Abstract
Amblyopia is the most common cause of monocular visual impairment affecting 2-5% of the general population. Amblyopia is a developmental cortical disorder of the visual pathway essentially due to abnormal visual stimulus, reaching the binocular cortical cells, which may be multivariate. Ganglion cells are of two types: parvocellular (P cells) and magnocellular (M cells); they are the first step where the light energy is converted in to neural impulse. P cells are involved in fine visual acuity, fine stereopsis, and color vision and M cells are involved in gross stereopsis and movement recognition. Strabismus, refractive error, cataract, and ptosis, occurring during critical period are highly amblyogenic. The critical period extends from birth to 7--8 years. The earlier the clinically significant refractive error and strabismus are detected and treated, the greater the likelihood of preventing amblyopia. Treatment for amblyopia in children includes: optical correction of significant refractive errors, patching, pharmacological treatment, and alternative therapies which include: vision therapy, binocular therapy, and liquid crystal display eyeglasses are newer treatment modalities for amblyopia. Age of starting the treatment is not predictive of outcome, instituting treatment on detection and early detection plays a role in achieving better outcomes. This review aims to give a simplified update on amblyopia, which will be of use to a clinician, in understanding the pathophysiology of the complex condition. We also share the cortical aspects of amblyopia and give recent developments in the treatment of amblyopia.
Keywords: Amblyopia, atropine penalization, optical correction, patching, vision therapy
Amblyopia is a cortical developmental disorder, secondary to abnormal visual inputs to each eye occurring early in life (during the cortical plasticity stage) where in dissimilar action potentials (in amplitude or time, or both) generated in the retina reach the cortex. These cortical changes entice the visual cortex to prefer one eye over the other, leading to a number of functional deficiencies in the eye, altered visual function like decreased vernier acuity, and impaired contrast sensitivity, particularly to detect high spatial frequency stimuli and impaired motor signs like hand-eye coordination and spatial localization, and it can be either unilateral or bilateral.[1] Amblyopia is the most common cause of monocular visual impairment affecting 2–5% of the general population.
Prevalence estimates from population-based studies in children age 6--71 months range from 0.73 to 1.9%,[2] whereas school-based studies of older children typically report higher rates (range: 1.0--5.5%)[2] depending on the population studied and the definition used.[2,3,4,5,6,7,8,9,10,11,12,13,14] Bilateral amblyopia is less frequent than unilateral, but the reported proportion varies considerably, from as low as 5% up to 41% of all cases of amblyopia,[4,11,12,13,14,15,16] and 2.7-18 times greater when strabismus is present.[12,15,17,18,19]
Risk factors for amblyopia are premature birth, small for gestational age,[20,21,22,23,24] developmental delay,[24] or having a first-degree relative with amblyopia.[25,26] Environmental factors, including maternal substance abuse during pregnancy, have been reported to be associated with an increased risk of amblyopia or strabismus in some studies.[27,28,29,30,31,32] However, some studies have refuted the same.[12,15,33]
In this review, we attempt to give an insight in to the various aspects of the normal cortical development during early postnatal life. The topic is covered in different headings to make it convenient for the practitioner, to comprehend the various treatment modalities, when to initiate, or terminate, and what to expect.
Anatomy and physiology of the retino-geniculo-cortical pathway
A clear understanding of the retino--geniculo--cortical path way is very essential for the proper understanding of amblyopia. We have included a brief note about the passage of electrical impulse from retinal ganglion cell to the primary visual cortex and beyond. In amblyopia, the cortical changes are not limited to V1 and extend beyond. The so-called critical period extends from birth to 7--8 years. Strabismus, refractive error, cataract, and ptosis, occurring during this critical period are highly amblyopiogenic. The normal visual experience during the critical period is essential for the cortical development, and this continues postnatally till the age of 7 years. After this, the cortical plasticity decreases, but is never fully lost till early 50s.[34] The ganglion cell is the first step where the light energy is converted in to neural impulse.[35] Hence, for the purposes of this review, we shall confine ourselves from ganglion cells onwards.
Simply put, ganglion cells are of two types: parvocellular (P cells) and magnocellular (m cells) types.[35] The former are in the foveal and parafoveal areas and the latter in the perifoveal and peripheral areas of retina. There are many direction-sensitive retinal ganglion cells, which fire when the image moves in one direction and not in the other direction.[35] P cells are involved in fine visual acuity, fine stereopsis, and color vision and M cells are involved in gross stereopsis and movement recognition.[35] P cells have a larger representation in the sensory cortical areas, compared with M cells.[36] There is again dichotomy of the ganglion cells. Those axons of the ganglion cells on the nasal side of a line splitting the fovea (in to nasal and temporal halves) cross over to the opposite side and those on temporal side go to the geniculate nucleus on the same side.[36]
Retinal fibers end in the lateral geniculate nucleus (a part of the thalamus and distinctly stratified) in specific layers. The right and left fibers are distinctly separate. The parvocellular and magnocellular fibers end in different layers in the lateral geniculate nucleus. Nearly 90% geniculate afferents are concerned with the vision.[35,36] For the purposes of discussion here, we shall ignore the rest of the 10% of the fibers. In the lateral geniculate nucleus, there is some modification of the stimulus from each eye, although topographical representation is exactly the same as retina. The adjacent retinal ganglion cells discharge on to the adjacent geniculate cells.
Cortical development
In their classical studies of normal visual development and the effects of deprivation, Hubel and Wiesel showed that the axons of relay cells in the lateral geniculate nucleus (LGN) that terminate in layer IV of the primate primary visual cortex (area 17) segregated over the first 3 weeks of life to form the ocular dominance columns (ODC) in which the inputs from the two eyes alternate equally.[37] However, if one eye was closed at birth, its ocular dominance bands became very narrow and those from the fellow eye expand. This enlargement of the LGN cell bodies is thought to occur because they must sustain more extensive axonal arborizations in their enlarged ocular dominance bands in the visual cortex.
When closure of an eye was delayed until 6--7 months of age, this produced only a very small difference in size between cells in the deprived and undeprived LGN laminae after 2 months of closure indicating that there is a second sensitive period in which the pattern of changes produced by visual deprivation is different.[38] The number of synapse in the primate visual cortex continues to increase until at least 6 months of age before progressively falling back to adult levels.[39] It may be the elaboration of these connections during development that leads to their increasing susceptibility to visual deprivation and the different effects of monocular deprivation at later ages.[40] Such connections are more important for parvocellular than magnocellular cells. In all likelihood, the shrinkage of parvocellular cell bodies in the LGN occurs because monocular closure prevents binocular cooperation and the cortical axonal arborizations of parvocellular cells related to both eyes make fewer connections and are smaller than normal.[41]
During the postnatal period, the cortical circuitry is not mature. The ocular dominance columns are amenable to alteration. Plasticity is a developmental necessity and the maturity of the ODC continues till the age of 36 months postnatally and during this maturation process visual experience from the two eyes must be matched[42] The maturation is completed by the GABAergic interneurons in the layer 2 and 3 of V1.[43] These interneurons are inhibitory in nature and inhibition of these interneurons can theoretically prolong the plasticity period.[44] The maturation process makes the cortical circuitry immune to altered visual experience as happens after the age of 10 years. However, the cortical plasticity is never fully lost in adults, and if there are any ways of restoring plasticity of the cortical circuitry, amblyopia is curable.
