In this study, Schumann et al. identify the penta-EF-hand protein PEF1 of the genetic model fungus Neurospora crassa as part of the cellular response to different types of membrane injury...
Keywords: membrane repair, penta-EF-hand protein, cell fusion, Neurospora crassa, antifungal drug
Abstract
Plasma membrane damage commonly occurs during cellular growth and development. To counteract these potentially lethal injuries, membrane repair mechanisms have evolved, which promote the integrity of the lipid bilayer. Although the membrane of fungi is the target of important clinical drugs and agricultural fungicides, the molecular mechanisms which mediate membrane repair in these organisms remain elusive. Here we identify the penta-EF-hand protein PEF1 of the genetic model fungus Neurospora crassa as part of a cellular response mechanism against different types of membrane injury. Deletion of the pef1 gene in the wild type and different lysis-prone gene knockout mutants revealed a function of the protein in maintaining cell integrity during cell–cell fusion and in the presence of pore-forming drugs, such as the plant defense compound tomatine. By fluorescence and live-cell imaging we show that green fluorescent protein (GFP)-tagged PEF1 accumulates at the sites of membrane injury in a Ca2+-dependent manner. Site-directed mutagenesis identified Ca2+-binding domains essential for the spatial dynamics and function of the protein. In addition, the subcellular localization of PEF1 revealed that the syncytial fungal colony undergoes compartmentation in response to antifungal treatment. We propose that plasma membrane repair in fungi constitutes an additional line of defense against membrane-disturbing drugs, thereby expanding the current model of fungal drug resistance mechanisms.
THE plasma membrane of any living cell constitutes the prime barrier between the cytosol and the outer environment. It controls the import and export of ions and molecules, thereby establishing chemical and electrical gradients required for normal physiological functioning. Membrane disintegration destroys this selective permeability, allows the mixture of inner and outer constituents and ultimately results in cell death. While membrane integrity is essential for cell survival, damage of this outer barrier frequently occurs in all organisms during physiological or pathophysiological conditions (Horn and Jaiswal 2018; Nakamura et al. 2018). Most common causes of membrane rupture include mechanical shear stress or exposure to pore-forming drugs. For example, cells in mechanically active tissue, such as mammalian skeletal and cardiac muscle, frequently encounter membrane rupture through physical force (McNeil and Khakee 1992; Clarke et al. 1995). Similarly, cells undergoing sexual or somatic fusion are at risk of rupture during plasma membrane merger as observed in yeast or filamentous fungi (Jin et al. 2004; Palma-Guerrero et al. 2014). Mounting evidence also points to important roles of membrane damage during various pathophysiological conditions including muscular dystrophies, diabetes, or ischemia-reperfusion injuries associated with strokes or heart attacks (Howard et al. 2011; Tzeng et al. 2014; Barthélémy et al. 2018). Membrane disruption by pore-forming drugs is common in host–pathogen interactions, where the cellular armory involves attack or defense compounds, such as plant saponins, which target the membrane and cause its disintegration (Dal Peraro and van der Goot 2016; Mondal et al. 2018). The saponin tomatine, for example, belongs to the group of steroidal glycoalkaloids and is produced by Solanum species, such as tomato (Solanum lycopersicum), as a preformed defense compound against fungal infections (Friedman 2002). In clinic and in agriculture, pore-forming drugs also represent important weapons for fighting pathogenic microbes. Examples include the polyene antibiotics for treating fungal infections of human, animals and plants (Bolard 1986; Chandrasekar 2011).
To survive membrane damage, cells must quickly and efficiently reseal the wounded area and restore the preinjury state. Depending on the size and nature of the injury, different repair mechanisms are employed, including the formation of membrane patches, removal of the injured area by exo- and/or endocytosis, membrane blebbing, and closing of the wound by clotting (Horn and Jaiswal 2018; Nakamura et al. 2018). Membrane patches, which allow the repair of larger wounds such as ruptures, are formed via the fusion of vesicles which accumulate in masses at the injured side followed by merger of the patch with the plasma membrane (McNeil and Kirchhausen 2005; Davenport and Bement 2016). Endo- and exocytosis remove the damaged area by internalization or detachment and efficiently repair pore-like injuries (Idone et al. 2008a,b). The exocytosis of internal membrane structures also releases plasma membrane tension, which promotes spontaneous sealing of small injuries. This process can be furthered by the disassembly of the underlying cortical cytoskeleton, resulting in tension relief (Togo et al. 2000). Membrane blebbing involves protruding of the injured membrane from the cell body followed by shedding of the damaged section (Babiychuk et al. 2011). Clotting, finally, describes a colloidal reaction of the protoplasm, in which similar to blood clotting, proteins, organelles, and/or vesicles aggregate into three-dimensional structures, which can plug larger wounds and prevent cytoplasmic loss even before the plasma membrane has been reconstituted (Eddleman et al. 2000; Horn and Jaiswal 2018).
While these different repair mechanisms vary in their underlying principles, they all depend on factors and structures present in the cell before injury occurrence. Membrane damage always threatens the survival of the cell and necessitates responses faster than the de novo synthesis of proteins. Immediate sensing and localization of an injury therefore represents the first and foremost step of any membrane repair system. A conserved trigger of plasma membrane repair mechanisms is the influx of Ca2+ ions through the wound into the cell. Intact cells usually maintain a Ca2+ gradient across their plasma membrane, with the external concentration exceeding the internal one. The rapidly increasing internal Ca2+ concentration after injury is detected by Ca2+-responsive proteins, which function as sensors within the repair machinery (Horn and Jaiswal 2018; Nakamura et al. 2018). In animal cells, for example, the Ca2+-binding penta-EF-hand protein ALG-2 (apoptosis-linked gene-2) initiates the accumulation of ALG-2-interacting protein X (ALIX), and the ESCRT-III and Vps4 complex at the injured cell membrane, resulting in cell recovery by shedding of the damaged site (Scheffer et al. 2014; la Cour et al. 2018).
So far, membrane repair mechanisms have mostly been studied in animal cells. Besides hints to conserved functions of individual factors, such as synaptotagmins, in plants, animals, and fungi (Aguilar et al. 2007; Schapire et al. 2009), it remains unclear if the broader repair machineries are conserved throughout the different kingdoms. In recent years, the filamentous fungus Neurospora crassa has advanced as an attractive model organism for studying general eukaryotic and specifically fungal cell biology. Topics addressed in this experimental system include cell polarity, directed growth, signal transduction, and cell–cell fusion (Riquelme et al. 2011; Daskalov et al. 2016). Earlier studies reported different types of membrane damage occurring in this fungus. First, as many fungi, N. crassa is inhibited by polyene antibiotics, such as nystatin, which induce pore formation in the plasma membrane (Kinsky 1961). Second, during colony establishment of this fungus, germinating vegetative spores, so-called conidia, undergo fusion to form a supracellular unit, which further develops into the mycelial colony. These fusion events bear the risk of lysis, especially in strains exhibiting aberrant cell–cell fusion, such as the ΔPrm1 mutants of Saccharomyces cerevisiae or N. crassa (Jin et al. 2004; Palma-Guerrero et al. 2014). The PRM1 protein possesses a conserved function in fungal cell–cell fusion, and its absence results in fusion failure of ∼50% of fusion pairs and increased cell lysis (Heiman and Walter 2000; Fleissner et al. 2009; Palma-Guerrero et al. 2014). Time-lapse microscopy of mating between ΔPrm1 mutant cells of baker’s yeast revealed a strong correlation between membrane merger and cell lysis, suggesting that lysis was the consequence of membrane rupture during engagement of the cell fusion machinery (Jin et al. 2004; Aguilar et al. 2007). A third type of membrane injury occurs in mature colonies of N. crassa. The fungus grows as a syncytial network of filament-like hyphae, which are compartmentalized by cross-walls containing a central pore. Injury of the syncytium, for example by the transsection of hyphae, results in clogging of the septal pore next to the injured compartment, followed by membrane sealing (Tenney et al. 2000; Yuan et al. 2003; Fleissner and Glass 2007).
In this study, we identify the ALG-2 homologous penta-EF-hand protein PEF1 as part of the cellular response to the above-described various types of membrane injury. In contrast to animal cells, this function seems to be independent of the ESCRT-III complex, suggesting a so far undescribed membrane repair mechanism involving PEF1. Interestingly, lack of the pef1 gene results in an increased sensitivity to the pore-forming plant defense compound tomatine. We hypothesize that membrane repair might constitute a fungal defense response against membrane-disturbing drugs and might therefore also contribute to pathogenic growth and development.
Materials and Methods
N. crassa strains and growth media
Fungal strains employed in this study are listed in Table 1. Strains were cultivated on Vogel’s minimal medium (MM) (Vogel 1956) supplemented with 2% sucrose as the carbon source and with 1.5% agar for solid media. For auxotrophic strains, the required supplements were added to MM (http://www.fgsc.net/neurospora/neurosporaprotocolguide.htm). For media lacking Ca2+, CaCl2 was omitted from MM, and agarose was used instead of agar for solidifying the medium. If not indicated otherwise, cultures were incubated at 30°.
Table. 1. Strains used in this study.
| Strain | Genotype | Origin of reference |
|---|---|---|
| A29 | matA, Prm1::hph | Fleissner et al., 2009 |
| A32 | mata, Prm1::hph | Fleissner et al., 2009 |
| C9-15 | matA, het-c2 pin-c2 thr2 | Xiang and Glass, 2002 |
| CR73-1 | mata, his-3::Pccg1-h1-dsRed | Rasmussen et al., 2008 |
| FGSC 988 | mata | FGSC |
| FGSC 2489 | matA | FGSC |
| FGSC 6103 | matA, his- | FGSC |
| FGSC15890 | mata, pef1::hph | FGSC |
| FGSC17273 | mata, fig-1::hph | FGSC |
| FGSC19267 | mata, lfd-2::hph | FGSC |
| GN2-50 | mata ,pef1::hph, his- | This study |
| GN2-78 | matA, his-3::dsRed-hex1 | This study |
| GN3-9 | matA, Prm1::hph, his-3::Pccg-1-pef1-gfp | This study |
| GN3-17 | mata, pef1::hph, his-3::Pccg1 pef1-gfp | This study |
| GN3-36 | matA, het-c1, pin-c1, his-3::Pccg-1-pef1-gfp, pyr4 | This study |
| GN6-42 | mata, Prm1::hph syt-1::hph | This study |
| GN6-47 | matA, pef1::hph syt-1::hph | This study |
| GN6-48 | matA, Prm1::hph pef1::hph syt-1::hph | This study |
| GN6-62 | matA,his-3::Pccg-1-syt-1-mcherry | This study |
| GN7-50 | mata, Prm1::hph, pef1::hph, his- | This study |
| GN7-54 | mata, Prm1::hph, pef1::hph, his-3:Pccg-1 pef1-gfp | This study |
| GN8-76 | matA, pef1::hph fig-1::hph | This study |
| GN8-80 | matA, pef1::hph lfd-2::hph | This study |
| GN9-1 | mata, Prm1::hph pef1::hph his-3::Pccg-1-pef1 E233A- gfp | This study |
| GN9-3 | mata, pef1::hph his-3::Ppef1-pef1-gfp | This study |
| GN9-22 | matA, pef1::hph his-3::Ptef-1-pef1-gfp | This study |
| GN9-34 | mata, Prm1::hph pef1::hph his-3::Pccg-1-pef1 E164A, E166A-gfp | This study |
| GN9-48 | matA, his-3::Pccg-1 sec-31-gfp | This study |
| GN9-50 | matA, his-3::Pccg1 chmp-1-gfp | This study |
| GN10-10 | mata, pef1::hph lfd-1::hph | This study |
| JPG6 | mata, lfd-1::hph | Palma-Guerrero et al., 2015 |
| N5-20 | mata Prm1::hph pef1::hph | This study |
| S10 | matA, trp-1 his-3::Pccg-1-pef1-gfp | This study |
| S20 | matA, nic-3 his-3:Pccg1-dsRed-hex-1 | This study |
| S48 | matA, hex-1::hph his-3::Pccg1-pef1-gfp | This study |
FGSC, Fungal Genetics Stock Center.
