Key Points
Question
What are the patterns of disease recurrence after resection of pancreatic cancer followed by systemic chemotherapy?
Findings
In this secondary analysis of a randomized clinical trial, median recurrence-free survival, median survival after recurrence, and the median overall survival were similar. Adjuvant gemcitabine plus capecitabine was associated with reduced rate of local recurrence compared with gemcitabine monotherapy and improved overall survival.
Meaning
Pancreatic cancer can be regarded as a systemic disease, irrespective of site of recurrence, requiring adjuvant systemic therapy after resection for effective treatment.
Abstract
Importance
The patterns of disease recurrence after resection of pancreatic ductal adenocarcinoma with adjuvant chemotherapy remain unclear.
Objective
To define patterns of recurrence after adjuvant chemotherapy and the association with survival.
Design, Setting, and Participants
Prospectively collected data from the phase 3 European Study Group for Pancreatic Cancer 4 adjuvant clinical trial, an international multicenter study. The study included 730 patients who had resection and adjuvant chemotherapy for pancreatic cancer. Data were analyzed between July 2017 and May 2019.
Interventions
Randomization to adjuvant gemcitabine or gemcitabine plus capecitabine.
Main Outcomes and Measures
Overall survival, recurrence, and sites of recurrence.
Results
Of the 730 patients, median age was 65 years (range 37-81 years), 414 were men (57%), and 316 were women (43%). The median follow-up time from randomization was 43.2 months (95% CI, 39.7-45.5 months), with overall survival from time of surgery of 27.9 months (95% CI, 24.8-29.9 months) with gemcitabine and 30.2 months (95% CI, 25.8-33.5 months) with the combination (HR, 0.81; 95% CI, 0.68-0.98; P = .03). The 5-year survival estimates were 17.1% (95% CI, 11.6%-23.5%) and 28.0% (22.0%-34.3%), respectively. Recurrence occurred in 479 patients (65.6%); another 78 patients (10.7%) died without recurrence. Local recurrence occurred at a median of 11.63 months (95% CI, 10.05-12.19 months), significantly different from those with distant recurrence with a median of 9.49 months (95% CI, 8.44-10.71 months) (HR, 1.21; 95% CI, 1.01-1.45; P = .04). Following recurrence, the median survival was 9.36 months (95% CI, 8.08-10.48 months) for local recurrence and 8.94 months (95% CI, 7.82-11.17 months) with distant recurrence (HR, 0.89; 95% CI, 0.73-1.09; P = .27). The median overall survival of patients with distant-only recurrence (23.03 months; 95% CI, 19.55-25.85 months) or local with distant recurrence (23.82 months; 95% CI, 17.48-28.32 months) was not significantly different from those with only local recurrence (24.83 months; 95% CI, 22.96-27.63 months) (P = .85 and P = .35, respectively). Gemcitabine plus capecitabine had a 21% reduction of death following recurrence compared with monotherapy (HR, 0.79; 95% CI, 0.64-0.98; P = .03).
Conclusions and Relevance
There were no significant differences between the time to recurrence and subsequent and overall survival between local and distant recurrence. Pancreatic cancer behaves as a systemic disease requiring effective systemic therapy after resection.
Trial Registration
ClinicalTrials.gov identifier: NCT00058201, EudraCT 2007-004299-38, and ISRCTN 96397434.
This secondary analysis of a randomized clinical trial investigates patterns of recurrence after adjuvant chemotherapy in pancreatic cancer and the association with survival.
Introduction
The effective treatment of pancreatic ductal adenocarcinoma remains hugely challenging.1 However, there has also been considerable progress toward extending overall survival by improving surgical outcomes2,3,4,5 and the development of better adjuvant6,7,8 and neoadjuvant9,10,11 therapies. The incidence of pancreatic cancer is rising, and it is likely to be the second leading cause of cancer death by 2030.12,13
In specialized centers, resection rates of 15% can be achieved1,2 with a 5-year survival rate around 10% without adjuvant therapy,7,14,15 increasing to 16% to 18% with single-agent adjuvant chemotherapy14,15,16 and 30% to 50% with combination gemcitabine and capecitabine or modified 5-fluorouracil, folinic acid, irinotecan, and oxliplatin combination (FOLFIRINOX), respectively.7,8 The patterns of disease recurrence following resection include both locoregional failure and distant metastases. Estimates of these patterns have been derived from several small postmortem analyses, retrospective single-center studies,3,17,18,19,20,21,22,23 and prospective data from the European Study Group for Pancreatic Cancer (ESPAC) 1 trial.15 In a large retrospective study3 from the Johns Hopkins Medical School, 692 of 1103 patients (62.7%) had sufficient data for analysis. Of these, 531 (76.7%) had a recurrence, of whom 126 (23.7%) had local-only recurrence, 307 (57.8%) had distant-only metastases, and 98 (19%) had both local recurrence and distant metastases.3 Key findings were that liver-only recurrence, which was found in 134 patients (25.2%), occurred relatively early after a median of 6.9 months while lung-only recurrence, which was found in 78 patients (15%), occurred much later at a median of 18.6 months, and patients with a positive lymph node ratio greater than 0.2 were most likely to develop distant metastatic disease, although this and other retrospective series are limited by a significant amount of missing data and other potential biases.3,17,18,19 These limitations are minimized in large prospective multicenter studies. We therefore investigated the patterns of disease recurrence after resection of pancreatic ductal adenocarcinoma in the large, multicenter randomized ESPAC-4 adjuvant study.7
Methods
Study Design
The pattern of pancreatic cancer recurrence was recorded prospectively at the Liverpool Clinical and Cancer Research UK Trials Unit, University of Liverpool, as part of the ESPAC-4 trial.7 This was an international phase 3 randomized clinical trial to compare overall survival after pancreatic adenocarcinoma resection followed by adjuvant gemcitabine (control arm) or combination gemcitabine plus capecitabine (experimental arm). Ethical approval was obtained from the Liverpool Adult Research Ethics Committee on March 4, 2008. Ethical approval was also obtained in each of the other participating countries. The study conformed to the principles of the International Conference on Harmonization on Good Clinical Practice. Informed consent was obtained in writing from each study participant.
