Abstract
Although peripheral nerve injuries secondary to angiography and endovascular interventions are uncommon and usually not permanent, they can result in significant functional impairment. Most arteries used in access for angiography and endovascular therapies lie in close proximity to a nerve. The nerve may be injured by needle puncture, or by compression from hematoma, pseudoaneurysm, hemostasis devices, or by manual compression with incidence in literature ranging from as low as 0.04% for femoral access in a large retrospective study to 9% for brachial and axillary access. Given the increasing frequency of endovascular arterial procedures and the increasing use of nontraditional access points, it is important that the interventionalist have a working knowledge of peripheral nerve anatomy and function as it relates to relevant arterial access sites to avoid injury.
Although peripheral nerve injuries secondary to angiography and endovascular interventions are uncommon and usually are not permanent, they can result in significant functional impairment. Most arteries used in access for angiography and endovascular therapies lie in close proximity to a nerve. The paired nerve may be injured by needle puncture, or by compression from hematoma, pseudoaneurysm, hemostasis devices, or manual pressure.
Nerve injuries have been reported most frequently with axillary and brachial arterial access due to the anatomic proximity of the vessels and nerves at this location in combination with anatomic challenges for hemostasis. Given the higher rate of complications, axillary and brachial arterial access is typically reserved for situations where the interventionalist needs upper extremity arterial access, but the radial or ulnar arteries are not options due to anatomic or other factors. Subclavian arterial access is rarely used owing to high complication rates due to hemostasis challenges as it traverses the thoracic inlet (1). Femoral nerve injury, associated with common femoral artery access, is the second most frequently encountered. This is likely due to the high frequency of use of this access site in combination with the proximity of the femoral nerve just lateral to the common femoral artery in the femoral triangle.
It has been suggested that nerve injuries related to angiography may be under-reported due to delayed onset of symptoms, their impermanent nature, lack of recognition, or reluctance of operators to report complications (2–5).
Given the increasing frequency of endovascular arterial procedures and the increasing use of non-traditional access points, it is important that interventionalists have a working knowledge of peripheral nerve anatomy and function as it relates to arterial access sites.
Upper limb
Radial artery
Radial artery access has gained popularity as a safe and technically useful technique, particularly for coronary, upper limb, mesenteric, renal, and neurovascular interventions since it has been associated with a lower incidence of major access site related complications compared to the traditional transfemoral approach (6–9). Although transient sensory impairment with radial artery access at the wrist has been reported, it is extremely rare likely due to the low incidence of pseudoaneurysms and hematomas which have reported incidence of 0.1%–1% as the superficial nature of the radial artery allows for easy access site management (10–14). Minor digital numbness has been reported from median or superficial radial nerve injury (9, 15). The median nerve is nearest in proximity to the radial artery but is contained in the carpal tunnel several centimeters in the ulnar direction (Fig. 1). The superficial branch of the radial nerve is several centimeters dorsal-lateral and has already splayed into small cutaneous branches at the level of the wrist (Table 1). The use of pneumatic compression devices such as the TR band (Terumo Interventional Systems) allow for non-occlusive hemostasis and helps to minimize radial access complications (16–18). However, too tight application of such devices could theoretically compress the superficial radial or median nerve and may be one explanation in the reported cases of nerve injury.
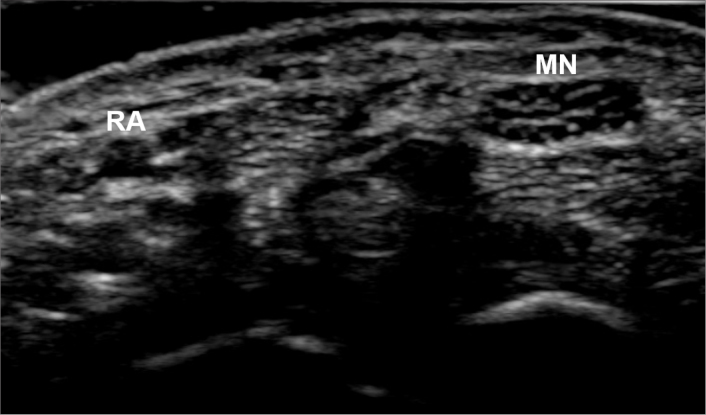
Figure 1.
Grayscale ultrasound image of the radial artery (RA) and median nerve (MN) at the wrist.
Table 1.
Motor deficits associated with upper extremity peripheral nerve injuries
| Access site | Possible nerve injury | Muscles involved | Motor impairment |
|---|---|---|---|
| Ulnar artery – wrist | Ulnar nerve | Abductor digiti minimi | Small finger flexion/abduction |
| Flexor digiti minimi | All MCP joint abduction/adduction | ||
| Interosseous muscles | MCP joint flexion with IP joint extension (small and ring fingers) | ||
| Lumbricales 3 and 4 | |||
|
| |||
| Brachial or axillary artery | Median nerve (variable impairment depending on relationship of injury to site motor branches) | Hand: | |
| Opponens pollicis | Thumb abduction/flexion/opposition | ||
| Abductor pollicis brevis | |||
| Flexor pollicis brevis | MCP joint flexion with IP joint extension (index and middle fingers) | ||
| Lumbricales 1 and 2 | |||
| Palmaris brevis | |||
| Forearm: | |||
| Pronator teres | Forearm pronation | ||
| Pronator quadratus* | Wrist flexion/radial deviation | ||
| Flexor carpi radialis | MCP and PIP joint flexion | ||
| Palmaris longus | DIP joint flexion (index and middle fingers) | ||
| Flexor digitorum superficialis | |||
| Flexor digitorum profundus (index and middle fingers)* | |||
| Flexor pollicis longus* | Thumb IP joint flexion | ||
| Ulnar nerve (variable impairment depending on relationship of injury to site motor branches) | Hand: See ulnar nerve involvement from injury at the wrist | Hand: See ulnar nerve involvement from injury at the wrist | |
| Forearm: | |||
| Flexor carpi ulnaris | Wrist flexion/ulnar deviation | ||
| Flexor digitorum profundus (ring and small finger) | DIP joint flexion (ring and small fingers) | ||
| Radial nerve (variable impairment depending on relationship of injury to site motor branches) | Upper arm: | ||
| Triceps | Elbow extension | ||
| Anconeus | |||
| Brachioradialis | Elbow flexion | ||
| Brachialis** | |||
| Extensor carpi radialis longus | Wrist extension | ||
| Extensor carpi radialis brevis | |||
| Extensor carpi ulnaris | |||
| Supinator | Forearm supination | ||
| Abductor pollicis longus | Thumb abduction/extension | ||
| Extensor pollicis longus | |||
| Extensor pollicis brevis | |||
| Extensor indicis | MCP joint extension of all digits | ||
| Extensor digitorum communis | |||
| Extensor digiti minimi | |||
| Musculocutaneous nerve (variable impairment depending on relationship of injury to site motor branches) | Upper arm: | ||
| Coracobrachialis | Shoulder flexion | ||
| Biceps brachii | Shoulder flexion, elbow flexion, supination | ||
| Brachialis** | Elbow flexion | ||
|
| |||
| Subclavian artery | Brachial supplying the lower trunk, medial cord and components of the posterior cord. Ultimately impairing C8-T1 dominant components of the median, ulnar, radial, and medial pectoral nerves | Pectoralis major | |
| Pectoralis minor | |||
| Median and ulnar muscle impairments as described with brachial/axillary involvement except with sparing of the Pronator teres, | Shoulder flexion and adduction | ||
| Flexor carpi radialis, and Palmaris longus | Scapular protraction and depression | ||
| Radial nerve components: Abductor and extensor pollicis longus | Thumb abduction and extension | ||
| Less weakness involving the extensor digitorum communis, extensor indicis, and extensor digiti minimi | Extension of the metacarpophalangeal joints of the lesser 4 digits | ||
MCP, metacarpophalangeal; IP, interphalangeal; PIP, proximal IP; DIP, distal IP.
Branches of the anterior interosseous nerve.
Brachialis shares innervation from the radial and musculocutaneous nerves.
Rare cases of complex regional pain syndrome, a disorder of the extremities also known as reflex sympathetic dystrophy, characterized by allodynia, swelling, range of motion limitation, and vasomotor disturbances have been reported and eventually resolved in the setting of transradial access (19–21). However, this entity is not thought to be related to injury to a specific neural structure in the region.
