Abstract
Urodele amphibians such as the axolotl regenerate complete limbs as adults, and understanding how the “blueprint”, or pattern, of the regenerate is established and manipulated are areas of intense interest. Nutrient signaling plays an important role in pattern formation during regeneration. Retinoic acid signaling is the most characterized pathway during this process. Exogenous retinoic acid (RA) reprograms the pattern information in regenerating cells to a more posterior, ventral, and proximal identity. Vitamin D signaling shares several molecular similarities with RA and has been shown to alter pattern formation during zebrafish pectoral fin regeneration. To determine if exogenous Vitamin D signaling is capable of reprograming pattern in the axolotl limb blastema, we treated regenerating limbs with a potent Vitamin D agonist. Under the studied conditions, exogenous Vitamin D did not act in a manner similar to RA and failed to proximalize the pattern of the resulting regenerates. The Vitamin D treatment did result in several skeletal defects during regeneration, including carpal fusions along the A/P axis; failure to integrate the newly regenerated tissue with the existing tissue, formation of ectopic nodules of cartilage at the site of amputation, and altered bone morphology in uninjured skeletal tissue.
Keywords: integration, regeneration, patterning, Ambystoma mexicanum, vitamin D signaling, skeleton
1. Introduction:
Nutrient signaling plays key roles in the patterning and differentiation of cells during embryogenesis and regeneration. One of most well-characterized nutrient pathways involved in these processes is retinoic acid (RA) signaling, which has been extensively reviewed elsewhere (Cunningham & Duester 2015; Rhinn & Dollé 2012). In the absence of RA, Retinoic Acid Receptors (RAR) form heterodimers with retinoid X receptor (RXR) and together this complex represses transcription of RA-responsive genes. The addition of RA leads to conformational changes in RAR, causing the heterodimer complex to either activate or further repress transcription of its associated genes. RA signaling is critical in the establishment of positional information during primary and secondary field formation in developing vertebrate embryos (as reviewed by (Cunningham & Duester 2015)), and in regenerating amphibian limbs (Rincón & Scadding 2002; Nguyen-yamamoto et al. 2010; Monaghan & Maden 2013). Additionally, exogenous RA reprograms the positional information within regenerating limb tissue resulting in proximalization and posteriorization of the pattern of the regenerate (Maden 1996; Mccusker et al. 2014; Bryant & Gardiner 1992).
The co-receptor to RAR, RXR, binds to other co-receptors that are involved with nutrient signaling as well. One of these receptors is the Vitamin D receptor (VDR), a participant in receptor-mediated Vitamin D signaling (Kliewer et al. 1992). Similar to RAR-associated signaling, VDR is believed to act together with RXR as a transcriptional co-repressor until it is bound by a metabolically active form of Vitamin D. This binding induces a conformational change in the nuclear receptor which results in transcriptional activation (Ohyama et al. 1994; Ohyama et al. 1996) or further suppression (Alroy et al. 1995) of the associated genes.
The involvement of Vitamin D signaling in development and regeneration has not been comprehensively studied. Vitamin D signaling contributes to normal limb formation as components of the vitamin D signaling pathway are expressed in developing limb buds (Liu et al. 2008) and alterations of this pathway negatively effects long bone formation in VDR-knockout mice (Zheng et al. 2004). In the context of regeneration, exogenous Vitamin D, like RA signaling, has been observed to affect pattern formation. The normal anterior/posterior (A/P) pattern in the zebrafish pectoral fin depends on a graded expression of Vitamin D signaling along this axis; however, overexpression of Hand2, an upstream inhibitor of Vitamin D signaling, disrupted pattern formation during pectoral fin regeneration by shortening all the fin rays along the A/P axis (Nachtrab et al. 2013), suggestive of axis posterization. Exogenous Vitamin D treatment, in conjunction with RA signaling, also disrupts A/P patterning in the regenerating axolotl limb (Washabaugh & Tsonis 1995); although, the effect of exogenous Vitamin D signaling (alone) on pattern formation in this system has not been fully characterized. Thus, a potential role for vitamin D signaling in positional reprogramming during regeneration exists.
Exogenous retinoic acid alters positional identity during limb regeneration in the axolotl in a reproducible manner whereby the regenerating cells, known as blastema cells, assume a more proximalized, posteriorized and ventralized identity (Mccusker et al. 2014). RA signaling is known to slow the cell cycle (Chen & Ross 2004) and it has been hypothesized that the length of the cell cycle plays a key role in the patterning of embryos and regenerating structures (Bryant & Gardiner 2016). Similar to RA signaling, Vitamin D regulates transcription by a RXR-associated heterodimer complex and this nutrient molecule also slows the cell cycle (Akutsu et al. 2001). Therefore, we hypothesize that exogenous Vitamin D reprograms positional identity during regeneration in a similar manner as RA.
To test this hypothesis, we sought to better characterize the effect of exogenous Vitamin D signaling on anterior/posterior patterning by treating axolotl with blastemas located on different locations of the limb axis with a potent Vitamin D agonist. Unlike RA, exogenous Vitamin D did not cause the proximalization of the pattern of the regenerates. However, the treatment did result in several skeletal defects during regeneration, including carpal fusions along the A/P axis; the failure to integrate the newly regenerated tissue with the existing tissue; and the formation of ectopic nodules of cartilage. In addition, growth plate morphology and skeletal homeostasis was negatively affected in uninjured, treated tissue.
2. Results:
2.1. Exogenous Vitamin D signaling alters the A/P pattern of the carpals
Nachtrab et al discovered that an A/P gradient of Vitamin D signaling played a role in A/P patterning in regenerating zebrafish fins (Nachtrab et al. 2013). We speculated that the role of Vitamin D signaling in regeneration could be conserved in amphibians. We used RT-PCR to analyze the expression of the vitamin D receptor (VDR) and downstream transcriptional target of VDR, cyp24a1 (Pike et al. 2014; Hahn et al. 1994), in differently staged regenerating axolotl limbs - early to mid bud stage, late bud stage, and tiny limb stage, compared with mature (uninjured limb tissue). We observed that VDR is expressed both in regenerating and mature tissues (Figure 1A), and the expression of cyp24a1 is significantly increased in early to mid bud staged regenerating blastema tissue (Figure 1B). Thus, VDR is present, and apparently activated during normal limb regeneration.
Figure 1: Vitamin D signaling is activated during regeneration and Doxercalciferol further enhances this signaling in the axolotl.
