randomized study of family-focused psychoeducation and pharmacotherapy in the outpatient management of bipolar disorder. Archives of General Psychiatry, 60, 904–911. [DOI] [PubMed] [Google Scholar]
- Miklowitz DJ, & Goldstein MJ (1997). Bipolar disorder:A family-focused treatment approach. New York: Guilford. [Google Scholar]
- Miklowitz DJ, Otto MW, Frank E, Reilly-Harrington NA, Wisniewski SR, Kogan JN, et al. (2007). Psychosocial treatments for bipolar depression: A 1-year randomized trial from the systematic treatment enhancement program. Archives of General Psychiatry, 64, 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklowitz DJ, Simoneau TL, George EL, Richards JA, Kalbag A, Sachs-Ericsson N, et al. (2000). Family- focused treatment of bipolar disorder: 1-year effects of a psychoeducational program in conjunction with pharmacotherapy. Biological Psychiatry, 48, 582–592. [DOI] [PubMed] [Google Scholar]
- Molnar GJ, Feeney MG, & Fava GA (1988). Duration and symptoms of bipolar prodromes. American Journal of Psychiatry, 145, 1576–1578. [DOI] [PubMed] [Google Scholar]
- Monk TH, Kupfer DJ, Frank E, & Ritenour AM (1991). The Social Rhythm Metric (SRM): Measuring daily social rhythms over 12 weeks. Psychiatry Research, 36,195–207. [DOI] [PubMed] [Google Scholar]
- Morris CD, Miklowitz DJ, & Waxmonsky JA (2007). Family-focused treatment for bipolar disorder in adults and youth. Journal of Clinical Psychology, 63, 433–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Ritchey M, Adcock RA, LaBar KS. (2010). fMRI studies of successful emotional memory encoding: a quantitativemeta-analysis. Neuropsychologia,48, 3459–3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R Abramson LY, Harmon-Jones E, Alloy LB & Coan JA (2009). Psychosocial interventions for bipolar disorder:Perspective from the behavioral approach system (BAS) dysregulation theory. Clinical Psychology: Science and Practice, 16, 449–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Abramson LY, Harmon-Jones E, Alloy LB & Hogan ME (2007). A goal-striving life event and the onset of hypomanic and depressive episodes and symptoms: perspective from the behavioral approach system (BAS) dysregulation theory. Journal of Abnormal Psychology, 116, 105–115. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Almeida JRC, Forbes EE, Versace A, LaBarbara EJ, Klein C, & Phillips ML (2012a). Waiting to win: Elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar adults. Bipolar Disorders, 14, 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Harmon-Jones E, Alloy LB, Urosevic S, Goldstein KE, & Abramson LY (2012b). Elevated left mid-frontal cortical activity prospectively predicts conversion to bipolar I disorder. Journal of Abnormal Psychology, 121,592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, & Frank E (2011). Subthreshold bipolarity: Diagnostic Issues and Challenges. Bipolar Disorders, 13, 587–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, Shackman AJ, Coan JA, Harmon-Jones E, Alloy LB, & Abramson LY (2011). Cognitive vulnerability and frontal brain asymmetry: common predictors of first prospective depressive episode. Journal of Abnormal Psychology, 120, 497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R, & Young C, Pornpattananangkul N & Damme K (in press). Neurophysiological and neuroimaging approaches to clinical psychological research In Cautin R & Lilienfeld S (Eds.), Encyclopedia of Clinical Psychology.New Jersey: Wiley-Blackwell. [Google Scholar]
- O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ (2002). Neural responses during anticipation of a primary taste reward. Neuron 33, 815–826. [DOI] [PubMed] [Google Scholar]
- Osuch EA, Bluhm RL, Williamson PC, Theberge J, Densmore M, Neufeld RW. (2009). Brain activation to favorite music in healthy controls and depressed patients. Neuroreport, 20, 1204–8. [DOI] [PubMed] [Google Scholar]