Ulnar artery
Although ulnar arterial access is less commonly performed than radial, there are instances where it may be useful. Such instances may include upper limb, coronary, neurointerventional, mesenteric, or renal arterial procedures when radial or common femoral arterial access is technically difficult or inadequate. The ulnar artery is closely related to the ulnar nerve at the wrist with the artery slightly radial and superficial to the nerve (Fig. 2). Both pass through the canal of de Guyon at the wrist, bordered superficially by the palmar carpal ligament and deeply by the flexor retinaculum. Despite this close relationship, ulnar nerve injury with ulnar artery access is exceedingly rare (22–25).
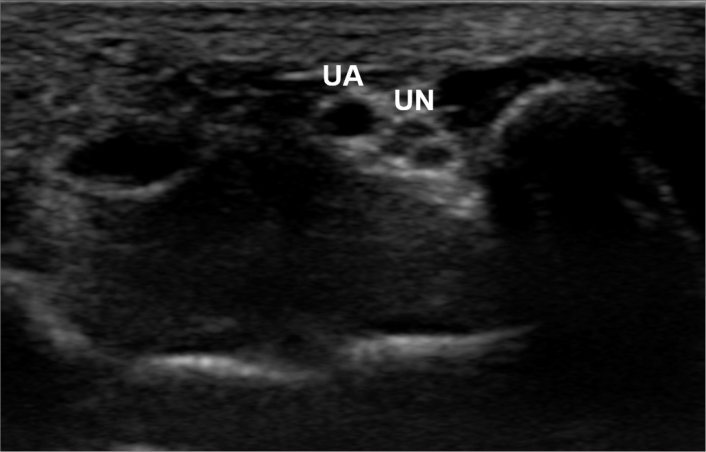
Figure 2.
Grayscale ultrasound image of the ulnar artery (UA) and ulnar nerve (UN) at the wrist.
Ulnar nerve injury at the wrist causes impairment of the hypothenar and most of the intrinsic hand muscles (the interosseous muscles, 3rd and 4th lumbricals, and the adductor pollicis). Sensory impairment involves the hypothenar aspect of the palm, the small finger, and ulnar side of the ring finger. These impairments are functionally significant, particularly given the importance of the intrinsic hand muscle function (Table 1).
Brachial/axillary artery
Axillary arterial access was once commonly performed by angiographers. Although it is sometimes necessary to use brachial or axillary arterial access, particularly when common femoral or radial access is inadequate or technically challenging, these access points have demonstrated significantly higher neurologic complications compared to other access sites (Table 1), with a reported incidence as high as 9% (2, 4). Arterial cutdown is sometimes necessary when the artery is too small and/or for placement of large sheaths; moreover, cutdown is associated with lower complication rates for brachial and axillary access site hematoma with reported complication rates ranging from 0%–4.1% (26–28).
From the axilla to just above the elbow, the medial brachial fascial compartment (MBFC) is formed by a thick brachial fascia covering a thin, axillary sheath which contains the axillary and brachial vessels (Fig. 3) as well as the median and ulnar nerves. These anatomic factors make hemostasis challenging and nerve injury more likely. Median nerve injury occurs more frequently than ulnar nerve injury. Although the radial and musculocutaneous nerves exit the compartment well proximal to the location of axillary arterial access, there have also been reports of injury involving these nerves. Furthermore, it appears that brachial artery access is similarly risky to axillary access because the anatomy of the MBFC still allows hematoma to form within the compartment and to compress the nerves (3–5, 29–31). Paradoxically, it appears that the size of hematoma does not correlate well with the severity of nerve injury (4, 5). Although brachial artery access with placement of a sheathless 4F catheter is relatively safe, this essentially limits the operator to diagnostic angiography without intervention from this access point. Additionally, brachial artery access complications may be reduced with the off-label employment of the Angioseal (St. Jude Medical) device in adequately sized vessels. Smaller studies support the off-label use of multiple other closure devices, including Perclose and StarClose (Abbott Laboratories) (32, 33).
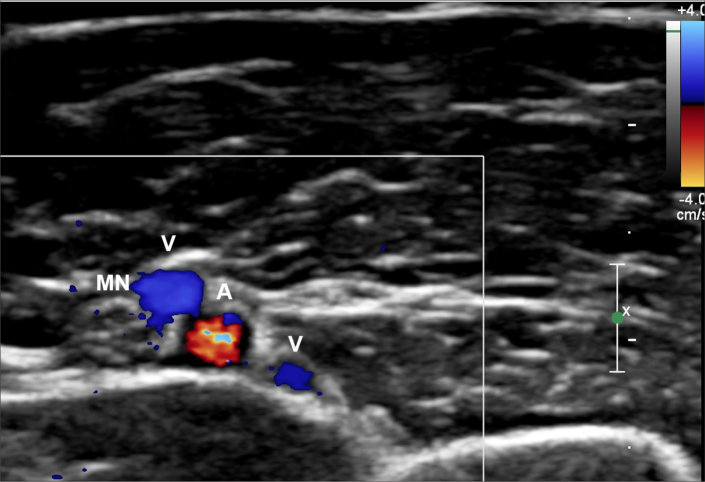
Figure 3.
Color doppler ultrasound image of the brachial artery (A), paired brachial veins (V), and median nerve (MN) above the elbow.
Injury to the median nerve in the axillary/brachial region results in motor impairment (Table 1) of nearly the entire anterior compartment of the forearm (sparing only the two ulnar innervated muscles: flexor carpi ulnaris and flexor digitorum profundus branches supplying the small and ring fingers). Additionally, the thenar muscles of the hand and 1st and 2nd lumbricals will be weak. Motor examination will demonstrate weakness in wrist and finger flexion, pronation, and thumb opposition and flexion. Sensory impairment will include much of the palmar surface of the hand (with sparing over the hypothenar region) as well as the thumb, index finger, middle finger, and radial half of the ring finger.
Injury to the ulnar nerve in the axillary/brachial region results in the same motor deficits described previously with injury at the wrist (Table 1), in addition to motor impairment of the flexor carpi ulnaris and flexor digitorum profundus branches supplying the small and ring fingers. The sensory deficits will also be the same with the addition of sensory impairment involving the posterior aspect of the ulnar side of the hand owing to involvement of sensory fibers supplying the dorsal ulnar cutaneous nerve.
Injury to the radial nerve in the axillary/brachial region results in impairment of the muscles of the entire posterior compartment of the forearm (Table 1). Motor exam will demonstrate weakness in supination, wrist extension and extension of the digits. There may also be subtle weakness of elbow flexion due to involvement of the brachioradialis and dual innervation of the brachialis by the radial and musculocutaeous nerves. If the nerve is injured far proximally, the triceps and anconeus will also be affected, yielding weakness in elbow extension. Sensory impairment will include the posterior, radial aspect of the hand, posterior forearm, and possibly the posterior arm.
Injury to the musculocutaneous nerve in the axillary/brachial region results in weakness in elbow flexion and forearm supination due to impairment in the biceps brachii and brachialis (Table 1). If the nerve is injured proximally enough, there may be weakness of shoulder flexion due to involvement of both the coracobrachialis and biceps brachii. Additionally, the terminal sensory branch of the musculocutaneous nerve, the lateral antebrachial cutaneous nerve, may yield sensory impairment in the anterior-radial aspect of the forearm.
Subclavian
Subclavian arterial access is rarely used. Although it has been used for transcatheter aortic valve replacement, endoluminal therapy and angiography when other access could not be obtained, it is typically avoided as hemostasis may be challenging and surgical repair may be complex (1, 34). Similar to axillary and brachial access, cut-down has been shown to reduce complications (35, 36). The artery is accessed infraclavicularly and visualization of the artery with ultrasound and bony landmarks with fluoroscopy can be helpful.
The subclavian artery is not enclosed within a fascial sheath with the accompanying nerves theoretically decreasing nerve injuries from small sheath hematomas (37, 38). However, the close anatomic relationship between brachial plexus and subclavian artery in the thoracic inlet can result in pseudoaneurysm and hematoma compression injury to the brachial plexus (39–42). The brachial plexus is formed by the lower four cervical nerves and first thoracic nerve (C5, C6, C7, C8, and T1). This network of nerves is divided into five roots, three trunks, six divisions, three cords, and five branches. The subclavian artery lies in close proximity to the roots and trunks in particular the C8 and T1 nerve root and inferior trunk. C8 and T1 contributes mostly to the medial cord, which forms the ulnar nerve, medial brachial and antebrachial cutaneous nerves, medial pectoral nerve, lower trunk and medial cord contributions to the median nerve, and the posterior cord contributions to the radial nerve. Hence neurologic symptoms from subclavian access would most likely involve these nerves. Functionally, injury to the inferior trunk can result in sensory impairment in the skin of the arm and forearm, inability to completely contract the pectoralis muscles (partial contraction would occur due to innervation from the lateral pectoral nerve), and most prominent impairment in the hand. Since the intrinsic hand muscles are all innervated via the C8-T1 lower trunk/medial cord components of the median and ulnar nerves, the thenar muscles, hypothenar, lumbricales, and interosseous muscles of the hand would all be weak. Additionally, C8-T1/lower trunk/posterior or medial cord dominant median, radial, and ulnar innervated muscles of the forearm would also be impaired such as the flexor digitorum profundus and superficialis, flexor carpi ulnaris, and extensor and abductor pollicis longus.