Patients were followed up every 3 months from surgery by standard practice, and suspected recurrence was confirmed by cross-sectional imaging. Local recurrence was defined as radiologic evidence of recurrent disease in the remnant pancreas, the surgical bed, or in locoregional nodes. Distant recurrence was defined as radiologic evidence of recurrence outside these areas. Distant recurrence was stratified by the organ of recurrence. Only the site or sites of first recurrence were analyzed.
The primary outcome measure was a competing risk covariate that measured the time from surgery until either local recurrence, distant recurrence, synchronous local, and distant recurrence or death without recurrence. Patients alive and without evidence of recurrence at the time of analysis were included as censored observations. Before randomization, patients were stratified by country and R0 or R1 status.24 An R0 resection was defined as the absence of any cancer cells within 1 mm of any cut surface of the resected specimen. An R1 resection was defined as at least 1 cancer cell within 1 mm of any surface of the removed specimen. Evidence of ascites, intra-abdominal, or distant metastasis precluded enrollment, as did an R2 resection. Patients who had received previous neoadjuvant therapy were not eligible for inclusion. A triple-phase contrast computed tomography scan of chest, abdomen, and pelvis was required in the 3 months before surgery to exclude preexisting metastatic disease. Tumor staging was undertaken prospectively using the Union for International Cancer Control TNM, 7th edition, classification of malignant tumors.25 Demographic and pathologic variables for the study inclusion were prespecified. A pathology proforma was completed, and the full pathology report submitted to the Liverpool Clinical and Cancer Research UK Trials Unit before randomization could take place. For the purposes of this study, pathology reporting was reexamined and restaged using the updated American Joint Committee on Cancer Staging Manual, 8th edition.26 The full trial protocol is available in Supplement 1.
Statistical Analysis
Competing risks regression modeling was performed to assess the impact of clinical and demographic factors on the time to the first event of interest, local recurrence vs distant recurrence vs death without known recurrence as well as median and overall survival. Clinical and demographic covariates considered for inclusion were prespecified and included those identified in the main trial analysis as predictive of overall survival.7 Further clinical and demographic factors with a significance level of P less than .25 on univariate modeling were considered for inclusion in the multivariable analysis with models constructed using backward selection and evaluated using the Akaike information criterion.27,28 Key variables, such as treatment arm and resection margin status, were forced into all multivariable models. Proportionality of subhazards assumption was evaluated after fitting Schoenfeld residuals. Results are reported in terms of the cause-specific hazard ratios (HR) with 95% confidence intervals. Power analysis for the original clinical trial has been described previously.7 All analyses were conducted using 2-sided significance tests at the .05 significance level. Stata, version 15 (StataCorp), and R, version 3.3.3 (R Foundation), were used to perform all statistical analyses.
Results
Patient Demographics
Between November 2008 and September 2014, 732 patients were randomized, 367 patients (50.1%) to receive gemcitabine alone and 365 (49.9%) to receive combination gemcitabine plus capecitabine. Two patients were excluded from the full analysis set because they withdrew consent between randomization and starting therapy (1 in each group); (Figure 1; the CONSORT diagram is also included in the original publication).7
Figure 1. CONSORT Diagram of European Study Group for Pancreatic Cancer 4.
Overall Survival
The median time from surgery to randomization was 65 (interquartile range [IQR], 23-111) days in the gemcitabine group and 64 (IQR, 21-111) days for the combination treatment arm. The median follow-up time from randomization was 43.2 months (95% CI, 39.7-45.5 months). The median overall survival from the time of surgery was 27.9 months (95% CI, 24.8-29.9 months) in the gemcitabine group and 30.2 months (95% CI, 25.8-33.5 months) in the gemcitabine plus capecitabine group (HR, 0.81; 95% CI, 0.68-0.98; P = .03). The 5-year survival estimates were 17.1% (95% CI, 11.6%-23.5%) in the gemcitabine group and 28.0% (95% CI, 22.0%-34.3%) in the gemcitabine plus capecitabine group.
Patterns of Recurrence
Disease recurred in 479 of 730 patients (65.6%). Baseline clinical demographics and pathologic variables are described in Table 1. Local recurrence occurred in 238 of these 479 patients (49.7%), distant-only recurrence in 193 patients (40.3%), and simultaneous local and distant recurrence in 48 patients (10.0%), while a further 78 patients (10.7%) died without any identifiable recurrence. The overall median time to recurrence was 12.65 months (IQR, 11.86-13.50 months). Recurrence within 2 years of randomization occurred in 416 of 479 patients (86.8%) with recurrences, in 202 of 238 patients (84.9%) with local recurrence, and in 214 of 241 patients (88.8%) with distant recurrence. Of the 458 patients who died, 380 (83.0%) had local recurrence and/or metastases prior to death. Patient groups were comparable, with no significant differences in the types and extent of surgical resection between groups.
