Abstract
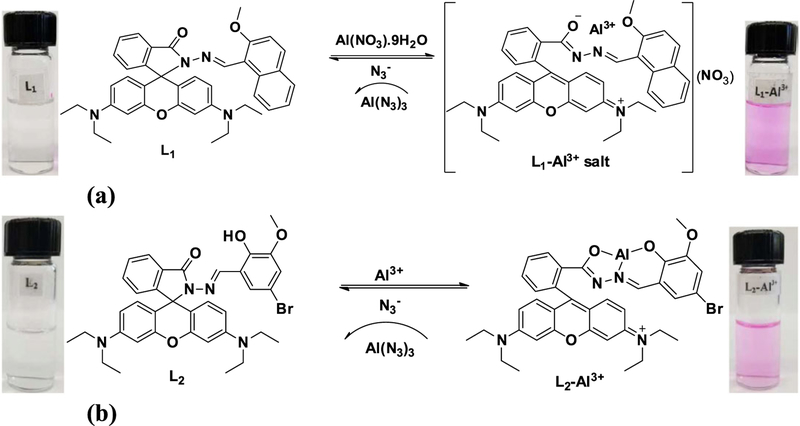
Two new rhodamine derivative L1 and L2 bearing 2-methoxy-1-naphthaldehyde and 5-bromo-3-methoxy salicylaldehyde units were designed and synthesized using microwave-assisted organic synthesis and utilized towards sequential fluorescence detection of aluminum ion (Al3+) and azide (N3−) in aqueous acetonitrile solution. Aluminum ion (Al3+) triggers the formation of highly fluorescent ring-open spirolactam. The fluorescence and colorimetric response of the L1-Al3+ and L2-Al3+ complexes were quenched by the addition of N3−, which extracting the Al3+ from the complexes and turn-off the sensors, confirming that the recognition process is reversible. The recognition ability of the sensors was investigated by fluorescence titration, Job’s plot, 1H-NMR spectroscopy and density functional theory (DFT) calculations.
Keywords: Al3+ and N3−, colorimetric, fluorescence, rhodamine
1 |. INTRODUCTION
Aluminum is the most abundant metallic element in the earth and is found in its ionic form Al3+ in most animal and plant tissues and in natural waters because of acidic rain and human activities.[1] The wide-spread use of aluminum in pharmaceuticals, cooking utensils, aluminum foil, vessels, and trays results in moderate increase in Al3+ concentration in food. The iron binding protein is known to be the main carrier of Al3+ in plasma, and Al3+ can enter the brain and reach the placenta and fetus. In addition, Al3+ has been implicated as a causative factor of Alzheimer’s disease and associated with damage to the central nervous system in humans.[2,3] The World Health Organization (WHO) listed Al3+ as one of the prime food pollutants and limited its concentration to 200 μg L−1 in drinking water.[4] WHO recommended tolerable weekly dietary human intake of Al3+ is 7.0 mg kg−1 body weight.[5] Due to the potential impact of Al3+ on the environment and human health, the effective detection of Al3+ ions is needed. Fluorescent chemosensor has been regarded as an effective method for tracing relevant ions and shows its unique potential advantages. Unfortunately, the determination of Al3+ is complicated by its poor coordination ability, a strong tendency to hydration, and lack of suitable spectroscopic characteristics.[6] It is well known that Al3+ prefers a coordination sphere containing N and O as hard-base donor sites. Schiff bases are known to be good ligands which provide a hard-base environment for the hard-acid Al3+. Most of the reported Al3+ sensors involve complicated synthetic routes with harsh reaction conditions and expensive chemicals.[7] Therefore, it is important to develop an easily synthesizable selective and sensitive chromo/fluorogenic dual signaling sensor for Al3+ in aqueous media.
In recent years the field of anion recognition has grown exponentially due to the significance of anions in environmental, biological and industrial systems.[8] Among several anions, azide ion (N3−) is equally important as they are widely used in automobile airbags, airplane escape chutes, pest control, agriculture, and laboratory research. It resembles carbon monoxide in its irreversible binding to heme cofactors and inhibits mitochondrial respiration causing impaired memory blocking the cytochrome c oxidase.[9,10] A number of chemosensors have appeared in the literature for either Al3+[11] or N3− ions,[12] but sensors for both Al3+ and N3− ions are scarce. The few sensors for both ions suffer from a tedious synthesis procedure, low sensitivity or slow response, turn-off fluorescence response, poor water solubility, and interference from other ions.