Lower limb
Common femoral artery
Common femoral access is performed most frequently for arterial interventions. Despite the close proximity of the femoral nerve just lateral to the common femoral artery in the femoral triangle (Fig. 4), nerve injury at this location is uncommon, likely from a combination of factors including interventionalists’ familiarity with the regional anatomy, use of ultrasound guidance allowing for avoidance of nerves, and the recognized morbidity associated with access site complications have emphasized proper access site management to reduce vascular complications which often are the culprits of neurologic symptoms (43–46). One large study in the United States pooling Nationwide Inpatient Sample data from 2002 to 2010 showed a femoral nerve injury incidence of 3.8 per 100,000 procedures, though there may be underestimation of actual occurrence from under-reporting and other variables (47). Another study by Kent et al. (48) reported a 0.2% incidence of femoral neuropathy. Furthermore, complications are reduced when ultrasound guided access is performed (45). However, femoral nerve injury has been reported secondary to hematoma, pseudoaneurysm, or via injury during cannulation (43, 49–51).
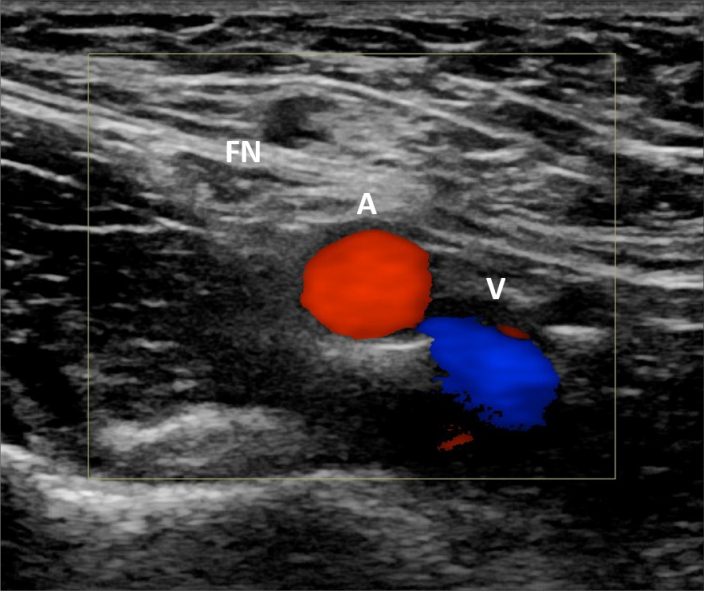
Figure 4.
Color Doppler image of the femoral triangle structures from lateral to medial: femoral nerve (FN), common femoral artery (A), and common femoral vein (V).
Injury to the common femoral nerve at this location causes weakness of the quadriceps muscles (Table 2). Sensory impairment involves the anterior-medial thigh and medial calf, owing to involvement of the anterior femoral cutaneous and saphenous nerves (52–54). Femoral neuralgia is generally characterized by post-procedural groin pain radiating down the anteromedial thigh (anterior femoral cutaneous nerve) with hyperesthesia. Sparing of the motor components of the femoral nerve in femoral neuralgia is likely explained by the deeper and safer anatomic course of the motor fibers (43). Prognosis of femoral neuralgia can range from near complete resolution to chronic pain requiring pain clinic visits and/or physical therapy (43, 49–51).
Table 2.
Motor deficits associated with lower extremity peripheral nerve injuries
| Access site | Possible nerve injury | Muscles involved | Motor impairment |
|---|---|---|---|
| Common femoral artery | Femoral nerve (variable impairment depending on relationship of injury to site motor branches) | Quadriceps | Knee extension |
| Pectineus | Hip flexion | ||
| Sartorius | |||
|
| |||
| Popliteal artery | Tibial nerve (variable impairment depending on relationship of injury to site motor branches) | Foot: | |
| Flexor hallucis brevis | Flexion of all digits | ||
| Flexor digitorum brevis | |||
| Quadratus plantae | |||
| Abductor hallucis | Abduction/Adduction of all digits | ||
| Adductor hallucis | |||
| Abductor digiti minimi | |||
| Interossei | IP joint extension with MCP joint flexion | ||
| Lumbricals | |||
| Leg: | Plantar flexion | ||
| Gastrocnemius | Flexion of all digits | ||
| Soleus | |||
| Tibialis posterior | |||
| Flexor hallucis longus | |||
| Flexor digitorum longus | |||
| Common peroneal nerve | Foot: | ||
| Extensor digitorum brevis | Extension of all digits | ||
| Extensor hallucis brevis | Extension of all digits | ||
| Leg: | |||
| Tibialis anterior | |||
| Extensor digitorum longus | Ankle dorsiflexion | ||
| Extensor digitorum brevis | |||
| Peroneus longus | |||
| Peroneus brevis | Ankle eversion | ||
|
| |||
| Posterior tibial artery-ankle/foot | Tibial nerve | As listed in the foot related to posterior tibial nerve | |
|
| |||
| Anterior tibial artery/Dorsalis pedis-ankle/foot | Deep peroneal nerve | As listed in foot related to common peroneal nerve | |
IP, interphalangeal; MCP, metacarpophalangeal.
Percutaneous vascular closure devices (VCD) such as Angio-Seal® (St. Jude Medical), Perclose® (Abbott Vascular) are most commonly used at the common femoral arteriotomy site and have been shown to achieve rapid hemostasis leading to early mobilization and discharge (55). Despite the advantages, device failure rates range from 1.5% to 20% in contemporary studies (56). Overall, literature suggests that VCDs are safe and effective, but equivocal complication rates compared to manual compression range from better to worse (55, 57–63). Nerve damage associated with vascular closure device occurs when the patient tolerated the procedure well until closure device deployment or there is failure of deployment with one study reporting a 5.4 fold increase risk of groin bleeding, hematoma, pseudoaneurysms that can result in nerve injury (52, 56). In one report, the patient’s pain and localized hypoesthesia were suspicious for injury and/or entrapment of the anterior cutaneous branches of the femoral nerve by the Angio-Seal collagen plug (52). In addition, VCDs may provide interventionalists with a false sense of security and decreased vigilance for the sequelae of bleeding complications. Studies have shown increased risk of groin bleeding, hematoma, pseudoaneurysms from failed VCD deployment (52, 56, 64–66).
Popliteal artery
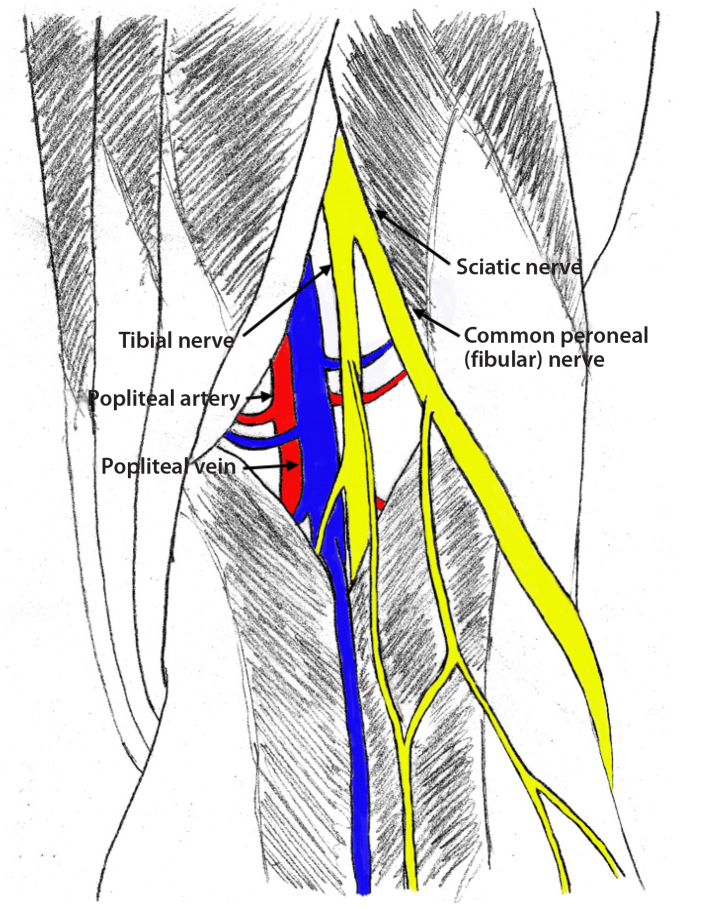
Popliteal artery access has gained popularity in lower extremity interventions in recent years, especially when anterograde access to treat superficial femoral artery disease is unsuccessful. Operators should be aware of the important anatomy in this region. Most commonly the sciatic nerve divides into the tibial nerve and common peroneal nerve in the popliteal fossa (Fig. 5), although the sciatic nerve may divide further proximally. Although somewhat variable, the popliteal artery is positioned anterior to the popliteal vein and anteromedial to the tibial nerve (Fig. 6). The common peroneal nerve courses further laterally in the popliteal fossa. Popliteal artery is accessed at the P2 segment (extending from proximal part of patella to center of knee joint space) via a media approach to avoid the nerve and vein (Fig. 5). Despite the close anatomic proximity of the nerves to the artery, nerve injuries from popliteal access are rare as ultrasound guidance allows for avoidance of the associated nerves (46, 67–69). In addition, antegrade balloon occlusion can be used to help obtain hemostasis at the popliteal access site once a lesion has been traversed in a retrograde fashion; when the lesion cannot be traversed retrograde, the relatively low pressure of the popliteal artery distal to the proximal occlusion helps maintain hemostasis during compression.