Cortical changes causing amblyopia
Orssaud et al. in their article on amblyopia state that amblyopia is a developmental disorder of the entire visual system, including the extrastriate cortex, although it manifests as impaired visual acuity in the amblyopic eye, other abnormalities of visual function such as decreased contrast sensitivity and stereoscopic vision are observed, and some abnormalities can be found in the “good” eye. Since amblyopia occurs during the critical period of brain development, it may be due to organic pathology of the visual pathways, visual deprivation, or functional abnormalities, mainly anisometropia or strabismus.[45]
The suppression of the visual input from the weaker eye has been suggested as the underlying reason of the amblyopic syndrome, although it is still an unresolved question as to what extent neural responses to the visual information coming from the amblyopic eye are suppressed during binocular viewing.[46] To address this question, Körtvélyes et al. measured event-related potentials (ERPs) to foveal face stimuli in amblyopic patients, both in monocular and binocular viewing conditions. They found early ERP components were reduced and delayed in the case of monocular stimulation of the amblyopic eye as compared with the fellow eye stimulation or to binocular viewing and the input from the amblyopic eye is completely suppressed already at the earliest stages of visual cortical processing during binocular viewing.
What are other features affected in amblyopic eye?
Prior studies by McKee et al. have found a reduction in contrast sensitivity in eyes with amblyopia using sinusoidal gratings, whereas minimal loss has been reported with Pelli-Robson charts. Most studies have evaluated contrast sensitivity at the time of diagnosis of amblyopia or after short-term treatment.[47] Repka et al. have evaluated the contrast sensitivity using Pelli-Robson low contrast letter charts at age 10 years, several years after treatment of amblyopia and found that the distribution of contrast sensitivity in the amblyopic eye was similar to that reported for monocular testing of normal 10-year olds.[48]
The younger the patients at enrollment into the randomized trial (3 to <5 years compared with those 5 to <7 years), the more likely to have slightly better contrast sensitivity in the amblyopic eye at 10 years of age This effect, if substantiated, could be due to a number of factors like a younger age at treatment allowing more complete cortical development, and a shorter duration of the vision deficit. Each of these circumstances might allow a more complete treatment effect or alternatively a shorter period of insult and thus less profound insult to the developing visual sensory system. Nevertheless, it seems likely that mild residual amblyopia is associated with only a mild reduction in contrast sensitivity after treatment of moderate amblyopia from strabismus, anisometropia, or both.
Slyshalova in their study recorded different types of electroretinograms (ERG) as part of the International Society for Clinical Electrophysiology of Vision (ISCEV) standard, as well as macular (15%) pattern and multifocal ERG in 41 children aged 5--17 years, who had high amblyopia with varying gaze fixation and a visual acuity of 0.03--0.1. In high amblyopia, the mixed, macular, and flicker (30 Hz) ERGs were unchanged; however, some patients had supernormal a-wave of a mixed ERG, subnormal a-a- and b-waves of a macular ERG, and a moderately subnormal ERG pattern. Recording of a multifocal ERG showed lower retinal density values in the first and second rings.[49] Karlica et al. correlated visual evoked potential (VEP) parameters (amplitude and latency) with visual acuity of the amblyopic eye and found that VEP may be a valid method to determine amblyopia.[50] Thus, in high amblyopia, there are characteristic retinal bioelectrical activity impairments recorded under different conditions of stimulation and adaptation, which suggest that there are impaired interreceptor relations at the retinal level. These changes statistically significantly differ from those in organic retinal defects, which may be a criterion for their differential diagnosis.
Effect on stereopsis: In individuals with amblyopia, the relationship between the visual acuity of the amblyopic eye and stereoacuity is complex, as illustrated by Fig. 1, replotted from a large-scale study.[51] Overall, worse visual acuity seems to correlate with worse stereoacuity. However, this relationship seems mostly driven by anisometropic subjects (blue symbols). Indeed, over the entire range of amblyopic eye visual acuities, there are amblyopes who are essentially stereo-blind (red and gray symbols plotted along the top of the graph). These are mainly strabismic amblyopes, whether purely strabismic or mixed (strabismic and anisometropic). It is worth noting that constant strabismics with good acuity in both eyes are generally stereoblind.[52] However, recent work suggests that coarse stereopsis may be selectively spared in stereo-deficient children with a history of amblyopia.[53]
Figure 1.
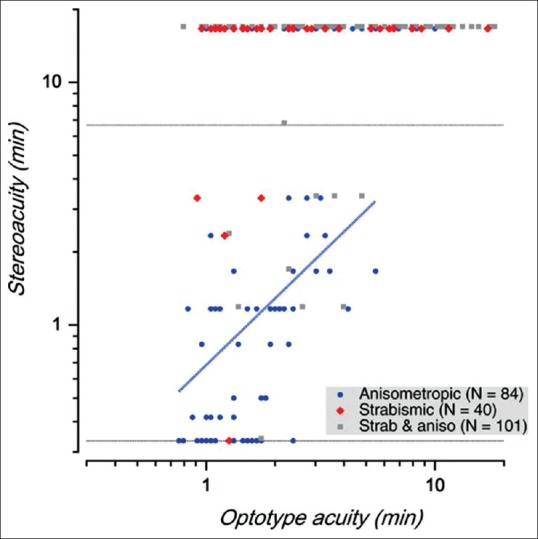
The relationship between the visual acuity of the amblyopic eye and stereoacuity
Fig. 1 indicates stereoacuity vs. visual acuity. The dotted lines show the upper and lower limits of the test. The data for strabismic anisometropes (gray squares) have been slightly displaced for clarity.[51] The blue regression line suggests that worse visual acuity goes hand in hand with worse stereoacuity in anisometropic amblyopes; however, this relationship does not hold in strabismic amblyopes or strabismic anisometropes.[51]
Are all amblyopias the same?