Gene deletion mutants used in this study were obtained from the Neurospora gene knockout collection (Dunlap et al. 2007), provided by the Fungal Genetics Stock Center (FGSC).
To generate double mutants, crosses were performed on Westergaard’s medium as described earlier (Westergaard and Mitchell 1947). To test the mating type of progeny, strains were crossed with the reference strains FGSC 2489 and FGSC 988.
Plasmid construction
To tag PEF1 with the green fluorescent protein (GFP), the pef1 open reading frame was amplified by PCR using primers 356 and 357 (Supplemental Material, Table S1). The obtained fragment was cloned into the expression plasmid pMF272, which contains the overexpression promoter Pccg-1 (Freitag et al. 2004), through the restriction sites XbaI and PacI. The resulting plasmid was integrated into the his-3 locus of strain GN2-50 (Δpef1, his-3−). To express the pef1-gfp construct under control of the native promoter, the pef1 open reading frame plus a 1-kb fragment upstream of the start codon was amplified by PCR with primers 633 and 357. The obtained fragment was cloned through the NotI and PacI restriction sites into pMF272, resulting in replacement of the Pccg-1 promoter by the Ppef1-pef fragment. The resulting construct was also integrated at the his-3 locus of strain GN2-50.
To tag the ESCRT-III complex proteins CHMP1 and SEC-31 with GFP, the chmp1 open reading frame was PCR amplified using primers 1106 and 1107, and the sec-31 open reading frame by primers 1110 and 1111. Both fragments were cloned individually into plasmid pMF272 via the XbaI and PacI restriction sites. The resulting plasmids were integrated at the his-3 locus of strain FGSC 6103 (his-3−).
To obtain pef1 expression constructs carrying point mutations in the EF-1 or EF-3 domain encoding regions, the pef1 open reading frame was amplified as two fragments to introduce the point mutations through the primers. For the EF-1 construct primer pairs 944/598 and 597/945 were used, and for the EF-3 construct pairs 595/598 and 597/596. The fragments were assembled by yeast recombinational cloning using the yeast vector pRS426 as described in Colot et al. (2006). The resulting constructs were cloned into pMF272 via the XbaI and PacI restriction sites. The final plasmids were transformed into strain GN7-50 (ΔPrm1, Δpef1, his-3−).
Transformation of N. crassa
N. crassa strains were transformed by electroporation of macroconidia according to an earlier described protocol (Margolin et al. 1997).
Phenotypical analysis of aerial hyphal growth, growth rate, and sporulation
The growth rate of aerial hyphae was determined by inoculating 2 ml of liquid MM with 3 × 106 conidia. The culture tubes were incubated for 5 days in complete darkness at 30°. For each strain four replicates were measured.
To address linear hyphal growth, race tubes containing 12 ml of solid MM were inoculated with 3 × 106 conidia. The linear growth was measured from 24 to 72 hr at 25° in constant light. For each strain, four replicates were tested.
For determining macroconidia production, slant tubes with 3 ml solid MM were inoculated with 3 × 106 conidia. The cultures were incubated for 3 days at 30° in the dark and for 4 more days at room temperature in daylight conditions. The conidia were harvested by vortexing the slant tube with 2 ml ddH2O, and hyphal fragments were removed by filtering through cheesecloth. The number of conidia from four independent replicates was counted for each strain.
Sample preparation for microscopy and quantitative germling fusion assay
To quantify fusion and lysis rates, 3 × 106 spores were plated on solid MM in 9-cm Petri dishes. Cultures were incubated for 4 hr at 30°. Squares of agar measuring 1 cm2 were excised and observed on a Zeiss Observer 2.1 microscope using Nomarski optics with a Plan-Neofluar 100×/1.30 oil immersion objective (420493-9900). Cell lysis was apparent by strong vacuolization of the germlings (Palma-Guerrero et al. 2014). For microscopy of hyphae, cultures were grown overnight at 30°. One square centimeter agar squares were cut from the edge of the colony where hyphal density was low and analyzed by microscopy as described above.
Live-cell imaging and fluorescence microscopy were conducted as described earlier (Serrano et al. 2018).
To analyze PEF1-GFP recruitment in response to the antifungal drugs nystatin and tomatine, germlings were incubated as mentioned above. Five microliters of 0.1 mg/ml nystatin (CAS-Nr. [1400-61-9]) and 2 mg/ml tomatine (CAS-Nr. [17406-45-0]) solutions (diluted in DMSO) were added directly on top of the agar slides and incubated for up to 5 min before analysis by Nomarski and fluorescent microscopy. For quantitative assays, 100 lysed germlings were tested for PEF1-GFP recruitment. Each test was independently repeated two more times.
For time-lapse microscopy of PEF1 recruitment in response to nystatin, 5 × 105 spores were incubated in 200 μl of liquid MM in ibidi eight-well μ-slides (www.ibidi.com, ordering number 80826) for 3 hr at 30°. After imaging of the untreated cells, 30 μl of a 0.1 mg/ml nystatin solution was carefully added to the side of the chamber to avoid movement of the germlings. Cells were further imaged by fluorescence microscopy.
Analysis of septal pore plugging
To analyze PEF1 recruitment to the septal plug and to quantify septal plugging, the fungus was cultivated as described above for the microscopy of hyphae. To cut the hyphae, a laser microdissection system (CellCut Plus System, Molecular Machines and Industries) equipped with a UV laser (355 nm) and combined with an Olympus IX81 microscope was used. In quantitative assays, which did not require the direct observation of the injury occurrence, hyphae were cut with a razor blade and imaged with the above-described Zeiss Observer 2.1 microscope set up.
Analysis of PEF1 localization during vegetative incompatibility
Incompatible heterokaryons between strains GN3-36 and C9-15 were formed and analyzed as described earlier (Fleissner and Glass 2007). In brief, spores of the two auxotrophic strains were mixed and plated on MM, allowing only the growth of heterokaryotic colonies. The culture medium contained 0.003% methylene blue to stain dead hyphal compartments indicating the activation of programmed cell death. Septa delimiting dead compartments were analyzed for PEF1 accumulation by fluorescence microscopy.
Stress tests
To test the sensitivity of N. crassa strains to EGTA, SDS, nystatin, and tomatine, spore serial dilution assays were conducted. To restrict hyphal growth and promote the formation of individual, distinct colonies, BDES medium was used (Brockman and De Serres 1963). A serial dilution of 105–101 spores were spotted in 5-μl droplets on BDES plates. Cultures were incubated for 2–3 days at 30°, and growth of the reference strain and the mutants was compared.
Data availability
All strains and plasmids are available on request. We confirm that all data and information necessary to confirm the conclusions of this study are present in the article, figures, tables, and supplemental material. Supplemental material available at Figshare: https://doi.org/10.25386/genetics.8239490.
Results
The N. crassa penta EF-Hand protein PEF1 is dispensable for vegetative growth and cell–cell fusion
The original motivation of this study was not to identify membrane repair mechanisms in N. crassa, but to find Ca2+-responsive proteins potentially involved in signaling processes mediating vegetative cell–cell fusion, since earlier studies revealed that Ca2+ is essential for this process (Palma-Guerrero et al. 2013). As one candidate, the penta EF-Hand protein PEF1 (NCU02738) was chosen. An earlier study described PEF1 as the only homolog of animal ALG-2. Its role and function in fungal growth and development remained, however, unknown (Cano-Domínguez et al. 2008).
The predicted N. crassa pef1 encoding open reading frame consists of 1266 bp and contains three introns, whose positions were confirmed by complementary DNA (cDNA) sequencing. The gene encodes a protein of 311 amino acid residues, with a predicted molecular weight of 35,581 Da (http://fungidb.org). Sequence comparison by BLAST analysis revealed that the N-terminal third of the protein (aa 1–111) is not conserved outside the taxon fungi, while the N-terminal two-thirds (aa 112–311), including the five putative calcium-binding EF domains, show high conservation (e.g., 44% identity to ALG-2 of Mus musculus).
To test the role of PEF1 for the growth and development and specifically vegetative cell–cell fusion of N. crassa, we obtained the Δpef1 gene deletion strain FGSC 15890 from the N. crassa gene knockout collection (Dunlap et al. 2007) and verified the integration of the gene replacement cassette at the pef1 locus by PCR analysis using primers 317 and 374 (data not shown). Compared to the wild-type reference strain, the mutant shows normal hyphal growth and formation of aerial hyphae (Figure S1, A and B), indicating that PEF1 is dispensable for general vegetative growth. It produces, however, significantly higher numbers of vegetative spores (Figure S1C), confirming the observations made for an independently constructed Δpef1 mutant analyzed in an earlier study (Cano-Domínguez et al. 2008). The only macroscopically discernable difference between the wild type and Δpef1 is a dark pigmentation occurring robustly between the mycelia and the glass surface of the culture tubes (Figure S1D). This phenotype is reminiscent of the Δhex-1 mutant of N. crassa, which is unable to seal the cross-walls of its syncytial hyphae after injury, resulting in cytoplasmic leakage indicated by the brown pigmentation of mutant cultures (Jedd and Chua 2000). The extent of cytoplasmic leakage can be measured by the amount of extracellular protein, which can be recovered from the culture. When tested, however, the amount of protein obtained from wild-type and Δpef1 cultures was comparable, indicating that the observed pigmentation is not caused by cytoplasmic leakage (Figure S1D).
Consistent with the overall lack of growth defects, the absence of PEF1 did not affect the tropic interaction of germinating spores resulting in cell–cell fusion, indicating that PEF1 is dispensable for this process (Figure S1E).