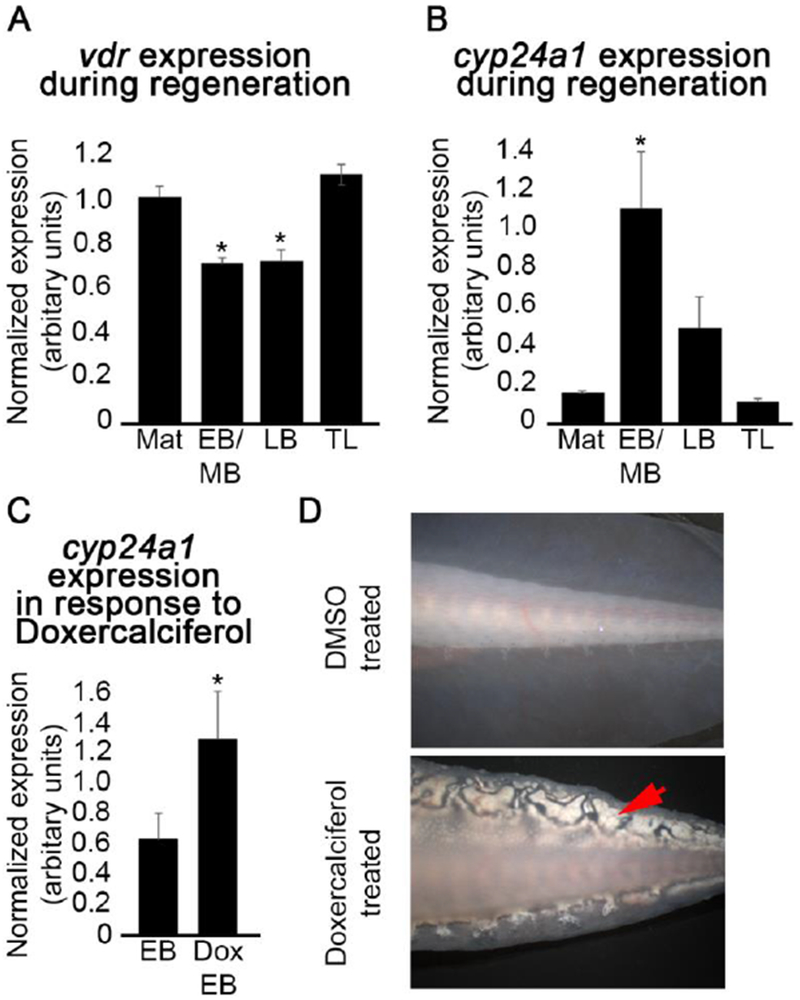
(A) Semi-quantitative analysis by RT-PCR of (A) vdr and (B) cyp24a1 gene expression during the course of regeneration, relative to the expression of the house keeping gene, ef1 α. Mat - mature, uninjured limb tissue; EB/MB - early to mid bud stage blastema tissue; LB – late bud stage blastema tissue; TL – tiny limb tissue; p < 0.05 (one way Anova; Tukey post hoc test relative to mature, uninjured tissue; N = 3). (C) Semi-quantitative analysis by RT-PCR of cyp24a1 revealed a significant increase in the expression of this gene in early bud stage blastema tissue in response to Doxercalciferol treatment (10mg/kg; Dox EB); p < 0.05 (t-test, N = 3). Error bars represent standard error of the mean. (D) Representative images of vehicle (top) and Doxercalciferol treated (10mg/kg, bottom) axolotl tail flaps. Doxcercaliferol treatment resulted in aberrant structures (red arrow) in the tail flap.
To test whether exogenous Vitamin D signaling alters A/P patterning in the axolotl limb regenerate, we performed a gain-of-function study. Axolotl limbs were amputated either through the humorous (proximal amputation) or between the distal radius and ulna and proximal carpals (distal amputation); allowed to develop mid-bud staged blastema, and then treated with the Vitamin D analogue Doxercalciferol. Blastemas were treated at the mid-bud stage, because we had previously shown that treatment with RA had the greatest effect on the pattern of the regenerate when applied at this stage (Mccusker et al. 2014). The Vitamin D analogue Doxercalciferol has proven biological activity in mammalian systems (Kubodera 2009; Nguyen-yamamoto et al. 2010), and here we show that cyp24a1 expression is significantly increased in treated blastema tissue relative to untreated blastema tissue, indicating that this analogue is also active in amphibian systems (Figure 1C). We also observed that within two weeks, the treated animals developed what looked like deposits in the skin (Figure 1D). We suspect that these deposits may be similar to those observed in mammals that are afflicted with Calcinosis Cutis, which are mineral deposits in the skin that have been associated with an excess of Vitamin D (Buffenstein et al. 1995). Together these observations indicate that Doxercalciferol treatment activates Vitamin D signaling in the axolotl.
We observed several different carpal patterning phenotypes within the treated regenerates (Figure 2). Limbs were scored by the location of the fusion along the A/P axis (Figure 2, Table 1). We observed that the frequency and type of carpal fusions greatly depended on whether the treated blastema was located in a proximal or distal limb location (0 - 75% for proximal amputations versus 70 - 100% in distal amputations). Carpal fusions have previously been found to occur at high frequency during normal (untreated) regeneration in response to distal amputation (Rincón & Scadding 2002). In the current study, fusions were more regularly observed in distally located regenerates regardless of Doxercalciferol treatment, as expected. However, more fusions were observed in the regenerates with the highest dosage of Doxercalciferol (Figure 2B). Additionally, both low and high doses of Doxercalciferol resulted in an increase in the number of fusions in regenerates from proximal located amputations (Figure 2C). Interestingly, the highest dose of Doxercalciferol resulted in anterior-located fusions in over 50% of the regenerates.
Figure 2: A concentration dependent effect of Doxercalciferol on carpal fusion in regenerates.
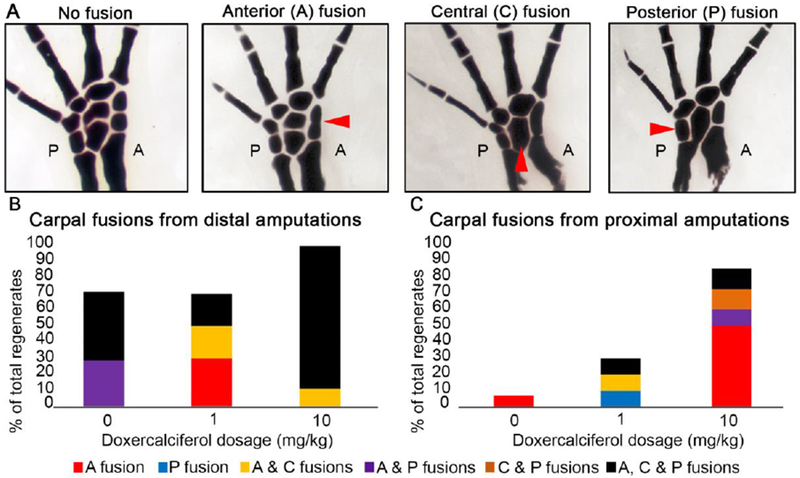
(A) Representative anterior, central and posterior carpal fusions observed during this study. Red arrow heads indicate the various types of carpal fusions along the anterior and posterior (A and P in the images respectively) axis. Quantification of carpal fusions in distal (B), and proximal (C) located regenerates in limbs treated with 0 (DMSO treated), 1, or 10 mg/kg of Doxercalciferol. Refer to Table 1 for statistics.
