An 80-year-old lady in good health with primary angle closure glaucoma (OU) since 15 years and on topical levobunalol 0.5%, bimatoprost 0.03%, and dorzolomide 2% had her intraocular pressure (IOP) under control. During her visit in January 2019, her visual fields showed progression and IOP recorded was 20 and 26 mmHg in OD and OS, respectively. Bimatoprost 0.03% was stopped and changed to brimonidine 0.2%. Two weeks after the use of brimonidine, she presented with raised IOP (26 mmHg) in both eyes with granulomatous anterior uveitis [Fig. 1a and b], with no posterior segment involvement.
Figure 1.
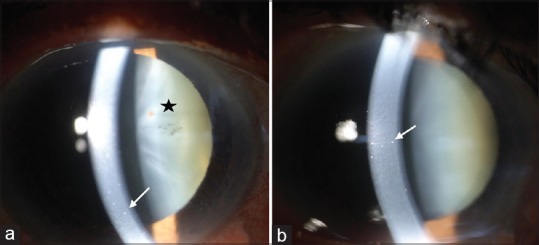
(a) Anterior segment photograph of the right eye showing medium-sized keratic precipitates on the endothelium (white arrow). Pigments can be seen over the anterior capsule of the lens (star). (b) Anterior segment photograph of the left eye showing medium-sized keratic precipitates on the endothelium (white arrow)
What is Your Next Step?
Investigate for systemic causes of granulomatous uveitis
Start on topical steroids
Start on systemic steroids or immunosuppressants if the inflammation is not under control
Stop brimonidine.
Correct Answer: D
Findings
The patient was asked to stop brimonidine eye drops and was started on prednisolone acetonide 1% eye drops. Investigations to rule out systemic causes of granulomatous uveitis like Mantoux test, chest X-ray, serum angiotensin converting enzyme, and venereal disease research laboratory test were not contributory. One week later, she had no signs of ocular inflammation. She had a similar history in the past on changing the antiglaucoma medication. At 3 months of follow-up after stopping the drug, there were no signs of inflammation in both eyes.
Diagnosis
Brimonidine-induced granulomatous uveitis
Discussion
Allergic conjunctivitis and skin excoriation are the most common side effects of brimonidine.[1] Although rare, brimonidine can cause granulomatous uveitis in the elderly.[2,3] It usually occurs 7–12 months after starting the drug and is seen in elderly patients. The uveitis usually resolves with drug discontinuation. In our case, the uveitis occurred 2 weeks after starting the drug. It has been hypothesized that brimonidine induces T-cell/macrophage activation and interaction.[3] Thus, though rare, granulomatous uveitis can occur with brimonidine and clinicians need to be aware of its usage in elderly.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Katz LJ. Brimonidine tartrate 0.2% twice dailyvstimolol 0.5% twice daily: 1-year results in glaucoma patients. Brimonidine Study Group. Am J Ophthalmol. 1999;127:20–6. doi: 10.1016/s0002-9394(98)00286-4. [DOI] [PubMed] [Google Scholar]
- 2.Goyal R, Ram AR. Brimonidine tartrate 0.2% (Alphagan) associated granulomatous anterior uveitis. Eye (Lond) 2000;14:908–10. doi: 10.1038/eye.2000.250. [DOI] [PubMed] [Google Scholar]
- 3.Beltz J, Zamir E. Brimonidine induced anterior uveitis. Ocul Immunol Inflamm. 2016;24:128–33. doi: 10.3109/09273948.2015.1037845. [DOI] [PubMed] [Google Scholar]