Rhodamine-based compounds have been widely used as chemosensors due to their remarkable spectroscopic properties including high absorption coefficients, high fluorescent quantum yields, and excitation and emission with the visible wavelength region.[13] The non-fluorescent spirolactam of rhodamine derivatives can undergo a ring opening in the presence of metal ions to give the highly fluorescent form.[13,14] The ring-open form is pink in color with orange or sometimes green fluorescence.[15] The metal ion sensing behavior of these rhodamine-based optical sensors is very interesting. This is an excellent mechanism which we can use to detect metal ions and several rhodamine based sensors have been reported for ions.[16–23]
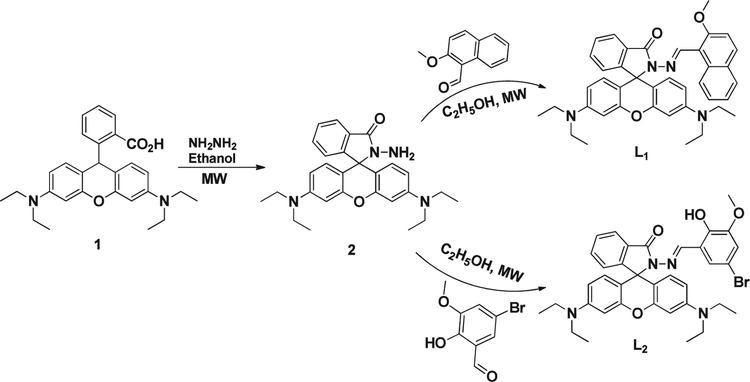
We are now able to report a newly designed and structurally characterized rhodamine Schiff base compounds L1 and L2 which are able to sense micromolar levels of Al3+ ions by chelation-enhanced fluorescence (CHEF) process, and Al3+ complexes L1-Al3+ and L2-Al3+ behave as highly selective chemosensors for N3− ions by quenching of the fluorescence in acetonitrile/water (CH3CN/H2O) medium at 25°C. The competitive ions do not affect the selectivity and specificity of the sensors in the detection of Al3+ and N3− ions. To the best of our knowledge, reports of Al3+ bound sensor for the detection of N3− ions are quite rare. In this work, we introduce a microwave-assisted organic synthesis (MAOS) method to synthesis L1 and L2 in a simple approach; the strategy is shown in Scheme 1.
SCHEME 1.
Chemical structure and synthetic route of L1 and L2
2 |. EXPERIMENTAL
2.1 |. Chemicals and instruments
All the reagents and solvents were purchased as analytical-grade and used without further purification unless otherwise stated. The stock solutions of metal ions were prepared from their nitrate and chloride salts and anion species from their tetrabutylammonium salts. Distilled deionized water was used throughout the experiments. 1H-NMR and 13C-NMR spectra were recorded using an Avance 400 MHz spectrometer (Bruker Billerica, Karlsruhe, Germany) with tetramethylsilane (TMS) as internal standard and deuterated chloroform (CDCl3) as solvent. NMR spectra were analyzed using MestReNova software (version 10, Mestrela Research, Feliciano Barrera-Bajo, Spain). The IR spectrum was obtained using FT-IR spectrometer (Shimadzu, IRAffinity-1S, Columbia, MD, USA). High resolution electrospray ionization mass spectrometry (ESI-MS) was acquired with a Bruker Apex-Qe instrument. All UV-vis spectroscopy experiments were recorded using a Cary UV/vis spectrophotometer 5000 (Varian, Walnut Creek, CA, USA). Fluorescence emission spectra experiments were measured using a Cary 60 series spectrometer (Agilent, Walnut Creek, CA, USA), with excitation and emission slit widths of 5 nm and excitation wavelength at 510 nm. MAOS reactions were carried out in a single mode Biotage Initiator 2.0 (Biotage, Uppsala, Sweden).
2.2 |. Microwave-assisted synthesis and characterization of L1 and L2
Sensors L1 and L2 were synthesized from the parent rhodamine B and aromatic aldehydes (2-methoxy-1-naphthaldehyde and 5-bromo-3-methoxy salicylaldehyde) in a two-step Schiff base condensation using MAOS heating protocols, as described in Scheme 1. Compound 2 was synthesized according to the reported procedure.[24]
2.2.1 |. Synthesis of sensor L1
Using microwave heating protocol: A mixture of compound 2 (105 mg, 0.230 mmol), 2-methoxy-1-naphthaldehyde (41 mg, 0.220 mmol) and ethanol (2 ml) was placed in a 10 ml reaction vial. The resulting mixture was stirred to make it homogeneous and it was placed in the cavity of a biotage microwave reactor. The closed reaction vessel was run under pressure and irradiated for 10 min at 100°C. After cooling to room temperature, the resulting solid was filtered and washed three times with cold ethanol. After drying, the ligand L1 was isolated to give in 92% yield. Melting point: 244–246°C; 1H-NMR (CDCl3), δ (ppm):9.63 (1H, s, N=C-H); 8.77 (1H, d, J = 7.4 Hz, H-Ar), 7.74 (1H, d, J = 8.4 Hz, H-Ar), 7.71 (1H, d, J = 8.0 Hz, H-Ar), 7.63 (1H, d, J =7.7 Hz, H-Ar), 7.48–7.51 (2H, m, H-Ar), 7.15–7.27 (2H, m, H-Ar),7.12 (1H, d, J = 8.4 Hz), 7.09 (1H, d, J = 4.9 Hz), 6.63 (2H, d, J =8.8 Hz), 6.44 (2H, d, J = 2.2 Hz), 6.28 (2H, dd, J = 8.8 Hz, 2.6 Hz), 3.82 (3H, s, OCH3), 3.31 (8H, q, J = 6.9 Hz, NCH2CH3), 1.14 (12H, t, J = 6.9 Hz, NCH2CH3). 13C-NMR (CDCl3), δ (ppm): 164.6, 157.8, 153.4, 151.7, 148.8, 147.6 (N=C-H), 137.6, 133.1, 131.9, 130.3, 129.2, 128.1, 127.0, 126.7, 124.0, 123.2, 116.8, 112.9, 108.1, 107.9, 106.5, 104.6, 79.9, 66.3 (spiro carbon), 56.7, 44.3 (NCH2CH3), 12.7(NCH2CH3); HRMS (ESI): m/z calcd for C40H40N4O3: 625.3173; Found: 625.3176 [M + H]+.