PEF1 accumulates at the fusion point of lysing cell–cell fusion pairs
To test a potential nonessential contribution of PEF1 to the process of germling fusion during colony establishment, we tagged the protein with GFP to determine its subcellular localization. The gfp-encoding DNA sequence was fused to the 3′ end of the pef1 open reading frame. This fusion construct was expressed under control of the native pef1 promoter and alternatively under control of the overexpression promoters Pccg-1 and Ptef-1, which are routinely used in protein localization studies in N. crassa (Freitag et al. 2004; Berepiki et al. 2010). All constructs were transformed into the Δpef1 mutant strain, resulting in the isolates GN9-3, GN3-17, and GN9-22, respectively. Expression controlled by the native promoter yielded no discernable fluorescence above the level of autofluorescence of the cells, suggesting that pef1 is expressed at low levels. In contrast, germlings and growing mature hyphae of the two overexpressing strains exhibited comparable cytoplasmic fluorescence with sometimes increased signal intensity around nuclei (Figure 1 and Figure S2). The presence of GFP at the C-terminus of PEF1 did not affect the function of the protein, indicated by the complementation of the above-described hyper-sporulation phenotype of the Δpef1 mutant (Figure S1C). To test the dynamics of PEF1-GFP during germling fusion, spores of strains GN3-17 (Pccg-1-pef-1-gfp) and GN9-22 (Ptef-1-pef-1-gfp) were plated on MM agar plates, incubated for 3 to 4 hr at 30°, and analyzed by fluorescence microscopy. At this time point, the majority of spore germlings were undergoing pairwise interactions, indicated by the mutual attraction of germ tubes, or cell pairs were already merged. Also, networks of more than two cells formed by successive fusion events were readily observed. In the vast majority of growing, fusing, and fused cells no altered or specific localization pattern of PEF1 was observed, supporting the notion that PEF1 seems not to be involved in these processes (Figure 2 and data not shown). In rare cases, however, the PEF1-GFP signal clearly and strongly accumulated at the contact point of cell pairs (Figure 2). Interestingly, DIC imaging revealed that in these pairings the cells appeared highly vacuolized, which earlier studies identified as an indication of cell lysis (Palma-Guerrero et al. 2014, 2015). Live-cell imaging supported this finding, showing rapid vacuolization of some fusion pairs minutes after cell–cell contact, consistent with fusion-induced cell lysis (Figure 2). To corroborate the potential correlation between fusion pair lysis and PEF1 recruitment to the contact zone, the lysed cell pairs within the cell population were quantified and tested for PEF1-GFP presence at the fusion point. As a result, ∼1% of all formed cell pairs exhibited lysis, and ca. 90% of these lysed fusion pairings showed PEF1 recruitment to the cell–cell contact zone (Figure 2). Based on this strong correlation, we hypothesized that PEF1 is recruited to the cell–cell fusion point in response to aberrant plasma membrane merger resulting in cell rupture. Since in the observed PEF1-GFP aggregates the protein is concentrated, we revisited the isolate expressing pef1-gfp under control of the native promoter. However, still no discernable GFP signal was detected.
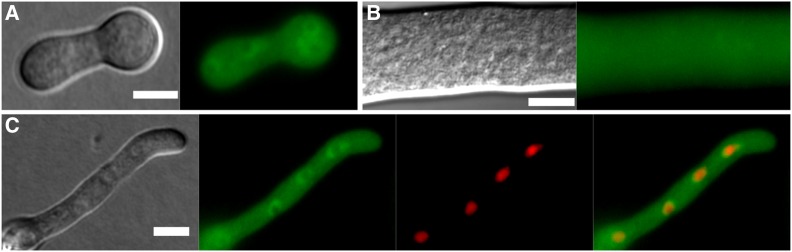
Figure 1.
PEF1-GFP is a cytoplasmic protein. Subcellular localization of PEF1-GFP in germlings (A) and hyphae (B) of strain GN9-22 (Ptef-1-pef-1-gfp). Left: DIC image; right: GFP fluorescence image. (C) Heterokaryon of strains GN9-22 expressing pef1-gfp and CR73-1 expressing the nuclear marker H1-dsRed. From the left: (1) DIC, (2) GFP fluorescence, (3) dsREd fluorescence, and (4) merged image of 2 and 3. Bar, 5 µm (A and C) and 10 µm (B).
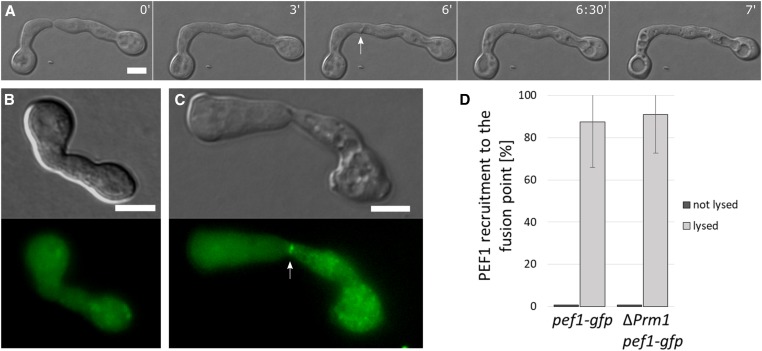
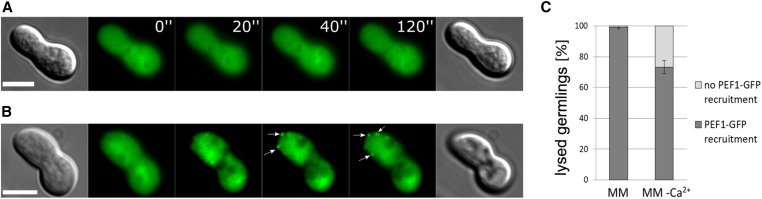
Figure 2.
PEF1-GFP accumulates at the contact point of fusing cells undergoing lysis. (A) Time course of two germlings undergoing mutual attraction and fusion. At time point 6 min, note the formation of a membrane protuberance (arrow) followed by rapid vacuolization of both fusion partners indicating lysis. Time = min. (B) PEF1-GFP localization during successful cell fusion (strain GN9-22, Ptef-1-pef-1-gfp). (C) PEF1-GFP accumulation at the contact point of a fusion pair undergoing lysis (arrow) (strain GN9-22, Ptef-1-pef-1-gfp). (B and C) Top images: DIC; bottom images: GFP fluorescence. (D) Quantification of PEF1-GFP recruitment in healthy and lysed cell pairs of strains GN9-22 (∆pef1, Ptef-1-pef1-gfp) and GN3-9 (∆Prm1, Pccg-1-pef1-gfp). Error bars indicate the SD calculated from three independent experiments (n = 100 each). Note that while the percentage of lysed cell pairs showing PEF1-GFP accumulation at the fusion point is comparable for both strains, the overall number of lysed cell pairs is significantly higher in ΔPrm-1 compared to the wild-type background strain (33 lysed pairs out of 300 total, compared to 8 out of 300 total, respectively). Bar, 5 µm.
To further corroborate the correlation between cell lysis and PEF1 aggregation, we expressed PEF1-GFP (from construct Pccg-1-pef-1-gfp) in the ΔPrm1 mutant, which is prone to fusion-induced cell lysis. In this mutant ca. 50% of germling pairs fail to fuse their plasma membranes, and ∼10% of cell pairs undergo cell lysis (Fleissner et al. 2009; Palma-Guerrero et al. 2014). We hypothesized that the majority of these lysed cell pairs would also exhibit PEF1-GFP accumulation at the fusion point. This expectation was fully met with ∼90% of lysed cell pairs exhibiting an increased GFP signal at the cell contact zone. Although the overall number of lysed pairs was significantly higher in ΔPrm1 than in the wild-type background, the correlation between lysis and PEF1-GFP recruitment was comparable (Figure 2D).
The lack of PEF1 results in increased lysis rates in lysis-prone mutants
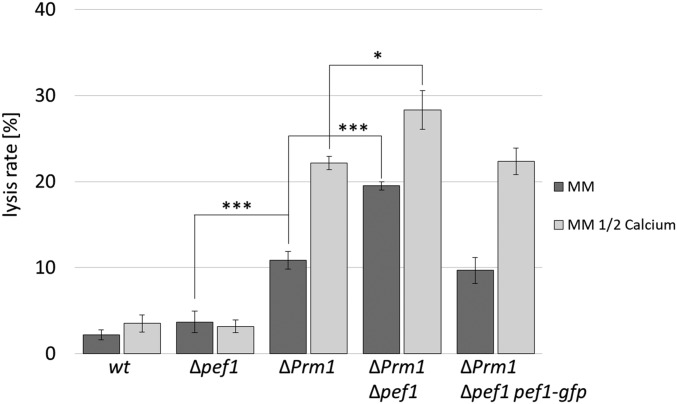
To test if the observed accumulation of PEF1 at the contact point of lysing fusion pairs represents a functional response to cell injury, we compared the lysis rates of the wild type and the ΔPrm1 mutant to those of the Δpef1 strain and the ΔPrm1/Δpef1 double mutant. While the frequency of fusion-induced lysis was comparable between wild type and Δpef1, the lysis rate of ΔPrm1/Δpef1 doubled compared to the ΔPrm1 single mutant. This increase could be reversed by the expression of Pccg-1-pef-1-gfp in the double mutant (Figure 3). Together, these data indicate that PEF1 is dispensable in the wild type but significantly promotes cell survival in the lysis-prone ΔPrm1 mutant. Earlier studies revealed that a reduction of Ca2+ in the growth medium increases the lysis rates of fusion pairs in N. crassa and S. cerevisiae, leading to the hypothesis that Ca2+-mediated membrane repair mechanisms counteract the effect of a lack of PRM1 (Palma-Guerrero et al. 2014) (Aguilar et al. 2007). We therefore hypothesized that PEF1 is part of this proposed membrane repair mechanism. To test this idea, we compared the lysis rates of the different isolates on standard growth medium and on medium containing only 50% of the usual Ca2+ [on medium with zero Ca2+, cell–cell interactions and fusion are not observed and fusion-induced lysis cannot be tested (Palma-Guerrero et al. 2013)]. While in the wild type and the Δpef1 mutant the reduced Ca2+ had no significant effect, the lysis rate of ΔPrm1 increased to the level observed in the ΔPrm1/Δpef1 double mutant on standard medium, indicating that either deleting pef1 or reducing the extracellular Ca2+ amount had similar effects. However, the ΔPrm1/Δpef1 double mutant also exhibited a further significant increase in lysis on Ca2+-reduced medium compared to standard growth conditions, suggesting that additional PEF1-independent Ca2+-mediated repair mechanisms exist (Figure 3).
Figure 3.
Lack of PEF1 increases the lysis rate during germling fusion in the lysis-prone ∆Prm1 mutant. Quantification of the lysis rate during germling fusion of the wild-type strain (FGSC 988) compared to ∆pef1 and ∆Prm1 deletion and double mutants (FGSC 15890, A32, N5-20, respectively) and the complemented isolate GN7-54 (∆Prm1/∆pef1, Pccg-1-pef1-gfp). Strains were incubated on MM or MM with 50% Ca2+. Error bars indicate the SD calculated from three independent experiments (n = 100 each). The asterisks represent statistically significant differences determined by the Student’s t-test (*P ≤ 0.05; ***P ≤ 0.001).