- Pacheco-Unguetti AP, Acosta A, Marqués E, & Lupiáñez J (2011). Alterations of the attentional networks in patients with anxiety disorders. Journal of Anxiety Disorders, 25(7), 888–895. [DOI] [PubMed] [Google Scholar]
- Pereira F, Mitchell T, & Botvinick M (2009). Machine learning classifiers and fMRI: A tutorial overview. Neuroimage, 45, S199–S209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis RH, Dennehy EB, Miklowitz DJ, DelBello MP, Ostacher M, Calabresem JR, Ametrano RM, Wisniewski SR, Bowden CL, Thase ME, Nierenberg AA, & Sachs G (2009). Retrospective age at onset of bipolar disorder and outcome during two-year follow up: Results from the STEP-BD study. Bipolar Disorders, 11, 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A, Tarrier N, Morriss R, McCarthy E, & Limb K (1999). Randomised controlled trial of efficacy of teaching patients with bipolar disorder to identify early symptoms of relapse and obtain treatment. BMJ, 318, 149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, & Vieta E (2007). Identifying Functional Neuroimaging Biomarkers of Bipolar Disorder: Toward DSM-V. Schizophrenia Bulletin, 33, 893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, & Fava M (2008). Reduced hedonic capacity in major depressive disorder: Evidence from a proabilistic reward task. Journal of Psychiatric Research, 43, 76–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Sherwood RJ, Henriques JB, & Davidson RJ (2005), Frontal brain asymmetry and reward responsiveness: A source localization study. Psychological Science, 16, 805–813. [DOI] [PubMed] [Google Scholar]
- Pompei F, Jogia J, Tatarelli R et al. (2011). Familial and disease specific abnormalities in the neural correlates of the Stroop Task in Bipolar Disorder. Neuroimage, 56, 1677–1684. [DOI] [PubMed] [Google Scholar]
- Post RM, Altshuler LL, Leverich GS, Frye MA, Nolen WA, Kupka RW, Suppes T, McElroy S, Keck PE, Denicoff KD, Grunze H, Walden J, Kitchen CMR, & Mintz J (2006). Mood switch in bipolar depression: Comparison of adjunctive venalfaxine, buproprion, and sertraline. British Journal of Psychiatry, 189, 124–131. [DOI] [PubMed] [Google Scholar]
- Rea MM, Tompson MC, Miklowitz DJ, Goldstein MJ, Hwang S, & Mintz J (2003). Family-focused treatment versus individualized treatment for bipolar disorder: Results of a randomized clinical trial. Journal of Consulting and Clinical Psychology, 71, 482–492. [DOI] [PubMed] [Google Scholar]
- Robinson JL, Monkul ES, Tordesillas-Gutierrez D et al. (2008). Fronto-limbic circuitry in euthymic bipolar disorder: evidence for prefrontal hyperactivation. Psychiatry Research, 164: 106–113. [DOI] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (1993). The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research Reviews, 18, 247–291. [DOI] [PubMed] [Google Scholar]
- Rubinsztein JS, Fletcher PC, Rogers RD, Ho LW, Aigbirhio FI, Paykel ES, Robbins TW, Sahakian BJ (2001). Decision-making in mania: a PET study. Brain, 124, 2550–2563. [DOI] [PubMed] [Google Scholar]
- Sachs GS, Nierenberg AA, Calabrese JR, Marangell LB, Wisniewski SR, Gyulai L, Friedman ES, Bowden C, Fossey MD, Ostacher MJ, Ketter TA, Patel. J, Hauser P, Rapport D, Martinez JM, Allen MH, Miklowitz DJ, Otto MW, Dennehy EB, & Thase ME (2007). Effectiveness of adjunctive antidepressant treatment for bipolar depression. The New England Journal of Medicine, 356, 1711–1722. [DOI] [PubMed] [Google Scholar]
- Salavert J, Caseras X, Torrubia R, Furest S, Arranz B, Duenas R, & San L (2007). The functioning of the Behavioral Activation and Inhibition Systems in bipolar I euthymic patients and its influence in subsequent episodes over an 18-month period. Personality and Individual Differences, 42, 1323–1331. [Google Scholar]