Classification of amblyopia is based on etiology, which is heterogeneous, may be caused by stimulus deprivation, strabismus, refractive error, or a combination of these. Amblyopia is usually unilateral, but it may be bilateral in cases of bilateral high refractive error or bilateral ocular pathology, such as cataract.[54]
Keech et al. in their paper mention that the commonest risk factors for amblyopia are constant strabismus and different refractive errors in each eye and age of the child when exposed to an amblyopia-inducing condition is the most important determinant for the development of amblyopia.[54]
Vojnosanit Pregl have published in their work that differences exist in psychophysical functions between the fovea and the retinal periphery in human strabismic amblyopia, on the one hand, and in anisometropic and visual deprivation amblyopia, on the other. There are also differences in the severity and reversibility of the various types of amblyopia. The Pediatric Subcommittee of the Royal College of Ophthalmologists on Management of amblyopia states that the basic amblyogenic mechanisms are the same even though their contribution to each type of amblyopia varies.[54] Stimulus deprivation amblyopia occurs when a physical obstruction along the line of sight prevents the formation of a well-focused, high-contrast image on the retina. The time of onset and extent of form deprivation are two important factors which determine the degree to which amblyopia develops. Unilateral form deprivation leads to denser amblyopia than bilateral form deprivation in the first 3 months of age as against first 6 months of age in bilateral cases. Early and vigorous institution of treatment for such cases is necessary for better prognosis for normal vision development.
When the onset of the cause of deprivation occurs after the first 6–12 months, the prognosis for vision recovery is improved with early treatment.[54] Unilateral strabismic amblyopia can develop in a child with either constant unilateral squint or a monocular fixation defect. It occurs far more often in esotropes. Anisometropic amblyopia occurs when an interocular difference in spherical or cylindrical refractive error exceeds certain limits. In spherical anisometropia, a minimum difference of 1.25 DS may be significant.[55,56]
What are the treatment options?
Prevention
Vision screening is important to identify factors that predispose to amblyopia.[57,58] The earlier the clinically significant refractive error and strabismus are detected and treated, the greater the likelihood of preventing amblyopia.[59] When amblyopia is present, it appears that the potential for successful treatment is greatest in young children, although improvement in visual acuity can reasonably be expected in older children and teenagers.[60,61,62]
A study by Pediatric Eye Disease Investigator Group of treatment of moderate strabismic and/or anisometropic amblyopia demonstrated that the visual acuity of the amblyopic eye improved to 20/30 or better 6 months after initiating treatment in approximately three-quarters of children under 7 years of age.[63]
Choice of therapy
Success rates of amblyopia treatment decline with increasing age.[57,64,65] However, treatment should be offered to all regardless of age. The prognosis for attaining normal vision in an amblyopic eye depends on many factors, including the age of onset; the cause, severity, and duration of amblyopia; the history of and response to previous treatment;[57] adherence to treatment recommendations and coexisting conditions.
Correction of the cause of amblyopia, correction of refractive error, and promotion of use of the amblyopic eye over the normal eye forms the basis of the treatment strategy. The goal of treatment is equal visual acuity between the two eyes, which may or may not be achieved in all cases. The treatment should be based on the child's age, visual acuity, and compliance and response to previous treatment as well as the child's physical, social, and psychological status.
Treatment for amblyopia in children includes:
Optical correction of significant refractive errors
Patching
Pharmacological treatment
Refractive surgery
Alternative therapies
Optical correction
Treatment of refractive error alone is the initial step in care of children 0--17 years of age with amblyopia.[10,57,60] Refractive error correction and compliance with the refractive correction is a challenge for patients with one eye having good visual acuity compared with other as many patients with this anisometropic or ametropic amblyopia reject the use of glasses. In such cases where the consistent use of glasses is difficult, surgical correction of refractive error is successful in achieving visual improvement.
Patching[63,66,67] is initiated for children who do not improve with eye glasses alone.[66] The amblyopia treatment study (ATS) found that 6 h of prescribed daily patching produces an improvement in visual acuity that is similar in magnitude to full time occlusion therapy prescribed for treating severe amblyopia (20/100 to 20/400) in children under 7 years of age.[68] In children who have moderate amblyopia (20/40 to 20/80), initial therapy of 2 h of prescribed daily patching produces an improvement in visual acuity that is similar in magnitude to the improvement produced by 6 h of daily patching.[66] The treatment benefit achieved by the patching appears stable through at least 15 years of age. Patching should be considered for older children and teenagers, particularly if they have not previously been treated.[57]
Pharmacological treatment
Cycloplegia
Pharmacological treatment that produces cycloplegia of the nonamblyopic eye can be considered for children who do not improve with eye glasses alone or compliance to patching is low due to various reasons, presence of latent nystagmus, or maintenance therapy.[62,63,64,69] It works best when the nonamblyopic eye is hyperopic. The cycloplegia optically defocuses the nonamblyopic eye.[62] The benefit achieved by pharmacologic treatment remains stable through 15 years of age.[70]
Pharmacological treatment has been prescribed using a variety of dosage schemes to the fellow eye. Traditionally, daily dosing was used and has been shown to be as effective as patching for initial treatment.[62] Atropine 1% given on two consecutive days per week for 4 months was as effective as once daily atropine 1% for moderate amblyopia, treated for 4 months.[69] Modest improvement of 4.5 lines (95% CI, 3.2–5.8 lines) from twice weekly dosing has been reported for children from 3 to 12 years of age with severe amblyopia.[71] There may be a small benefit to augmenting atropine therapy with a plano lens over the hyperopic fellow eye for children who have stopped improving with atropine 1%.[72]
Levodopa-Carbidopa
Iuvone et al. have proposed a theory that increasing levels of dopamine may improve vision in the context of amblyopia. Some investigators have reported that levels of retinal dopamine are decreased in deprivation amblyopia.[73] There have been several clinical trials that have evaluated the use of levodopa across a range of patients. PEDIG investigators organized a randomized trial of levodopa for the treatment of amblyopia in an older cohort of patients (children aged 7–12 years). When prescribed daily levodopa with carbidopa in addition to continued 2 h/day of patching, no clinically significant or meaningful improvement in VA was seen in a different prospective trial with a larger cohort of patients. Sofia et al. assessed children who had previously received spectacles but were otherwise treatment-naïve were prescribed full-time patching and then randomized to levodopa or placebo. They reported statistically significant visual gains sustained at 1 year of follow-up for children; however, the levodopa dosage was three times higher than in the PEDIG study.[74]
Citicoline
Citicoline confers both cholinergic and neuroprotective properties. Initial work in adult patients demonstrated improvement in VA with citicoline augmentation of patching that was not sustained following cessation of the medication. Early studies in amblyopic children were promising, showing treatment effect with citicoline both alone and in addition to patching. A study of treatment-naïve participants randomized to added citicoline after a run-in patching phase showed a significant treatment effect at 90 days for the citicoline-augmented group. However, failure to demonstrate improvement in the control group (2 h a day of patching) was unexpected and therefore results from this study should be cautiously interpreted. Research into the use of citicoline is arguably behind that of levodopa and at the time of this review, all the studies of citicoline failed to include follow-up periods beyond 3–6 months.[75,76]
Drawbacks to medical therapy
Medical therapy for amblyopia appears to be well tolerated. A liquid suspension of levodopa is available, although has an unpleasant bitter taste. Side effects are mild, with mild nausea, vomiting and headache being described. The addition of carbidopa to the prescribed formulation reduces these gastrointestinal side effects by inhibiting peripheral conversion of levodopa to dopamine. Because carbidopa cannot cross the blood--brain barrier, it only prevents levodopa conversion peripherally and allows more central activity of levodopa. The PEDIG study showed regression of treatment effect with drug cessation. Therefore, randomized controlled trials with ample follow-up still remain necessary. Side effects of citicoline were negligible in all studies. And oral as well as intramuscular formulations are available. Medical therapy, in isolation or in addition to conventional therapy, is in the research and development stages.[77]
Other drugs
Ongoing clinical trials with drugs targeting the neuromodulatory systems show promise for amblyopia treatment in adult patients. Selective serotonin reuptake inhibitors (SSRI) treatment has been shown to augment visually-evoked potentials (VEPs) in normal human subjects. In a few adult patients with amblyopia, SSRI (citalopram) enhanced visual acuity improvements when combined with two weeks of occlusion therapy, but effects in the population were not significantly different from placebo.[78] Another study pairing SSRIs with video game training demonstrated that while video games improved visual acuity, no added value of the SSRI treatment was observed.[79] It is possible that such behavioral and pharmacological manipulations reach a ceiling effect if they engage similar neuromodulatory pathways. Stryker et al. in their paper mention an ongoing clinical study at Boston Children's Hospital is using donepezil, a cholinesterase inhibitor that is typically used to treat Alzheimer's disease, to boost cholinergic signaling, and recover vision in amblyopic patients is mentioned.