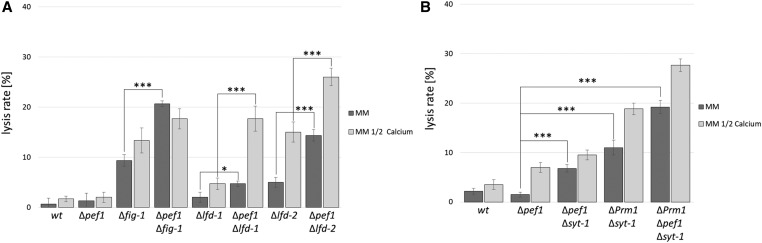
In addition to PRM1, earlier studies identified three additional factors, whose absence results in aberrant fusion and increased lysis rates during germling fusion, LFD-1, LFD-2, and FIG1 (Palma-Guerrero et al. 2014, 2015). To test if the observed role of PEF1 is specific for ΔPrm1-caused defects or if it is of broader significance, we introduced the Δpef1 gene knockout into the Δlfd-1, Δlfd-2 and Δfig1 mutants and compared the lysis rates of the single and the respective double mutants on standard and Ca2+-reduced growth medium. The lack of PEF1 resulted in increased lysis rates in all double mutants compared to the single mutants (Figure 4A). Interestingly, similar to the observation made for the ΔPrm1 and ΔPrm1/Δpef1 mutants, the Δlfd-1/Δpef1 and Δlfd-2/Δpef1 double mutants exhibited similar lysis rates on standard medium to the single mutants under Ca2+-reduced conditions. Again, the reduction of Ca2+ also further increased the frequency of lysis in the double mutants. In contrast, while deletion of pef1 in Δfig1 significantly increased the occurrence of lysis on standard medium, the reduction of Ca2+ did not further increase lysis of the double mutant (Figure 4A).
Figure 4.
Lack of PEF1 increases the lysis rate in mutants with membrane merger defects. (A) Quantification of the lysis rate during germling fusion of the wild-type strain (FGSC 988) compared to ∆pef1 (FGSC 15890), ∆fig-1 (FGSC 17273), ∆lfd-1 (JPG6), ∆lfd-2 (FGSC 19267), and double mutants (GN8-76, GN10-10, GN8-80, respectively). (B) Quantification of the lysis rate during germling fusion of the wild-type strain (FGSC 988) compared to ∆pef1 (FGSC 15890), the double mutants ∆pef1/∆syt-1 (GN6-47) and ∆Prm1/∆syt-1 (GN6-42), and the triple mutant ∆Prm1/∆pef1/∆syt-1 (GN6-48). Strains were grown on MM or MM with 50% Ca2+. Error bars indicate the SD calculated from three independent experiments (n = 100 each). The asterisks represent statistically significant differences determined by the Student’s t-test (*P ≤ 0.05; ***P ≤ 0.001).
The earlier study describing the role of LFD-1 also identified the synaptotagmin SYT1 as a potential mediator of plasma membrane repair. Deletion of syt1 in ΔPrm1 resulted in a significant increase in lysed cell pairs, similar to our observation for pef1 (Palma-Guerrero et al. 2014). To test if SYT1 and PEF1 function in a common pathway or have independent functions, we constructed a Δpef1/Δsyt1 double and a ΔPrm1/Δpef1/Δsyt1 triple mutant and compared the fusion pair lysis rates to those of the Δsyt1 single and the ΔPrm1/Δsyt1 double mutant. In our hands, however, the ΔPrm1/Δsyt1 mutant did not exhibit increased lysis compared to ΔPrm1. Consistent with this finding, the triple mutant also behaved comparably to the ΔPrm1/Δpef1 double mutant (Figure 4B). Thus, under our test conditions, SYT1 seems to make no contribution to plasma membrane integrity during germling fusion.
PEF1 is recruited to the plasma membrane in response to the action of pore-forming drugs
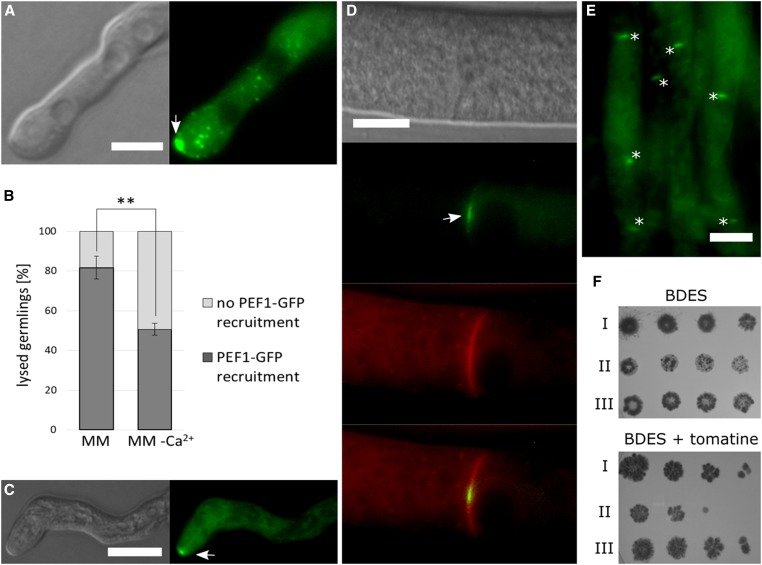
Cell lysis observed during aberrant plasma membrane fusion events is likely caused by membrane rupture. To investigate if PEF1 is also involved in the response to other types of membrane injuries, we sought different ways of harming the fungal plasma membrane. Most fungi, including N. crassa, are sensitive to polyene antibiotics, which bind to ergosterol in the plasma membrane, resulting in pore formation. The polyene nystatin is an important antifungal drug, which is commonly used to treat human fungal infections caused by Candida or Aspergillus species (Semis et al. 2010). One consequence of the exposure to nystatin is an increased Ca2+ influx into the cell (Eilam and Grossowicz 1982). To test the effect of nystatin, 3-hr-old spore germlings of the PEF1-GFP expressing strain GN9-22 (Ptef-1-pef1-gfp) were analyzed by light and fluorescence microscopy. Before the addition of nystatin, germlings had a healthy appearance and exhibited the typical cytoplasmic PEF1-GFP signal (Figure 5A). Within 40 sec after the addition of nystatin, cells became vacuolized, indicating lysis, and PEF1-GFP accumulated in puncta at the plasma membrane. Consistent with an enrichment of the nystatin target ergosterol at the growing cell tips, the GFP signal was also most apparent at this cellular region, where its intensity increased over time (Figure 5B). Quantification experiments revealed that this recruitment occurred in basically every germling. In growth medium, containing no Ca2+, this number was reduced by ∼30%, suggesting that extracellular Ca2+ is required for efficient PEF1 recruitment to the plasma membrane (Figure 5C).
Figure 5.
PEF1-GFP is recruited in response to nystatin-induced cell lysis. (A and B) Subcellular localization of PEF1-GFP in germlings of strain GN9-22 (Ptef-1-pef-1-gfp). (A) Control, without the addition of nystatin. (B) Nystatin was added directly after the photo for time point 0’ was taken. (A and B) DIC image on the left: 0 sec; DIC image on the right: 120 sec. Bar, 5 µm. Arrows in B indicate punctate PEF1-GFP aggregates. (C) Quantification of cells exhibiting PEF1-GFP recruitment to the plasma membrane after treatment with nystatin on MM and MM without Ca2+. Error bars indicate the SD calculated from three independent experiments (n = 100 each). The asterisks represent statistically significant differences determined by the Student’s t-test (*P ≤ 0.05; ***P ≤ 0.001).
To corroborate the observed response of PEF1 to pore-inducing drugs, we also tested the subcellular protein dynamics during treatment with the plant defense compound tomatine. Similar to the polyenes, tomatine binds ergosterol resulting in pore formation. Administering tomatine to 3-hr-old germlings of strain GN9-22 (Ptef-1-pef-1-gfp) resulted in cell lysis and accumulation of PEF1 at the plasma membrane, similar to the observations made for nystatin (Figure 6A). The signal intensity observed at the cell tips was, however, even more intense. Quantifications revealed PEF1 recruitment in the large majority of cells. In the absence of extracellular Ca2+, this number was significantly reduced (Figure 6B). Taken together these observations indicate that PEF1 is also recruited to the plasma membrane in response to the action of pore-forming drugs.
Figure 6.
PEF1-GFP is recruited in response to tomatine-induced cell lyses and contributes to the survival in presence of this pore-forming drug. (A) Recruitment of PEF1-GFP to the germling tips in response to tomatine-induced cell lysis (arrow). Left: DIC; right: GFP fluorescence. Bar, 5 µm. (B) Quantification of PEF1-GFP recruitment in germlings treated with tomatine on MM and MM without Ca2+. Error bars indicate the SD calculated from three independent experiments (n = 100 each). The asterisks represent statistically significant differences determined by the Student’s t-test (**P ≤ 0.01). (C) Recruitment of PEF1-GFP to a tip of a mature hypha in response to tomatine-induced cell lysis (arrow). Left: DIC; right: GFP fluorescence. (D) PEF1-GFP recruitment to the septal pore (arrow) after treatment with tomatine in mature hypha. The plasma membrane was stained in red with the lipophilic dye FM4-64. Top image: DIC; second image from top: GFP; third image from the top: FM4-64; bottom image: merger of GFP and FM4-64 images. (E) Overview of a group of hyphae exhibiting PEF1-GFP at septal pores after tomatine treatment (aggregates indicated by asterisks). (A–E) Strain GN9-22 (Ptef-1-pef-1-gfp). Bars in (A–E), 10 µm. (F) Fivefold serial spore dilutions (105–102) of wild type (I) (FGSC 988), ∆pef1 (II) (FGSC 15890), and the complemented strain ∆pef1 Ptef-1-pef1-gfp (III) (GN9-22) were spotted on BDES medium and on BDES medium containing 75 µg/ml tomatine. Growth was documented after 3 dpi.
To test if this recruitment is also observed in mature hyphae, strain GN9-22 (Ptef-1-pef1-gfp) was inoculated on solid MM and cultivated overnight, resulting in the formation of mature mycelial colonies. Treatment of these cultures with tomatine also resulted in a rapid accumulation of PEF1-GFP at the majority of tips of smaller branch hyphae in the inner parts of the colony (70%, n = 30) (Figure 6C). Surprisingly, no recruitment was observed at the tips of big leading hyphae (0%, n = 30). Studies on liposomes revealed that the concentration of sterols in the membrane must exceed a certain threshold before the interaction with saponins results in complex formation (Elias et al. 1979). To test for the presence of sterols, we stained the different hyphal types with filipin. The results indicated that only small branch hyphae but not leading hyphae carry a sterol-rich domain at their tip (data not shown). This observation suggests that the sterol-mediated membrane disturbing action of tomatine mostly affects the smaller hyphae in the inner parts of the colony, consistent with the recruitment pattern of PEF1. Interestingly, we also observed PEF1 accumulation at the septal pores in all hyphal types (PEF1-GFP at 94% of all septa, n = 100), suggesting their closure by clogging (Figure 6, D and E). To corroborate this finding, we also analyzed PEF1 dynamics at septal pores after addition of nystatin. As a result, comparable recruitment to the septal pores was observed (PEF1-GFP at 95% of all septa, n = 100).