Table 1. Demographic Data of ESPAC-4 Trial Patients Grouped According to Site of Initial Recurrencea.
| Characteristic | No. (%) | |||||
|---|---|---|---|---|---|---|
| Alive Without Recurrence (n = 173) | Local-Only Recurrence (n = 238) | Distant-Only Recurrence (n = 193) | Local/Distant Recurrence (n = 48) | Dead Without Recurrence (n = 78) | Total (n = 730) | |
| Age, median (IQR), y | 63 (57-68) | 63 (55-68) | 66 (56-70) | 66 (57-70) | 64 (58-71) | 64 (56-69) |
| Sex | ||||||
| Female | 76 (24) | 102 (32) | 104 (33) | 19 (6) | 34 (11) | 316 |
| Male | 97 (23) | 136 (33) | 137 (33) | 29 (7) | 44 (11) | 414 |
| WHO status | ||||||
| 0 | 80 (26) | 97 (31) | 103 (33) | 17 (5) | 28 (9) | 308 |
| 1 | 88 (22) | 134 (33) | 132 (33) | 30 (7) | 47 (12) | 401 |
| 2 | 5 (24) | 7 (33) | 6 (29) | 1 (5) | 3 (14) | 21 |
| Treatment allocation | ||||||
| Gemcitabine | 80 (22) | 130 (36) | 113 (31) | 22 (6) | 43 (12) | 366 |
| Gemcitabine plus capecitabine | 93 (26) | 108 (30) | 128 (35) | 26 (7) | 35 (10) | 364 |
| T stage | ||||||
| T1 | 25 (42) | 17 (29) | 12 (20) | 2 (3) | 5 (8) | 59 |
| T2 | 109 (23) | 150 (32) | 161 (34) | 32 (7) | 49 (10) | 469 |
| T3 | 39 (19) | 71 (35) | 68 (34) | 14 (7) | 24 (12) | 202 |
| N stage | ||||||
| N0 | 63 (46) | 32 (23) | 33 (24) | 4 (3) | 9 (7) | 137 |
| N1 | 87 (28) | 102 (33) | 92 (29) | 18 (6) | 31 (10) | 312 |
| N2 | 22 (8) | 102 (37) | 115 (42) | 25 (9) | 38 (14) | 277 |
| Stage | ||||||
| I | 53 (43) | 32 (26) | 29 (24) | 5 (4) | 8 (7) | 122 |
| II | 98 (30) | 104 (31) | 97 (29) | 18 (5) | 32 (10) | 331 |
| III | 22 (8) | 102 (37) | 115 (42) | 25 (9) | 38 (14) | 277 |
| Smoker | ||||||
| Never | 72 (24) | 97 (33) | 103 (35) | 21 (7) | 25 (8) | 297 |
| Past | 77 (27) | 88 (31) | 88 (31) | 17 (6) | 31 (11) | 284 |
| Present | 24 (20) | 39 (32) | 40 (33) | 7 (5) | 20 (16) | 123 |
| Diabetes mellitus | ||||||
| Insulin dependent | 24 (26) | 37 (40) | 23 (25) | 3 (3) | 9 (10) | 93 |
| No | 121 (22) | 171 (32) | 186 (35) | 32 (6) | 60 (11) | 538 |
| Non–insulin dependent | 28 (29) | 29 (30) | 31 (32) | 13 (13) | 9 (9) | 97 |
| Tumor grade | ||||||
| Poor | 48 (17) | 80 (28) | 125 (44) | 23 (8) | 34 (12) | 287 |
| Undifferentiated | 2 (50) | 2 (50) | 0 (0) | 0 (0) | 0 (0) | 4 |
| Moderately | 101 (28) | 127 (35) | 98 (27) | 19 (5) | 41 (11) | 367 |
| Well | 20 (32) | 23 (37) | 17 (27) | 6 (10) | 2 (3) | 62 |
| Maximum tumor size | ||||||
| Median (IQR), mm | 28 (21-35) | 31 (25-40) | 30 (24.5-40) | 30 (25-40) | 30 (25-40) | 30 (24-40) |
| Extent of lymph resection | ||||||
| Extended | 10 (22) | 17 (37) | 15 (33) | 3 (6) | 4 (9) | 46 |
| Radical | 26 (24) | 35 (32) | 39 (36) | 10 (9) | 9 (8) | 109 |
| Standard | 136 (24) | 183 (32) | 185 (33) | 34 (6) | 64 (11) | 568 |
| Portal vein resection | ||||||
| No | 154 (25) | 198 (32) | 206 (33) | 45 (7) | 63 (10) | 621 |
| Yes | 16 (16) | 38 (37) | 34 (33) | 3 (3) | 14 (14) | 102 |
| Local invasion | ||||||
| No | 101 (27) | 117 (31) | 125 (33) | 25 (7) | 35 (9) | 378 |
| Yes | 71 (20) | 121 (35) | 115 (33) | 23 (7) | 42 (12) | 349 |
| Postoperative complications | ||||||
| No | 124 (24) | 171 (33) | 170 (33) | 34 (6) | 56 (11) | 521 |
| Yes | 47 (23) | 66 (32) | 71 (34) | 14 (7) | 22 (11) | 206 |
| Type of resection | ||||||
| Distal pancreatectomy | 19 (32) | 20 (33) | 17 (28) | 5 (8) | 4 (7) | 60 |
| Pylorus preserving pancreatectomy | 60 (24) | 79 (31) | 81 (32) | 17 (7) | 31 (12) | 251 |
| Total pancreatectomy | 17 (35) | 20 (41) | 10 (20) | 2 (4) | 2 (4) | 49 |
| Classic whipple | 77 (21) | 119 (32) | 133 (36) | 24 (7) | 41 (11) | 370 |
| Resection margin | ||||||
| R0 | 88 (30) | 83 (29) | 93 (32) | 22 (8) | 26 (9) | 290 |
| R1 | 85 (19) | 155 (35) | 148 (34) | 26 (6) | 52 (12) | 440 |
| Toxicity ≥grade 3b | ||||||
| No | 74 (24) | 91 (29) | 118 (38) | 25 (8) | 26 (8) | 309 |
| Yes | 99 (24) | 147 (35) | 123 (29) | 23 (5) | 52 (12) | 421 |
