Abstract
Purpose:
The sub-retinal injections are not very commonly performed procedures in vitreoretina, but form a crucial step in any cell replacement therapy for retinal diseases. The purpose of this study is to describe the learning curve of a trained vitreo-retinal surgeon in sub-retinal injections in a rat model and its implications in future clinical trials.
Methods:
This is an in-vivo retrospective animal study using Wistar rats. All ARVO guidelines regarding animal handling were followed. After anesthetization, aspectic preparation and dilating the pupils with 1% tropicamide eye drops, subretinal injection of 10 μl saline was done via a limbal entry. Data recorded included time taken for the procedure, success of injection, associated complications, post-operative infections and complications. The rats were followed up for 1 month post procedure. A trend analysis was done for the above factors to look for improvement in ease of procedure, reduction in procedure time and reduction in complications for the clinician using a novel objective scale.
Results:
About 20 eyes were studied. Mean weight of the rats was 188 ± 12.82 gram. Mean time taken for the procedure was 14.1 ± 5.07 minutes. There was a significant inverse co-relation between the serial number of the eye and time taken for the procedure (r = −0.89, P < 0.0001). Comparative complications noted between the first ten and the last ten eyes were: conjunctival tear 30% versus 10% (P = 0.27), lens touch 50% versus 10% (P = 0.05), subretinal hemorrhage 40% versus 0% (P = 0.13), vitreous loss 30% versus 0% (P = 0.06). The successful subretinal injection without intraocular complications was achieved in 40% versus 90% (P = 0.02). There was a significant co-relation between the serial number of the eye and ease of the procedure (r = 0.87, P < 0.0001). Post operatively none of the eyes had any infection. Six eyes (12%) developed cataract and 3 eyes (6%) had non-resolving retinal detachment at the last examination visit.
Conclusion:
Subretinal injections in rats have a definite learning curve even for a trained vitreoretinal surgeon. This should be accounted for and resources allocated accordingly to achieve good technical comfort and negate confounding by the surgeon factor in the results of future clinical trials
Keywords: Rat model, retinal stem cells, subretinal injections
The subretinal space is an ideal target site for drug delivery and gene therapy purposes.[1,2,3,4,5,6] This is especially true for therapies intended at regeneration of the photoreceptors (PR) and/or the retinal pigment epithelium (RPE). In comparison to an intravitreal injection, subretinal injections have a greater direct effect on the target cells in the subretinal space. Currently diseases like age related macular degeneration and retinitis pigmentosa do not have any definitive curative therapy. But, several studies have shown that there is promise in sub-retinal cellular replacement therapy in these conditions.[7,8,9,10,11,12] To test the survival, safety and functionality of the injected cells, it is imperative to perform sub-retinal injections in a rodent model and acquire technical expertise. The techniques of subretinal injection of cells are multiple and vary from trans-scleral injections,[13,14] subretinal implants[15,16] and subretinal injection following vitrectomy.[17,18] Attempting subretinal implants or a vitrectomy can be a very challenging situation and often leads to complications. A trans-scleral approach via a hypodermic needle is a relatively safer technique in small animals.
In clinical vitreoretinal practice, subretinal injections are not uncommon, for clearance of subretinal hemorrhages. While the procedure has a relatively small learning curve in human eyes due to familiar anatomy and larger size, it has a different set of challenges in a small animal eye. As attempts in translational regenerative therapy are being made around the world, it is increasingly imperative for the clinician to be able to participate in animal research to further the cause. This requires the clinician to be well versed with the technique of subretinal injections in a small animal eye and be able to do it safely and effectively. In the current communication, we describe our experience and learning curve of a trained vitreo-retinal surgeon in sub-retinal injections in a rat model which would have implications for future clinical trials.
Methods
This was an in-vivo retrospective interventional animal study. The study was conducted at the National Institute of Nutrition and at the LV Prasad Eye Institute, Hyderabad, India with appropriate Institutional Review Board approval. All animal handling was done according to the Statement of the use of animals in ophthalmic and visual research as suggested by the Association for Research in Vision and Ophthalmology (ARVO).[19] About 20 eyes of 20 Wistar rats were included in the study. All rats were anesthetized by a trained veterinarian using 80 mg/kg ketamine and 12 mg/kg xylazine. Adequate anesthesia was confirmed after 5 minutes by observing the wince reflex by pinching the ear lobe or the tail. Post anesthesia, the pupils were dilated using 1% tropicamide eye drops.