The research strongly supports the need to combine a pharmacological approach with personalized behavioral training, with the goal of targeting plasticity within specific brain regions or specific cortical circuits. However, more research into the matter is awaited.
Role of physical activity
Lunghi and Sale found that adult subjects who intermittently cycled on a stationary bicycle while watching a movie showed enhanced effects of transient eye patching compared with those subjects who watched the movie while sitting still. Moreover, recovery from amblyopia is expedited by tasks requiring coordination of hand and eye movements, such as having patients manipulate objects during visual training as reviewed in Daw, in 2013. Several researchers have pointed out that patients with amblyopia exhibit oculomotor impairments including problems with saccadic eye movements,[80] smooth pursuit,[81] fixation stability,[82] hand--eye coordination,[83] thus suggesting that targeting visuomotor circuits during treatment may help to alleviate some of these deficits.[84]
Refractive surgery
Refractive surgery has demonstrated benefits for the population of children with refractive amblyopia who are noncompliant with spectacle wear or nonresponsive to standard treatment in multiple case series. Evidence also suggests that correction of ametropia in children with neurobehavioral disorders that preclude spectacle correction improves not only vision but also global functioning. Clear lens extraction has shown some benefit, but not the robust gains that PRK and pIOL treatments have demonstrated. While there are no randomized controlled trials to support widespread adoption of these techniques, PEDIG is currently planning Amblyopia Treatment Study 19, which is a controlled randomized clinical trial that will compare PRK versus nonsurgical treatment of anisometropic amblyopia in children who have failed conventional treatment. The results from this trial may provide yet more evidence for the use of refractive surgery in the management of amblyopia.[85]
Alternative Therapies
Vision therapy
Vision therapy (also termed “orthoptics” or eye exercises) is defined as a doctor-prescribed, nonsurgical program of visual activities to improve visual acuity and binocularity.[86] These include computer programs, prisms, filters, metronomes, vergence activities, accommodation activities, antisuppression activities, and eye--hand coordination exercises.[87] These are often conducted in an office setting with a therapist, supplemented with home exercises. These treatments have also been promoted for the treatment of amblyopia as an adjunct to patching.[88] However, there is insufficient evidence to recommend vision therapy techniques.[87,89]
Perceptual learning
Perceptual learning was defined in 1963 by Eleanor Gibson simply put as performance on simple visual tasks shows improvement with practice in adults.
Studies by Polat et al. suggest that perceptual learning in adult amblyopes can augment visual function. Improved pretest to posttest performance and gains in visual acuity (VA) were reported when subjects participated in a learnt trial of Gabor signals in a series of 77 adult amblyopes. The neural basis for this is postulated to result from a reduction in lateral inhibition within the brain with training.[89,90] The criticism of this approach is that gains on test outcome measures in the amblyopic eye do not transfer to novel situations—improvement is only seen for the task practiced. Perceptual learning has yet to gain widespread support. Most studies contained very small numbers of participants. Perceptual learning effects have been demonstrated to last hours to months without continued practice, but long-term follow-up is lacking. Additionally, implementation of a successful clinical programme of treatment would require the ability to perform training at home while the aforementioned studies required perceptual learning tasks to be in a laboratory setting.[77]
Binocular therapy/dichoptic therapy
Binocular therapy has been used to treat amblyopia in children with no strabismus or small-angle strabismus with some binocularity. Images are presented dichoptically; high-contrast images are presented to the amblyopic eye and low-contrast images are presented to the fellow eye. The binocular treatment was adapted to an iPad® (Apple, Inc., Cupertino, CA) device as a “falling blocks” game, which uses red--green anaglyphic eyeglasses to allow dichoptic presentation. Although early nonrandomized studies were promising,[91,92,93,94] results from a recent randomized trial failed to demonstrate that game play prescribed 1 h per day was as good as patching prescribed 2 h per day.[95] Although research is ongoing, there is insufficient evidence to recommend binocular therapy for treatment of amblyopia.
Liquid Crystal Display Eyeglasses
Intermittent occlusion therapy using liquid crystal eyeglasses has been introduced as an alternative treatment for amblyopia that may be associated with better treatment compliance. The eyeglasses alternate between a clear and opaque lens before the fellow eye. Spierer et al. and Erbagci et al. and Wang et al. in their respective publications have found them efficacious to patching.[95,96,97]
Use of microsensor therapy
There is a commercially available 8 × 12 mm small Thera Mon microsensor (TheraMon®-Chip, MC Technology GmbH). This sensor allows a simple objective documentation of the therapy compliance of patches and glasses. It samples the surrounding temperature in regular intervals. Due to the specific temperatures, it is possible to detect the time of application and, therefore, the compliance. Therefore, TheraMon microsensor could be a study-related approach for monitoring the compliance and further leading to possible improvement of application time protocols in amblyopia therapy.[98]
Comparison between various treatment modalities
There are no studies comparing the conventional modalities of amblypia management like patching and penalization versus the newer modalities like dichoptic therapy, liquid crystal, glasses etc.