| Preoperative CA19.9 level, kU/L | ||||||
| <150 | 66 (29) | 75 (33) | 65 (29) | 16 (7) | 22 (10) | 228 |
| ≥150 | 42 (18) | 80 (35) | 81 (35) | 15 (7) | 27 (12) | 230 |
| Postoperative CA19.9 level, kU/L | ||||||
| <18.7 | 107 (32) | 102 (31) | 93 (28) | 18 (5) | 29 (9) | 331 |
| ≥18.7 | 51 (15) | 115 (35) | 121 (37) | 22 (7) | 44 (13) | 331 |
| Preoperative CRP level, mg/L | ||||||
| <7 | 63 (24) | 85 (32) | 90 (34) | 19 (7) | 29 (11) | 267 |
| ≥7 | 60 (22) | 93 (33) | 97 (35) | 21 (8) | 29 (10) | 279 |
| Postoperative CRP level, mg/L | ||||||
| <5 | 70 (23) | 112 (37) | 97 (32) | 16 (5) | 27 (9) | 306 |
| ≥5 | 90 (23) | 114 (30) | 134 (35) | 29 (8) | 46 (12) | 384 |
Abbreviations: CRP, C-reactive protein; CA19-9, carbohydrate antigen 19-9; ESPAC-4, European Study Group for Pancreatic Cancer 4; WHO, World Health Organization.
SI conversion factor: To convert C-reactive protein to nanomoles per liter, multiply by 9.524.
Disease recurrence was observed in 479 patients (65.6%).
Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria, version 4.03.
Local recurrence occurred at a median of 13.57 months (95% CI, 12.61-14.06 months) and was statistically significantly different from those with distant recurrence with a median of 11.27 months (95% CI, 10.38-12.55 months) (HR, 1.20; 95% CI, 1.01-1.44; P = .04). The most common oligometastatic site among the 241 patients with distant recurrence was the liver found in 99 patients (41%) (or 20.7% of all recurrences), followed by lung-limited disease in 52 patients (22%) (or 10.9% of all recurrences) (Table 2). Liver metastatic disease occurred soonest with a median of 9.66 months (95% CI, 8.11-11.14 months) compared with lung metastases, which occurred at 15.31 months (95% CI, 11.76-20.00 months) (HR, 0.47; 95% CI, 0.33-0.68; P < .001).
Table 2. Sites of First Recurrence and Median Overall Survival From Surgery and Median Survival After Diagnosis of Recurrence by Site.
| Site of Recurrence | No. | Median (95% CI) | ||
|---|---|---|---|---|
| Recurrence-Free Survival, mo | Survival After Recurrence, mo | Overall Survival, mo | ||
| Local only | 238 | 13.57 (12.61-14.06) | 9.36 (8.08-10.48) | 24.83 (22.96-27.863) |
| Local and distant recurrence | 48 | 11.99 (10.28-15.83) | 8.11 (5.22-11.79) | 23.82 (17.48-28.32) |
| Distant only | 193 | 11.14 (10.05-12.32) | 9.23 (7.82-11.43) | 20.61 (18.12-23.80) |
| Patients with any distant recurrence | 241 | 11.27 (10.38-12.55) | 8.94 (7.82-11.17) | 23.06 (20.43-25.36) |
| Oligometastatic | ||||
| Bone | 6 | NA | NE | NE |
| Distant nodal | 16 | 10.61 (7.85-15.14) | 13.17 (4.86-NE) | 25.36 (14.36-NE) |
| Liver | 99 | 9.66 (8.11-11.14) | 8.54 (7.03-9.62) | 20.43 (16.59-23.85) |
| Lung | 52 | 15.31 (11.76-20.00) | 15.04 (12.25-23.65) | 33.47 (24.77-48.72) |
| Other intra-abdominal | 26 | 14.06 (9.62-19.32) | 11.17 (5.85-13.40) | 29.11 (20.79-49.28) |
| Ovarian | 2 | NE | NE | NE |
| Polymetastatic | ||||
| Distant nodal and bone | 1 | NE | NE | NE |
| Liver and bone | 1 | NE | NE | NE |
| Liver and lung | 18 | 10.61 (8.97-12.94) | 4.53 (2.13-6.41) | 14.91 (11.83-20.99) |
| Liver, lung, and bone | 1 | NE | NE | NE |
| Liver, lung, and nodal | 2 | NE | NE | NE |
| Liver and nodal | 2 | NE | NE | NE |
| Liver and peritoneal | 1 | NE | NE | NE |
| Lung and nodal | 7 | NE | NE | NE |
| Lung and peritoneal | 7 | NE | NE | NE |
Abbreviation: NE, not estimable.
Following identification of recurrence, median survival was 9.36 months (95% CI, 8.08-10.48 months) for local recurrence and 8.94 months (95% CI, 7.82-11.17 months) with distant recurrence with no significant difference (HR, 0.89; 95% CI, 0.73-1.09; P = .27) (Figure 2A). Patients with lung-limited metastatic disease had significantly longer survival from time of recurrence than those with liver-only metastases (HR, 0.60; 95% CI, 0.40-0.90; P = .01) (Figure 2B).