Table 1:
Phenotypic alterations observed in limb regenerates in response to Doxercalciferol treatment.
| Site of amputation | Dosage (mg/kg) | Total number of limbs amputated | Total number of limbs evaluatedβ | Regenerates exhibiting*¥ | ||||
|---|---|---|---|---|---|---|---|---|
| Carpal fusions | Carpal duplications | Cartilage nodules** | Autopods with a reduced number of digits | Normal limbs | ||||
| Proximal | 0 (vehicle treated) | 14 | 14 | 1 (7.14%) | 3 (21.43%) | 3 (21.43%) | 0 | 8 (57.14%) |
| 1 | 10 | 10 | 2 (20%) | 1 (10%) | 4 (40%) | 0 | 4 (40%) | |
| 10 | 10 | 8 | 6 (75%) | 0 | 2 (25%) | 0 | 2 (25%) | |
| Distal | 0 (vehicle treated) | 9 | 7 | 5 (71.43%) | 0 | 1 (14.29%) | 1 (11.11%) | 1 (11.11%) |
| 1 | 10 | 10 | 7 (70%) | 0 | 5 (50%) | 3 (30%) | 0 | |
| 10 | 10 | 10 | 10 (100%) | 0 | 8 (80%) | 2 (20%) | 0 | |
# of regenerated limbs exhibiting a specific phenotype. The % of total regenerates is provided in parentheses
At the site of amputation
Some limbs exhibited multiple abnormalities
A small number of animal deaths and the failure of limbs to regenerate reduced the number of limbs that were ultimately evaluated between experimental groups
In zebrafish fins, the over expression of Hand2 (a posterior marker) increases the expression of Cyp24a1, a Vitamin D inactivating enzyme, and results in the “posteriorization” of the fins (Nachtrab et al. 2013). Thus, it is noteworthy that the least frequent fusions in the treated blastema were observed in the posterior-located carpals, and that the anterior-located carpals appear to be more sensitive to exogenous Vitamin D signaling. However, it is possible that this may be linked to the anterior to posterior specification of the axolotl limb axis (Shubin & Alberch 1986), since the specification of the anterior carpals occurs closer in time to the Doxercalciferol treatment. Treatment with Doxercalciferol was not found to have an effect on digit number or structure (bifurcations) in the regenerates of this study.
2.2. Exogenous Vitamin D signing decreases the proximal/distal length of distally amputated regenerates
Washabaugh and Tsonis (1995) detected proximalization of the regenerate when distal blastema tissue was treated with Vitamin D metabolites and RA simultaneously; however, this observation was infrequent in the experimental population, less than 20% of the experimental group (Washabaugh & Tsonis 1995). The pattern of the regenerate is considered proximalized when either the pattern of the regenerate is more proximal than the amputation site, for example if a humerous grows from a carpal-level amputation, or the length of the most proximal regenerated skeletal elements is increased. As the dosage of RA used was less than what would normally result in proximalization, the authors suggest that the Vitamin D and RA metabolites could function synergistically.
In the current study, Doxercalciferol did not appear to proximalize the regenerated structure (Figure 3). First, the Doxercalciferol treated regenerates did not form structures that were proximal to the site of amputation, as what occurs in RA treated animals (Maden 1983). We also measured the length of the regenerated skeletal elements to determine whether they were elongated in comparison to control animals, since this would also indicate that the limbs were proximalized. To compensate for the differently sized axolotl, the length of the regenerated radius and ulna was calculated relative to the length of the regenerated second digit. This method of quantification facilitated direct comparison between proximal and distal limb amputations (Figure 3D). No effect on the relative length of the regenerated radius and ulna was observed when distal blastemas were treated with Doxercalciferol relative to DMSO treatment (Figure 3C). Additionally, the combined length of the stump and regenerated portions of the radius and ulna of distal amputations was smaller in both treatment groups relative to untreated controls (Figure 3C), and is likely a result of Doxercalciferol slowing regenerate (Washabaugh & Tsonis 1995) and body growth (Figure 3D) at different rates. Since increased skeletal length is a result of proximalization, our observation that the treatment decreased the length of the regenerate indicates that these limbs are not proximalized.
Figure 3: Doxercalciferol had minimal effect on regenerating bone length.
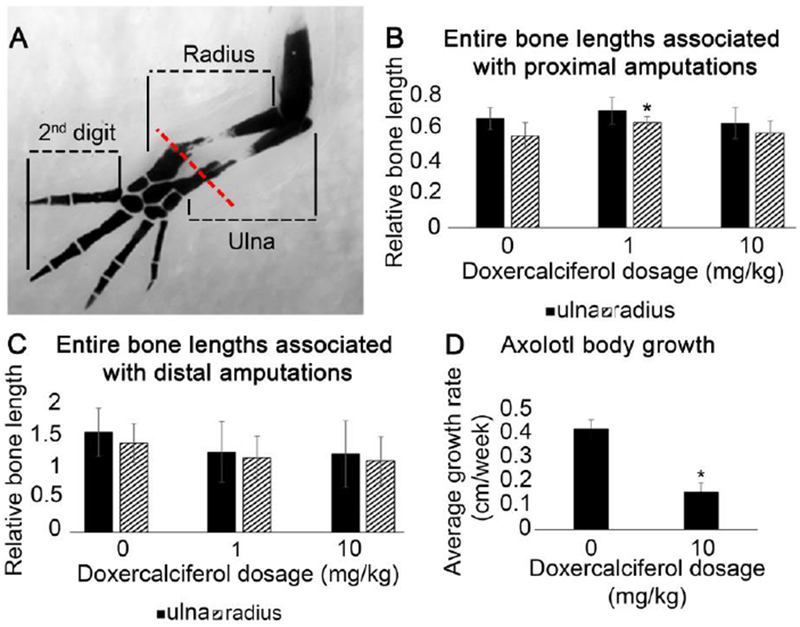
(A) Representative image showing how the entire radius and ulna bone were quantified, along with the second digit, in DMSO and Doxercalciferol treated forelimb regenerates. Red line represents the site of amputation. Histogram representing the proportional length of the radius and ulna (measured as in “A”) resulting from (B) proximal amputations (N = 13 for DMSO treated amputations, N = 10 for 1mg/kg and 8 for 10mg/kg Doxercalciferol treatment groups; one-way Anova analysis p<0.05, post hoc Tukey tests revealed that DMSO treated radia were significantly shorter than 1mg/kg Doxercalciferal treated radia) or (C) distal amputations (N = 7 for DMSO treated amputations, N = 10 for both Doxercalciferol treatment groups, one-way Anova analysis p>0.05). Error bars represent standard deviation of the mean. (D) Body length (snout-to-tail tip) was measured at the day of injection and then 8 weeks later. The average change in body length over this time period was significantly smaller for Doxercalciferol treated axolotl (N=5) compared to vehicle treated animals (N=9) (t-test, p<0.05).
We additionally observed that Doxercalciferol treatment of blastema tissue resulted in a non-linear, often angular, integration of the existing with the newly generated skeletal structures (Figure 4). This phenomenon was less frequent in proximal amputations, which may suggest that distal tissues in the limb are more susceptible to the effects of Vitamin D signaling in terms of integration relative to proximal counterparts. This may also be due to whether the amputation was performed through bone versus cartilage; as distal amputations occurred mostly through cartilaginous regions of the stylopod, while proximal amputations occurred through ossified bone of the zeugopod.
Figure 4: Doxercalciferol treatment results in defective bone integration at distal sites of amputation.