PEF1 is involved in septal pore sealing in response to injury and during induced cell death
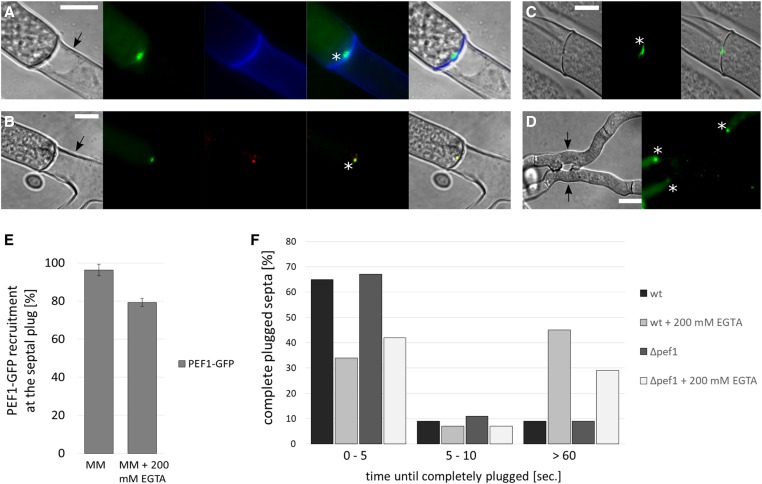
In young and healthy growing hyphae, septal pores are typically open and allow the rapid exchange of cytoplasm and organelles between the hyphal compartments. In response to injury or during aging and apoptotic-like reactions the pores are closed by a clogging mechanism (Fleissner and Glass 2007). During injury, this reaction involves a specific organelle, the Woronin body, which translocates into the septal pore and becomes surrounded by aggregating proteins (Jedd and Chua 2000; Tenney et al. 2000; Lai et al. 2012). In contrast, the developmental compartmentation is Woronin body independent (Markham 1994; Fleissner and Glass 2007). To test the role of PEF1 in these different processes, we tested the PEF1-GFP localization during hyphal injury and during programmed cell death. For the former, mature hyphae of strain GN3-17 (Pccg-1-pef1-gfp) were cut with a UV pulse laser at a laser microdissection microscope, and the septa next to the injury were observed by fluorescence microscopy. The injured hyphae showed the typical plugging reaction, and PEF1 accumulated at the majority of closed septal pores. Chelating of Ca2+ by the addition of EGTA resulted in a reduced number of septa exhibiting the PEF1-GFP signal, suggesting that Ca2+ promotes aggregation of the protein (Figure 7, A and E). To corroborate the position of the PEF1 aggregate at the septal plug, we co-localized PEF1-GFP with the Woronin body, which was visualized by tagging its main constituent, the HEX-1 protein, with the red fluorescent protein dsRED. Co-localization of both proteins was detected (Figure 7B). To test if the PEF1-GFP accumulation at the septal plug is Woronin body dependent, the GFP fusion protein was expressed in a Δhex-1 mutant strain. Δhex-1 hyphae lack Woronin bodies, resulting in an inability to quickly seal the septal pore after injury and significant cytoplasmic leakage. However, eventually Δhex-1 pores also become plugged by a clogging mechanism. PEF1 was also present at these sealed septa, indicating that the PEF1 recruitment to the septal pore is Woronin body independent (Figure 7C). To test if PEF1 is required for septal sealing, the plugging efficiency of wild-type and Δpef1 hyphae was compared by measuring the time required for complete sealing after hyphal transsection. No differences were found indicating that PEF1 is dispensable for septal sealing. Interestingly, however, chelating of Ca2+ resulted in delayed plugging of septal pores in both strains, suggesting that Ca2+ also promotes clogging as a wound response (Figure 7F).
Figure 7.
PEF1-GFP localizes to the septal plug after injury and during programmed cell death. (A) PEF1-GFP recruitment to the septal pore after cutting of the hypha with a laser (strain GN3-17, Pccg-1-pef-1-gfp). Arrow: injured, empty compartment. Asterisk indicates the septal plug. The cell wall was stained with the chitin-binding dye calcofluor white (CFW, 100 µg/ml) (false colored in blue). Images from the left: (1) DIC; (2) GFP fluorescence; (3) CFW; (4) merged image of GFP and CFW; (5) merged image of 1–3. (B) Co-localization of PEF1-GFP and the Woronin body, which is visualized by dsRED-HEX-1, in a heterokaryon of strains S10 (Pccg-1-pef-1-gfp) and S20 (Pccg-1-dsred-hex-1). The asterisk indicates the septal plug, the arrow the injured compartment. Images from the left: (1) DIC; (2) GFP fluorescence; (3) dsRED fluorescence; (4) merged image of GFP and dsRED; (5) merged image of 1–3. (C) Recruitment of PEF1-GFP to the septal pore in the ∆hex-1 strain (S48, Pccg-1-pef-1-gfp), which does not possess Woronin bodies, after cutting the hypha with a laser. The asterisk indicates the septal plug. Left: DIC; center: GFP; right: merged image of DIC and GFP. (D) PEF1-GFP recruitment to septal plugs in hyphae undergoing programmed cell death induced by heterokaryon incompatibility [forced heterokaryon of C9-15 and GN3-36 (Pccg-1-pef-1-gfp)]. Arrows indicate compartments undergoing cell death. Asterisks indicate septal plugs. Note the absence of GFP fluorescence in the dead compartments. Left: DIC; right: GFP. Bar, 10 µm (A–D). (E) Quantification of PEF1-GFP recruitment to the septal plug after cutting with a laser on MM and MM + 200 mM EGTA (strain GN3-17, Pccg-1-pef-1-gfp). Error bars indicate the SD calculated from three independent experiments (n = 100 each). (F) Comparison of the time required for complete sealing of the septal pore in the wild type (FGSC 988) and the ∆pef1 strain (FGSC 15890) on MM and MM + 200 mM EGTA. Two hundred hyphae of each strain were cut with a laser and the time until cytoplasmic leakage through the septal pore ceased was measured.
Hyphae of different N. crassa colonies are able to fuse; however, this fusion between different individuals is subject to a genetically encoded control system. Fusion between hyphae that differ in their allelic specificity of at least one of 11 heterokaryon incompatibility (het) loci results in septal pore plugging in the fused compartments and subsequent programmed cell death (Glass and Kaneko 2003). To test if PEF1 is also involved in these Woronin body-independent clogging processes, PEF1-GFP (expressed from Pccg-1-pef1-gfp) was localized in an incompatible heterokaryon of two strains carrying different het-c alleles. In these cultures, two incompatible strains with different auxotropic markers are forced into a heterokaryon. Because of their auxotrophic requirements, the strains can only grow on MM when fused, but at the same time the permanent induction of the incompatibility reaction results in high numbers of hyphal compartments undergoing programmed cell death. The obtained heterokaryons exhibited a typical incompatibility phenotype, including slow growth, complete absence of sporulation, and high numbers of dying compartments, which were visualized by methylene blue staining. PEF1-GFP was readily observed at the plugged septa of compartments undergoing cell death, indicating that it is also involved in this clogging mechanism (Figure 7D).
PEF1 promotes resistance against the phytoanticipine tomatine
Based on the observed translocation of PEF1 to the plasma membrane in response to pore-forming drugs, we hypothesized that PEF1 promotes resistance against these substances. In addition, an earlier study described that mutants of S. cerevisiae lacking the pef1 homologous gene exhibit growth defects on medium containing SDS or EGTA (Vernarecci et al. 2007). We therefore compared growth of the wild type, the Δpef1 mutant, and the Δpef1 mutant expressing the functional PEF1-GFP fusion protein (expressed from Ptef-1-pef1-gfp) on medium containing nystatin, tomatin, SDS, and EGTA. While no growth differences between the tested strains were detected on nystatin, SDS, and EGTA, growth of the Δpef1 mutant was significantly reduced on the plant saponin tomatine, suggesting that PEF1 contributes to the stress response against this pore-forming drug (Figure 6F and Figure S3).
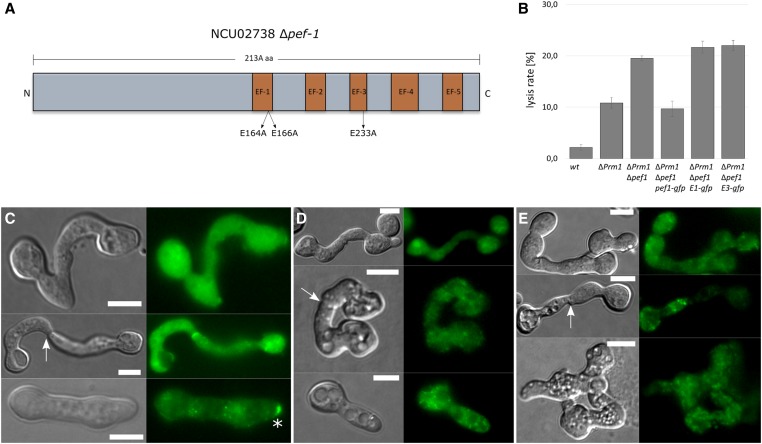
The EF-hand domains are essential for PEF1 function
The reduced PEF1 plasma membrane recruitment in response to lysis on medium with reduced amounts of Ca2+ suggested a role of binding to Ca2+ in the protein dynamics. We therefore hypothesized that the mutation of Ca2+-binding domains would also result in reduced or absent membrane recruitment of the protein. Members of the PEF protein family contain five conserved EF-hand domains comprising helix–loop–helix Ca2+-binding motifs, which exhibit different Ca2+-binding affinities. In S. cerevisiae the EF-1-hand and EF-3-hand domains of the PEF1 homologous Pef1p possess the highest affinity to Ca2+ (Vernarecci et al. 2007). To test the role of these conserved domains for PEF1 of N. crassa, we introduced E164A, E166A mutations into EF-1 and in an independent construct an E233A change into EF-3 (Figure 8A). Both mutated gene constructs were fused with gfp and were expressed in the ΔPrm1/Δpef1 double mutant under control of the ccg-1 promoter. The subcellular dynamics of both variants were compared to wild-type PEF1-GFP in healthy spore germlings and during drug-induced and fusion-induced cell lysis. The overall signal intensity was comparable in all strains indicating that the mutations had no negative effect on transcription, translation, or protein stability. In the absence of the pore-forming drugs and in healthy fusing germlings, no differences were observed for the three protein variants, and all showed the typical cytoplasmic localization (Figure 8). In contrast, after treatment with nystatin, wild-type PEF1 accumulated at the plasma membrane in the above-described manner, while no membrane recruitment was observed for the mutated variant. Similarly, the mutated proteins did not accumulate at the contact point of lysing fusion pairs (Figure 8). Consistent with these findings, the mutated variants did not complement the increased lysis rates of fusing ΔPrm1/Δpef1 germlings (Figure 8B). Taken together, these data indicate that the EF-1- and the EF-3-hand domains are essential for normal PEF1 functioning, suggesting that Ca2+ binding is mediating the dynamics of this protein during drug- and fusion-induced lysis.
Figure 8.
The EF-hand domains EF-1 and EF-3 are essential for PEF1 function. (A) Cartoon illustrating the structure of PEF1. The five EF-Hand domains and their relative positions are highlighted in orange. The replaced amino acids in EF-1 and EF-3 are indicated. (B) Quantification of the lysis rate during germling fusion in the ∆Prm1/∆pef1 strains expressing pef1-gfp (GN7-54), pef1E164A/E166A-gfp (GN9-34), and pef1E233A-gfp (GN9-1). The wild type (FGSC 988) and ∆Prm1 (A32) were used as controls. Error bars indicate the SD calculated from three independent experiments (n = 100 each). Localization of (C) PEF1-GFP [GN9-22 (Ptef-1-pef-1-gfp)], (D) PEF1E164A/E166A-GFP [GN9-34 (Ptef-1-pef-1E164A/E166A-gfp)], and (E) PEF1E233A-GFP [GN9-1 (Ptef-1-pef-1E233A-gfp)] during successful fusion (top), fusion failure (middle), and during nystatin-induced cell lysis (bottom). The arrows point to the fusion point and the asterisk indicates PEF1 aggregates at the plasma membrane. Left images: DIC; right images: GFP. Bar, 5 µm.