Painting and preparation of the eye was done using 5% povidone iodine eye drops and solution. [Fig. 1]. The animal was laid under a dissecting microscope and the eyelids were retracted using a custom-made eye clamp. A glass cover slip was secured on the cornea after instilling viscoelastic to allow visualization of the fundus during the procedure. Using a micro-vitreoretinal blade, the sclera was incised to access the vitreous cavity. Through the entry, a hypodermic needle was inserted and advanced to the subretinal space. In the subretinal space, 10 μl of saline was injected to raise a small bleb using a Hamilton syringe and 27G needle. [Figs. 1 and 2] Data recorded included time taken for the procedure, success of injection, associated complications, post-operative infections and complications. The rats were followed up for 1 month post procedure. A trend analysis was done for the above factors to look for improvement in ease of procedure, reduction in procedure time and reduction in complications for the clinician using a novel objective scale [Table 1].
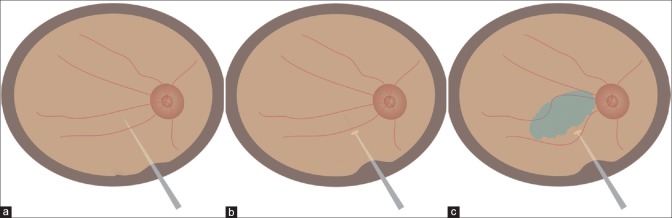
Figure 1.
Cartoon showing (a) entry of a micro-vitreoretinal blade into the vitreous cavity via an incision behind the limbus. (b) A retinotomy created by the MVR blade. (c) Sub retinal injection done by 27G needle with Hamilton syringe
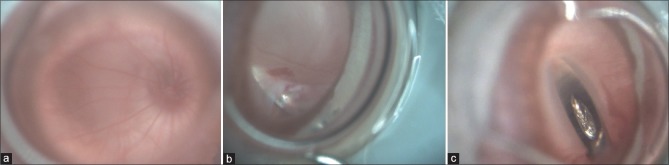
Figure 2.
Panel showing (a) a normal focused rat retina. (b) A retinotomy created by the MVR blade. (c) Sub retinal injection done by 27G needle with Hamilton syringe
Table 1.
Table showing calculation of comfort score for subretinal injections in a rat eye
| Present | Absent | |
|---|---|---|
| Conjunctival tears | 0 | 1 |
| Lens touch | 0 | 1 |
| Cataract formation on follow up | 0 | 1 |
| Subretinal/viteous hemorrhage | 0 | 1 |
| Retinal detachment | 0 | 1 |
| Time taken <10 min | 3 | - |
| Time taken 10<->20 min | 2 | - |
| Time taken >20 min | 1 | - |
A score of ≥5 was assigned as good comfort
Statistical analysis
Statistical analysis was done using MedCalcVer 18.11 (Ostend, Belgium). Mean with standard deviation was calculated for all continuous parametric variables whereas, median was reported for non-parametric variables. Pearson's co-relation coefficient was calculated to assess the effect of the serial number of the eye operated on the ease of the procedure and the time taken for the surgery. A P value of <0.05 was assigned to be statistically significant.
Results
About 20 eyes were studied. Mean weight of the rats was 188 ± 12.82 gram. Mean time taken for the procedure was 14.1 ± 5.07 minutes. There was a significant inverse co-relation between the serial number of the eye and time taken for the procedure (r = −0.89, P < 0.0001) [Graph 1]. Comparative complications noted between the first ten and the last ten eyes were: conjunctival tear 30% versus 10% (P = 0.27), lens touch 50% versus 10% (P = 0.05), subretinal hemorrhage 40% versus 0% (P = 0.13), vitreous loss 30% versus 0% (P = 0.06). Successful subretinal injection without intraocular complications was achieved in 40% versus 90% (P = 0.02) [Table 2]. There was a significant co-relation between the serial number of the eye and ease of the procedure (r = 0.87, P < 0.0001) [Graph 2]. Post operatively none of the eyes had any infection. Six eyes (12%) developed cataract and 3 eyes (6%) had non-resolving retinal detachment at the last examination visit.