Figure 2. Kaplan-Meier Curves Showing Survival From Time of Recurrence.
A, Recurrence stratified by local vs distant disease. B, Recurrence stratified by organ of recurrence.
Factors Associated With Patterns of Recurrence
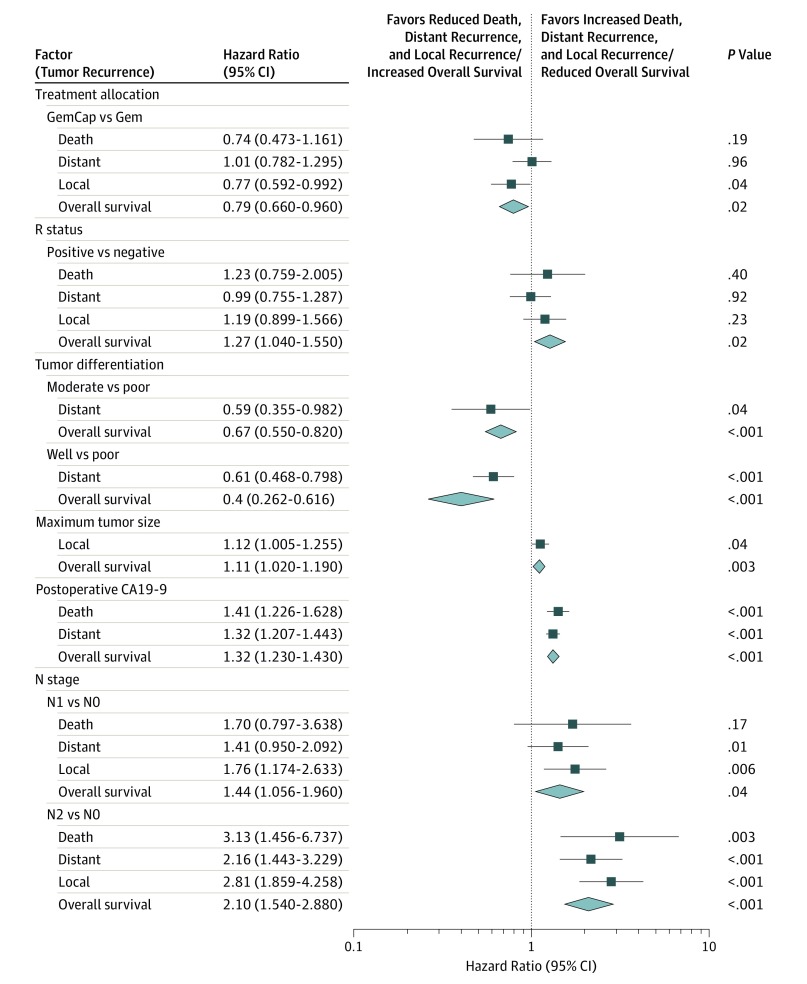
Univariate competing risk analyses of clinical and demographic factors on the risk of recurrence or death along with the forest plot are described in eTable 2 in the Supplement and Figure 3. Multivariable analyses identified independent factors significantly associated with local recurrence, distant recurrence, or death without recurrence. For local recurrence adjuvant treatment (HR, 0.77; 95% CI, 0.592-0.992; P = .04), N1 status (HR, 1.76; 95% CI, 1.174-2.633; P = .006) and N2 status (HR, 2.81; 95% CI, 1.859-4.258; P < .001) were significant but R status was not; for distant recurrence, moderately differentiated grade (HR, 0.59; 95% CI, 0.355-0.982; P = .04), well-differentiated grade (HR, 0.61; 95% CI, 0.468-0.798; P < .001), log-adjusted postoperative carbohydrate antigen (CA) 19-9 levels (HR, 1.32; 95% CI, 1.207-1.443; P < .001), and N2 stage (HR, 2.16; 95% CI, 1.443-3.229; P < .001) were significant; for death without recurrence, postoperative CA19-9 levels (HR, 1.41; 95% CI, 1.226-1.628; P < .001) and N2 status (HR, 3.13; 95% CI, 1.456-6737; P = .003) were significant; and for overall survival, adjuvant treatment (HR, 0.79; 95% CI, 0.66-0.96; P = .16), R status (HR, 1.27; 95% CI, 1.04-1.55; P = .02), moderately differentiated grade (HR, 0.67; 95% CI, 0.55-0.82; P < .001), well-differentiated grade (HR, 0.4; 95% CI, 0.262-0.616; P < .001), maximum tumor size (HR, 1.11; 95% CI, 1.02-1.19; P = .003), postoperative CA19-9 levels (HR, 1.32; 95% CI, 1.23-1.43; P < .001), N1 status (HR, 1.44; 95% CI, 1.056-1.96; P = .04), and N2 status (HR, 2.10; 95% CI, 1.54-2.88; P < .001) were all significant.
Figure 3. Forest Plot Comparing Competing Risks Results for Local Recurrence, Distant Recurrence, and Overall Survival (OS).
The cumulative incidence plot showing the accumulation of local and distant recurrence and deaths without recurrence, as well as the accumulation of distant recurrences stratified by organ, is presented in eFigure 1 in Supplement 2. The small number of patients with local and distant recurrence did not allow competing risk or cumulative incidence analysis to be performed.