Atropine penalization and occlusion were well tolerated by child and family but compliance was found better with atropine penalization as the cost of atropine penalization is less than that of conventional patching. Studies have recommended that atropine penalization should be used as first-line treatment for amblyopia.[99]
Conclusion
Amblyopia is a developmental cortical disorder of the visual path way that contributes to amblyopia formation, essentially due to abnormal visual stimulus, reaching the binocular cortical cells, which may be multivariate. Screening prior to age of 2--3 years may help in early detection and prompt treatment may reduce the prevalence of amblyopia. While age of starting the treatment is not predictive of outcome, instituting treatment on detection and early detection plays a role in achieving better outcomes for amblyopia. Successful treatment has been reported to 63--83% of patients.[57] Refractive correction alone may be successful in treating anisometropic amblyopia and minimal occlusion, and/or atropine penalization can provide initial vision improvement that may improve compliance with subsequent long duration of treatment. Refractive correction in infants substantially reduces the incidence of accommodative esotropia and amblyopia without interference with emmetropization.[99,100] However, interpretation of the currently available literature is made difficult due to inaccurate measurement of visual acuity at the initial visit, improper refractive correction, and paucity of long-term follow-up results.[100]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Martin PR, Lee BB, White AJ, Solomon SG, Rüttiger L. Chromatic sensitivity of ganglion cells in the peripheral primate retina. Nature. 2001;410:933–6. doi: 10.1038/35073587. [DOI] [PubMed] [Google Scholar]
- 2.Snowdon S, Stewart-Brown S. Effectiveness of preschool vision screening. Nurs Stand. 1997;12:44–5. doi: 10.7748/ns.12.15.44.s51. [DOI] [PubMed] [Google Scholar]
- 3.Pediatric Eye Disease Investigator Group. A randomized trial of near versus distance activities while patching for amblyopia in children aged 3 to less than 7 years. Ophthalmology. 2008;115:2071–8. doi: 10.1016/j.ophtha.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pediatric Eye Disease Investigator Group Writing Committee. A randomized trial comparing Bangerter filters and patching for the treatment of moderate amblyopia in children. Ophthalmology. 2010;117:998–04. doi: 10.1016/j.ophtha.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhola R, Keech RV, Kutschke P, Pfeifer W, Scott WE. Recurrence of amblyopia after occlusion therapy. Ophthalmology. 2006;113:2097–100. doi: 10.1016/j.ophtha.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 6.Tacagni DJ, Stewart CE, Moseley MJ, Fielder AR. Factors affecting the stability of visual function following cessation of occlusion therapy for amblyopia. Graefes Arch Clin Exp Ophthalmol. 2007;245:811–6. doi: 10.1007/s00417-006-0395-2. [DOI] [PubMed] [Google Scholar]
- 7.Walsh LA, Hahn EK, LaRoche GR. The method of treatment cessation and recurrence rate of amblyopia. Strabismus. 2009;17:107–16. doi: 10.1080/09273970903126709. [DOI] [PubMed] [Google Scholar]
- 8.Scheiman MM, Hertle RW, Beck RW, Edwards AR, Birch E, Cotter SA, et al. Randomized trial of treatment of amblyopia in children aged 7 to 17 years. Arch Ophthalmol. 2005;123:437–47. doi: 10.1001/archopht.123.4.437. [DOI] [PubMed] [Google Scholar]
- 9.Repka MX, Kraker RT, Beck RW, Birch E, Cotter SA, Holmes JM, et al. Treatment of severe amblyopia with weekend atropine: Results from 2 randomized clinical trials. J AAPOS. 2009;13:258–63. doi: 10.1016/j.jaapos.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheiman MM, Hertle RW, Kraker RT, Beck RW, Birch EE, Felius J, et al. Patching vs atropine to treat amblyopia in children aged 7 to 12 years: A randomized trial. Arch Ophthalmol. 2008;126:1634–42. doi: 10.1001/archophthalmol.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pediatric Eye Disease Investigator Group. A randomized trial of atropine versus patching for treatment of moderate amblyopia: Follow-up at 10 years of age. Arch Ophthalmol. 2008;126:1039–44. doi: 10.1001/archopht.126.8.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medghalchi AR, Dalili S. A randomized trial of atropine vs patching for treatment of moderate amblyopia. Iran Red Crescent Med J. 2011;13:578–81. [PMC free article] [PubMed] [Google Scholar]
- 13.Menon V, Shailesh G, Sharma P, Saxena R. Clinical trial of patching versus atropine penalization for the treatment of anisometropic amblyopia in older children. J AAPOS. 2008;12:493–7. doi: 10.1016/j.jaapos.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Felius J, Chandler DL, Holmes JM, Chu RH, Cole SR, Hill M, et al. Evaluating the burden of amblyopia treatment from the parent and child's perspective. J AAPOS. 2010;14:389–95. doi: 10.1016/j.jaapos.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes JM Pediatric Eye Disease Investigator Group. A randomized pilot study of near activities versus non-near activities during patching therapy for amblyopia. J AAPOS. 2005;9:129–36. doi: 10.1016/j.jaapos.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Alotaibi AG, Fawazi SM, Alenazy BR, Abu-Amero KK. Outcomes of 3 hours part-time occlusion treatment combined with near activities among children with unilateral amblyopia. Saudi Med J. 2012;33:395–8. [PubMed] [Google Scholar]
- 17.Moseley MJ, Neufeld M, McCarry B, Charnock A, McNamara R, Rice T, et al. Remediation of refractive amblyopia by optical correction alone. Ophthalmic Physiol Opt. 2002;22:296–9. doi: 10.1046/j.1475-1313.2002.00034.x. [DOI] [PubMed] [Google Scholar]
- 18.Cleary M, Moody AD, Buchanan A, Stewart H, Dutton GN. Assessment of a computer-based treatment for older amblyopes: The Glasgow Pilot Study. Eye (Lond) 2009;23:124–31. doi: 10.1038/sj.eye.6702977. [DOI] [PubMed] [Google Scholar]
- 19.Li RW, Ngo C, Nguyen J, Levi DM. Video-game play induces plasticity in the visual system of adults with amblyopia. PLoS Biol. 2011;9:e1001135. doi: 10.1371/journal.pbio.1001135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herbison N, Cobb S, Gregson R, Ash I, Eastgate R, Purdy J, et al. Interactive binocular treatment (I-BiT) for amblyopia: Results of a pilot study of 3D shutter glasses system. Eye (Lond) 2013;27:1077–83. doi: 10.1038/eye.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Thompson B, Deng D, Chan LY, Yu M, Hess RF, et al. Dichoptic training enables the adult amblyopic brain to learn. Curr Biol. 2013;23:308–9. doi: 10.1016/j.cub.2013.01.059. [DOI] [PubMed] [Google Scholar]
- 22.Spiegel DP, Li J, Hess RF, Byblow WD, Deng D, Yu M, et al. Transcranial direct current stimulation enhances recovery of stereopsis in adults with amblyopia. Neurotherapeutics. 2013;10:831–9. doi: 10.1007/s13311-013-0200-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leguire LE, Rogers GL, Walson PD, Bremer DL, McGregor ML. Occlusion and levodopa-carbidopa treatment for childhood amblyopia. JAAPOS. 1998;2:257–64. doi: 10.1016/s1091-8531(98)90080-5. [DOI] [PubMed] [Google Scholar]