Figure 5.
Diagram of sciatic nerve dividing into the common peroneal and tibial nerves. A medial approach is used to avoid the nerve and vein. Popliteal artery is accessed at the P2 segment shown in figure.
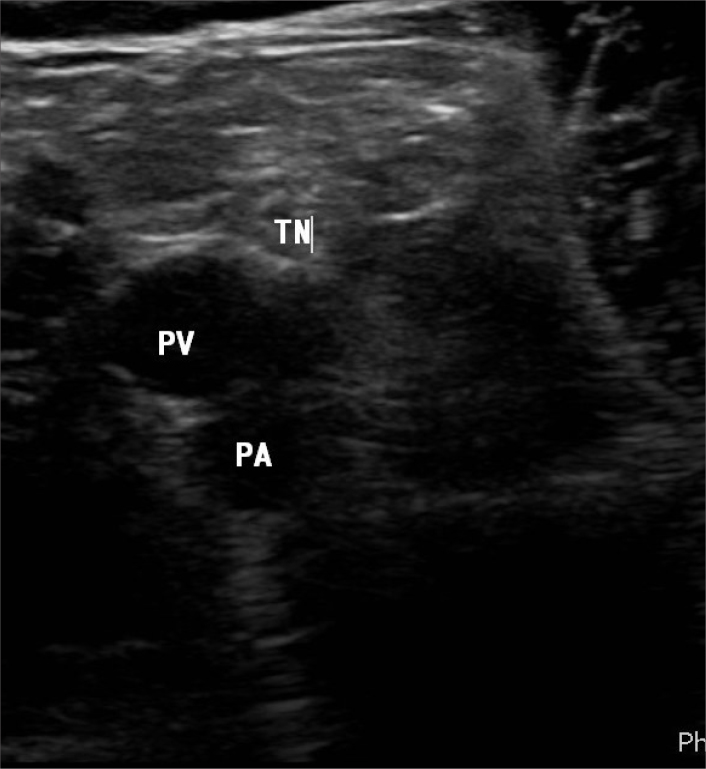
Figure 6.
Grayscale ultrasound image demonstrating the tibial nerve (TN) posterior laterally, popliteal vein (PV), and popliteal artery (PA). The common peroneal nerve is further lateral and not included on this image.
The tibial nerve courses through the mid portion of the popliteal fossa into the deep posterior compartment of the leg, whereas the common peroneal nerve courses laterally in the popliteal fossa to wrap laterally around the fibular neck where it divides into the deep and superficial peroneal nerve branches.
Injury of the tibial nerve in the popliteal fossa will affect the motor function of the muscles of the posterior compartments of the leg and plantar foot (Table 2). Therefore, there will be weakness in plantar flexion, ankle inversion, and toe flexion. Sensory impairment will involve the plantar aspect of the foot.
Injury of the common peroneal nerve at the popliteal fossa will affect motor function of the muscles of the anterior and lateral compartments of the leg as well as the dorsal foot (Table 2). Therefore, weakness of ankle dorsiflexion (“drop foot” during gait), ankle eversion, and toe extension may be present. Sensory impairment will involve the dorsal foot and ankle and first webspace (Table 2).
Posterior tibial artery
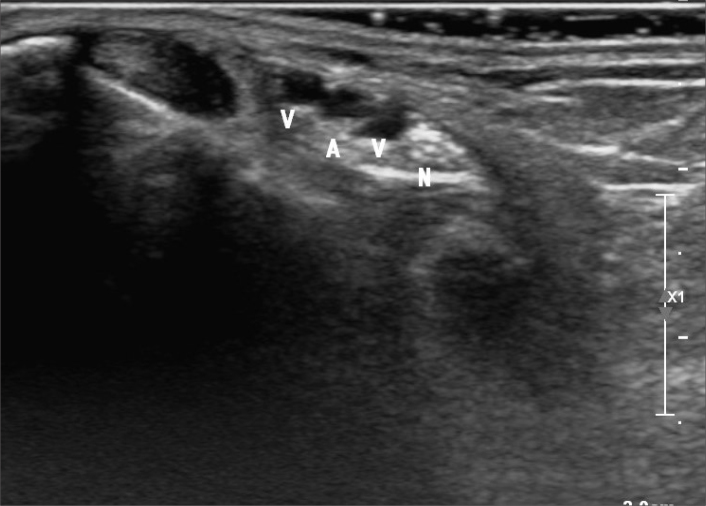
Since Botti et al. (70) described recanalization of tibial arteries using retrograde access, these techniques have gained increasing popularity. When performing posterior tibial artery access at the ankle, operators should be aware that the posterior tibial artery lies just medial to the tibial nerve in the region of the tarsal tunnel (Fig. 7). Despite this close proximity, we are unaware of any reported cases of tibial nerve injury from posterior tibial artery access.
Figure 7.
Grayscale ultrasound of the tarsal tunnel region demonstrating the posterior tibial artery (A) with paired veins (V) and the tibial nerve (N) medially.
As it courses distally into the foot, the tibial nerve divides into the medial and lateral plantar nerves and supplies the muscles and skin of the plantar foot. Therefore, injury of the tibial nerve at this location may cause weakness of toe flexion and impaired sensation on the plantar foot (Table 2).
Dorsalis pedis/anterior tibial artery
Dorsalis pedis or anterior tibial arterial access at the ankle has also gained popularity in recent years. Nerve injury from access at this location is exceedingly unlikely as the deep peroneal nerve has already given off all except the most distal of its motor branches in the anterior compartment. A tiny caliber deep peroneal nerve at this location only contains motor fibers to the extensor digitorum brevis and extensor hallucis brevis on the dorsal foot and a sensory branch to the first webspace. In the unlikely event of an injury at this location, the functional impairment would be miniscule owing to the redundant function of the extensor digitorum longus and extensor hallucis longus, which would remain intact (Table 2).
Treatment
Nerve injuries may result from direct needle puncture, compression from hematoma, pseudoaneurysm, manual compression, or compression device use. Most commonly, they are the result of hematoma with or without pseudoaneurysm formation. The hematoma may compress the adjacent nerve(s) directly or by raising the pressure within a confined anatomic compartment and causing compartment syndrome in its most severe form.
Hematoma is usually treated with manual compression to stop the site of extravasation. Vascular Quality Initiative classifies access site hematomas with or without pseudoaneurysms into four types: minor without treatment, moderate necessitating blood transfusion, moderate necessitating thrombin injection, or major requiring a surgical procedure (71). Surgical repair may be necessary when minimally invasive techniques are unsuccessful (27, 72, 73). In the event of compartment syndrome, fasciotomy is required to relieve the pressure.
Pseudoaneurysm is a false aneurysm contained by fewer than all the layers of the vessel wall. Treatment of pseudoaneurysms have evolved from the traditional surgical option to a less invasive approach including ultrasound-guided compression at the pseudoaneurysm, ultrasound-guided percutaneous thrombin injection, and endovascular management such as embolization or stent-graft placement when feasible (74–81). Very rarely is surgical neck ligation repair necessary. Most of the literature for treatment of pseudoaneurysm is based on the more traditional femoral artery access site; however, compression, thrombin injection, endovascular management have been shown to be successful in upper extremity pseudoaneurysms (82–89). In cases of radial access, successful treatment of pseudoaneurysms via compression with the Terumo TR Band™ (Terumo Medical Corporation) has also been successfully demonstrated (90).