2.2.2 |. Synthesis of sensor L2
Using microwave heating protocol: A mixture of compound 2 (100 mg, 0.220 mmol), 5-bromo-3-methoxy salicylaldehyde (51 mg, 0.221 mmol) and ethanol (2 ml) was placed in a 10 ml reaction vial. The resulting mixture was stirred to make it homogeneous and it was placed in the cavity of a biotage microwave reactor. The closed reaction vessel was run under pressure and irradiated for 10 min at 100°C. After cooling to room temperature, the resulting solid was filtered and washed three times with cold ethanol. After drying, the ligand L2 was isolated to give in 88% yield. 1H-NMR (CDCl3), δ (ppm):11.11 (1H, s, −OH), 8.94 (1H, s, −CH=N), 7.96 (1H, t, J = 6.6 Hz, −Ar), 7.49 (2H, m, −Ar), 6.86 (1H, d, J = 6.6 Hz, −Ar), 7.50 (2H, s, −Ar), 6.51–6.43 (4H, m, −Ar), 6.25 (2H, d, J = 7.5 Hz, −Ar), 3.82 (3H, s, −OCH3), 3.31 (8H, q, NCH2CH3), 1.16 (12H, t, J = 6.6 Hz, NCH2CH3) 13C-NMR (CDCl3), δ (ppm): 163.6, 152.7, 148.5, 146.6 (− CH=N), 138.5, 138.1, 137.7, 134.0, 128.9, 128.5, 127.5, 123.1, 121.8, 121.3, 108.1, 108.0, 106.5, 104.8, 97.3, 80.9, 65.5 (spiro carbon), 56.1, 43.6 (NCH2CH3), 12.4 (NCH2CH3). HRMS (ESI): m/z calcd for C36H37BrN4O4: 669.2071; Found: 669.2076 [M + H] +.
2.3 |. General procedure for the spectroscopic studies
All the spectroscopic measurements were carried out in aqueous CH3CN medium at room temperature. Stock solutions of ligands L1 and L2 (1 × 10−3 M), selected salts of cations (1 × 10−3 M) and anions (1 × 10−4 M) were prepared in CH3CN/H2O. Thus, L1-Al3+ and L2-Al3+ solutions for N3− detection were prepared by addition of 1.0 equivalent of Al3+ to the solution of both L1 and L2 (20 μM) in Tris-HCl (10 mM, pH = 7.2) buffer containing CH3CN/H2O (7:3, v/v) solution. The resulting solution was shaken well before recording the spectra. Each and every fluorescence titration was repeated at least thrice until consistent values were obtained. Jobs continuous variation method was used for determining the binding stoichiometry of the complexation reaction. The association constant (K) was calculated from absorbance studies by the linear Benesi–Hildebrand equation.[25] Color changes in solution phase were observed visually under normal light and under a hand-held UV lamp upon addition of various metal ions at room temperature.
3 |. RESULTS AND DISCUSSION
3.1 |. Synthesis of sensors L1 and L2
The synthesis of L1 and L2 were prepared in two steps with 92% and 88% overall yields respectively (Scheme 1). The results obtained indicate that, unlike classical heating, MAOS results in higher yields, shorter reaction time, mild reaction condition, simple work-up procedure and better purity offer privilege over other methods where complex chromatographic techniques are required for purification of the target compounds. The structure of sensors were fully characterized by 1H-NMR, 13C-NMR, FT-IR and HRMS spectroscopy and all data are in accordance with the proposed structure. Detailed synthetic process and structure characterization are given in the experimental section and in the Supporting Information.