- 24.Mohan K, Dhankar V, Sharma A. Visual acuities after levodopa administration in amblyopia. J Pediatr Ophthalmol Strabismus. 2001;38:62–7. doi: 10.3928/0191-3913-20010301-05. [DOI] [PubMed] [Google Scholar]
- 25.Repka MX, Kraker RT, Beck RW, Atkinson CS, Bacal DA, Bremer DL, et al. Pilot study of levodopa dose as treatment for residual amblyopia in children aged 8 years to younger than 18 years. Arch Ophthalmol. 2010;128:1215–7. doi: 10.1001/archophthalmol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dadeya S, Vats P, Malik KP. Levodopa/carbidopa in the treatment of amblyopia. J AAPOS. 2009;46:87–90. doi: 10.3928/01913913-20090301-07. [DOI] [PubMed] [Google Scholar]
- 27.Morishita H, Miwa JM, Heintz N, Hensch TK. Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science. 2010;330:1238–40. doi: 10.1126/science.1195320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao J, Lam DS, Chen LJ, Wang Y, Zheng C, Lin Q, et al. Randomized controlled trial of patching vs acupuncture for anisometropic amblyopia in children aged 7 to 12 years. Arch Ophthalmol. 2010;128:1510–7. doi: 10.1001/archophthalmol.2010.306. [DOI] [PubMed] [Google Scholar]
- 29.Lam DS, Zhao J, Chen LJ, Wang Y, Zheng C, Lin Q, et al. Adjunctive effect of acupuncture to refractive correction on anisometropic amblyopia: One-year results of a randomized crossover trial. Ophthalmology. 2011;118:1501–11. doi: 10.1016/j.ophtha.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 30.Althomali TA. Posterior chamber toricphakic IOL implantation for the management of pediatric anisometropicamblyopia. J Refract Surg. 2013;29:396–400. doi: 10.3928/1081597X-20130410-01. [DOI] [PubMed] [Google Scholar]
- 31.Paysse EA, Coats DK, Hussein MA, Hamill MB, Koch DD. Long-term outcomes of photorefractive keratectomy for anisometropic amblyopia in children. Ophthalmology. 2006;113:169–76. doi: 10.1016/j.ophtha.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Joslin CE, Mcmahon TT, Kaufman LM. The effectiveness of occluder contact lenses in improving occlusion compliance in patients that have failed traditional occlusion therapy. Optom Vis Sci. 2002;79:376–80. doi: 10.1097/00006324-200206000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Pradeep A, Proudlock FA, Awan M, Bush G, Collier J, Gottlob I, et al. An educational intervention to improve adherence to high-dosage patching regimen for amblyopia: A randomised controlled trial. Br J Ophthalmol. 2014;98:865–70. doi: 10.1136/bjophthalmol-2013-304187. [DOI] [PubMed] [Google Scholar]
- 34.Sloper J. The other side of amblyopia. J AAPOS. 2016;20:1.e1–13. doi: 10.1016/j.jaapos.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Denison RN, Vu AT, Yacoub E, Feinberg DA, Silver MA. Functional mapping of the magnocellular and parvocellular subdivisions of human LGN. Neuroimage. 2014;102:358–69. doi: 10.1016/j.neuroimage.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purves D, Augustine GJ, Fitzpatrick D, Hall WC, LaMantia AS, McNamara JO, et al. Neuroscience 2008. De Boeck, Sinauer, Sunderland: Mass; 2014. [Google Scholar]
- 37.Le Vay S, Wiesel TN, Hubel DH. The development of ocular dominance columns in normal and visually deprived monkeys. J Comp Neurol. 1980;191:1–51. doi: 10.1002/cne.901910102. [DOI] [PubMed] [Google Scholar]
- 38.Headon MP, Sloper JJ, Hiorns RW, Powell TP. Sizes of neurons in the primate lateral geniculate nucleus during normal development. Brain Res. 1985;18:51–6. doi: 10.1016/0165-3806(85)90249-4. [DOI] [PubMed] [Google Scholar]
- 39.O'Kusky J, Colonnier M. Postnatal changes in the number of neurons and synapses in the visual cortex (area 17) of the macaque monkey: A stereological analysis in normal and monocularly deprived animals. J Comp Neurol. 1982;210:291–306. doi: 10.1002/cne.902100308. [DOI] [PubMed] [Google Scholar]
- 40.Headon MP, Sloper JJ, Hiorns RW, Powell TP. Effects of monocular closure at different ages on deprived and undeprived cells in the primate lateral geniculate nucleus. Brain Res. 1985;18:57–78. doi: 10.1016/0165-3806(85)90250-0. [DOI] [PubMed] [Google Scholar]
- 41.Horton JC. Cytochrome oxidase patches: A new cytoarchitectonic feature of monkey visual cortex. Philos Trans R Soc Lond B Biol Sci. 1984;304:199–253. doi: 10.1098/rstb.1984.0021. [DOI] [PubMed] [Google Scholar]
- 42.Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron. 2012;75:230–49. doi: 10.1016/j.neuron.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Magueresse C, Monyer H. GABAergic interneurons shape the functional maturation of the cortex. Neuron. 2013;77:388–405. doi: 10.1016/j.neuron.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 44.Sengpiel F. Plasticity of the visual cortex and treatment of amblyopia. Curr Biol. 2014;24:R936–40. doi: 10.1016/j.cub.2014.05.063. [DOI] [PubMed] [Google Scholar]
- 45.Orssaud C. Amblyopia. J Fr Ophtalmol. 2014;37:486–96. doi: 10.1016/j.jfo.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Körtvélyes J, Bankó É, Andics A, Rudas G, Németh J, Hermann P, et al. Visual cortical responses to the input from the amblyopic eye are suppressed during binocular viewing. Acta Biol Hung. 2012;63(Suppl 1):65–79. doi: 10.1556/ABiol.63.2012.Suppl.1.7. [DOI] [PubMed] [Google Scholar]
- 47.Moseley MJ, Stewart CE, Fielder AR, Stephens DA MOTAS cooperative. Intermediate spatial frequency letter contrast sensitivity: Its relation to visual resolution before and during amblyopia treatment. Ophthalmic Physiol Opt. 2006;26:1–4. doi: 10.1111/j.1475-1313.2005.00343.x. [DOI] [PubMed] [Google Scholar]
- 48.Repka MX, Kraker RT, Beck RW, Cotter SA, Holmes JM, Arnold RW, et al. Contrast sensitivity following amblyopia treatment in children. Arch Ophthalmol. 2009;127:1225–7. doi: 10.1001/archophthalmol.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slyshalova NN, Shamshinova AM. Retinal bioelectrical activity in amblyopia. Vestn Oftalmol. 2008;124:32–6. [PubMed] [Google Scholar]
- 50.Karlica D, Galetović D, Bućan K, Znaor L, Skelin S. Changes in visual evoked potentials curve parameters in children with amblyopia. Acta Med Croatica. 2009;63:321–4. [PubMed] [Google Scholar]
- 51.Levi DM, McKee SP, Movshon JA. Visual deficits in anisometropia. Vision Res. 2011;51:48–57. doi: 10.1016/j.visres.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levi DM, Knill DC, Bavelier D. Stereopsis and amblyopia: A mini-review. Vision Res. 2015;114:17–30. doi: 10.1016/j.visres.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giaschi D, Lo R, Narasimhan S, Lyons C, Wilcox LM. Sparing of coarse stereopsis in stereodeficient children with a history of amblyopia? J Vis. 2013;13 doi: 10.1167/13.10.17. doi: 10.1167/13.10.17. [DOI] [PubMed] [Google Scholar]
- 54.Keech RV, Kutschke PJ. Upper age limit for the development of amblyopia. J PediatrOphthalmol Strabismus. 1995;32:89–93. doi: 10.3928/0191-3913-19950301-07. [DOI] [PubMed] [Google Scholar]
- 55.Powell C, Wedner S, Richardson S. Screening for correctable visual acuity deficits in school-age children and adolescents. Cochrane Database Syst Rev. 2005;25:CD005023. doi: 10.1002/14651858.CD005023.pub2. [DOI] [PubMed] [Google Scholar]
- 56.American Academy of Ophthalmologists. Amblyopia is a medical condition. [Last accessed on 2009 Jul 08]. Available from: http://one.aao.org .