Independent factors associated with poorer survival following recurrence were resection margin status (HR, 1.39;95% CI, 1.106-1.744; P = .005), moderately (HR, 0.51; 95% CI, 0.406-0.64; P < .001) and well-differentiated tumor grades (HR, 0.47; 95% CI, 0.303-0.732; P < .001), local invasion (HR, 1.26; 95% CI, 1.018-1.554; P = .03), current smoking status (HR, 1.46; 95% CI, 1.087-1.957; P = .01), and preoperative C-reactive protein levels (HR, 1.22; 95% CI, 1.095-1.361; P < .001) (eTable 1 in Supplement 2). In this model, patients who received combination gemcitabine plus capecitabine had a 21% reduction of death following recurrence compared with patients treated with gemcitabine alone (HR, 0.79; 95% CI, 0.64-0.98; P = .03).
Overall Survival by Patterns of Recurrence
The median overall survival of patients with distant only (P = .85) or local with distant recurrence were not significantly different from those with only local recurrence (Table 2; eFigure 2A in Supplement 2). Using distant nodal disease as the reference between the distant metastasis subgroups, there were no significant differences in overall median survival compared with patients with liver only, lung only, or other intra-abdominal recurrence, but patients with combined liver and lung metastases had significantly shorter survival (P = .02) (Table 2; eFigure 2B in the Supplement). The median survival of patients with lung-only metastases was 33.47 months (95% CI, 24.77-48.72 months), which was significantly longer than these with liver-only metastases, which was 20.43 months (95% CI, 16.59-23.85 months) (HR, 0.50; 95% CI, 0.33-0.76; P = .001).
Discussion
This study showed there were no differences attributable to the combination regimen compared with gemcitabine monotherapy in either development of distant metastases or death without recurrence. However, importantly, there was a 23% reduction in the risk of developing local recurrence, a 21% reduction of death following recurrence, and an 18% increase in overall survival using the combination of gemcitabine with capecitabine compared with gemcitabine alone. Almost 90% of distant recurrences occurred within 2 years of surgery, with half of patients who developed liver metastases doing so within 12 months. This implies that most patients had already developed distant metastases prior to resection,29,30,31 a finding consistent with the significant independent association of distant metastases with N2 lymph node involvement, elevated postoperative CA19-9 levels, and poorly differentiated tumors. These findings are also supportive of the notion that micrometastases develop early in the pathogenesis of pancreatic ductal adenocarcinoma.29,30,31 Furthermore, this might explain why R status is associated with survival but not with local recurrence. Previous studies have shown that even low-grade pancreatic intraepithelial neoplasms with oncogenic KRAS mutations can migrate away from the glandular preneoplasm into the surrounding tissue and circulatory system representing early epithelial-to-mesenchymal transition.30 Using autopsy and radiological data from 101 patients, Haeno et al31 proposed that pancreatic cancer grows at an exponential rate and that cells with high metastatic competency were generated during tumor expansion in 1 in a million pancreatic cancer cells. From this modeling, they predicted that even very small primary tumors frequently produced microscopic metastasis prior to surgical removal. The autopsy series also revealed that a small subset of patients died with only locally advanced disease, suggesting that some tumors may lack metastasis-promoting factors (or have metastasis-suppressing factors) or have metastases that are especially sensitive to systemic therapy.31 In this study, we found that 78 of 458 patients (17.0%) who died (or 10.7% of all 730 patients) did so without evidence of recurrence or metastases. This compares with 161 of 692 patients (23.3%) in the study by Groot et al,3 and in 13 of 81 patients (16%) in 4 autopsy studies collectively.20,21,22,23 Although the association between N2 status, CA19-9, and death without identified recurrence implies a proportion had unconfirmed recurrence, it is not possible to further elucidate this based on the trial data.
Of particular importance, we found that there were no statistically significant differences between the time to recurrence and subsequent and overall survival between local and distant recurrence. It has been assumed that patients with local recurrence have a less aggressive tumor biology and slower growing tendency than those patients with distant metastases and might benefit from additional local treatment of recurrence such as stereotactic body radiation therapy.3,32 The lack of survival differences between local and distant recurrence in this study challenge this hypothesis. The LAP07 randomized trial33 in patients with locally advanced pancreatic cancer with disease controlled after 4 months of induction chemotherapy found no overall survival with chemoradiotherapy compared with chemotherapy alone but with added toxicity. Moreover, in an autopsy study Iacobuzio-Donahue et al20 found that 30% of patients died with localized pancreatic cancer and 70% died with metastatic disease and that primary tumor DPC4 expression was associated with limited metastatic disease burden (<10 metastases), while loss of DPC4 expression was associated with widespread metastatic disease (>1000 metastases). Although these observations suggest a degree of clonality to explain the divergent patterns of failure, they were unrelated to clinical stage at initial presentation, treatment history, or histopathologic features. Similarly, in this study, there were variances in the determinates predicting local and distant recurrence and death without recurrence suggesting clonality but without significant differences in survival patterns. However, within the group of patients with distant metastases, there were significant survival differences. Patients with liver and lung metastases had the shortest survival of any group or subgroup. Lung metastases occurred much later than liver metastases. Patients with lung metastases also had longer survival from time of recurrence as well as longer overall survival than those with lung-only metastases. This is in keeping with 2 clinical studies3,34 and supported by experimental evidence.35,36 It now seems apparent that most lung and liver metastases from pancreatic cancer are monophyletic, with subclones giving rise to both liver and lung metastases in parallel.35 Nevertheless, pancreatic cancer metastases often involve seeding by more than 1 clone, and subsequent metastatic tumor growth may actually be more dependent on the stromal environment of the metastatic site.36 The development of specific management strategies for patients with metastatic lung cancer are warranted.