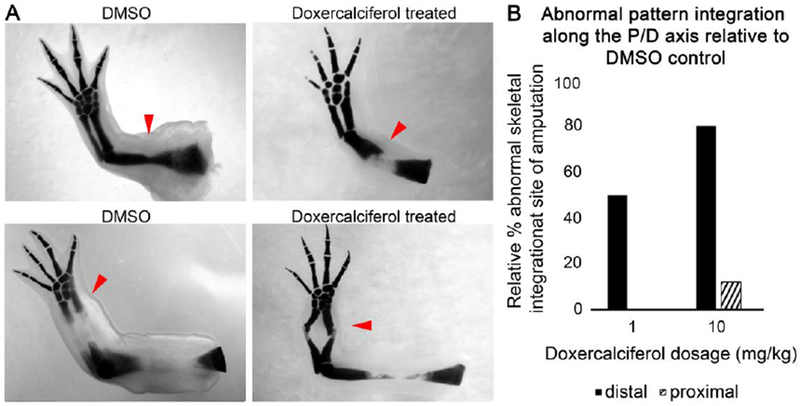
(A) Representative images of DMSO and doxercalciferol treated regenerating structures from proximal and distal amputations (red arrows indicate the sites of amputation). (B) Histogram representing the percentage of doxercalciferol treated regenerates, relative to DMSO treated controls, which exhibited abnormal skeletal integration between the newly regenerated and existing structures.
2.3. Exogenous Vitamin D signaling results in abnormal morphology of the growth plates in uninjured skeletal tissues
Bone formation occurs by a process of endochondral ossification in the amphibian limb. This process is initiated by aggregating mesenchymal cells, which condense in appropriate positions to become chondrocytes and thus generating a cartilaginous skeleton. Once the cartilage framework is laid down, the chondrocytes are replaced by osteoblasts that are responsible for the mineralization of the structure (Miura et al. 2008). The mechanism by which exogenous Vitamin D affects bone formation is uncertain and appears to involve species-specific differences (as reviewed by (Eisman & Bouillon 2014)); however, there is data to support the role of Vitamin D positively regulating bone formation and mineralization.
In the current study we found that the exogenous activation of Vitamin D signaling appears to alter the function of the growth plates in the uninjured skeletal elements (Figure 5). First, we observed that growth rate of the Doxercalciferol treated animals was significantly slower than control animals (Figure 3D). Since the axolotl were treated at the juvenile stage, when they are growing relatively quickly, we interpret this difference in size to indicate that Doxercalciferol treatment stunted the growth of the animals (Figure 3D). By comparing the whole-mount cartilage staining in Doxercalciferol- versus DMSO-treated uninjured limbs (Figure 5A and B), we additionally observed that analogue-treated uninjured autopods had a significant reduction in the amount of cartilage staining (Figure 5D), particularly in the regions of the skeletal elements where the growth plates are located. The epiphyseal growth plate is responsible for bone growth (as reviewed by (Pines & Hurwtiz, 1999)). Interestingly, we did not observe changes in cartilage staining in the carpal elements, which do not have growth plates. Older, adult animals that were not treated with Doxercalciferol were found to exhibit similar autopod cartilage staining as the control animals (Figure 5B and C), suggesting that Doxercalciferol was not simply accelerating the natural development of the skeletal elements in the digits of the axolotl.
Figure 5: Doxercalciferol treatment altered epiphysis structure in uninjured limb tissue.
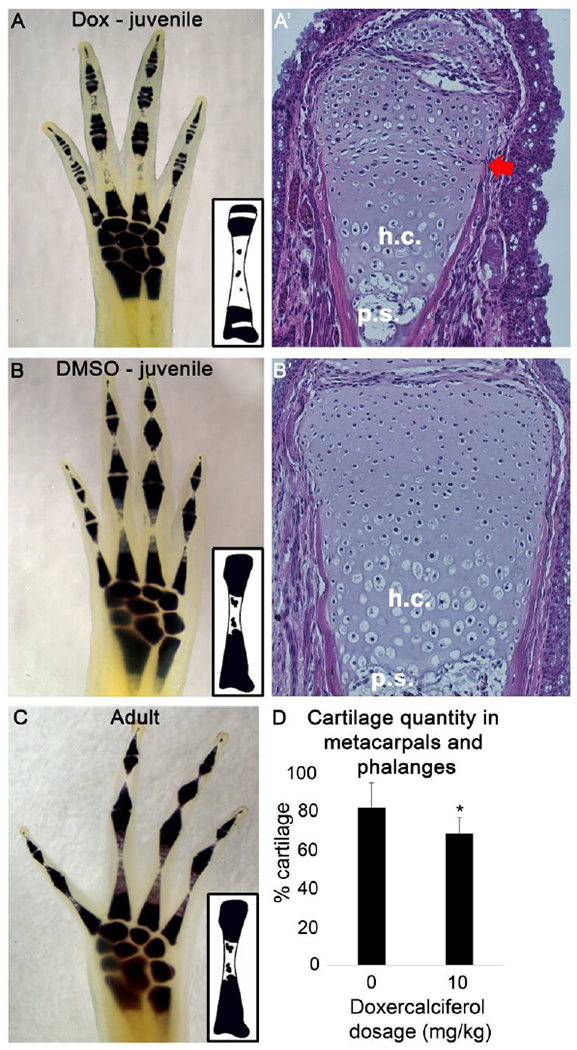
Whole mount cartilage staining revealed that Doxercalciferol decreased metacarpal and phalange cartilage staining in mature autopods (A) relative to age matched, DMSO-treated controls (B). This observation was observed in all analogue treated autopods (N = 9). Hematoxylin and Eosin staining of the distal joint of the metacarpal in Doxcercalciferol- (A’) and DMSO-treated (B’) animals revealed unusual morphology in the epiphysis with Doxicalciferol tretament (red arrow). h.c. – hypertrophic chondrocyte,.s. - primary spongiosa. (C) Older, untreated animals (1 year old, N = 4), had a similar cartilage staining pattern as DMSO treated, juvenile animals. Insets: graphical representations of cartilage staining in the metacarpals. (D) Histogram representing the % of the metacarplas and phalanges that stains positive for cartilage. Doxercalciferol treatment significantly decreased the cartilage staining of the metacarpals and phalanges of mature autopods (t-test, p<0.05, N = 9 for both groups).
To better characterize the changes that we observed in the autopods, we sectioned the Docercalciferol- and DMSO-treated limbs and stained them with Hematoxalin and Eosin (Figure 5A’ and B’). Histological evaluation of the metacarpals and phalanges revealed an unusual morphology in the epiphysis of of Doxicalciferol treated compared to control animals. In particular, the region below the most distal end of the epiphysis, which is likely to be composed of the growth plate, appears to be compacted. We additionally observed that the cells in the epiphysis of the Doxicalciferol treated tissue appear to have more cytoplasm than the control animals. This may indicate that cartilage in the epiphyses if these animals are hypertrophic, a phenomenon that occurs in cartilage prior to the invasion of osteoblasts to generate calcified bone. Given that the overall growth of the Doxicalciferol treated animals was stunted, it is possible that exogenous Vitamin D activity is negatively affecting the growth plates in the skeletal tissue throughout the body of the animal.