- Scarna A, Gijsman HJ, Mctavish SF et al. (2003). Effects of a branched-chain amino acid drink in mania. British Journal of Psychiatry,182, 210–213. [DOI] [PubMed] [Google Scholar]
- Schmidt L, Clery-Melin ML, Lafargue G et al. (2009). Get aroused and be stronger: emotional facilitation of physical effort in the human brain. Journal of Neuroscience, 29, 9450–9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W (2000). Multiple reward signals in the brain. Nature Review Neuroscience, 1, 199–207. [DOI] [PubMed] [Google Scholar]
- Schultz W (2002). Getting formal with dopamine and reward. Neuron 36, 241–263. [DOI] [PubMed] [Google Scholar]
- Schultz W Tremblay L, Hollerman JR (2000). Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex 10, 272–284. [DOI] [PubMed] [Google Scholar]
- Scott J, Colom F, & Vieta E (2007). A meta-analysis of relapse rates with adjunctive psychological therapies compared to usual psychiatric treatment for bipolar disorders. International Journal of Neuropsychopharmacology, 10, 123–129. [DOI] [PubMed] [Google Scholar]
- Scott J, Garland A, & Moorhead S (2001). A pilot study of cognitive therapy in bipolar disorders. Psychological Medicine,31, 459–467. [DOI] [PubMed] [Google Scholar]
- Scott J, & Pope M (2003). Cognitive styles in individuals with bipolar disorders. Psychological Medicine, 33, 1081–1088. [DOI] [PubMed] [Google Scholar]
- Scott J, Stanton B, Garland A, & Ferrier IN (2000). Cognitive vulnerability in patients with bipolar disorder. Psychological Medicine, 30, 467–472. [DOI] [PubMed] [Google Scholar]
- Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M (2001). Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain, 124, 1720–1733. [DOI] [PubMed] [Google Scholar]
- Smit DJA, Posthuma D, Boomsma DI, De Geus EJ (2007). The relation between frontal EEG asymmetry and the risk for anxiety and depression. Biological Psychology, 74, 26–33. [DOI] [PubMed] [Google Scholar]
- Smith JA, & Tarrier N (1992). Prodromal symptoms in manic depressive psychosis. Social Psychiatry and Psychiatric Epidemiology, 27, 245–248. [DOI] [PubMed] [Google Scholar]
- Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, et al. (2009). fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. Journal of Affective Disorders, 118, 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoy M, Schlagenhauf F, Sterzer P, Bermpohl F, Hagele C, Suchotzki K, et al. (2012). Hyporeactivity of ventral striatum towards incentive stimuli in unmedicated depressed patients normalizes after treatment with escitalopram. Journal of Psychopharmacology, 26, 677–88. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Zimmerman ME, Getz GE, Mills NP, Ret J, Shear P, Adler CM (2002). Ventricular and periventricularstructural volumes in first- versus multiple-episode bipolar disorder. American Journal of Psychiatry, 159, 1841–1847. [DOI] [PubMed] [Google Scholar]
- Sutton SK, & Davidson RJ (1997). Prefrontal brain asymmetry: A biological substrate of the behavioral approach system and inhibition systems. Psychological Science, 8, 204–210. [Google Scholar]
- Swann AC, Lafer B, Perugi G, Frye MA, Bauer M, Bahk WM, Scott J, Ha K, Suppes T (2013). Bipolar mixed states: an international society for bipolar disorders task force report of symptom structure, course of illness, and diagnosis. American Journal of Psychiatry, 170 31–42 [DOI] [PubMed] [Google Scholar]
- Tanda G, Carboni E, Frau R, Di Chiara G. (1994). Increase of extracellular dopamine in the prefrontal cortex: a trait of drugs with antidepressant potential? Psychopharmacology 115, 285–288. [DOI] [PubMed] [Google Scholar]
- Thibodeau R, Jorgensen RS, & Kim S (2006). Depression, anxiety, and resting frontal EEG asymmetry: A meta-analytic review. Journal of Abnormal Psychology, 115, 715–729. [DOI] [PubMed] [Google Scholar]