In the setting of upper extremity access complicated by nerve injury, an aggressive approach is advocated as surgical evacuation of compressive hematoma within 48 hours yields improvement in nearly all patients whereas delaying surgery beyond 48 hours yields improvement in only about half of patients (91–93). Complex regional pain syndrome has been encountered rarely after arterial access. Management is multidisciplinary and may necessitate consultation with pain management, rehabilitation, and mental health specialists. Sympathetic nerve block has been shown to alleviate symptoms in complex regional pain syndrome to a significant degree in reported cases associated with transarterial access (19, 20).
The different types/etiologies of nerve injuries and their treatments are summarized in Table 3.
Table 3.
Different nerve injury types and treatment
| Type/etiology of nerve injury | Treatment options |
|---|---|
| Hematoma | Observation (if hemostasis is achieved) |
| Manual or ultrasound-guided compression for hemostasis | |
| Endovascular means to achieve hemostasis (embolization or stent-graft if feasible/appropriate) | |
| Surgical evacuation/repair | |
|
| |
| Pseudoaneurysm | Ultrasound-guided compression |
| Ultrasound-guided thrombin injection | |
| Endovascular management (embolization and stent-graft placement if feasible/appropriate) | |
| Surgical repair | |
|
| |
| Compartment syndrome | Fasciotomy |
|
| |
| Neuralgia (i.e., femoral neuralgia) | Observation |
| Physical therapy | |
| Nerve block | |
| Analgesic and pain medications | |
|
| |
| Palsies (i.e., brachial palsy) | Early surgical intervention for decompression |
| Physical therapy | |
|
| |
| Regional complex pain syndrome | Physical therapy |
| Psychotherapy | |
| Medications such as opioids, tricyclic antidepressants, anticonvulsants | |
| Sympathetic nerve block | |
| Surgical sympathectomy | |
Conclusion
Although nerve injuries associated with angiography and endovascular interventions are rare, they may result in significant functional impairment and are largely avoidable. Nerve injuries may result from direct needle puncture, compression from hematoma, pseudoaneurysm, manual compression, or compression device use, with hematoma being the most common cause.
The severity of nerve injury is multifactorial and varies from transient paresthesia and/or pain to severe, prolonged disability. When nerve injury persists, key emphasis to patients should be time and reassurance; in addition, multidisciplinary evaluation and treatment is appropriate and may necessitate involvement of specialists in pain management, neurology, and rehabilitation (43). Fortunately, vascular access related peripheral nerve symptoms are most often transient and relatively minor. In terms of prevention, understanding the anatomical relationship between the arteries and nerves will decrease the possibility of nerve injury. Ultrasound guidance allows for proper visualization of the access needle and its trajectory to avoid nearby nerves (44, 45, 67). Using a smaller sheath when technically feasible diminishes the risk of vascular complications and subsequent neurologic injury (10, 15, 94–96). Utilizing smaller access needle selection may be preferred, particularly for difficult access or inexperienced operators as increased needle size is associated with increased frequency of intraneural hematoma causing neurologic deficits (97–99). A good understanding of nerve and vessel anatomic relationships can also help prevent direct compression of the nerve itself; there are very few reported cases of nerve injury from direct compression likely due to the relatively short duration of manual compression, rarity of the injury, the transient nature of most such nerve injuries, under-recognition, and under-reporting. One tangentially relevant case report showed manual compression that resulted in nerve injury resolved after cessation of compression (100).
As most nerve injuries are associated with pseudoaneurysms and hematomas, proper access, hemostasis, and periprocedural care are the mainstay of prevention. Manual compression has been accepted as the gold standard for access site hemostasis. In the event of vascular complications, close monitoring and early/proper treatment of pseudoaneurysms and hematomas, before they expand to large sizes, are essential to reduce risk of nerve injury and other morbidity and mortality. Though not always feasible, making sure the patient is in optimal health with good control of underlying disease decreases the risks of vascular complications. Diabetes, hypertension, and infection are some common entities that increase the risk of pseudoaneurysm and hematoma formation (101, 102).
Knowledge of the relative risks of various access sites, relevant anatomy, employment of ultrasound-guided access, and thorough attention to achieving hemostasis can minimize the risks. Although rare, neurologic complications ranging from mild transient sensory to disabling motor neuropathies can be avoided.
Main points.
Nerve injuries associated with angiography and endovascular interventions are rare and usually transient but may result in significant functional impairment and are largely avoidable.
Nerve injuries more often result from hematoma and pseudoaneurysm formation. Less commonly they may result from direct needle puncture or external/manual compression.
Knowledge of the relative risks of various access sites, relevant anatomy, employment of ultrasound-guided access, and thorough attention to achieving hemostasis can minimize the risks.
Nerve injuries are most frequently reported with axillary and brachial arterial access due to the anatomic proximity of the vessels and nerves at this location in combination with anatomic challenges for hemostasis.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Caceres M, Braud R, Roselli EE. The axillary/subclavian artery access route for transcatheter aortic valve replacement: a systematic review of the literature. Ann Thorac Surg. 2012;93:1013–1018. doi: 10.1016/j.athoracsur.2011.10.056. [DOI] [PubMed] [Google Scholar]
- 2.Hessel SJ, Sequeira JC. Femoral artery catheterization and vessel tortuosity. Cardiovasc Interv Radiol. 1981;4:80–82. doi: 10.1007/BF02552381. [DOI] [PubMed] [Google Scholar]
- 3.Kennedy AM, Grocott M, Schwartz MS, Modarres H, Scott M, Schon F. Median nerve injury: an underrecognised complication of brachial artery cardiac catheterisation? J Neurol Neurosurg Psychiatry. 1997;63:542–546. doi: 10.1136/jnnp.63.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith DC, Mitchell DA, Peterson GW, Will AD, Mera SS, Smith LL. Medial brachial fascial compartment syndrome: anatomic basis of neuropathy after transaxillary arteriography. Radiology. 1989;173:149–154. doi: 10.1148/radiology.173.1.2675180. [DOI] [PubMed] [Google Scholar]
- 5.Tsao BE, Wilbourn AJ. The medial brachial fascial compartment syndrome following axillary arteriography. Neurology. 2003;61:1037–1041. doi: 10.1212/01.WNL.0000089488.35632.08. [DOI] [PubMed] [Google Scholar]
- 6.Agostoni P, Biondi-Zoccai GG, de Benedictis ML, et al. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures; Systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol. 2004;44:349–356. doi: 10.1016/j.jacc.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 7.Bhat T, Teli S, Bhat H, et al. Access-site complications and their management during transradial cardiac catheterization. Expert Rev Cardiovasc Ther. 2012;10:627–634. doi: 10.1586/erc.12.16. [DOI] [PubMed] [Google Scholar]
- 8.Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J. 2009;157:132–140. doi: 10.1016/j.ahj.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 9.Stella PR, Kiemeneij F, Laarman GJ, Odekerken D, Slagboom T, van der Wieken R. Incidence and outcome of radial artery occlusion following transradial artery coronary angioplasty. Cathet Cardiovasc Diagn. 1997;40:156–158. doi: 10.1002/(sici)1097-0304(199702)40:2<156::aid-ccd7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 10.Kanei Y, Kwan T, Nakra NC, et al. Transradial cardiac catheterization: a review of access site complications. Cathet Cardiovasc Interv. 2011;78:840–846. doi: 10.1002/ccd.22978. [DOI] [PubMed] [Google Scholar]