3.2 |. Absorption spectra studies
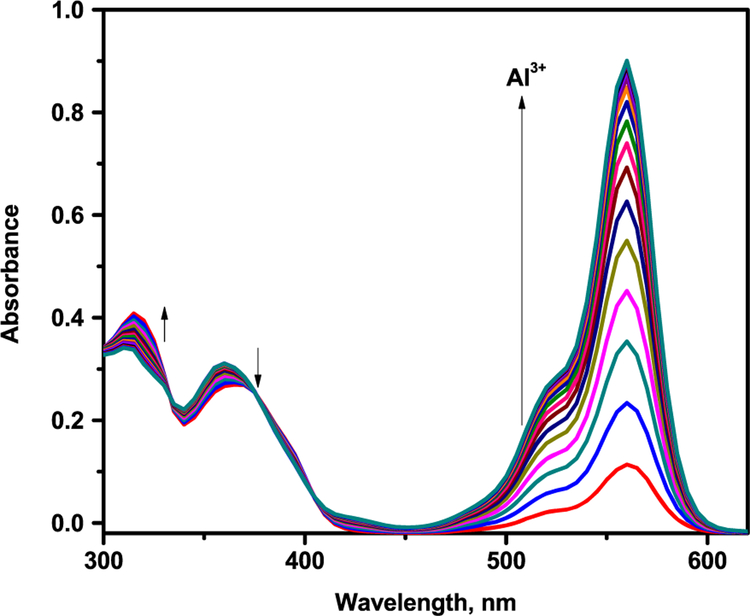
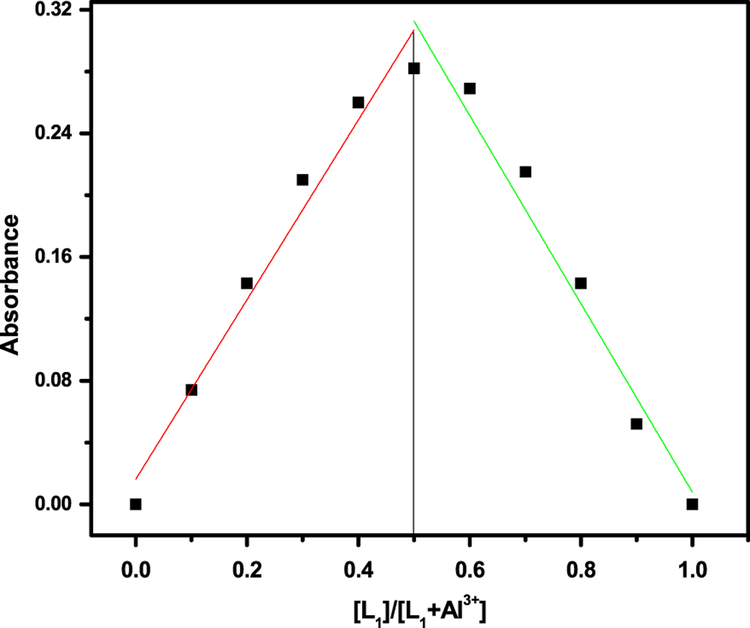
The metal ion sensing of L1 and L2 were first investigated by UV-vis absorption spectra. The colorless solutions were very weakly fluorescent and showed no absorption above 450 nm, properties which are characteristic of the predominant ring-closed spirolactam. The predominant spirolactam form was further confirmed by observation of the characteristic carbon resonance near 66 ppm for each of the sensors. The UV-vis spectrum of sensors were recorded in buffer at 25°C and showed an absorption maximum at λ = 315 nm, which may be attributed to the intramolecular π-π* charge transfer transition. On incremental addition of Al3+ ions, the absorption intensity at 315 nm increased gradually and a new absorption peak at 565 nm with a shoulder at 525 nm was generated by ring opening with a visual color change from colorless to pink. The well-defined isosbestic points at 340 and 375 nm clearly indicates the formation of a new complex species between L1 and Al3+ ion (Figure 1). The absorption enhancement is high compared to other metal ions. Selectivity of L1 was checked in the presence of other metal ions. No significant change in the UV-vis spectrum was observed upon the addition of a 10 equivalent excess of other metal ions of interest: Na+, K+, Mg2+, Ca2+, Ni2+, Zn2+, Co2+, Hg2+, Pb2+, Fe2+, Fe3+, Cr2+ and Cu2+. Absorption spectra of sensors recorded with the continuous addition of Al3+ showed a continuous increase in the absorption at 565 nm and that was employed to calculate binding constants for L1 and L2 with Al3+ using the Benesi–Hildebrand method. The plot of absorbance of L1 at 565 nm as a function of mole fraction of added Al3+ metal ion reveals that these probes bind to the metal ion in 1:1 stoichiometry (Figure 2). The complex association constant (K) calculated through the Benesi–Hildebrand equation for Al3+ with L1 and L2 were found to be3.82 × 104 M−1 and 2.41 × 104 M−1respectively.
FIGURE 1.
UV-vis spectra of L1 (10 μM) with Al3+ (0–23 μM) in CH3CN/H2O (7:3 v/v) solution
FIGURE 2.