- Tohen M, Goldberg JF, Gonzalez-Pinto Arrillaga AM et al. (2003a). A 12-week, double-blind comparison of olanzapine vs. haloperidol in the treatment of acute mania. Archives of General Psychiatry, 60, 1218–1226. [DOI] [PubMed] [Google Scholar]
- Tohen M, Vieta E, Calabrese J et al. (2003b). Efficacy of olanzapine and olanzapine–fluoxetine combination in the treatment of bipolar I depression. Archives of General Psychiatry, 60, 1079–1088. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, & Doss RC (1992). Individual differences in anterior brain asymmetry and fundamental dimensions of emotion. Journal of Personality and Social Pscyhology, 62, 676–687. [DOI] [PubMed] [Google Scholar]
- Trost S, Diekhof EK, Zvonik K, Lewandowski M, Usher J, Keil M, Zilles D, Falkai P, Dechart D, & Gruber O (2014). Disturbed anterior prefrontal control of the mesolimbic reward system and increased impulsivity in bipolar disorder. Neuropsychopharmacology, epub aherad of press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urosevic S, Abramson LY, Harmon-Jones E, & Alloy LB (2008). Dysregulation of the behavioral approach system (BAS) in bipolar spectrum disorders: Review of theory and evidence. Clinical Psychology Review, 28, 1188–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag HM, Korf J. (1975). Central monoamine deficiency indepressions: causative of secondary phenomenon? Pharmakopsychiatr Neuropsychopharmakol, 8, 322–326. [DOI] [PubMed] [Google Scholar]
- Valenti M, Pacchiarotti I, Bonnin CM, Rosa AR, Popovic D, Nivoli AM, et al. (2012): Risk factors for antidepressant-related switch to mania. Journal of Clinical Psychiatry, 73, 271–276. [DOI] [PubMed] [Google Scholar]
- Vieta E, Ros S, Goikolea JM et al. (2005). An open-label study of amisulpride in the treatment of mania. Journal of Clinical Psychiatry, 66, 575–578. [DOI] [PubMed] [Google Scholar]
- Visser HM, Van Der Mast RC. (2005). Bipolar disorder, antidepressants and induction of hypomania or mania. A systematic review. World Journal of Biological Psychiatry, 6, 231–241. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Wong C et al. (1999). Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. Journal of Pharmacology and Experimental Therapeutics, 291, 409–415. [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, Fowler JS, Zhu W, Logan J, Ma Y, Pradhan K, Wong C, & Swanson JM (2009). Evaluating dopamine reward pathway in ADHD, Journal of American Medical Association, 302, 1084–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker J, Dillon DG, Pizzagalli DA. (2009). The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. NeuroImage. 46, 327–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. (2008). Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron, 59, 1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G, Sinsel E, Sobanski T, Köhler S, Marinou V, Mentzel H, Sauer H, & Schlösser RGM (2006). Cortical inefficiency in patients with unipolar depression: An event-related fMRI study with the stroop task. Biological Psychiatry, 59, 958–965. [DOI] [PubMed] [Google Scholar]
- Wise RA (2002). Brain reward circuitry: insights from unsensed incentives. Neuron, 36, 229–240. [DOI] [PubMed] [Google Scholar]
- Yang H, Liu J, Sui J, Pearlson G, & Calhoun VD (2010). A hybrid machine learning method for fusing fMRI and genetic data: Combining both improves classification of schizophrenia. Frontiers in Human Neurosceience, 4, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Holroyd CB, & Cohen JD (2005). ERP correlates of feedback and reward processing in the presence and absence ofresponse choice. Cerebral Cortex, 15, 535–544. [DOI] [PubMed] [Google Scholar]
- Zaretsky A, Lancee W, Miller C, Harris A, & Parikh SV (2008). Is cognitive-behavioural therapy more effective than psychoeducation in bipolar disorder. Canadian Journal of Psychiatry, 53, 441–448. [DOI] [PubMed] [Google Scholar]