- 11.Sanmartin M, Cuevas D, Moxica J, et al. Transradial cardiac catheterization in patients with coronary bypass grafts: feasibility analysis and comparison with transfemoral approach. Cathet Cardiovasc Interv. 2006;67:580–584. doi: 10.1002/ccd.20633. [DOI] [PubMed] [Google Scholar]
- 12.Calvino-Santos RA, Vazquez-Rodriguez JM, Salgado-Fernandez J, et al. Management of iatrogenic radial artery perforation. Cathet Cardiovasc Interv. 2004;61:74–78. doi: 10.1002/ccd.10698. [DOI] [PubMed] [Google Scholar]
- 13.Rao SV, Ou FS, Wang TY, et al. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv. 2008;1:379–386. doi: 10.1016/j.jcin.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Sanmartin M, Cuevas D, Goicolea J, Ruiz-Salmeron R, Gomez M, Argibay V. Vascular complications associated with radial artery access for cardiac catheterization. Rev Esp Cardiol. 2004;57:581–584. doi: 10.1016/S1885-5857(06)60634-8. [DOI] [PubMed] [Google Scholar]
- 15.Jang HJ, Kim JY, Han JD, et al. Numbness after transradial cardiac catheterization: the results from a nerve conduction study of the superficial radial nerve. Korean Circ J. 2016;46:161–168. doi: 10.4070/kcj.2016.46.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cubero JM, Lombardo J, Pedrosa C, et al. Radial compression guided by mean artery pressure versus standard compression with a pneumatic device (RACOMAP) Cathet Cardiovasc Interv. 2009;73:467–472. doi: 10.1002/ccd.21900. [DOI] [PubMed] [Google Scholar]
- 17.Pancholy S, Coppola J, Patel T, Roke-Thomas M. Prevention of radial artery occlusion-patent hemostasis evaluation trial (PROPHET study): a randomized comparison of traditional versus patency documented hemostasis after transradial catheterization. Cathet Cardiovasc Interv. 2008;72:335–340. doi: 10.1002/ccd.21639. [DOI] [PubMed] [Google Scholar]
- 18.Gilchrist IC. Laissez-faire hemostasis and transradial injuries. Cathet Cardiovasc Interv. 2009;73:473–474. doi: 10.1002/ccd.22006. [DOI] [PubMed] [Google Scholar]
- 19.Papadimos TJ, Hofmann JP. Radial artery thrombosis, palmar arch systolic blood velocities, and chronic regional pain syndrome 1 following transradial cardiac catheterization. Cathet Cardiovasc Interv. 2002;57:537–540. doi: 10.1002/ccd.10367. [DOI] [PubMed] [Google Scholar]
- 20.Sasano N, Tsuda T, Sasano H, Ito S, Sobue K, Katsuya H. A case of complex regional pain syndrome type II after transradial coronary intervention. J Anesth. 2004;18:310–312. doi: 10.1007/s00540-004-0266-0. [DOI] [PubMed] [Google Scholar]
- 21.Subramanian R, White CJ, Sternbergh WC, 3rd, Ferguson DL, Gilchrist IC. Nonhealing wound resulting from a foreign-body reaction to a radial arterial sheath. Cathet Cardiovasc Interv. 2003;59:205–206. doi: 10.1002/ccd.10468. [DOI] [PubMed] [Google Scholar]
- 22.de Andrade PB, Tebet MA, Nogueira EF, et al. Transulnar approach as an alternative access site for coronary invasive procedures after transradial approach failure. Am Heart J. 2012;164:462–467. doi: 10.1016/j.ahj.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Hahalis G, Tsigkas G, Xanthopoulou I, et al. Transulnar compared with transradial artery approach as a default strategy for coronary procedures: a randomized trial. The Transulnar or Transradial Instead of Coronary Transfemoral Angiographies Study (the AURA of ARTEMIS Study) Circ Cardiovasc Interv. 2013;6:252–261. doi: 10.1161/CIRCINTERVENTIONS.112.000150. [DOI] [PubMed] [Google Scholar]
- 24.Kedev S, Zafirovska B, Dharma S, Petkoska D. Safety and feasibility of transulnar catheterization when ipsilateral radial access is not available. Cathet Cardiovasc Interv. 2014;83:E51–60. doi: 10.1002/ccd.25123. [DOI] [PubMed] [Google Scholar]
- 25.Dobson PF, Purushothaman B, Michla Y, England S, Krishnan MK, Tourret L. Delayed ulnar nerve palsy secondary to ulnar artery pseudoaneurysm distal to Guyon’s canal following penetrating trauma to the hand. Ann R Coll Surg Engl. 2013;95:e75–76. doi: 10.1308/003588413X13511609955850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarez-Tostado JA, Moise MA, Bena JF, et al. The brachial artery: a critical access for endovascular procedures. J Vasc Surg. 2009;49:378–385. doi: 10.1016/j.jvs.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Kret MR, Dalman RL, Kalish J, Mell M. Arterial cutdown reduces complications after brachial access for peripheral vascular intervention. J Vasc Surg. 2016;64:149–154. doi: 10.1016/j.jvs.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 28.Wooster M, Powell A, Back M, Illig K, Shames M. Axillary artery access as an adjunct for complex endovascular aortic repair. Ann Vasc Surg. 2015;29:1543–1547. doi: 10.1016/j.avsg.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 29.Treitl KM, Konig C, Reiser MF, Treitl M. Complications of transbrachial arterial access for peripheral endovascular interventions. J Endovasc Ther. 2015;22:63–70. doi: 10.1177/1526602814564363. [DOI] [PubMed] [Google Scholar]
- 30.Lijftogt N, Cancrinus E, Hoogervorst EL, van de Mortel RH, de Vries JP. Median nerve neuropraxia by a large false brachial artery aneurysm. Vascular. 2014;22:378–380. doi: 10.1177/1708538113516321. [DOI] [PubMed] [Google Scholar]
- 31.Chuang YM, Luo CB, Chou YH, Cheng YC, Chang CY, Chiou HJ. Sonographic diagnosis and treatment of a median nerve epineural hematoma caused by brachial artery catheterization. J Ultrasound Med. 2002;21:705–708. doi: 10.7863/jum.2002.21.6.705. [DOI] [PubMed] [Google Scholar]
- 32.Lupattelli T, Clerissi J, Clerici G, et al. The efficacy and safety of closure of brachial access using the AngioSeal closure device: experience with 161 interventions in diabetic patients with critical limb ischemia. J Vasc Surg. 2008;47:782–788. doi: 10.1016/j.jvs.2007.11.050. [DOI] [PubMed] [Google Scholar]
- 33.Sheth RA, Ganguli S. Closure of alternative vascular sites, including axillary, brachial, popliteal, and surgical grafts. Tech Vasc Interv Radiol. 2015;18:113–121. doi: 10.1053/j.tvir.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Andros G, Harris RW, Dulawa LB, Oblath RW, Schneider PA. Subclavian artery catheterization: a new approach for endovascular procedures. J Vasc Surg. 1994;20:566–576. doi: 10.1016/0741-5214(94)90281-X. [DOI] [PubMed] [Google Scholar]
- 35.Modine T, Obadia JF, Choukroun E, et al. Transcutaneous aortic valve implantation using the axillary/subclavian access: feasibility and early clinical outcomes. J Thorac Cardiovasc Surg. 2011;141:487–491. doi: 10.1016/j.jtcvs.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 36.Petronio AS, De Carlo M, Bedogni F, et al. Safety and efficacy of the subclavian approach for transcatheter aortic valve implantation with the CoreValve revalving system. Circ Cardiovasc Interv. 2010;3:359–366. doi: 10.1161/CIRCINTERVENTIONS.109.930453. [DOI] [PubMed] [Google Scholar]
- 37.AbuRahma AF, Robinson PA, Boland JP, et al. Complications of arteriography in a recent series of 707 cases: factors affecting outcome. Ann Vasc Surg. 1993;7:122–129. doi: 10.1007/BF02001005. [DOI] [PubMed] [Google Scholar]
- 38.Hessel SJ, Adams DF, Abrams HL. Complications of angiography. Radiology. 1981;138:273–281. doi: 10.1148/radiology.138.2.7455105. [DOI] [PubMed] [Google Scholar]
- 39.Mol TN, Gupta A, Narain U. Brachial plexus compression due to subclavian artery pseudoaneurysm from internal jugular vein catheterization. Indian J Nephrol. 2017;27:148–150. doi: 10.4103/0971-4065.179334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kosmadakis G, Pappas P, Gobou A, Smirloglou D, Michail S. Severe upper extremity polyneuropathy due to inferior brachial plexus compression as a result of left subclavian artery pseudoaneurysm. Saudi J Kidney Dis Transpl. 2012;23:1028–1031. doi: 10.4103/1319-2442.100941. [DOI] [PubMed] [Google Scholar]