Graph 1.
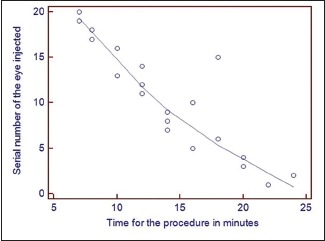
Graph showing serial number of eye injected plotted against the time taken for the procedure
Table 2.
Comparison of complications and success between the first and the last ten rat eyes operated
| Complication | First 10 eyes (%) | Last 10 eyes (%) | P |
|---|---|---|---|
| Conjunctival tear | 30 | 10 | 0.27 |
| Lens touch | 50 | 10 | 0.05 |
| Subretinal hemorrhage | 40 | 0 | 0.13 |
| Vitreous loss | 30 | 0 | 0.06 |
| Successful subretinal injection | 40 | 90 | 0.02 |
Graph 2.
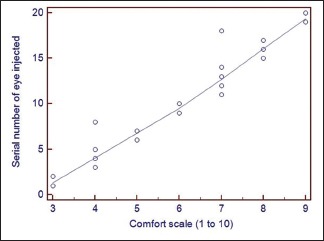
Graph showing serial number of the eye injected plotted against the comfort scale
Discussion
Sub-retinal injection technique in a small animal model has a definite learning curve for a clinician but can be overcome and mastered well by repetitive performance of the procedures. In the current technique, we describe approach to the subretinal space using a limbal incision with a transvitreal approach. Various workers have described techniques of subretinal injection via a transcorneal approach.[20,21,22] Though these techniques are simpler to perform, a relatively high rate of cataract was described in these studies (25%-40%). These cataracts resulted from damage inflicted on the lens as the cornea was punctured and/or as the blunt needle was directed toward the subretinal matrix. In our technique the risk is circumvented as the approach is via the pars plana. The cataracts noted in our study were seen mainly in the initially operated eyes. This can be attributed to the learning curve that was required to get a judgment of the relative position of the posterior lens capsule with respect to the retina. This occurs due to a peculiar anatomy of the rat eye where the lens occupies almost half of the space in the vitreous cavity.
It has been seen in previous studies that irrespective of the technique used in subretinal injections, there can be complications noted at the level of the retinal pigment epithelium and the retinal photoreceptors which can lead to progressive degeneration of the photoreceptors.[23,24] These observations indicate the importance of a correct technique to ensure minimal damage to the sensitive ocular structures during the process of injection. The clinicians trained in vitreoretinal surgeries have been performing subretinal injections over the past decade successfully for treating macular diseases especially submacular hemorrhage following trauma or an underlying choroidal neovascularization.[25,26] In spite of it being a not uncommonly performed procedure, complications like retinal detachments and choroidal hemorrhage are known with these procedures which can limit the final favorable outcome.[27,28] If we take into perspective the comparative schematic eye sizes of a rat eye and a human eye, it shows that a rat eye has an axial length which is 1/4th and the vitreous chamber depth which is 1/10th of that of a human eye.[29,30] Given such large differences in the ocular sizes, it natural that for an uninitiated clinician there would be a definite learning curve in carrying out these procedures. The current study shows how the learning curve can be overcome by repeating the procedure in a sustained manner to get a good outcome. This would avoid the effect of technique-related confounding factors on the final procedure outcome.
Conclusion
In conclusion, subretinal injections in rats have a definite learning curve even for a trained vitreoretinal surgeon as clinicians are not well-versed with surgical maneuvers in a small animal eye. This should be accounted for and resources allocated accordingly to allow adequate practice of these injection techniques. This can help achieve good technical comfort and negate confounding by the surgeon factor in the results of future clinical trials.