Absorbance Job’s plot for determination of L1-Al3+ complex (10 μM) in CH3CN/H2O (7:3 v/v) solution
3.3 |. Fluorescence spectral response of sensors
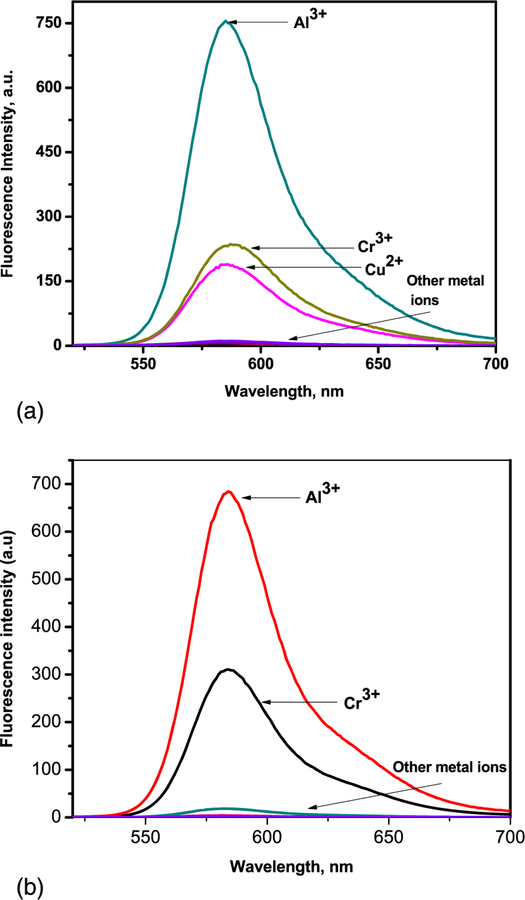
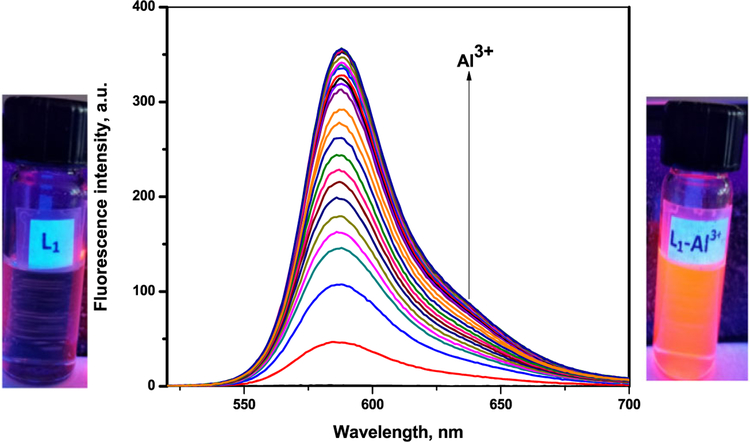
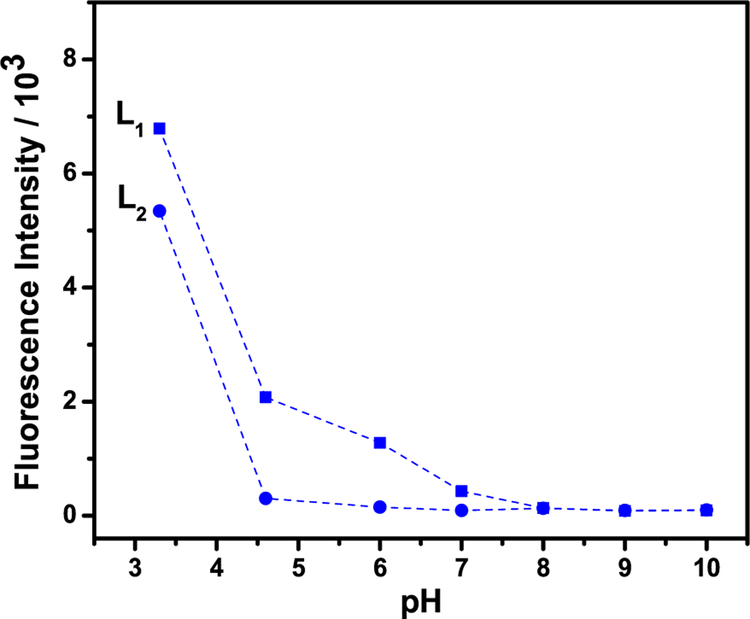
To further explore the sensing behavior of L1 for Al3+ ion, the fluorescence spectra of L1 in CH3CN with various metal ions were examined. The fluorescence spectra were obtained by excitation at 510 nm, and both the excitation and emission slit were 5 nm. The fluorescence intensity of L1 upon the additions of metal ions in CH3CN showed a remarkable sensitivity and selectivity towards Al3+, even though there were relatively small effects with Cu2+ and Cr3+ (Figure 3a). There was a significant emission intensity enhancement with 1.0 equivalent of Al3+ which indicate sensor L1 is an excellent turn-on sensor for Al3+. This very high fluorescence enhancement is attributed to the formation of ring-open spirolactum in the presence of Al3+. This selectivity for Al3+ ions over all other ions is due to selective chelate formation with L1 to afford an L1-Al3+ complex (Scheme 2). When illuminated with a hand-held UV lamp, the addition of Al3+ ions to sensor solution resulted in orange fluorescence emission from L1 solution (Figure 4 and see also Supporting Information Figure S5). The fluorescence profile of L2 were very similar to those for sensor L1: again Al3+ registered the highest fluorescence enhancement while other metal ions showed no significant enhancement (Figure 3b). The fluorescence spectrum of sensors L1 and L2 showed a peak at 585 nm upon the addition of Al3+ corresponding to the delocalization in the xanthenes moiety of rhodamine. It is assumed that the spirolactam form was opened upon the addition of Al3+ to sensors and makes a highly delocalized π-conjugated stable complexes with Al3+ through their active donor sites (e.g. N and O atoms) of receptor part, though other ions failed which basically indicates that the coordinate moiety of L1 and L2 matches perfectly with Al3+ ions instead of the other ions. The detection limits of L1 and L2 for Al3+ ions were estimated based on the fluorescence titration experiment as 32 μM and 47 μM respectively. Furthermore, the effect of pH values on the fluorescence of L1 and L2 were also investigated in a pH range from 3 to 10. Figure 5 shows that for free L1 and L2 at pH < 5, due to protonation of the open-ring of spirolactam, an obvious color change and fluorescence turn-on appeared. Thus, all the optical measurements were performed in buffer solution with a pH of 7 to keep the sensors in their ring closed form.