Last, Doxercalciferol treated regenerates exhibited cartilage nodules (Table 1, Figure 6), with a maximum frequency of 80%. Presentation of the feature varied more according to the site of amputation than Doxercalciferol dosage (Table 1), such that nodules were more frequently observed in distally located regenerates, and was only observed in one control distal regenerate. We speculate that these nodules may be a result of aberrant chondrocyte formation and proliferation, which are processes that are naturally affected by Vitamin D signaling. The site-specific nature of this observation may relate to the tissues in the different amputation locations; distal amputations occurred mostly through cartilaginous tissue, relative to proximal sites.
Figure 6: Doxercalciferol treatment results in ectopic cartilage nodule formation at distal sites of amputation.
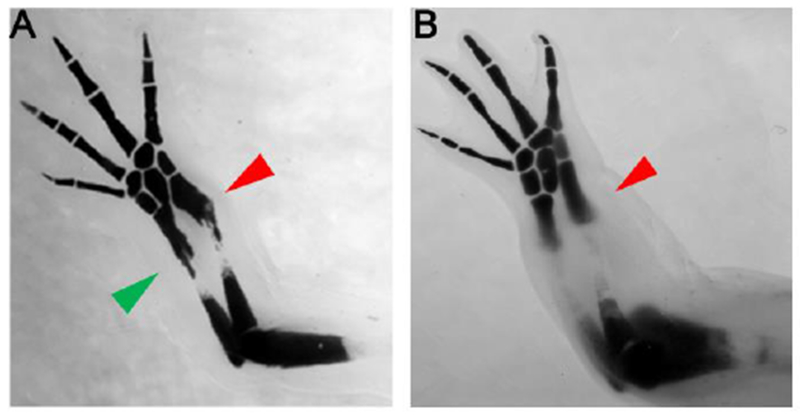
Representative images of a treated regenerating structure (A), showing cartilage nodule formation (green arrow) at the site of amputation (red arrow), and an DMSO treated regenerating structure (B), lacking cartilage nodule formation at the site of amputation (red arrow. Refer to Table 1 for statistics.
3. Discussion:
Exogenous RA is capable of reprogramming positional identity at the site of amputation, altering the pattern of the resulting regenerate (as reviewed by (Maden 1996)). Vitamin D signaling shares several molecular similarities to Vitamin A signaling including the use of the same co-receptor, RXR, a similar consensus sequence for transcriptional regulation (Kliewer et al. 1992), and slowing of the cell cycle (Chen & Ross 2004; Akutsu et al. 2001), a process hypothesized to play a pivotal role in pattern formation (Bryant & Gardiner 2016). Although limited, regeneration studies in the zebrafish pectoral fin (Nachtrab et al. 2013) have shown that altering Vitamin D signaling disrupts pattern formation along the A/P axis. Therefore, in the current study we tested whether exogenous Vitamin D signaling reprograms positional identity in the axolotl limb blastema.
3.1. Exogenous Vitamin D signaling alters A/P, but not P/D limb pattern
To test the above hypothesis, we treated proximal and distal located blastemas with varying concentrations of a potent Vitamin D agonist. Although we observed the up-regulation of VDR transcriptional targets, this treatment failed to result in proximalized regenerates. Thus, our data indicates that Vitamin D does not have the same ability as RA to the reprogram positional information in blastema cells to a more proximal positional identity, under the studied conditions. However, we did observe several skeletal defects both along the A/P and proximal/distal (P/D) axis.
In the current study, the anterior side of the limb axis, at least in terms of carpal patterning, was more susceptible to defects when subjected to exogenous Vitamin D signaling (Figure 2). Similar carpal patterning defects have been observed in regenerating axolotl limbs, although at much lower frequencies, when blastema tissue was treated with RARα- or RAR pan-antagonists (Ro41-5253 or Ro61-8431 respectively) (Rincón & Scadding 2002). It is unknown if these two pathways complement each other by potentially competing for the co-receptor RXR or a shared consensus sequence to facilitate appropriate A/P skeletal patterning. Additionally, the morphology and integration of the regenerated skeletal tissue was abnormal along the P/D axis. Vitamin D signaling contributes to skeleton formation (Driel & Leeuwen 2017; Eisman & Bouillon 2014; Lin et al. 2002; Woeckel et al. 2010; Jiang et al. 2017) and thus the skeletal abnormalities documented within this study, as well as by others (Nachtrab et al. 2013; Washabaugh & Tsonis 1995), may be due to effects on skeletal formation rather than positional reprogramming.
3.2. Region-specific differences in the effect of exogenous Vitamin D signaling on skeletal tissue regeneration.
In the current study, we performed amputations through the distal stylopod (distal amputation), or the mid-humerous (proximal amputation). Thus, the distal amputation site was composed of precursor- and proliferative chondrocytes, while the proximal site of injury was composed of mostly post-mitotic chondrocytes, osteoblasts, precursor osteoblasts and osteoclasts (Mackie et al. 2008). As cartilage nodule formation and regenerate bone integration were more susceptible to exogenous Vitamin D at distal, relative to proximal, amputation sites, this could indicate that this treatment had a greater effect on undifferentiated and proliferative chondrocytes. Although contradicting observations exist in literature (as reviewed by (Driel & Leeuwen 2017; Eisman & Bouillon 2014)), Vitamin D has been shown to promote bone formation and ossification by a variety of mechanisms – promoting murine growth plate vascularization (Lin et al. 2002); mineralization of cultured human osteoblasts (Woeckel et al. 2010), and chondrocyte differentiation of rodent bone marrow-derived mesenchymal stem cells (Jiang et al. 2017). Studies in developing mice have shown that Vitamin D receptor (VDR) signaling in chondrocytes promotes osteoclastogenesis (Masuyama 2006). Any of these mechanisms may, in part, contribute to the effect of exogenous Vitamin D on the regenerating and uninjured skeletal elements observed within the current study.
3.3. Exogenous Vitamin D disrupts skeletal tissue homeostasis
Not only did exogenous Vitamin D affect skeletal elements during regeneration, but altered tissue homeostasis in uninjured autopod phalange and metacarpal bones (Figure 5). We observed that the abundance of whole mount cartilage staining decreased in the treated, uninjured skeletal tissue. Intriguingly this was observed in the diaphysis, which naturally undergoes ossification, and the bone ends, an area enriched in precursor and proliferating chondrocytes (Mackie et al. 2008). The phenotype of this latter site may represent epiphyseal plate closure, explaining the stunted growth observed within treated axolotl (Figure 3D). Exogenous RA alone (Luca et al. 2000; Noyes et al. 2016) or in combination with Vitamin D (Woodard et al. 1997) has been reported to cause long bone pre-mature growth plate closure and stunted growth in humans and calves. Thus, the effect on growth plate morphology that we have observed in the current study may represent a conserved response to exogenous Vitamin D treatment. Overall the axolotl may, therefore, represent a novel system for the understanding of basic bone homeostasis process, in a site and cell specific context, as well as diseases of bone mineralization and density.