Financial support and sponsorship
This study was supported by the Hyderabad Eye Research Foundation, Department of Biotechnology Government of India and the Cognizant Foundation.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Peng Y, Tang L, Zhou Y. Subretinal injection: A review on the novel route of therapeutic delivery for vitreoretinal diseases. Ophthalmic Res. 2017;58:217–26. doi: 10.1159/000479157. [DOI] [PubMed] [Google Scholar]
- 2.Yasukawa T, Ogura Y, Sakurai E, Tabata Y, Kimura H. Intraocular sustained drug delivery using implantable polymeric devices. Adv Drug Deliv Rev. 2005;57:2033–4. doi: 10.1016/j.addr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Schorderet DF, Manzi V, Canola K, Bonny C, Arsenijevic Y, Munier FL. Maurer FD-TAT transporter as an ocular peptide delivery system. Clin Exp Ophthalmol. 2005;33:628–35. doi: 10.1111/j.1442-9071.2005.01108.x. [DOI] [PubMed] [Google Scholar]
- 4.Maia M, Kellner L, de Juan E, Jr, Smith R, Farah ME, Margalit E, et al. Effects of indocyanine green injection on the retinal surface and into the subretinal space in rabbits. Retina. 2004;24:80–91. doi: 10.1097/00006982-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Shen WY, Rakoczy PE. Uptake dynamics and retinal tolerance of phosphorothioate oligonucleotide and its direct delivery into the site of choroidal neovascularization throughsubretinal administration in the rat. Antisense Nucleic Acid Drug Dev. 2001;11:257–64. doi: 10.1089/108729001317022250. [DOI] [PubMed] [Google Scholar]
- 6.Kimura H, Spee C, Sakamoto T, Hinton DR, Ogura Y, Tabata Y, et al. Cellular response in subretinal neovascularization induced by bFGF-impregnated microspheres. Invest Ophthalmol Vis Sci. 1999;40:524–8. [PubMed] [Google Scholar]
- 7.Algvere PV, Berglin L, Gouras P, Sheng Y. Transplantation of fetal retinal pigment epithelium in age-related macular degeneration with subfoveal neovascularization. Graefes Arch Clin Exp Ophthalmol. 1994;232:707–16. doi: 10.1007/BF00184273. [DOI] [PubMed] [Google Scholar]
- 8.Binder S, Krebs I, Hilgers RD, Abri A, Stolba U, Assadoulina A, et al. Outcome of transplantation of autologous retinal pigment epithelium in age-related macular degeneration: A prospective trial. Invest Ophthalmol Vis Sci. 2004;45:4151–60. doi: 10.1167/iovs.04-0118. [DOI] [PubMed] [Google Scholar]
- 9.Binder S, Stolba U, Krebs I, Kellner L, Jahn C, Feichtinger H, et al. Transplantation of autologous retinal pigment epithelium in eyes with foveal neovascularization resulting from age-related macular degeneration: A pilot study. Am J Ophthalmol. 2002;133:215–25. doi: 10.1016/s0002-9394(01)01373-3. [DOI] [PubMed] [Google Scholar]
- 10.de Juan E, Jr, Loewenstein A, Bressler NM, Alexander J. Translocation of the retina for management of subfovealchoroidal neovascularization II: A preliminary report in humans. Am J Ophthalmol. 1998;125:635–46. doi: 10.1016/s0002-9394(98)00018-x. [DOI] [PubMed] [Google Scholar]
- 11.Falkner-Radler CI, Krebs I, Glittenberg C, Povazay B, Drexler W, Graf A, et al. Human retinal pigment epithelium (RPE) transplantation: Outcome after autologous RPE-choroid sheet and RPE cell-suspension in a randomised clinical study. Br J Ophthalmol. 2011;95:370–5. doi: 10.1136/bjo.2009.176305. [DOI] [PubMed] [Google Scholar]
- 12.Lam TT, Fu J, Tso MO. Ahistopathologic study of retinal lesions inflicted by transscleraliontophoresis. Graefes Arch Clin Exp Ophthalmol. 1991;229:389–94. doi: 10.1007/BF00170699. [DOI] [PubMed] [Google Scholar]
- 13.Ambati J, Gragoudas ES, Miller JW, You TT, Miyamoto K, Delori FC, et al. Transscleral delivery of bioactive protein to the choroid and retina. Invest Ophthalmol Vis Sci. 2000;41:1186–91. [PubMed] [Google Scholar]