The updated American Joint Committee on Cancer Cancer Staging Manual, 8th edition, staging system for pancreatic cancer makes a new distinction between N1 (<4 nodes) and N2 (≥4 nodes) disease.26 In this series, N2 disease was associated with more distant recurrence but not more local recurrence, supporting the clinical utility of this updated staging system. A positive resection margin was strongly associated with poorer overall survival in the main study group. Point estimates (data not shown) suggest this association is maintained in patients who develop local recurrence or death prior to recurrence but not in patients who develop distant recurrence. In this study, N1 status and N2 status were each independent factors significantly associated with local recurrence along with adjuvant treatment allocation, while N1 status was also an independent determinate for distant recurrence. Interestingly, Honselmann et al37 found that lymph node status was predictive of time to recurrence but not location of recurrence.
Limitations
A number of potential confounders exist in this analysis. Because follow-up was performed according to local protocol, not all patients were routinely imaged in the same way, but the detection rate for recurrence in patients who died (19.7%) approximated the rate in historical autopsy studies (16.1%).20,21,22,23 Additional treatment was given to 94 of 243 patients (39%) in the gemcitabine group with relapse and 77 of 236 patients (33%) in the gemcitabine plus capecitabine group,7 but it is unclear whether early detection and treatment of recurrence confers an overall survival benefit. Trial data only captured site of first recurrence, which was subsequently stratified as local, distant, or synchronous local and distant. Subsequent sites of recurrence were not recorded and so the patterns of progression from local recurrence to eventual distant recurrence and/or death were not evaluable. It may be that capturing only the first site of recurrence also partly explains the lower rates of combined local/distant recurrence seen in this series compared with others.3 In future, more detailed data capture on patterns of first (and subsequent) recurrence would help better define the arc of the disease. In addition, no patients in the ESPAC 4 trial received neoadjuvant therapy. The growing use of neoadjuvant treatments are likely to affect patterns of postresection disease recurrence, and trials of neoadjuvant therapy should therefore capture these data to allow comparison.
Conclusions
Pancreatic cancer can still be regarded as a systemic disease despite resection and irrespective of site of recurrence. This supports the further development of adjuvant systemic therapy after resection to increase the long-term survival rate.
Trial protocol
eTable 1. Multivariable Analysis of Overall Survival After Identification of Recurrence
eTable 2. Competing Risks Analysis for Local Recurrence Versus Distant Recurrence Versus Death Without Recurrence
eFigure 1. Postprogression Survival as Cumulative Incidence Plots From Time of Surgery
eFigure 2. Kaplan–Meier Curves Showing Survival from Time of Surgery
References
- 1.Kleeff J, Korc M, Apte M, et al. . Pancreatic cancer. Nat Rev Dis Primers. 2016;2:16022. doi: 10.1038/nrdp.2016.22 [DOI] [PubMed] [Google Scholar]
- 2.Strobel O, Neoptolemos J, Jäger D, Büchler MW. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. 2019;16(1):11-26. doi: 10.1038/s41571-018-0112-1 [DOI] [PubMed] [Google Scholar]
- 3.Groot VP, Rezaee N, Wu W, et al. . Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2018;267(5):936-945. doi: 10.1097/SLA.0000000000002234 [DOI] [PubMed] [Google Scholar]
- 4.Winter JM, Brennan MF, Tang LH, et al. . Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol. 2012;19(1):169-175. doi: 10.1245/s10434-011-1900-3 [DOI] [PubMed] [Google Scholar]
- 5.Konstantinidis IT, Warshaw AL, Allen JN, et al. . Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? what is a “true” R0 resection? Ann Surg. 2013;257(4):731-736. doi: 10.1097/SLA.0b013e318263da2f [DOI] [PubMed] [Google Scholar]
- 6.Khorana AA, Mangu PB, Berlin J, et al. . Potentially curable pancreatic cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35(20):2324-2328. doi: 10.1200/JCO.2017.72.4948 [DOI] [PubMed] [Google Scholar]
- 7.Neoptolemos JP, Palmer DH, Ghaneh P, et al. ; European Study Group for Pancreatic Cancer . Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011-1024. doi: 10.1016/S0140-6736(16)32409-6 [DOI] [PubMed] [Google Scholar]
- 8.Conroy T, Hammel P, Hebbar M, et al. ; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group . FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379(25):2395-2406. doi: 10.1056/NEJMoa1809775 [DOI] [PubMed] [Google Scholar]
- 9.Katz MH, Shi Q, Ahmad SA, et al. . Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg. 2016;151(8):e161137. doi: 10.1001/jamasurg.2016.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hackert T, Sachsenmaier M, Hinz U, et al. . Locally advanced pancreatic cancer: neoadjuvant therapy with FOLFIRINOX results in resectability in 60% of the patients. Ann Surg. 2016;264(3):457-463. doi: 10.1097/SLA.0000000000001850 [DOI] [PubMed] [Google Scholar]
- 11.Murphy JE, Wo JY, Ryan DP, et al. . Total neoadjuvant therapy with FOLFIRINOX followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2018;4(7):963-969. doi: 10.1001/jamaoncol.2018.0329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 13.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi: 10.1158/0008-5472.CAN-14-0155 [DOI] [PubMed] [Google Scholar]