- 41.Melas N, Saratzis A, Saratzis N, Kiskinis D. Endovascular repair of inadvertent subclavian artery perforation during cannulation for dialysis access: case report and review of the literature. Eur J Emerg Med. 2009;16:323–326. doi: 10.1097/MEJ.0b013e32832a0851. [DOI] [PubMed] [Google Scholar]
- 42.Guilbert MC, Elkouri S, Bracco D, et al. Arterial trauma during central venous catheter insertion: Case series, review and proposed algorithm. J Vasc Surg. 2008;48:918–925. doi: 10.1016/j.jvs.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 43.Hallett JW, Jr, Wolk SW, Cherry KJ, Jr, Gloviczki P, Pairolero PC. The femoral neuralgia syndrome after arterial catheter trauma. J Vasc Surg. 1990;11:702–706. doi: 10.1016/0741-5214(90)90217-X. [DOI] [PubMed] [Google Scholar]
- 44.Maecken T, Grau T. Ultrasound imaging in vascular access. Crit Care Med. 2007;35(Suppl 5):S178–185. doi: 10.1097/01.CCM.0000260629.86351.A5. [DOI] [PubMed] [Google Scholar]
- 45.Seto AH, Abu-Fadel MS, Sparling JM, et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access With Ultrasound Trial) JACC Cardiovasc Interv. 2010;3:751–758. doi: 10.1016/j.jcin.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 46.Yilmaz S, Altinbas H, Senol U, Sindel T, Mete A, Luleci E. Common peroneal nerve palsy after retrograde popliteal artery puncture. Eur J Vasc Endovasc Surg. 2002;23:467–469. doi: 10.1053/ejvs.2002.1629. [DOI] [PubMed] [Google Scholar]
- 47.El-Ghanem M, Malik AA, Azzam A, Yacoub HA, Qureshi AI, Souayah N. Occurrence of femoral nerve injury among patients undergoing transfemoral percutaneous catheterization procedures in the United States. J Vasc Interv Neurol. 2017;9:54–58. [PMC free article] [PubMed] [Google Scholar]
- 48.Kent KC, Moscucci M, Gallagher SG, DiMattia ST, Skillman JJ. Neuropathy after cardiac catheterization: incidence, clinical patterns, and long-term outcome. J Vasc Surg. 1994;19:1008–1014. doi: 10.1016/S0741-5214(94)70212-8. [DOI] [PubMed] [Google Scholar]
- 49.Kent KC, McArdle CR, Kennedy B, Baim DS, Anninos E, Skillman JJ. A prospective study of the clinical outcome of femoral pseudoaneurysms and arteriovenous fistulas induced by arterial puncture. J Vasc Surg. 1993;17:125–133. doi: 10.1067/mva.1993.41707. [DOI] [PubMed] [Google Scholar]
- 50.Graham AN, Wilson CM, Hood JM, Barros D’Sa AA. Risk of rupture of postangiographic femoral false aneurysm. Brit J Surg. 1992;79:1022–1025. doi: 10.1002/bjs.1800791012. [DOI] [PubMed] [Google Scholar]
- 51.Hajarizadeh H, LaRosa CR, Cardullo P, Rohrer MJ, Cutler BS. Ultrasound-guided compression of iatrogenic femoral pseudoaneurysm failure, recurrence, and long-term results. J Vasc Surg. 1995;22:425–433. doi: 10.1016/S0741-5214(95)70010-2. [DOI] [PubMed] [Google Scholar]
- 52.Jabara B, Punch G, Ching B. Neurologic complication after use of a percutaneous vascular closure device. J Vasc Interv Radiol. 2012;23:1099–1101. doi: 10.1016/j.jvir.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 53.Barcin C, Kursaklioglu H, Kose S, Isik E. Transient femoral nerve palsy after diagnostic coronary angiography. Anatol J Cardiol. 2009;9:248–249. [PubMed] [Google Scholar]
- 54.Kalapatapu VR, Ali AT, Masroor F, Moursi MM, Eidt JF. Techniques for managing complications of arterial closure devices. Vasc Endovasc Surg. 2006;40:399–408. doi: 10.1177/1538574406293760. [DOI] [PubMed] [Google Scholar]
- 55.Cox T, Blair L, Huntington C, Lincourt A, Sing R, Heniford BT. Systematic review of randomized controlled trials comparing manual compression to vascular closure devices for diagnostic and therapeutic arterial procedures. Surg Technol Int. 2015;27:32–44. [PubMed] [Google Scholar]
- 56.Bangalore S, Arora N, Resnic FS. Vascular closure device failure: frequency and implications: a propensity-matched analysis. Circ Cardiovasc Interv. 2009;2:549–556. doi: 10.1161/CIRCINTERVENTIONS.109.877407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wagner SC, Gonsalves CF, Eschelman DJ, Sullivan KL, Bonn J. Complications of a percutaneous suture-mediated closure device versus manual compression for arteriotomy closure: a case-controlled study. J Vasc Interv Radiol. 2003;14:735–741. doi: 10.1097/01.RVI.0000079982.80153.D9. [DOI] [PubMed] [Google Scholar]
- 58.Koreny M, Riedmuller E, Nikfardjam M, Siostrzonek P, Mullner M. Arterial puncture closing devices compared with standard manual compression after cardiac catheterization: systematic review and meta-analysis. JAMA. 2004;291:350–357. doi: 10.1001/jama.291.3.350. [DOI] [PubMed] [Google Scholar]
- 59.Camenzind E, Grossholz M, Urban P, Dorsaz PA, Didier D, Meier B. Collagen application versus manual compression: a prospective randomized trial for arterial puncture site closure after coronary angioplasty. J Am Coll Cardiol. 1994;24:655–662. doi: 10.1016/0735-1097(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 60.Walter J, Vogl M, Holderried M, et al. Manual compression versus vascular closing device for closing access puncture site in femoral left-heart catheterization and percutaneous coronary interventions: a retrospective cross-sectional comparison of costs and effects in inpatient care. Value Health. 2017;20:769–776. doi: 10.1016/j.jval.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 61.Schulz-Schupke S, Helde S, Gewalt S, et al. Comparison of vascular closure devices vs manual compression after femoral artery puncture: the ISAR-CLOSURE randomized clinical trial. JAMA. 2014;312:1981–1987. doi: 10.1001/jama.2014.15305. [DOI] [PubMed] [Google Scholar]
- 62.Fokkema TM, Minnee RC, Kock GA, Blomjous JG, Vahl AC, Leijdekkers VJ. Comparison of a collagen plug arterial closure device with manual compression after endovascular interventions for peripheral artery disease. J Vasc Surg. 2016;64:104–108. doi: 10.1016/j.jvs.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 63.Wu PJ, Dai YT, Kao HL, Chang CH, Lou MF. Access site complications following transfemoral coronary procedures: comparison between traditional compression and angioseal vascular closure devices for haemostasis. BMC Cardiovasc Disord. 2015;15:34. doi: 10.1186/s12872-015-0022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johanning JM, Franklin DP, Elmore JR, Han DC. Femoral artery infections associated with percutaneous arterial closure devices. J Vasc Surg. 2001;34:983–985. doi: 10.1067/mva.2001.120033. [DOI] [PubMed] [Google Scholar]
- 65.Nehler MR, Lawrence WA, Whitehill TA, Charette SD, Jones DN, Krupski WC. Iatrogenic vascular injuries from percutaneous vascular suturing devices. J Vasc Surg. 2001;33:943–947. doi: 10.1067/mva.2001.115002. [DOI] [PubMed] [Google Scholar]
- 66.Sprouse LR, 2nd, Botta DM, Jr, Hamilton IN., Jr The management of peripheral vascular complications associated with the use of percutaneous suture-mediated closure devices. J Vasc Surg. 2001;33:688–693. doi: 10.1067/mva.2001.112324. [DOI] [PubMed] [Google Scholar]
- 67.Yilmaz S, Sindel T, Luleci E. Ultrasound-guided retrograde popliteal artery catheterization: experience in 174 consecutive patients. J Endovasc Ther. 2005;12:714–722. doi: 10.1583/05-1576MR.1. [DOI] [PubMed] [Google Scholar]
- 68.Brountzos EN, Moulakakis KG, Avgerinos ED, et al. Retrograde transpopliteal approach of iliofemoral lesions. Vasc Endovasc Surg. 2011;45:646–650. doi: 10.1177/1538574411414308. [DOI] [PubMed] [Google Scholar]
- 69.Ye M, Zhang H, Huang X, et al. Retrograde popliteal approach for challenging occlusions of the femoral-popliteal arteries. J Vasc Surg. 2013;58:84–89. doi: 10.1016/j.jvs.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 70.Botti CF, Jr, Ansel GM, Silver MJ, Barker BJ, South S. Percutaneous retrograde tibial access in limb salvage. J Endovasc Ther. 2003;10:614–618. doi: 10.1177/152660280301000330. [DOI] [PubMed] [Google Scholar]