PEF1 appears to function independently of the ESCRT complex during fusion-induced lysis
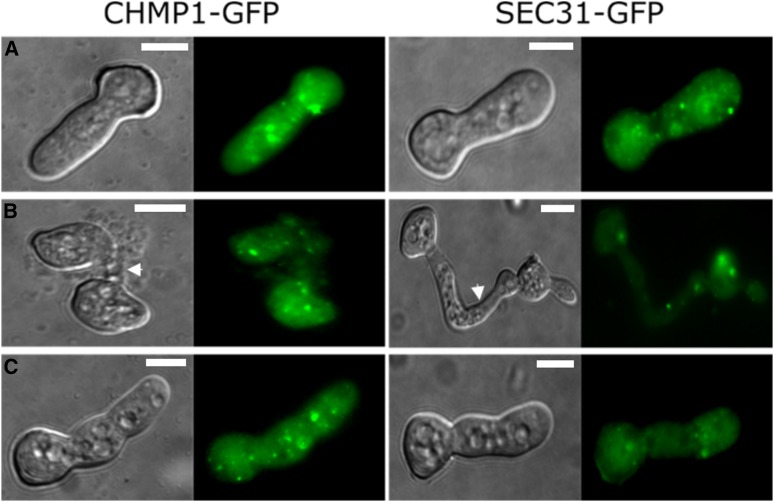
A recent study describing a role of the mammalian PEF1 homolog ALG-2 in membrane repair linked its function to the ESCRT-III complex. After laser-induced injury of the plasma membrane of myoblasts, the ESCRT complex and accessory proteins accumulated at the wound site in an ALG-2-dependent manner, resulting in membrane repair (Scheffer et al. 2014). To test if PEF1 mediates a similar process in N. crassa, we localized the conserved and essential ESCRT-III constituents CHMP1 and SEC31 via GFP tagging. Spores of the resulting strains, GN9-50 and GN9-48, respectively, were incubated on solid medium for 3 hr at 30°. The spore germlings showed cytoplasmic fluorescence with some increased signal intensity in dot-like structures. No accumulation of the signal at the fusion point or the plasma membrane was observed in lysed fusion pairs or after treatment with nystatin (Figure 9). Together these data suggest that unlike in mammalian cells, PEF1 is not mediating the assembly of the ESCRT complex at wound sites and might therefore mediate a different so far unknown mechanism of fungal plasma membrane repair.
Figure 9.
The ESCRT-III complex proteins CHMP1-GFP and SEC31-GFP do not accumulate at the plasma membrane during fusion-induced lysis or after nystatin treatment. Subcellular localization of CHMP1-GFP (GN9-50) and SEC31-GFP (GN9-48) in untreated healthy germlings (A), during fusion failure (B), and during nystatin-induced cell lysis (C). Fusion points are indicated (arrows). Left images: DIC; right images: GFP. Bar, 5 µm.
Discussion
PEF1 dynamics depend on Ca2+
Plasma membrane injury commonly occurs during growth and development of all organisms, posing the risk of rapid cell death. To counteract the life-threatening membrane disintegration, efficient membrane repair mechanisms have evolved, which commonly include the activity of Ca2+-responsive proteins. In this study we identified the penta-EF-hand protein PEF1 as a response mediator for different types of membrane injury, including fusion-induced membrane rupture and drug-induced pore formation. Fungal PEF1 homologs consist of a conserved C-terminal part with homology to plant and animal proteins, including the mammalian ALG-2. The N-terminal third of the protein is specific for the taxon fungi, suggesting some fungal-specific function. Similar to ALG-2 in mammals, PEF1 rapidly translocates to the plasma membrane after injury in a Ca2+-dependent manner, suggesting a conserved reaction to binding of the EF-hand domains to Ca2+. Translocation of repair-mediating factors to the wound site in response to a local increase of Ca2+ is common in different plasma membrane repair models. For example, in injured human myotubes, calpain, a Ca2+-dependent cysteine protease, which acts as a primary mediator of membrane repair, rapidly accumulates at the site of injury (Redpath et al. 2014). Similarly, membrane-binding annexins, which mediate wound sealing through vesicle fusion, aggregate at wound sites in different types of mammalian cells (Carmeille et al. 2016; Koerdt and Gerke 2017). This quick activation and translocation of preformed proteins allows a very rapid response independent of time-consuming gene transcription or protein biosynthesis, which an injured cell cannot afford. Our data demonstrate that in fungi PEF1 belongs to these preformed response factors.
The rapidly increasing intracellular Ca2+ concentration at the wound site has mostly been attributed to the influx of extracellular Ca2+. Similar to other eukaryotic organisms, fungi maintain a low cytosolic Ca2+ concentration and higher concentrations of this ion in the extracellular space or within organelles [reviewed in Cyert and Philpott (2013)]. Disintegration of the plasma membrane therefore quickly results in collapsing of this gradient and local cytoplasmic Ca2+ increase. When in our study calcium was reduced or depleted in the growth medium, recruitment of PEF1 to the plasma membrane in response to injury was significantly reduced, fully consistent with these current models of membrane repair. A recent study investigating sarcolemma repair in murine muscle fibers also identified lysosomal Ca2+ as an essential intracellular source during Ca2+-mediated membrane injury responses (Cheng et al. 2014). Since a lack of Ca2+ in the growth medium can also result in depletion of intracellular Ca2+ storages, we cannot exclude the possibility that also the cytoplasmic increase in Ca2+ through influx from organelles, such as vacuoles, might control PEF1 dynamics. A more detailed understanding of the Ca2+ fluxes throughout healthy and injured fungal cells will be a vital prerequisite for fully understanding membrane repair in this group of organisms.
PEF1 mediates protection against membrane fusion-induced cell lysis
The loss of pef1 results in increased fusion-induced lysis only in lysis-prone mutants but not in a wild-type background. This observation suggests that in the wild type, cell fusion is robust and repair mechanisms are dispensable. In the ΔPrm1 and Δlfd-1 mutants, however, the onset of plasma membrane fusion has three different outcomes. First, cell pairs fuse in a wild-type like manner. Second, the fusion process arrests after cell wall deconstruction, and third, the cells lyse because of membrane rupture. Cell lysis is strongly correlated to the moment of cytoplasmic mixing, suggesting that lysis is caused by the engagement of the cell fusion machinery. So far the molecular functions of PRM1 and LFD-1 remain unknown. They appear to ensure fusion fidelity, probably by organizing or spatially restricting the fusion machinery (Fleissner et al. 2009; Palma-Guerrero et al. 2014). The lack of LFD-2 or FIG1 also causes increased lysis rates, but no adhering membranes are observed, indicating distinct functions from PRM1 and LFD-1 (Palma-Guerrero et al. 2015). Interestingly, the lysis rates in Δfig1 do not further increase in the absence of pef1. FIG1 is a low affinity Ca2+ uptake system, facilitating Ca2+ influx, which is mediating mating fusion in S. cerevisiae and C. albicans (Muller et al. 2003; Yang et al. 2011). While PRM1, LFD-1, and LFD-2 are involved in fusion pore formation, FIG1 might play a role in an earlier step of the fusion process, before engagement of the fusion machinery. The observed cell lysis in Δfig1 might not be caused by fusion-induced membrane rupture and might therefore not activate the PEF1-mediated response. Studying the interplay of the different fusion factors and also the related membrane repair mechanisms in N. crassa represents one very promising strategy to further illuminate the molecular mechanisms mediating eukaryotic cell–cell fusion.
PEF1 appears to function independently of the ESCRT-III complex
While our data strongly support a role of PEF1 in mediating the response to different types of membrane injuries in fungi, the exact molecular repair mechanisms remain unknown. In mammalian muscle cells, the PEF1 homologous ALG-2 accumulates at membrane injury sites in a Ca2+-dependent manner and acts as an initiator of ALIX recruitment and ESCRT-III–Vps4 complex formation. As a consequence, the injured membrane region undergoes shedding and repair (Scheffer et al. 2014). While the involved proteins are conserved in fungi, our data suggest that in N. crassa, PEF1 functions in an ESCRT-III-independent way. No recruitment of the ESCRT proteins to the plasma membrane or co-localization with PEF1 was observed. In addition to its interaction with the ESCRT complex, ALG-2 and homologous proteins are involved in various different cellular processes, including ER-to-Golgi vesicular transport or signal transduction from the membrane to downstream factors [reviewed in Maki et al. (2016)]. It is therefore conceivable that an ESCRT-III independent membrane repair mechanism is controlled by PEF1. Fungal PEF1 shares only homology with ALG-2 in the C-terminal two-thirds of the protein. The N-terminal third is specific for fungi. This part might mediate the interaction with other, probably fungal-specific, proteins. An attractive hypothesis is that PEF1 might serve as a conserved upstream sensor of membrane injury mediating divergent downstream molecular processes. The potential existence of fungal-specific membrane repair mechanisms calls for further investigation, since they might provide highly interesting targets for controlling fungi. An earlier study identified the synaptotagmin SYT1 as part of a fungal membrane repair mechanism (Palma-Guerrero et al. 2014). Similar to our observations for pef1, the introduction of the syt1 knockout mutation in lysis-prone fusion mutants significantly increased the number of lysing cell fusion pairs. In our hands, however, the syt1 double mutants did not show this defect, suggesting that this phenotype is not robust. We hypothesize that different culture conditions, which could not be controlled for, resulted in the contradictory observations in the two studies.
Membrane repair could contribute to antifungal resistance
PEF1 accumulates at the plasma membrane during exposure to pore-forming drugs, and the lack of PEF1 results in an increased sensitivity to the plant defense compound tomatine. Plants possess two lines of chemical antimicrobial defense. First, numerous species accumulate preformed antifungals or phytoanticipines, such as the glycoalkaloids of nightshades including tomatine. Second, many plants produce defense compounds in response to an infection, so called phytoalexins. Both phytoanticipins and phytoalexins include pore-forming substances (Osbourn 1999). The current model of fungal resistance against plant defense compounds and antifungals includes two different mechanisms, which function in a step-wise manner: first, the activation of efflux pumps, which reduce the intracellular concentration of the toxic chemicals; and second, the enzymatic degradation of these compounds (Denny and VanEtten 1981, 1983; Fleissner et al. 2002). The gene expression of efflux pumps is induced within minutes after toxin exposure, allowing rapid protection and providing time for activation of the detoxification response (Del Sorbo et al. 2000; Fleissner et al. 2002). The main caveat of this model is, however, still a “time conundrum”: although the activation of efflux pumps is rapid, it might not be fast enough. Once a pore-forming drug is interacting with membrane lipids, the lipid bilayer is disintegrating, and the cell would quickly die. Based on our data, we suggest adding a third line of defense into the current model. We hypothesize that membrane repair mechanisms provide instant protection against pore-forming drugs, even before efflux pumps are activated. The model therefore comprises three successive steps: (1) Membrane repair, (2) Efflux, and (3) Detoxification. Since important clinical antifungals also target the plasma membrane, this model might also apply to the fungal cellular response against these drugs. It will be of great interest to test this hypothesis in clinically and agriculturally relevant fungal species.