3.3. Identification of a novel tissue integration phenotype
In addition to bone formation, vitamin D treatment of blastema tissue affected tissue integration of the regenerate. Integration is defined as the process by which the newly regenerated tissues are interconnected seamlessly with the existing tissues to form a fully-functional regenerated organ (Vieira & McCusker 2017; McCusker & Gardiner 2014). Under normal circumstances the regenerated portion of the axolotl limb will connect seamlessly with the existing stump tissue resulting in an overall structure that matches the original precisely. As depicted in Figure 7B, the new bone of the regenerate is fused with that of the stump in such a manner that the two portions form a continuous, correctly orientated and seamless connection which matches the original structure. Although appropriate integration is critical to proper limb function; there is little information about how this process occurs. Additionally, tissue integration does not automatically occur during regeneration, as the two processes can be uncoupled through specific surgical manipulations (as reviewed by (Vieira & McCusker 2017)).
Figure 7: A diagrammatic representation of different types of skeletal integration observed during salamander limb regeneration.
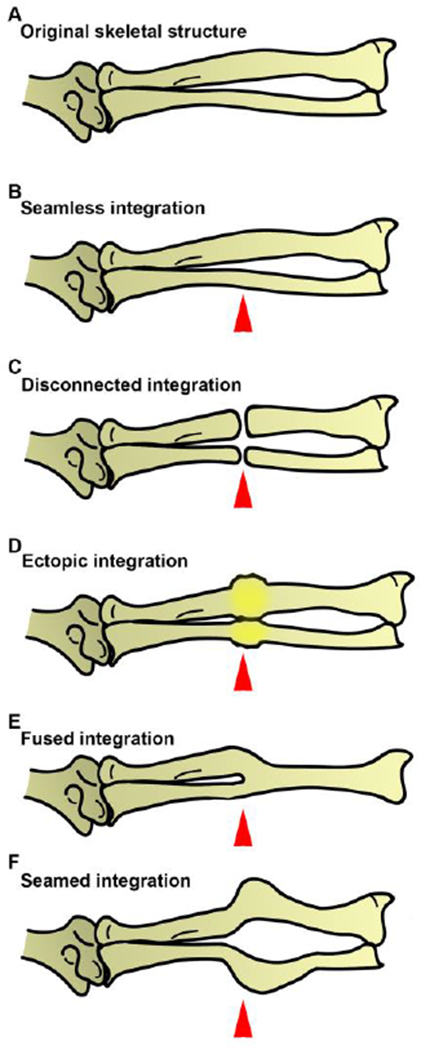
(A) The original limb was amputated mid-stylopod, through the radius and ulna, and allowed to regenerate under various experiential conditions, resulting in different types of skeletal integration. (B) Under normal circumstances the regenerating axolotl limb will connect seamlessly with the existing stump tissue resulting in an overall structure that matches the original precisely. Specific surgical manipulations can, however, result in failed integration between the stump and the regenerate. (C) As seen with the accessory limb model, there is a disconnection between the stump and regenerated bone tissue (Endo et al. 2004). Exogenous RA treatment of blastema tissue frequently results in (D) the formation of an ectopic skeletal element between the stump and regenerate or (E) a fused connection that gives rise to a novel structure relative to the original pattern (Maden 1996). (F) In the current study, exogenous vitamin D treatment induced an abnormal fusion between the stump and regenerated skeletal element, resulting in a “seamed” (visible point of) integration. Red arrowheads represent the plane of amputation.
A number of different skeletal integration phenotypes have already been observed in regeneration limbs, and in the current study we have identified a new integration phenotype as a result of vitamin D treatment. One type of integration phenotype occurs when the existing and newly regenerated skeletal elements do not fuse to make a continuous skeletal unit (Figure 7C). An example of this phenomenon is a hypertrophic nonunion that is a result of severe long bone breaks in humans (Gómez-Barrena et al. 2015). In the context of the amphibian limb regenerate this is similar to the nonunion, or disconnection, between the limb proper, and an ectopic limb structure that has formed on the lateral side of the limb as a result of the Accessory Limb Model (ALM) surgery (Endo et al. 2004). This surgery is performed by deviating the brachial nerve into a lateral wound site with a graft of tissue form the opposing side of the limb axis (Endo et al. 2004). In this instance, the most proximal region of the regenerate is the stylopod, which does not form a joint with the zeugopod in the limb proper.
Another integration phenotype that has been observed in amphibian limb regenerates is the formation of ectopic and amorphic skeletal tissue, known as a “bulbous mass”, between the existing skeletal elements and the newly regenerated elements (Figure 7D). This phenotype is often observed when the regenerating blastema is treated with exogenous RA (Mccusker et al. 2014; Niazi et al. 1985). RA treatment of the regenerating blastema can also cause a different integration phenotype that we have called “fused integration” (Figure 7 E). This phenotype occurs as a result of the direct fusion of the regenerated structures, which have a proximal skeletal structure as a result of the RA treatment, with the existing (distal) skeletal elements where a joint should have formed between these structures (Maden 1983).
In addition to these previously documented forms of integration failure discussed above, here we have observed that exogenous vitamin D treatment of blastema tissue induces a unique form of integration failure. In this instance the newly regenerated skeletal elements form an angled skeletal fusion (Figure 7F), as opposed to a continuous fusion (Figure 7B). We have named this phenomenon “seamed integration”. Therefore, appropriate abundances of both RA and Vitamin D signaling contribute towards tissue integration in the context of regeneration. The mechanisms by which these two nutrient molecules contribute to these different integration phenotypes, however, is unknown and thus requires further investigation.
4. Materials and methods
4.1. Ethics Statement
Ethical approval for this study was obtained from the Institutional Animal Care and Use Committee at the University of Massachusetts, Boston (Protocol # IACUC2015004) and all experimental undertakings were conducted in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
4.2. Animals
The Mexican axolotl (Ambystoma mexicanum) was obtained from the Ambystoma Genetic Stock Center, at the University of Kentucky, and were housed under standard conditions, being exposed to a 14-hour light day-night cycle and an ambient room temperature not exceeding 20°C.
4.3. Surgery
Juvenile animals, having an approximate snout to tail tip length of between 8-14 cm, were used for all experiments and anesthetized using 0.1% solution of MS222 (Ethyl 3-aminobenzoate methanesulfonate salt, Sigma), pH 7.0 before surgery. Regeneration was initiated by amputating the forelimb at one of two sites along the proximal-distal axis. Either the zuegopod, distal to the radius and ulna and proximal to the carpals (hereafter referred to as the distal amputation), or the mid-stylopod (hereafter referred to as the proximal amputation) was amputated. Mid-bud stage blastemas were treated with the Vitamin D homologue or vehicle (see Vitamin D treatment section).
4.4. Vitamin D treatment
A Vitamin D2 analogue, Doxercalciferol (Sigma Aldrich), was re-suspended in DMSO to a concentration of 10g/L and subsequently diluted in water. Early stage blastemas, generated as a result of amputation, were treated with one of two concentrations of the analogue, 1 mg/kg and 10 mg/kg of body weight by injecting anesthetized animals intraperitoneally in the flank with the analogue.
4.5. Tissue preparation for whole mount cartilage staining
Regenerates, once differentiated (7 weeks post amputation), were collected for further analysis. Whole-mount cartilage staining was performed using a 1% Victoria blue B solution (MP Biomedicals) as described by Bryant and Iten (Bryant & Iten 1974), but with modifications. In brief, tissue samples were fixed in 4% formaldehyde (RICCA) overnight, in acid alcohol (0.1% 12N hydrochloric acid in a 70% ethanol solution) overnight and then the cartilage was stained with Victoria blue B (1% in acid alcohol) for 3 hours. The tissue was de-stained with 70% ethanol, treated with 10% EDTA pH14 for 3 hours and then the tissue was cleared with increasing concentrations of glycerol (50, 75 and 95%).