FIGURE 3.
(a) Fluorescence spectra of L1 (10 μM) with metal ions (10 μM) in CH3CN/H2O (7:3 v/v) solution (λex = 510 nm). (b) Fluorescence spectra of L2 (10 μM) with metal ions (10 μM) in CH3CN/H2O (7:3 v/v) solution (λex = 510 nm)
SCHEME 2.
A possible proposed binding mechanism of sensor L1 (a) and L2 (b) towards Al3+ in the presence and absence of azide (N3−)
FIGURE 4.
Fluorescence spectral titration of L1 (10 μM) on the incremental addition of Al(NO3)3 (23 equivalents) (λex = 510 nm)
FIGURE 5.
Effect of pH values on fluorescence intensity of sensors L1 and L2 (10 μM)
3.4 |. Detection of azide (N3−)
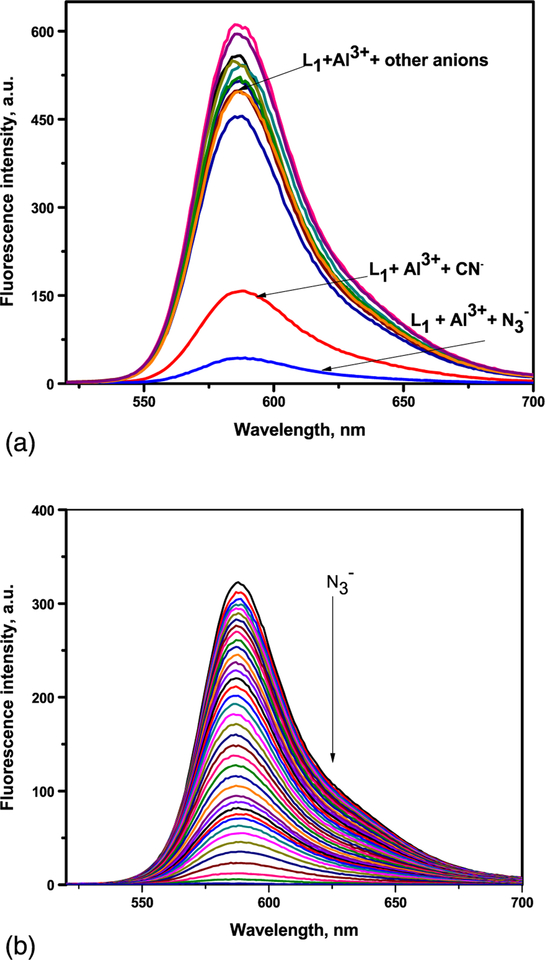
It was interesting to investigate the reversible binding nature of the sensors as shown in Figure 6 and Scheme 2. Due to the high stability of AlN3, the L1-Al3+ and L2-Al3+ complexes could serve as possible means to detect N3−. Figure 6(a) shows the addition of 20 μM of anions N3−, CN−, ClO4−, CH3COO−, HSO4−, H2SO42−, SCN−, Cl−, I−, F−, and OH− to L1-Al3+ (1:1) of which N3− alone quenches the fluorescence, with a slight effect for CN−, indicating high selectivity for N3−. High concentration of CN− contamination is likely to mislead the fluorescent selectivity of N3−. So, when L1-Al3+ is used as the sensor for N3−, high concentration of CN− interference must be eliminated by using mesoporous carbon based adsorbent.[26] The addition of N3− to the L1-Al3+ solution led to a change in color of the solutions from pink to colorless, which was observed with the naked eye. The addition of N3− to the solution containing L1-Al3+ complex resulted in the reversal of the Al3+ induced changes in the emission band at 585 nm in the fluorescence emission spectra. Gradual addition of N3− results in continuous decrease in the emission intensity at 585 nm (Figure 6b). Based on fluorescence data, the detection limit of L1-Al3+ for N3− was calculated as 12 μM. A similar finding was observed for complex L2-Al3+ towards N3− ions (Figure S5). The L2-Al3+ system revealed remarkably selective fluorescence “off” behavior exclusively with N3−. The limit of detection value for N3− ions was found at 18 μM. Thus, results strongly support that L1-Al3+ and L2-Al3+ binds N3− ions with higher selectivity and the process is reversible. The proposed binding mechanism of sensors with Al3+ in the presence and absence of azide (N3−) is shown in Scheme 2.