- 57.Scheiman MM, Hertle RW, Beck RW, Edwards AR, Birch E, Cotter SA, et al. Randomized trial of treatment of amblyopia in children aged 7 to 17 years. Arch Ophthalmol. 2005;123:437–47. doi: 10.1001/archopht.123.4.437. [DOI] [PubMed] [Google Scholar]
- 58.Donahue SP, Arthur B, Neely DE, Arnold RW, Silbert D, Ruben JB AAPOS Vision Screening Committee. Guidelines for automated preschool vision screening: A 10-year, evidence-based update. J AAPOS. 2013;17:4–8. doi: 10.1016/j.jaapos.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 59.Williams C, Northstone K, Harrad RA, Sparrow JM, Harvey I. Amblyopia treatment outcomes after screening before or at age 3 years: Follow up from randomised trial. BMJ. 2002;324:1549. doi: 10.1136/bmj.324.7353.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eibschitz-Tsimhoni M, Friedman T, Naor J, Eibschitz N, Friedman Z. Early screening for amblyogenic risk factors lowers the prevalence and severity of amblyopia. J AAPOS. 2000;4:194–9. doi: 10.1067/mpa.2000.105274. [DOI] [PubMed] [Google Scholar]
- 61.Kvarnström G, Jakobsson P, Lennerstrand G. Visual screening of Swedish children: An ophthalmological evaluation. Acta Ophthalmol Scand. 2001;79:240–4. doi: 10.1034/j.1600-0420.2001.790306.x. [DOI] [PubMed] [Google Scholar]
- 62.US Preventive Services Task Force. Vision screening for children 1 to 5 years of age: US Preventive Services Task Force Recommendation statement. Pediatrics. 2011;127:340–6. doi: 10.1542/peds.2010-3177. [DOI] [PubMed] [Google Scholar]
- 63.Glaser SR, Matazinski AM, Sclar DM, Sala NA, Vroman CM, Tanner CE, et al. A randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Arch Ophthalmol. 2002;120:268–78. doi: 10.1001/archopht.120.3.268. [DOI] [PubMed] [Google Scholar]
- 64.Mohan K, Saroha V, Sharma A. Successful occlusion therapy for amblyopia in 11-to 15-year-old children. J Pediatr Ophthalmol Strabismus. 2004;41:89–95. doi: 10.3928/0191-3913-20040301-08. [DOI] [PubMed] [Google Scholar]
- 65.Holmes JM, Lazar EL, Melia BM, Astle WF, Dagi LR, Donahue SP, et al. Effect of age on response to amblyopia treatment in children. Arch Ophthalmol. 2011;129:1451–7. doi: 10.1001/archophthalmol.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Repka MX, Beck RW, Holmes JM, Birch EE, Chandler DL, Cotter SA, et al. A randomized trial of patching regimens for treatment of moderate amblyopia in children. Arch Ophthalmol. 2003;121:603–11. doi: 10.1001/archopht.121.5.603. [DOI] [PubMed] [Google Scholar]
- 67.Repka MX, Wallace DK, Beck RW, Kraker RT, Birch EE, Cotter SA, et al. Two-year follow-up of a 6-month randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Arch Ophthalmol. 2005;123:149–57. doi: 10.1001/archopht.123.2.149. [DOI] [PubMed] [Google Scholar]
- 68.Pediatric Eye Disease Investigator Group. A randomized trial of atropine regimens for treatment of moderate amblyopia in children. Ophthalmology. 2004;111:2076–85. doi: 10.1016/j.ophtha.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 69.Repka MX, Cotter SA, Beck RW, Kraker RT, Birch EE, Everett DF, et al. Pediatric Eye Disease Investigator Group. A randomized trial of atropine regimens for treatment of moderate amblyopia in children. Ophthalmology. 2004;111:2076–85. doi: 10.1016/j.ophtha.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 70.Repka MX, Kraker RT, Holmes JM, Summers AI, Glaser SR, Barnhardt CN. Atropine vs patching for treatment of moderate amblyopia: Follow-up at 15 years of age of a randomized clinical trial. JAMA Ophthalmol. 2014;132:799–805. doi: 10.1001/jamaophthalmol.2014.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Repka MX, Kraker RT, Beck RW, Birch E, Cotter SA, Holmes JM, et al. Treatment of severe amblyopia with weekend atropine: Results from 2 randomized clinical trials. J AAPOS. 2009;13:258–63. doi: 10.1016/j.jaapos.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wallace DK, Lazar EL, Repka MX, Holmes JM, Kraker RT, Hoover DL, et al. A randomized trial of adding a plano lens to atropine for amblyopia. J AAPOS. 2015;19:42–8. doi: 10.1016/j.jaapos.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iuvone PM, Tigges M, Fernandes A, Tigges J. Dopamine synthesis and metabolism in rhesus monkey retina: Development, aging, and the effects of monocular visual deprivation. Vis Neurosci. 1989;2:465–71. doi: 10.1017/s0952523800012360. [DOI] [PubMed] [Google Scholar]
- 74.Sofi IA, Gupta SK, Bharti A, Tantry TG. Efficiency of the occlusion therapy with and without levodopa-carbidopa in amblyopic children-A tertiary care centre experience. Int J Health Sci. 2016;10:249–57. [PMC free article] [PubMed] [Google Scholar]
- 75.Campos EC, Schiavi C, Benedetti P, Bolzani R, Porciatti V. Effect of citicoline on visual acuity in amblyopia: Preliminary results. Graefes Arch Clin Exp Ophthalmol. 1995;233:307–12. doi: 10.1007/BF00177654. [DOI] [PubMed] [Google Scholar]
- 76.Fresina M, Dickmann A, Salerni A, De Gregorio F, Campos EC. Effect of oral CDP-choline on visual function in young amblyopic patients. Graefes Arch Clin Exp Ophthalmol. 2008;246:143–50. doi: 10.1007/s00417-007-0621-6. [DOI] [PubMed] [Google Scholar]
- 77.Kraus CL, Culican SM. New advances in amblyopia therapy I: Binocular therapies and pharmacologic augmentation. Br J Ophthalmol. 2018;102:1492–6. doi: 10.1136/bjophthalmol-2018-312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thompson B, Lagas AK, Stinear CM, Byblow WD, Russel BR, Kydd RR, et al. The use of selective serotonin reuptake inhibitors to treat amblyopia in adulthood. Invest Ophthalmol Vis Sci. 2014;55:801. [Google Scholar]