4.6. Tissue preparation for histology
Whole mount tissues were rehydrated in PBS, soaked in 10% EDTA for 3 days and then 30% sucrose overnight. Samples were embedded in OCT (Fisher Sci) and flash frozen before being sectioned at 5µm onto Superfrost slides (Fisher Sci).
4.7. Hematoxylin and Eosin staining
Tissue sections were soaked twice in citrisolv (Fisher Sci), rehydrated in ethonal (100%, 95% 70% ethanol and then water) before being incubated in Hematoxylin solution (Sigma-Aldrich) for 3 minutes. Slides were rinsed for 15 minutes under running tap water, soaked briefly in 70% ethanol, and then incubated in 0.25% Eosin Y for 3 minutes. Samples were rinsed in water and then dehydrated in ethanol (70%, 95% and 100% ethanol), soaked in citrisolv and then mounted with paramount.
4.8. Bone Measurements
With the aid of the measuring tool in Image J, on images obtained from the harvested limbs that were whole mount cartilage stained, the length of the entire ulna and radius was determined for each animal. As we had observed that the treatment with Doxercalciferol slowed the overall body growth of the axolotl (Figure 3D) and Vitamin D treatment is known to slow the growth of the regenerating structure (Washabaugh & Tsonis 1995), radius and ulna lengths were determined relative to the length of the regenerated, second forelimb digit. This allowed direct comparison of bone length between control and experimental groups.
4.9. RNA isolation and cDNA synthesis
Uninjured tissue and early to mid bud, late bud, and tiny limb staged regenerates were collected and pooled (3 of blasts per sample). Untreated and treated blastema tissue was harvested 24 hours post injection and RNA was extracted in Tripure Isolation Reagent (Sigma) and isolated with the Nucleospin RNA XS kit (Macherey-Nagel) according to the manufacturers’ specifications. Subsequently, cDNA libraries were generated with the Transcription first strand cDNA synthesis kit (Roche), using the anchored oligo(dT)18 primer as specified by the manufacturer. Three technical replicates of each of the three biological replicates per sample were performed.
4.10. RT-PCR
Using semi-quantitative, RTPCR the relative transcription levels of genes involved in the Vitamin D signaling pathway were determined between the different experimental groups. Forward and reverse primer sequences for efα, vdr, and cyp24a1 are in Table 2.
Table 2:
Primer sequences used for the validation of the biological activity of Doxercalciferol in the axolotl.
| Name | Sequence |
|---|---|
| ef1α Forward | 5’ – CGGGCACAGGGATTTCATC |
| ef1α Reverse | 5’ – TGCCGGCTTCAAACTCTCC |
| vdr Forward | 5’ – CAGACCACTGCCATTGTGAC |
| vdr Reverse | 5’ – TTGGATTTCCCTGCAAGAAG |
| cyp24a1 Forward | 5’ – CGCTGGCTTCAGGAGAATAG |
| cyp24a1 Reverse | 5’ – GACAAGCCAACACAGCGTTA |
ef1α primer sequences have been used previously (McCusker & Gardiner 2013), while the remaining primer sequences were generated using contig data derived from the Ambystoma EST (http://www.ambystoma.org/) and Axolotl-omics EST Databases (https://www.axolotl-omics.org/home).
KEY RESOURCES TABLE
| Reagent or resource | Source | Identifier |
|---|---|---|
| Chemicals, Peptides, and Recombinant Proteins | ||
| Doxercalciferol | Sigma Aldrich | PubChem Substance ID 329798592 |
| Experimental Models: Organisms/Strains | ||
| Ambystoma Mexicanum | Genetic Stock Center, UKY | RRID:AGSC_101E |
Highlights.
During limb regeneration exogenous vitamin D affects A/P carpal patterning
Exogenous vitamin D results in defects in the integration of the newly regenerated with the existing skeletal elements.
Exogenous vitamin D results in altered bone morphology in uninjured skeletal tissues.
Acknowledgments
Funding sources
Funding for this project was provided by the start-up funding to C.M from the Biology Department at the University of Massachusetts at Boston.
Footnotes
Declarations of interest
None
5. References
- 1.Akutsu N et al. , 2001. Regulation of Gene Expression by 1a , 25-Dihydroxyvitamin D 3 and Its Analog EB1089 under Growth- Inhibitory Conditions in Squamous Carcinoma Cells. Molecular Endocrinology, 25(3), pp.1127–1139. [DOI] [PubMed] [Google Scholar]
- 2.Alroy I, Towers TL & Freedman LP, 1995. Transcriptional Repression of the Interleukin-2 Gene by Vitamin D 3 : Direct Inhibition of NFATp / AP-1 Complex Formation by a Nuclear Hormone Receptor. Molecular and Cellular Biology, 15(10), pp.5789–5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryant SV & Gardiner DM, 1992. Retinoic acid, local cell-cell interactions, and pattern formation in vertebrate limbs. Developmental biology, 152(1), pp.1–25. [DOI] [PubMed] [Google Scholar]
- 4.Bryant SV & Gardiner DM, 2016. The relationship between growth and pattern formation. Regeneration, 3(2), pp.103–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryant SV & Iten LE, 1974. The regulative ability of the limb regeneration blastema of Notophlhalmus viridescens: Experiments in situ. Wilhelm Roux Archiv für Entwicklungsmechanik der Organismen, 174(1), pp.90–101. [DOI] [PubMed] [Google Scholar]
- 6.Buffenstein R et al. , 1995. Vitamin D3 Intoxication in Naked Mole-Rats (Heterocephalus glaber) Leads to Hypercalcaemia and Increased Calcium Deposition in Teeth with Evidence of Abnormal Skin Calcification. General and Comparative Endocrinology, 99(1), pp.35–40. [DOI] [PubMed] [Google Scholar]
- 7.Chen Q & Ross AC, 2004. Retinoic acid regulates cell cycle progression and cell differentiation in human monocytic THP-1 cells. Experimental cell research, 297, pp.68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham TJ & Duester G, 2015. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nature reviews. Molecular cell biology, 16(2), pp.110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Driel M. Van & Leeuwen J.P.T.M. Van, 2017. Molecular and Cellular Endocrinology Vitamin D endocrinology of bone mineralization. Molecular and Cellular Endocrinology, 453, pp.46–51. [DOI] [PubMed] [Google Scholar]
- 10.Eisman JA & Bouillon R, 2014. Vitamin D : direct effects of vitamin D metabolites on bone : lessons from genetically modified mice. BoneKEy Reports, 3(September 2013), pp.1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Endo T, Bryant SV & Gardiner DM, 2004. A stepwise model system for limb regeneration. Developmental Biology, 270(1), pp.135–145. [DOI] [PubMed] [Google Scholar]
- 12.Gómez-Barrena E et al. , 2015. Bone fracture healing: Cell therapy in delayed unions and nonunions. Bone, 70, pp.93–101. [DOI] [PubMed] [Google Scholar]