- 14.Neoptolemos JP, Dunn JA, Stocken DD, et al. ; European Study Group for Pancreatic Cancer . Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358(9293):1576-1585. doi: 10.1016/S0140-6736(01)06651-X [DOI] [PubMed] [Google Scholar]
- 15.Neoptolemos JP, Stocken DD, Friess H, et al. ; European Study Group for Pancreatic Cancer . A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350(12):1200-1210. doi: 10.1056/NEJMoa032295 [DOI] [PubMed] [Google Scholar]
- 16.Neoptolemos JP, Stocken DD, Bassi C, et al. ; European Study Group for Pancreatic Cancer . Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304(10):1073-1081. doi: 10.1001/jama.2010.1275 [DOI] [PubMed] [Google Scholar]
- 17.Johnstone PA, Sindelar WF. Patterns of disease recurrence following definitive therapy of adenocarcinoma of the pancreas using surgery and adjuvant radiotherapy:correlations of a clinical trial. Int J Radiat Oncol Biol Phys. 1993;27(4):831-834. doi: 10.1016/0360-3016(93)90456-6 [DOI] [PubMed] [Google Scholar]
- 18.Kayahara M, Nagakawa T, Ueno K, Ohta T, Takeda T, Miyazaki I. An evaluation of radical resection for pancreatic cancer based on the mode of recurrence as determined by autopsy and diagnostic imaging. Cancer. 1993;72(7):2118-2123. doi: [DOI] [PubMed] [Google Scholar]
- 19.Hishinuma S, Ogata Y, Tomikawa M, Ozawa I, Hirabayashi K, Igarashi S. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J Gastrointest Surg. 2006;10(4):511-518. doi: 10.1016/j.gassur.2005.09.016 [DOI] [PubMed] [Google Scholar]
- 20.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. . DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27(11):1806-1813. doi: 10.1200/JCO.2008.17.7188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnelldorfer T, Ware AL, Sarr MG, et al. . Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Ann Surg. 2008;247(3):456-462. doi: 10.1097/SLA.0b013e3181613142 [DOI] [PubMed] [Google Scholar]
- 22.Gnerlich JL, Luka SR, Deshpande AD, et al. . Microscopic margins and patterns of treatment failure in resected pancreatic adenocarcinoma. Arch Surg. 2012;147(8):753-760. doi: 10.1001/archsurg.2012.1126 [DOI] [PubMed] [Google Scholar]
- 23.Suenaga M, Fujii T, Kanda M, et al. . Pattern of first recurrent lesions in pancreatic cancer: hepatic relapse is associated with dismal prognosis and portal vein invasion. Hepatogastroenterology. 2014;61(134):1756-1761. [PubMed] [Google Scholar]
- 24.Campbell F, Smith RA, Whelan P, et al. . Classification of R1 resections for pancreatic cancer: the prognostic relevance of tumour involvement within 1 mm of a resection margin. Histopathology. 2009;55(3):277-283. doi: 10.1111/j.1365-2559.2009.03376.x [DOI] [PubMed] [Google Scholar]
- 25.Sobin LH, Gospodarowicz MK, Wittekind C, eds. TNM Classification of Malignant Tumours 7th ed. UICC, Oxford, England: Wiley-Blackwell; 2009. [Google Scholar]
- 26.Amin MB, Edge SB, Greene FL, et al. , eds. AJCC Cancer Staging Manual. 8th ed New York, NY: Springer; 2017. doi: 10.1007/978-3-319-40618-3 [DOI] [Google Scholar]
- 27.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 28.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716-723. doi: 10.1109/TAC.1974.1100705 [DOI] [Google Scholar]
- 29.Tuveson DA, Neoptolemos JP. Understanding metastasis in pancreatic cancer: a call for new clinical approaches. Cell. 2012;148(1-2):21-23. doi: 10.1016/j.cell.2011.12.021 [DOI] [PubMed] [Google Scholar]
- 30.Rhim AD, Mirek ET, Aiello NM, et al. . EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148(1-2):349-361. doi: 10.1016/j.cell.2011.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haeno H, Gonen M, Davis MB, Herman JM, Iacobuzio-Donahue CA, Michor F. Computational modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell. 2012;148(1-2):362-375. doi: 10.1016/j.cell.2011.11.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wild AT, Hiniker SM, Chang DT, et al. . Re-irradiation with stereotactic body radiation therapy as a novel treatment option for isolated local recurrence of pancreatic cancer after multimodality therapy: experience from two institutions. J Gastrointest Oncol. 2013;4(4):343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammel P, Huguet F, van Laethem JL, et al. ; LAP07 Trial Group . Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315(17):1844-1853. doi: 10.1001/jama.2016.4324 [DOI] [PubMed] [Google Scholar]
- 34.Van den Broeck A, Sergeant G, Ectors N, Van Steenbergen W, Aerts R, Topal B. Patterns of recurrence after curative resection of pancreatic ductal adenocarcinoma. Eur J Surg Oncol. 2009;35(6):600-604. doi: 10.1016/j.ejso.2008.12.006 [DOI] [PubMed] [Google Scholar]
- 35.Reiter JG, Makohon-Moore AP, Gerold JM, et al. . Reconstructing metastatic seeding patterns of human cancers. Nat Commun. 2017;8:14114. doi: 10.1038/ncomms14114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maddipati R, Stanger BZ. Pancreatic cancer metastases harbor evidence of polyclonality. Cancer Discov. 2015;5(10):1086-1097. doi: 10.1158/2159-8290.CD-15-0120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Honselmann KC, Pergolini I, Castillo CF, et al. . Timing but not patterns of recurrence is different between node-negative and node-positive resected pancreatic cancer. Ann Surg. 2019. doi: 10.1097/SLA.0000000000003123 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eTable 1. Multivariable Analysis of Overall Survival After Identification of Recurrence
eTable 2. Competing Risks Analysis for Local Recurrence Versus Distant Recurrence Versus Death Without Recurrence
eFigure 1. Postprogression Survival as Cumulative Incidence Plots From Time of Surgery
eFigure 2. Kaplan–Meier Curves Showing Survival from Time of Surgery