- 71.Ortiz D, Jahangir A, Singh M, Allaqaband S, Bajwa TK, Mewissen MW. Access site complications after peripheral vascular interventions: incidence, predictors, and outcomes. Circ Cardiovasc Interv. 2014;7:821–828. doi: 10.1161/CIRCINTERVENTIONS.114.001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Skagius E, Bosnjak M, Bjorck M, Steuer J, Nyman R, Wanhainen A. Percutaneous closure of large femoral artery access with Prostar XL in thoracic endovascular aortic repair. Eur J Vasc Endovasc Surg. 2013;46:558–563. doi: 10.1016/j.ejvs.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 73.Spitzer SG, Wilbring M, Alexiou K, Stumpf J, Kappert U, Matschke K. Surgical cut-down or percutaneous access-which is best for less vascular access complications in transfemoral TAVI? Cathet Cardiovasc Interv. 2016;88:E52–58. doi: 10.1002/ccd.26361. [DOI] [PubMed] [Google Scholar]
- 74.Fellmeth BD, Roberts AC, Bookstein JJ, et al. Postangiographic femoral artery injuries: nonsurgical repair with US-guided compression. Radiology. 1991;178:671–675. doi: 10.1148/radiology.178.3.1994400. [DOI] [PubMed] [Google Scholar]
- 75.Morgan R, Belli AM. Current treatment methods for postcatheterization pseudoaneurysms. J Vasc Interv Radiol. 2003;14:697–710. doi: 10.1097/01.RVI.0000071089.76348.6A. [DOI] [PubMed] [Google Scholar]
- 76.La Perna L, Olin JW, Goines D, Childs MB, Ouriel K. Ultrasound-guided thrombin injection for the treatment of postcatheterization pseudoaneurysms. Circulation. 2000;102:2391–2395. doi: 10.1161/01.CIR.102.19.2391. [DOI] [PubMed] [Google Scholar]
- 77.Badran MF, Gould DA, Sampson C, et al. Transluminal occlusion of a pseudoaneurysm arising from a thoracic aortic graft patch using catheter delivery of thrombin. J Vasc Interv Radiol. 2003;14:1201–1205. doi: 10.1097/01.RVI.0000086535.86489.59. [DOI] [PubMed] [Google Scholar]
- 78.Saad NE, Saad WE, Davies MG, Waldman DL, Fultz PJ, Rubens DJ. Pseudoaneurysms and the role of minimally invasive techniques in their management. Radiographics. 2005;25(Suppl 1):S173–189. doi: 10.1148/rg.25si055503. [DOI] [PubMed] [Google Scholar]
- 79.McConnell PI, Rehm J, Oltman DL, Lynch TG, Baxter BT. Thrombin injection for treating a subclavian artery pseudoaneurysm. Surgery. 2000;127:716–718. doi: 10.1067/msy.2000.105293. [DOI] [PubMed] [Google Scholar]
- 80.Pastores SM, Marin ML, Veith FJ, Bakal CW, Kvetan V. Endovascular stented graft repair of a pseudoaneurysm of the subclavian artery caused by percutaneous internal jugular vein cannulation: case report. Am J Crit Care. 1995;4:472–475. [PubMed] [Google Scholar]
- 81.Marin ML, Veith FJ, Panetta TF, et al. Transluminally placed endovascular stented graft repair for arterial trauma. J Vasc Surg. 1994;20:466–473. doi: 10.1016/0741-5214(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 82.Herold J, Brucks S, Boenigk H, Said SM, Braun-Dullaeus RC. Ultrasound guided thrombin injection of pseudoaneurysm of the radial artery after percutaneous coronary intervention. Vasa. 2011;40:78–81. doi: 10.1024/0301-1526/a000074. [DOI] [PubMed] [Google Scholar]
- 83.Mazzaccaro D, Malacrida G, Occhiuto MT, Stegher S, Tealdi DG, Nano G. Endovascular treatment of iatrogenic axillary artery pseudoaneurysm under echographic control: a case report. J Cardiothorac Surg. 2011;6:78. doi: 10.1186/1749-8090-6-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yamashita Y, Kimura S, Kurisu K, Ueno Y. Successful treatment of iatrogenic subclavian artery pseudoaneurysm by ultrasound-guided thrombin injection. Ann Vasc Dis. 2016;9:108–110. doi: 10.3400/avd.cr.15-00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saydam O, Serefli D, Atay M, Sert C. Endovascular management of right subclavian artery pseudoaneurysm due to war injury in adolescent patient. Case Rep Vasc Med. 2017;2017 doi: 10.1155/2017/9030457. 9030457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clark TW, Abraham RJ. Thrombin injection for treatment of brachial artery pseudoaneurysm at the site of a hemodialysis fistula: report of two patients. Cardiovasc Interv Radiol. 2000;23:396–400. doi: 10.1007/s002700010091. [DOI] [PubMed] [Google Scholar]
- 87.Patterson MC, Platt F. Therapy of Niemann-Pick disease, type C. Biochim Biophys Acta. 2004;1685:77–82. doi: 10.1016/j.bbalip.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 88.Kang SS, Labropoulos N, Mansour MA, et al. Expanded indications for ultrasound-guided thrombin injection of pseudoaneurysms. J Vasc Surg. 2000;31:289–298. doi: 10.1016/S0741-5214(00)90160-5. [DOI] [PubMed] [Google Scholar]
- 89.D’Achille A, Sebben RA, Davies RP. Percutaneous ultrasound-guided thrombin injection for coagulation of post-traumatic pseudoaneurysms. Australas Radiol. 2001;45:218–221. doi: 10.1046/j.1440-1673.2001.00906.x. [DOI] [PubMed] [Google Scholar]
- 90.Liou M, Tung F, Kanei Y, Kwan T. Treatment of radial artery pseudoaneurysm using a novel compression device. J Invasive Cardiol. 2010;22:293–295. [PubMed] [Google Scholar]
- 91.Raju S, Carner DV. Brachial plexus compression: complication of delayed recognition of arterial injuries of the shoulder girdle. Arch Surg. 1981;116:175–178. doi: 10.1001/archsurg.1981.01380140027006. [DOI] [PubMed] [Google Scholar]
- 92.O’Leary MR. Subclavian artery false aneurysm associated with brachial plexus palsy: a complication of parenteral drug addiction. Am J Emerg Med. 1990;8:129–133. doi: 10.1016/0735-6757(90)90200-J. [DOI] [PubMed] [Google Scholar]
- 93.Hansky B, Murray E, Minami K, Korfer R. Delayed brachial plexus paralysis due to subclavian pseudoaneurysm after clavicular fracture. Eur J Cardiothorac Surg. 1993;7:497–498. doi: 10.1016/1010-7940(93)90281-F. [DOI] [PubMed] [Google Scholar]
- 94.Doyle BJ, Ting HH, Bell MR, et al. Major femoral bleeding complications after percutaneous coronary intervention: incidence, predictors, and impact on long-term survival among 17,901 patients treated at the Mayo Clinic from 1994 to 2005. JACC Cardiovasc Interv. 2008;1:202–209. doi: 10.1016/j.jcin.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 95.Uhlemann M, Mobius-Winkler S, Mende M, et al. The Leipzig prospective vascular ultrasound registry in radial artery catheterization: impact of sheath size on vascular complications. JACC Cardiovasc Interv. 2012;5:36–43. doi: 10.1016/j.jcin.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 96.Berry C, Kelly J, Cobbe SM, Eteiba H. Comparison of femoral bleeding complications after coronary angiography versus percutaneous coronary intervention. Am J Cardiol. 2004;94:361–363. doi: 10.1016/j.amjcard.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 97.Steinfeldt T, Nimphius W, Werner T, et al. Nerve injury by needle nerve perforation in regional anaesthesia: does size matter? Brit J Anaesth. 2010;104:245–253. doi: 10.1093/bja/aep366. [DOI] [PubMed] [Google Scholar]
- 98.Scopel GP, Marques Faria JC, Orpheu SC, Alves HR, Dellon AL, Ferreira MC. Intraneural hematoma with extrinsic compression: experimental study in rats and therapeutic options. J Reconstr Microsurg. 2007;23:275–281. doi: 10.1055/s-2007-985209. [DOI] [PubMed] [Google Scholar]
- 99.Lundborg G, Myers R, Powell H. Nerve compression injury and increased endoneurial fluid pressure: a “miniature compartment syndrome”. J Neurol Neurosurg Psychiatry. 1983;46:1119–1124. doi: 10.1136/jnnp.46.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Komiyama M, Nakajima H, Nishikawa M, Yasui T. Brachial plexus and supraclavicular nerve injury caused by manual carotid compression for spontaneous carotid-cavernous sinus fistula. Surg Neurol. 1999;52:306–309. doi: 10.1016/S0090-3019(99)00088-9. [DOI] [PubMed] [Google Scholar]
- 101.Ganchi PA, Wilhelmi BJ, Fujita K, Lee WP. Ruptured pseudoaneurysm complicating an infected radial artery catheter: case report and review of the literature. Ann Plast Surg. 2001;46:647–650. doi: 10.1097/00000637-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 102.Tsao JW, Neymark E, Gooding GA. Radial artery mycotic pseudoaneurysm: an unusual complication of catheterization. J Clin Ultrasound. 2000;28:414–416. doi: 10.1002/1097-0096(200010)28:8<414::aid-jcu6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]