- 13.Hahn CN et al. , 1994. Identification of a vitamin D responsive element in the promoter of the rat cytochrome P450 24 gene. Nucleic Acids Research, 22(12), pp.2410–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang X et al. , 2017. TGF-β1 is Involved in Vitamin D-Induced Chondrogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells by Regulating the ERK / JNK Pathway. Cellular Physiology and Biochemistry, 42, pp.2230–2241. [DOI] [PubMed] [Google Scholar]
- 15.Kliewer S et al. , 1992. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature, 355(6359), pp.446–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubodera N, 2009. A New Look at the Most Successful Prodrugs for Active. , pp.3869–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin R et al. , 2002. 1a ,25-Dihydroxyvitamin D3 Promotes Vascularization ofthe Chondro-osseous Junction by Stimulating Expression of Vascular Endothelial Growth Factor and Matrix Metalloproteinase 9. Journal of Bone and Mineral Research, 17(9), pp.1604–1612. [DOI] [PubMed] [Google Scholar]
- 18.Liu W et al. , 2008. The Mn1 transcription factor acts upstream of Tbx22 and preferentially regulates posterior palate growth in mice. Development, 3968, pp.3959–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luca FDE et al. , 2000. Retinoic Acid Is a Potent Regulator of Growth Plate. Endocrinology, 141(1), pp.346–353. [DOI] [PubMed] [Google Scholar]
- 20.Mackie EJ et al. , 2008. Endochondral ossification : How cartilage is converted into bone in the developing skeleton. The International Journal of Biochemistry & Cell Biology, 40, pp.46–62. [DOI] [PubMed] [Google Scholar]
- 21.Maden M, 1996. Retinoic acid in development and regeneration. Journal of Biosciences, 21(3), pp.299–312. [Google Scholar]
- 22.Maden M, 1983. The effect of vitamin A on the regenerating amphibian limb. J Embryol Exp Morphol, 77, pp.273–295. [PubMed] [Google Scholar]
- 23.Masuyama R, et al. , 2006. Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. Journal of Clinical Investigations. 116(12):3150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mccusker C, Lehrberg J & Gardiner D, 2014. Position-specific induction of ectopic limbs in non-regenerating blastemas on axolotl forelimbs. , pp.27–34. [DOI] [PMC free article] [PubMed]
- 25.McCusker CD & Gardiner DM, 2013. Positional Information Is Reprogrammed in Blastema Cells of the Regenerating Limb of the Axolotl (Ambystoma mexicanum). PLoS ONE, 8(9), pp.1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCusker CD & Gardiner DM, 2014. Understanding positional cues in salamander limb regeneration: implications for optimizing cell-based regenerative therapies. Disease Models & Mechanisms, 7(6), pp.593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miura S, Hanaoka K & Togashi S, 2008. Skeletogenesis in Xenopus tropicalis : Characteristic bone development in an anuran amphibian. Bone, 43, pp.901–909. [DOI] [PubMed] [Google Scholar]
- 28.Monaghan JR & Maden M, 2013. Visualization of retinoic acid signaling in transgenic axolotls during limb development and regeneration. Developmental biology, 368(1), pp.63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nachtrab G et al. , 2013. Transcriptional components of anteroposterior positional information during zebrafish fin regeneration. , 3764, pp.3754–3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen-yamamoto L et al. , 2010. Comparison of Active Vitamin D Compounds and a Calcimimetic in Mineral Homeostasis. , pp.1713–1723. [DOI] [PMC free article] [PubMed]
- 31.Niazi IA, Pescitelli MJ & Stocum DL, 1985. Stage-dependent effects of retinoic acid on regenerating urodele limbs. Wilhelm Roux’s Archives of Developmental Biology, 194(6), pp.355–363. [Google Scholar]
- 32.Noyes JJ et al. , 2016. Premature Epiphyseal Closure of the Lower Extremities Contributing to Short Stature after cis-Retinoic Acid Therapy in Medulloblastoma: A Case Report. Hormone Research in Paediatrics, 85(1), pp.69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohyama Y et al. , 1996. Functional Assessment of Two Vitamin D-responsive Elements in the Rat 25-Hydroxyvitamin D 3 24-Hydroxylase Gene *. The Journal of Biological Chemistry, 271(48), pp.30381–30385. [DOI] [PubMed] [Google Scholar]
- 34.Ohyama Y et al. , 1994. Identification of a Vitamin D-responsive Element in the 5 ” Flanking Region of the Rat 25-Hydroxyvitamin D3 24-Hydroxylase Gene *. The Journal of Biological Chemistry, 269(14), pp. 10545–50. [PubMed] [Google Scholar]
- 35.Pine M, Hurwitz S 1991. The Role of the Growth Plate in Longitudinal Bone Growth. Poulrty Science. 70(8), pp.1806–1814 [DOI] [PubMed] [Google Scholar]
- 36.Pike JW, Lee SM & Meyer MB, 2014. Regulation of gene expression by 1,25-dihydroxyvitamin D3 in bone cells: exploiting new approaches and defining new mechanisms. BoneKEy reports, 3(JANUARY), p.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhinn M & Dollé P, 2012. Retinoic acid signalling during development. , 858, pp.843–858. [DOI] [PubMed] [Google Scholar]
- 38.Rincón SVDEL & Scadding SR, 2002. Retinoid Antagonists Inhibit Normal Patterning During Limb Regeneration in the Axolotl , Ambystoma mexicanum. , 443(October 2001), pp.435–443. [DOI] [PubMed] [Google Scholar]
- 39.Shubin NH & Alberch P, 1986. A Morphogenetic Approach to the Origin and Basic Organization of the Tetrapod Limb. Evolutionary Biology, 20, pp.319–387. [Google Scholar]
- 40.Vieira WA & McCusker CD, 2017. Developmental plasticity and tissue integration In Gardiner DM, ed. Regenerative Engineering and Developmental Biology: Principles and Applications. Boca Raton: CRC Press, pp. 411–432. [Google Scholar]
- 41.Washabaugh CH & Tsonis P. a, 1995. Effects on vitamin D metabolites on axolotl limb regeneration. Development, Growth & Differentiation, 37, pp.497–503. [DOI] [PubMed] [Google Scholar]
- 42.Woeckel VJ, Eijken M & Chiba H, 2010. 1 a , 25- ( OH ) 2 D 3 Acts in the Early Phase of Osteoblast Differentiation to Enhance Mineralization Via Accelerated Production of Mature Matrix Vesicles. Journal of Cellular Physiology, 225, pp.593–600. [DOI] [PubMed] [Google Scholar]
- 43.Woodard JC, Donovan GA & Fisher LW, 1997. Pathogenesis of Vitamin ( A and D ) -Induced Premature Growth-Plate Closure in Calves. Bone, 21(2), pp.171–182. [DOI] [PubMed] [Google Scholar]
- 44.Zheng W et al. , 2004. Critical role of calbindin-D28k in calcium homeostasis revealed by mice lacking both vitamin D receptor and calbindin-D28k. The Journal of biological chemistry, 279(50), pp.52406–13. [DOI] [PubMed] [Google Scholar]