Interestingly, we did not observe an increased sensitivity of the Δpef1 mutant to the polyene nystatin. Nystatin and tomatine are both pore-forming drugs and PEF1-GFP accumulated at the membrane after treatment with both substances, albeit to a lesser extent during nystatin application. So far, this difference is inconceivable. Our data support the notion that other membrane repair mechanisms exist in N. crassa, with partially overlapping functions with the PEF1 pathway. If the spatial and/or temporal dynamics of the membrane injuries caused by tomatine and nystatin differ, the different repair mechanisms might have different contributions, which could explain the observed differences. Also, if the nystatin treatment results in a significantly lower Ca2+ influx than the tomatine treatment, repair mechanisms might not be sufficiently activated to prevent cell death. This hypothesis is supported by the marked weaker PEF1 recruitment to the plasma membrane in response to nystatin compared to tomatine. In human cells, two annexins with different Ca2+ sensitivities mediate specific responses to different types of injuries (Potez et al. 2011). Similarly, PEF1 might be more sensitive to Ca2+ than the so far unknown repair mechanism required to cope with nystatin-induced injuries. This could explain why a recruitment of PEF1 to the plasma membrane in response to nystatin was observed but no changes in the nystatin sensitivity of the Δpef1 mutant were detected. Therefore, identifying the complete set of repair machineries, their interplay, and their activation modes will be of high relevance for fully understanding and controlling the cellular response to membrane-targeting antifungal drugs.
PEF1 is involved in the clogging of septal pores
PEF1 accumulates at septal pores in response to tomatine exposure. Similarly, the protein is part of the pore plug formed after hyphal transsection. While the occlusion of septal pores by Woronin bodies has already been described in the early days of mycology (Buller 1933), recent years have seen the identification of numerous proteins accumulating not only around the Woronin body, but also in Woronin body-independent pore occlusions (Fleissner and Glass 2007; Lai et al. 2012). The first protein identified to take place in these processes was the SO protein of N. crassa, which shows comparable dynamics to PEF1 (Fleissner and Glass 2007). A common characteristic of pore-occluding proteins is stretches of disordered structure (Lai et al. 2012). The fungal-specific N-terminal first third of PEF1 is meeting this requirement. It is likely that pore occlusion has evolved as a secondary role of proteins with different primary functions. The SO protein, for example, is involved in the signaling of fungal germlings and hyphae undergoing vegetative cell fusion (Fleissner et al. 2005). The aggregation of proteins at the septal pore promotes its closure through the Woronin body by a sealing mechanism. In Woronin body-independent septal pore sealing, e.g., during vegetative incompatibility or hyphal aging, the proteinous aggregates even seem to serve as the main plug for hyphal compartmentation (Fleissner et al. 2005; Lai et al. 2012). The recruitment of PEF1 to basically all septa within a hyphal network after exposure to tomatine might hint to an important mechanism employed by fungi to control their multicellular state. Fungal multicellularity has evolved independently of plant and animal tissues. So far, the functioning and adaptation of multicellular fungal colonies is only very poorly understood. Healthy hyphae of N. crassa are a syncytium, growing by tip extension and branching. While hyphal differentiation includes permanent compartmentation by septal pore plugging, there is evidence that pores can also be closed temporarily (Markham 1994; Bleichrodt et al. 2012). PEF1 might provide a suitable marker for further investigation of the still poorly understood functioning of multicellular fungal colonies.
Acknowledgments
We thank Ralf Schnabel and Christian Hennig for ongoing support in developing our microscopy. We are grateful to Ulrich Kück and Ines Teichert for hosting C.A. for laser dissection experiments. We thank Louise Glass and Javier Palma-Guerrero for providing various mutant strains. We gratefully acknowledge use of materials generated by PO1 GM068087, “Functional analysis of a model filamentous fungus.” This work was in part supported by funding from the German Research Foundation (Grants FL706/1-1 and FL706/1-2) to A.F.
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25386/genetics.8239490.
Communicating editor: M. Freitag
Literature Cited
- Aguilar P. S., Engel A., Walter P., 2007. The plasma membrane proteins Prm1 and Fig1 ascertain fidelity of membrane fusion during yeast mating. Mol. Biol. Cell 18: 547–556. 10.1091/mbc.e06-09-0776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiychuk E. B., Monastyrskaya K., Potez S., Draeger A., 2011. Blebbing confers resistance against cell lysis. Cell Death Differ. 18: 80–89. 10.1038/cdd.2010.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthélémy F., Defour A., Levy N., Krahn M., Bartoli M., 2018. Muscle cells fix breaches by orchestrating a membrane repair ballet. J. Neuromuscul. Dis. 5: 21–28. 10.3233/JND-170251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berepiki A., Lichius A., Shoji J. Y., Tilsner J., Read N. D., 2010. F-actin dynamics in Neurospora crassa. Eukaryot. Cell 9: 547–557. 10.1128/EC.00253-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleichrodt R. J., van Veluw G. J., Recter B., Maruyama J., Kitamoto K., et al. , 2012. Hyphal heterogeneity in Aspergillus oryzae is the result of dynamic closure of septa by Woronin bodies. Mol. Microbiol. 86: 1334–1344. 10.1111/mmi.12077 [DOI] [PubMed] [Google Scholar]
- Bolard J., 1986. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim. Biophys. Acta 864: 257–304. 10.1016/0304-4157(86)90002-X [DOI] [PubMed] [Google Scholar]
- Brockman H. E., De Serres F. J., 1963. Sorbose toxicity in neurospora. Am. J. Bot. 50: 709–714. 10.1002/j.1537-2197.1963.tb12246.x [DOI] [Google Scholar]
- Buller A., 1933. Researches on Fungi. Longman, London. [Google Scholar]
- Cano-Domínguez N., Alvarez-Delfin K., Hansberg W., Aguirre J., 2008. NADPH oxidases NOX-1 and NOX-2 require the regulatory subunit NOR-1 to control cell differentiation and growth in Neurospora crassa. Eukaryot. Cell 7: 1352–1361. 10.1128/EC.00137-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeille R., Bouvet F., Tan S., Croissant C., Gounou C., et al. , 2016. Membrane repair of human skeletal muscle cells requires Annexin-A5. Biochim. Biophys. Acta 1863: 2267–2279. 10.1016/j.bbamcr.2016.06.003 [DOI] [PubMed] [Google Scholar]
- Chandrasekar P., 2011. Management of invasive fungal infections: a role for polyenes. J. Antimicrob. Chemother. 66: 457–465. 10.1093/jac/dkq479 [DOI] [PubMed] [Google Scholar]
- Cheng X., Zhang X., Gao Q., Ali Samie M., Azar M., et al. , 2014. The intracellular Ca2+ channel MCOLN1 is required for sarcolemma repair to prevent muscular dystrophy. Nat. Med. 20: 1187–1192. 10.1038/nm.3611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M. S., Caldwell R. W., Chiao H., Miyake K., McNeil P. L., 1995. Contraction-induced cell wounding and release of fibroblast growth factor in heart. Circ. Res. 76: 927–934. 10.1161/01.RES.76.6.927 [DOI] [PubMed] [Google Scholar]
- Colot H. V., Park G., Turner G. E., Ringelberg C., Crew C. M., et al. , 2006. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc. Natl. Acad. Sci. USA 103: 10352–10357 (erratum: Proc. Natl. Acad. Sci. USA 103: 16614). 10.1073/pnas.0601456103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert M. S., Philpott C. C., 2013. Regulation of cation balance in Saccharomyces cerevisiae. Genetics 193: 677–713. 10.1534/genetics.112.147207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Peraro M., van der Goot F. G., 2016. Pore-forming toxins: ancient, but never really out of fashion. Nat. Rev. Microbiol. 14: 77–92. 10.1038/nrmicro.2015.3 [DOI] [PubMed] [Google Scholar]
- Daskalov A., Heller J., Herzog S., Fleißner A., Glass N. L., 2016. Molecular mechanisms regulating cell fusion and heterokaryon formation in filamentous fungi. Microbiol. Spectr. 5 10.1128/microbiolspec.FUNK-0015-2016 [DOI] [PubMed] [Google Scholar]
- Davenport N. R., Bement W. M., 2016. Cell repair: revisiting the patch hypothesis. Commun. Integr. Biol. 9: e1253643 10.1080/19420889.2016.1253643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Sorbo G., Schoonbeek H., De Waard M. A., 2000. Fungal transporters involved in efflux of natural toxic compounds and fungicides. Fungal Genet. Biol. 30: 1–15. 10.1006/fgbi.2000.1206 [DOI] [PubMed] [Google Scholar]
- Denny T. P., VanEtten H. D., 1981. Tolerance by Nectria haematococca M P VI of the chickpea (Cicer arietinum) phytoalexins medicarpin and maackiain. Physiol. Plant Pathol. 19: 419–437. 10.1016/S0048-4059(81)80073-2 [DOI] [Google Scholar]
- Denny T. P., VanEtten H. D., 1983. Tolerance of Nectria haematococca MP VI to the phytoalexin pisatin in the absence of detoxification. J. Gen. Microbiol. 129: 2893–2901. [Google Scholar]
- Dunlap J. C., Borkovich K. A., Henn M. R., Turner G. E., Sachs M. S., et al. , 2007. Enabling a community to dissect an organism: overview of the Neurospora functional genomics project. Adv. Genet. 57: 49–96. 10.1016/S0065-2660(06)57002-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddleman C. S., Bittner G. D., Fishman H. M., 2000. Barrier permeability at cut axonal ends progressively decreases until an ionic seal is formed. Biophys. J. 79: 1883–1890. 10.1016/S0006-3495(00)76438-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilam Y., Grossowicz N., 1982. Nystatin effects on cellular calcium in Saccharomyces cerevisiae. Biochim. Biophys. Acta 692: 238–243. 10.1016/0005-2736(82)90527-2 [DOI] [PubMed] [Google Scholar]
- Elias P. M., Friend D. S., Goerke J., 1979. Membrane sterol heterogeneity. Freeze-fracture detection with saponins and filipin. J. Histochem. Cytochem. 27: 1247–1260. 10.1177/27.9.479568 [DOI] [PubMed] [Google Scholar]
- Fleissner A., Glass N. L., 2007. SO, a protein involved in hyphal fusion in Neurospora crassa, localizes to septal plugs. Eukaryot. Cell 6: 84–94. 10.1128/EC.00268-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleissner A., Sopalla C., Weltring K. M., 2002. An ATP-binding cassette multidrug-resistance transporter is necessary for tolerance of Gibberella pulicaris to phytoalexins and virulence on potato tubers. Mol. Plant Microbe Interact. 15: 102–108. 10.1094/MPMI.2002.15.2.102 [DOI] [PubMed] [Google Scholar]