- 14.Martin DF, Parks DJ, Mellow SD, Ferris FL, Walton RC, Remaley NA, et al. Treatment of cytomegalovirus retinitis with an intraocular sustained-release ganciclovir implant. A randomized controlled clinical trial. Arch Ophthalmol. 1994;112:1531–9. doi: 10.1001/archopht.1994.01090240037023. [DOI] [PubMed] [Google Scholar]
- 15.Srivastava S, Taylor P, Wood LV, Lee SS, Robinson MR. Post-surgical scleritis associated with the ganciclovir implant. Ophthalmic Surg Lasers Imaging. 2004;35:254–5. [PubMed] [Google Scholar]
- 16.Baba T, Bhutto IA, Merges C, Grebe R, Emmert D, McLeod DS, et al. A rat model for choroidal neovascularization using subretinal lipid hydroperoxide injection. Am J Pathol. 2010;176:3085–97. doi: 10.2353/ajpath.2010.090989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hillenkamp J, Surguch V, Framme C, Gabel VP, Sachs HG. Management of submacular hemorrhage with intravitreal versus subretinal injection of recombinant tissue plasminogen activator. Graefes Arch Clin Exp Ophthalmol. 2010;248:5–11. doi: 10.1007/s00417-009-1158-7. [DOI] [PubMed] [Google Scholar]
- 18.Olivier S, Chow DR, Packo KH, MacCumber MW, Awh CC. Subretinal recombinant tissue plasminogen activator injection and pneumatic displacement of thick submacular hemorrhage in Age-Related macular degeneration. Ophthalmology. 2004;111:1201–8. doi: 10.1016/j.ophtha.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 19. [Last accessed on 2019 Jan 02]. Available from: http://www.arvo.org/AboutARVO/Policies/Statement for the Use of Animals in Ophthalmic and Visual Research/
- 20.Timmers AM, Zhang H, Squitieri A, Gonzalez-Pola C. Subretinal injections in rodent eyes: Effects on electrophysiology and histology of rat retina. Mol Vis. 2001;7:131–7. [PubMed] [Google Scholar]
- 21.Price J, Turner D, Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987;84:156–60. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987;328:131–6. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto H, Miller JW, Vavvas DG. Retinal detachment model in rodents by subretinal injection of sodium hyaluronate. J Vis Exp. 2013:e50660. doi: 10.3791/50660. doi: 10.3791/50660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westenskow PD, Kurihara T, Bravo S, Feitelberg D, Sedillo ZA, Aguilar E, et al. Performing subretinal injections in rodents to deliver retinal pigment epithelium cells in suspension. J Vis Exp. 2015:e52247. doi: 10.3791/52247. doi: 10.3791/52247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novelli FJD, Preti RC, Monteiro MLR, Nobrega MJ, Takahashi WY. A new method of subretinal injection of tissue plasminogen activator and air in patients with submacular hemorrhage. Retina. 2017;37:1607–11. doi: 10.1097/IAE.0000000000001491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bell JE, Shulman JP, Swan RJ, Teske MP, Bernstein PS. Intravitreal versus subretinal tissue plasminogen activator for submacular hemorrhage. Ophthalmic Surg Lasers Imaging Retina. 2017;48:26–32. doi: 10.3928/23258160-20161219-04. [DOI] [PubMed] [Google Scholar]
- 27.Haupert CL, McCuen II, BW, Jaffe GJ, Steuer ER, Cox TA, Toth CA, et al. Pars plana vitrectomy, subretinal injection of tissue plasminogen activator and fluid-gas exchange for displacement of thick sub-macular hemorrhage in age-related macular degeneration. Am J Ophthalmol. 2001;131:208–15. doi: 10.1016/s0002-9394(00)00734-0. [DOI] [PubMed] [Google Scholar]
- 28.Sharma S, Kumar JB, Kim JE, Thordsen J, Dayani P, Ober M, et al. Pneumatic displacement of submacular hemorrhage with subretinal air and tissue plasminogen activator. Ophthalmol Retina. 2018;2:180–6. doi: 10.1016/j.oret.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Massof RW, Chang FW. A revision of the rat schematic eye. Vision Res. 1972;12:793–6. doi: 10.1016/0042-6989(72)90005-3. [DOI] [PubMed] [Google Scholar]
- 30.Vojnikovic B, Tamajo E. Gullstrand's optical schematic system of the eye - modified by Vojnikovic and Tamajo. Coll Antropol. 2013;37:41–5. [PubMed] [Google Scholar]