- 79.Uusitalo H. Hermo Pharma Reports Topline Data with HER-801 from Clinical Study in Adult Amblyopia. Retrieved September 21. 2016 [Google Scholar]
- 80.Perdziak M, Witkowska DK, Gryncewicz W, Ober JK. Not only amblyopic but also dominant eye in subjects with strabismus show increased saccadic latency. J Vis. 2016;16:12. doi: 10.1167/16.10.12. [DOI] [PubMed] [Google Scholar]
- 81.Raashid RA, Liu IZ, Blakeman A, Goltz HC, Wong AM. The initiation of smooth pursuit is delayed in anisometropic amblyopia. Invest Ophthalmol Vis Sci. 2016;57:1757–64. doi: 10.1167/iovs.16-19126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chung ST, Kumar G, Li RW, Levi DM. Characteristics of fixational eye movements in amblyopia: Limitations on fixation stability and acuity.? Vision Res. 2015;114:87–99. doi: 10.1016/j.visres.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Niechwiej-Szwedo E, Goltz HC, Chandrakumar M, Wong AM. Effects of strabismic amblyopia and strabismus without amblyopia on visuomotor behavior: III. Temporal eye–hand coordination during reaching. Invest Ophthalmol Vis Sci. 2014;55:7831–8. doi: 10.1167/iovs.14-15507. [DOI] [PubMed] [Google Scholar]
- 84.Stryker MP, Loewel S. Amblyopia: New molecular/pharmacological and environmental approaches. Vis Neurosci. 2018;35:E018. doi: 10.1017/S0952523817000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kraus CL, Culican SM. New advances in amblyopia therapy II: Refractive therapies. Br J Ophthalmol. 2018;102:1611–4. doi: 10.1136/bjophthalmol-2018-312173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scheiman M, Wick B. Clinical Management of Binocular Vision: Heterophoric, Accommodative, and Eye Movement Disorders. Lippincott Williams and Wilkins; 2008. [Google Scholar]
- 87.Suttle CM. Active treatments for amblyopia: A review of the methods and evidence base. Clin Exp Optom. 2010;93:287–99. doi: 10.1111/j.1444-0938.2010.00486.x. [DOI] [PubMed] [Google Scholar]
- 88.Li RW, Young KG, Hoenig P, Levi DM. Perceptual learning improves visual performance in juvenile amblyopia. Invest Ophthal Vis Sci. 2005;46:3161–8. doi: 10.1167/iovs.05-0286. [DOI] [PubMed] [Google Scholar]
- 89.Helveston EM. Visual training: Current status in ophthalmology. Am J Ophthalmol. 2005;140:903–10. doi: 10.1016/j.ajo.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 90.Hussain Z, Webb BS, Astle AT, McGraw PV. Perceptual learning reduces crowding in amblyopia and in the normal periphery. J Neurosci. 2012;32:474–80. doi: 10.1523/JNEUROSCI.3845-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li SL, Jost RM, Morale SE, De La Cruz A, Dao L, Stager D, Jr, et al. Binocular iPad treatment of amblyopia for lasting improvement of visual acuity. JAMA Ophthalmol. 2015;133:479–80. doi: 10.1001/jamaophthalmol.2014.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Birch EE, Li SL, Jost RM, Morale SE, De La Cruz A, Stager D, Jr, et al. Binocular iPad treatment for amblyopia in preschool children. J AAPOS. 2015;19:6–11. doi: 10.1016/j.jaapos.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Knox PJ, Simmers AJ, Gray LS, Cleary M. An exploratory study: Prolonged periods of binocular stimulation can provide an effective treatment for childhood amblyopia. Invest Ophthalmol Vis Sci. 2012;53:817–24. doi: 10.1167/iovs.11-8219. [DOI] [PubMed] [Google Scholar]
- 94.Holmes JM, Manh VM, Lazar EL, Beck RW, Birch EE, Kraker RT, et al. Effect of a binocular iPad game vs part-time patching in children aged 5 to 12 years with amblyopia: A randomized clinical trial. JAMA Ophthalmol. 2016;134:1391–400. doi: 10.1001/jamaophthalmol.2016.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Spierer A, Raz J, BenEzra O, Herzog R, Cohen E, KarshaiI, et al. Treating amblyopia with liquid crystal glasses: A pilot study. Invest Ophthalmol Vis Sci. 2010;51:3395–8. doi: 10.1167/iovs.09-4568. [DOI] [PubMed] [Google Scholar]
- 96.Erbaǧcı I, Okumuş S, Öner V, Coşkun E, Çelik O, Ören B, et al. Using liquid crystal glasses to treat amblyopia in children. J AAPOS. 2015;19:257–9. doi: 10.1016/j.jaapos.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 97.Wang J, Neely DE, Galli J, Schliesser J, Graves A, Damarjian TG, et al. A pilot randomized clinical trial of intermittent occlusion therapy liquid crystal glasses versus traditional patching for treatment of moderate unilateral amblyopia. J AAPOS. 2016;19:257–9. doi: 10.1016/j.jaapos.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Januschowski K, Rickmann A, Emmerich C, Abaza A, Bechtold TE, Schott TC, et al. Der Einsatz eines Mikrosensors in der Amblyopietherapie. Klinische Monatsblätter für Augenheilkunde. 2017 doi: 10.1055/s-0043-118221. Nov 08. [DOI] [PubMed] [Google Scholar]
- 99.Li T, Shotton K. Conventional occlusion versus pharmacologic penalization for amblyopia. Cochrane Database Syst Rev. 2009;4:CD006460. doi: 10.1002/14651858.CD006460.pub2. doi: 10.1002/14651858.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Simons K. Amblyopia characterization, treatment, and prophylaxis. Surv Ophthalmol. 2005;50:123–66. doi: 10.1016/j.survophthal.2004.12.005. [DOI] [PubMed] [Google Scholar]