- Fleissner A., Sarkar S., Jacobson D. J., Roca M. G., Read N. D., et al. , 2005. The so locus is required for vegetative cell fusion and postfertilization events in Neurospora crassa. Eukaryot. Cell 4: 920–930. 10.1128/EC.4.5.920-930.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleissner A., Diamond S., Glass N. L., 2009. The Saccharomyces cerevisiae PRM1 homolog in Neurospora crassa is involved in vegetative and sexual cell fusion events but also has postfertilization functions. Genetics 181: 497–510. 10.1534/genetics.108.096149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag M., Hickey P. C., Raju N. B., Selker E. U., Read N. D., 2004. GFP as a tool to analyze the organization, dynamics and function of nuclei and microtubules in Neurospora crassa. Fungal Genet. Biol. 41: 897–910. 10.1016/j.fgb.2004.06.008 [DOI] [PubMed] [Google Scholar]
- Friedman M., 2002. Tomato glycoalkaloids: role in the plant and in the diet. J. Agric. Food Chem. 50: 5751–5780. 10.1021/jf020560c [DOI] [PubMed] [Google Scholar]
- Glass N. L., Kaneko I., 2003. Fatal attraction: nonself recognition and heterokaryon incompatibility in filamentous fungi. Eukaryot. Cell 2: 1–8. 10.1128/EC.2.1.1-8.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman M. G., Walter P., 2000. Prm1p, a pheromone-regulated multispanning membrane protein, facilitates plasma membrane fusion during yeast mating. J. Cell Biol. 151: 719–730. 10.1083/jcb.151.3.719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn A., Jaiswal J. K., 2018. Cellular mechanisms and signals that coordinate plasma membrane repair. Cell. Mol. Life Sci. 75: 3751–3770. 10.1007/s00018-018-2888-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A. C., McNeil A. K., Xiong F., Xiong W. C., McNeil P. L., 2011. A novel cellular defect in diabetes: membrane repair failure. Diabetes 60: 3034–3043. 10.2337/db11-0851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idone V., Tam C., Andrews N. W., 2008a. Two-way traffic on the road to plasma membrane repair. Trends Cell Biol. 18: 552–559. 10.1016/j.tcb.2008.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idone V., Tam C., Goss J. W., Toomre D., Pypaert M., et al. , 2008b. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J. Cell Biol. 180: 905–914. 10.1083/jcb.200708010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd G., Chua N. H., 2000. A new self-assembled peroxisomal vesicle required for efficient resealing of the plasma membrane. Nat. Cell Biol. 2: 226–231. 10.1038/35008652 [DOI] [PubMed] [Google Scholar]
- Jin H., Carlile C., Nolan S., Grote E., 2004. Prm1 prevents contact-dependent lysis of yeast mating pairs. Eukaryot. Cell 3: 1664–1673. 10.1128/EC.3.6.1664-1673.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsky S. C., 1961. Alterations in the permeability of Neurospora crassa due to polyene antibiotics. J. Bacteriol. 82: 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerdt S. N., Gerke V., 2017. Annexin A2 is involved in Ca2+-dependent plasma membrane repair in primary human endothelial cells. Biochim Biophys Acta Mol Cell Res 1864: 1046–1053. 10.1016/j.bbamcr.2016.12.007 [DOI] [PubMed] [Google Scholar]
- la Cour J. M., Winding Gojkovic P., Ambjorner S. E. B., Bagge J., Jensen S. M., et al. , 2018. ALG-2 participates in recovery of cells after plasma membrane damage by electroporation and digitonin treatment. PLoS One 13: e0204520 10.1371/journal.pone.0204520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J., Koh C. H., Tjota M., Pieuchot L., Raman V., et al. , 2012. Intrinsically disordered proteins aggregate at fungal cell-to-cell channels and regulate intercellular connectivity. Proc. Natl. Acad. Sci. USA 109: 15781–15786. 10.1073/pnas.1207467109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki M., Takahara T., Shibata H., 2016. Multifaceted roles of ALG-2 in Ca(2+)-regulated membrane trafficking. Int. J. Mol. Sci. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin B. S., Freitag M., Selker E. U., 1997. Improved plasmids for gene targeting at the his-3 locus of Neurospora crassa by electroporation. Fungal Genet. Newsl. 44: 34–36. [Google Scholar]
- Markham P., 1994. Occlusions of septal pores in filamentous fungi. Mycol. Res. 98: 1089–1106. 10.1016/S0953-7562(09)80195-0 [DOI] [Google Scholar]
- McNeil P. L., Khakee R., 1992. Disruptions of muscle fiber plasma membranes. Role in exercise-induced damage. Am. J. Pathol. 140: 1097–1109. [PMC free article] [PubMed] [Google Scholar]
- McNeil P. L., Kirchhausen T., 2005. An emergency response team for membrane repair. Nat. Rev. Mol. Cell Biol. 6: 499–505. 10.1038/nrm1665 [DOI] [PubMed] [Google Scholar]
- Mondal A. K., Sreekumar A., Kundu N., Kathuria R., Verma P., et al. , 2018. Structural basis and functional implications of the membrane pore-formation mechanisms of bacterial pore-forming toxins. Adv. Exp. Med. Biol. 1112: 281–291. 10.1007/978-981-13-3065-0_19 [DOI] [PubMed] [Google Scholar]
- Muller E. M., Mackin N. A., Erdman S. E., Cunningham K. W., 2003. Fig1p facilitates Ca2+ influx and cell fusion during mating of Saccharomyces cerevisiae. J. Biol. Chem. 278: 38461–38469. 10.1074/jbc.M304089200 [DOI] [PubMed] [Google Scholar]
- Nakamura M., Dominguez A. N. M., Decker J. R., Hull A. J., Verboon J. M., et al. , 2018. Into the breach: how cells cope with wounds. Open Biol. 8 10.1098/rsob.180135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osbourn A. E., 1999. Antimicrobial phytoprotectants and fungal pathogens: a commentary. Fungal Genet. Biol. 26: 163–168. 10.1006/fgbi.1999.1133 [DOI] [PubMed] [Google Scholar]
- Palma-Guerrero J., Hall C. R., Kowbel D., Welch J., Taylor J. W., et al. , 2013. Genome wide association identifies novel loci involved in fungal communication. PLoS Genet. 9: e1003669 10.1371/journal.pgen.1003669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma-Guerrero J., Leeder A. C., Welch J., Glass N. L., 2014. Identification and characterization of LFD1, a novel protein involved in membrane merger during cell fusion in Neurospora crassa. Mol. Microbiol. 92: 164–182. 10.1111/mmi.12545 [DOI] [PubMed] [Google Scholar]
- Palma-Guerrero J., Zhao J., Goncalves A. P., Starr T. L., Glass N. L., 2015. Identification and characterization of LFD-2, a predicted fringe protein required for membrane integrity during cell fusion in Neurospora crassa. Eukaryot. Cell 14: 265–277. 10.1128/EC.00233-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potez S., Luginbuhl M., Monastyrskaya K., Hostettler A., Draeger A., et al. , 2011. Tailored protection against plasmalemmal injury by annexins with different Ca2+ sensitivities. J. Biol. Chem. 286: 17982–17991. 10.1074/jbc.M110.187625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen C. G., Morgenstein R. M., Peck S., Glass N. L., 2008. Lack of the GTPase RHO-4 in Neurospora crassa causes a reduction in numbers and aberrant stabilization of microtubules at hyphal tips. Fungal Genet. Biol. 45: 1027–1039. 10.1016/j.fgb.2008.02.006 [DOI] [PubMed] [Google Scholar]
- Redpath G. M., Woolger N., Piper A. K., Lemckert F. A., Lek A., et al. , 2014. Calpain cleavage within dysferlin exon 40a releases a synaptotagmin-like module for membrane repair. Mol. Biol. Cell 25: 3037–3048. 10.1091/mbc.e14-04-0947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme M., Yarden O., Bartnicki-Garcia S., Bowman B., Castro-Longoria E., et al. , 2011. Architecture and development of the Neurospora crassa hypha: a model cell for polarized growth. Fungal Biol. 115: 446–474. 10.1016/j.funbio.2011.02.008 [DOI] [PubMed] [Google Scholar]
- Schapire A. L., Valpuesta V., Botella M. A., 2009. Plasma membrane repair in plants. Trends Plant Sci. 14: 645–652. 10.1016/j.tplants.2009.09.004 [DOI] [PubMed] [Google Scholar]
- Scheffer L. L., Sreetama S. C., Sharma N., Medikayala S., Brown K. J., et al. , 2014. Mechanism of Ca2+-triggered ESCRT assembly and regulation of cell membrane repair. Nat. Commun. 5: 5646 10.1038/ncomms6646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semis R., Polacheck I., Segal E., 2010. Nystatin-intralipid preparation: characterization and in vitro activity against yeasts and molds. Mycopathologia 169: 333–341. 10.1007/s11046-009-9271-z [DOI] [PubMed] [Google Scholar]
- Serrano A., Illgen J., Brandt U., Thieme N., Letz A., et al. , 2018. Spatio-temporal MAPK dynamics mediate cell behavior coordination during fungal somatic cell fusion. J. Cell Sci. 131 10.1242/jcs.213462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenney K., Hunt I., Sweigard J., Pounder J. I., McClain C., et al. , 2000. Hex-1, a gene unique to filamentous fungi, encodes the major protein of the Woronin body and functions as a plug for septal pores. Fungal Genet. Biol. 31: 205–217. 10.1006/fgbi.2000.1230 [DOI] [PubMed] [Google Scholar]
- Togo T., Krasieva T. B., Steinhardt R. A., 2000. A decrease in membrane tension precedes successful cell-membrane repair. Mol. Biol. Cell 11: 4339–4346. 10.1091/mbc.11.12.4339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng H. P., Evans S., Gao F., Chambers K., Topkara V. K., et al. , 2014. Dysferlin mediates the cytoprotective effects of TRAF2 following myocardial ischemia reperfusion injury. J. Am. Heart Assoc. 3: e000662 10.1161/JAHA.113.000662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernarecci S., Colotti G., Ornaghi P., Schiebel E., Chiancone E., et al. , 2007. The yeast penta-EF protein Pef1p is involved in cation-dependent budding and cell polarization. Mol. Microbiol. 65: 1122–1138. 10.1111/j.1365-2958.2007.05852.x [DOI] [PubMed] [Google Scholar]
- Vogel H. J., 1956. A convenient growth medium for Neurospora. Microb. Genet. Bull. 13: 42–46. [Google Scholar]
- Westergaard M., Mitchell H. K., 1947. Neurospora V. A synthetic medium favoring sexual reproduction. Am. J. Bot. 34: 573–577. 10.1002/j.1537-2197.1947.tb13032.x [DOI] [Google Scholar]
- Xiang Q., Rasmussen C., Glass N. L., 2002. The ham-2 locus, encoding a putative transmembrane protein, is required for hyphal fusion in Neurospora crassa. Genetics 160: 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Brand A., Srikantha T., Daniels K. J., Soll D. R., et al. , 2011. Fig1 facilitates calcium influx and localizes to membranes destined to undergo fusion during mating in Candida albicans. Eukaryot. Cell 10: 435–444. 10.1128/EC.00145-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P., Jedd G., Kumaran D., Swaminathan S., Shio H., et al. , 2003. A HEX-1 crystal lattice required for Woronin body function in Neurospora crassa. Nat. Struct. Biol. 10: 264–270. 10.1038/nsb910 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All strains and plasmids are available on request. We confirm that all data and information necessary to confirm the conclusions of this study are present in the article, figures, tables, and supplemental material. Supplemental material available at Figshare: https://doi.org/10.25386/genetics.8239490.