FIGURE 6.
(a) Fluorescence spectra of L1-Al3+ (1:1) with anions (10 μM) (λex = 510 nm). (b) Fluorescence spectral titration of L1-Al3+ (23 equivalents of Al3+) on the incremental addition of N3− (up to35 equivalents) (λex = 510 nm)
3.5 |. FT-IR and 1H-NMR study for elucidation of coordination mechanism between sensors and Al3+
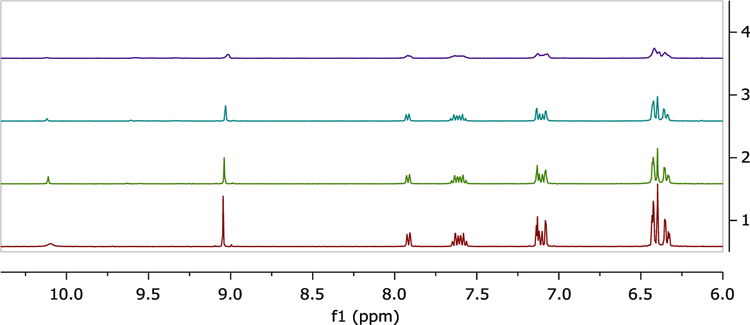
To elucidate the coordination mechanism of L1-Al3+ and L2-Al3+ complexes, the FT-IR spectrum of L1 and L2 were conducted in the absence and presence of Al3+ ion. The characteristic peak of the amide carbonyl γ(C=O) shifted from 1680 cm−1 to 1614 cm−1 in the presence of Al3+, indicating that carbonyl O atoms of the L1 and L2 are involved in the coordination of Al3+ (Figure S9 and S14). 1H-NMR was also performed by adding Al3+ to deuterated dimethyl sulfoxide (DMSO-d6) solution of L2 as shown in Figure 7. The L2-Al3+ complexes were prepared by the additions of 0.25, 0.5 and 1.0 equivalent AlCl3·6H2O to the DMSO solution of L2. The peaks observed at δ 10.10 and δ 9.07 are attributable to the phenolic OH and the imine proton (−CH=N−) in L2. Addition of 1 equivalent of Al3+ resulted in the disappearance of the hydroxyl proton indicating the binding of Al3+ ion through the phenoxide interaction. Further, the little unfilled-shifts from 9.07 to9.00 ppm and shortening of imine protons were observed because of the complex formation between nitrogen atoms and Al3+. The formation of the L2-Al3+ complex through normal ring opening was confirmed by performing the 13C-NMR experiment with L2 in the absence and presence of Al3+ions, from which it was observed that the signal at δ = 66 ppm attributable to the tertiary carbon of the spirolactam ring in L2 was absent from the spectrum of L2-Al3+ complex. Therefore, we propose that the O atom of phenolic OH, N atom of imine and O atom of spiro ring might coordinate to Al3+ as shown in Scheme 2.
FIGURE 7.
1H-NMR spectral changes of L2 (8 mM) in DMSO-d6 and titrated with 0–1.0 equivalents of Al3+ in deuterated water
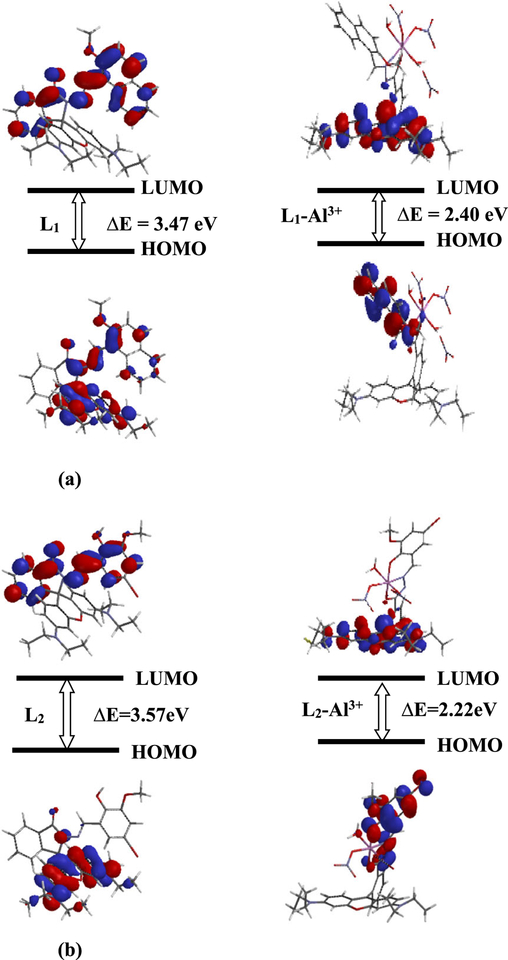
3.6 |. Geometry optimization
To better understand the nature of the coordination of Al3+ with sensors, theoretical calculations on structures L1, L2, L1-Al3+ and L2-Al3+ were carried out using Spartan’16 software. Density functional theory (DFT), employing the B3LYP functional and the 6–31G* basis set was used to obtain gas phase, optimized geometries of these structures. The optimized structures of L1, L2 and their respective Al-complexes are depicted in Figure 8(a) and 8(b). L1 and L2 can undergo rotation of c. 180° about the N-N bond, producing two prominent cis and trans conformations. For both L1 and L2, the trans conformation is more energetically stable than the respective cis one by c. 11.3 kJ mol−1, owing to anti arrangement of the methoxy (−OMe) group and the xanthene moiety in trans L1 and to the anti arrangement of the hydroxyl (−OH) group and the xanthene moiety in trans L2. Additionally, in trans L1 the energy gap between the highest occupied molecular orbital (HOMO) (−4.81 eV) and the lowest unoccupied molecular orbital (LUMO) (−1.34 eV) is 3.47 eV, and in cis L1 the gap, HOMO (−5.03 eV) and LUMO (−1.35 eV), is3.68 eV. In trans L2, the energy gap, HOMO (−4.86 eV) and LUMO (−1.29 eV) is 3.57 eV, and in cis L2 the energy gap, HOMO (−5.05 eV) and LUMO (−1.22 eV) is 3.83 eV, suggesting that trans L1 and trans L2 are the major equilibrium conformations available stereochemically for direct Al3+ coordination. Also, in trans L1, the electron density is delocalized over the entire xanthene moiety with some found on the spirolactam ring as well as on the imine and the ortho-methoxy naphthalene moieties (Figure 8a). In cis L1, the electron density is mainly localized on half of the xanthene moiety (Figure S15). In both trans L2 and cis L2, the electron density is mainly located over the entire xanthene moiety with some found on the lactam ring nitrogen of both. Moreover, some electron density is also found on the carbonyl oxygen in trans L2 but not on the carbonyl oxygen in cis L2 (Figure 8b and Figure S15).
FIGURE 8.
(a) The optimized structures and energy correlation of the HOMO-LUMO gap between L1 and L1-Al3+ salt. (b) The optimized structures and energy correlation of the HOMO-LUMO gap between L2 and L2-Al3+ complex
Density functional calculations of molecular interactions of trans-L1 and trans-L2 with aqueous aluminum (Al3+) nitrate solution revealed that both sensors are energetically stabilized on binding with Al3+ ions. For instance, upon formation of L1-Al3+ salt complex, the HOMO-LUMO energy gap in trans-L1 (ΔE = 3.47 eV) decreased to ΔE = 2.40 eV, and upon formation of L2-Al3+ complex, the HOMO-LUMO energy gap in trans-L2 (ΔE = 3.57 eV) decreased to 2.22 eV. In L1-Al3+ salt complex, formulated as [Al (L1) NO3)2(H2O)2][NO3], HOMO is primarily delocalized over the methoxy naphthalene moiety, while LUMO is primarily delocalized over the xanthene moiety. In L2-Al3+ complex, formulated as Al (L2) (NO3)2(H2O), HOMO is found over the tricyclic structure about Al3+ while LUMO is delocalized over the xanthene moiety (Figure 8a and 8b).
Vertical electronic excitations of optimized B3LYP/6–31G* trans-L1, trans-L2 and their respective complexes were computed using time-dependent-density functional theory (TD-DFT) Spartan’16 software calculations, formalized in water and using a conductor-like polarizable continuum model (CPCM). In the TD-DFT UV-vis spectrum of trans-L1, an absorption band at λ = 379.24 nm with a vertical excitation energy of 3.2693 eV and corresponding to HOMO-2 → LUMO excitation (oscillator strength = 0.4632) dominates as shown in Table S5. While in the TD-DFT UV-vis spectrum of trans-L1-Al3+ salt complex, an absorption band at λ = 422.57 nm dominates, corresponding to HOMO→ LUMO excitation (vertical excitation energy = 2.9341 eV and oscillator strength = 1.0951), (Table S6). In the case of trans-L2, an absorption band at λ = 344.32 nm dominates, corresponding to HOMO-2 → LUMO excitation with a vertical excitation energy of 3.6008 eV and an oscillator strength = 0.3152 as shown in Table S8. For trans-L2-Al3+ complex, an absorption band at λ = 456.19 nm dominates, corresponding to HOMO-1 → LUMO and HOMO→LUMO excitations with a vertical excitation energy of 2.7178 eV and an oscillator strength = 0.7824 (Table S9). The detailed theoretical studies, including the TD-DFT calculations (Tables S1–S9), are in good agreement with the experimental observation.
4 |. CONCLUSION
We have developed reversible fluorescent sensors L1 and L2 for the selective and sensitive sequential detections of Al3+ and N3− via the fluorescence spectral changes. Upon binding to Al3+, obvious detectable change in fluorescence was observed due to the CHEF effect. The in situ prepared L1-Al3+ and L2-Al3+ complexes were used to detect N3 − via the metal-displacement approach which displayed an excellent selectivity and sensitivity towards N3−. Thus, upon the addition of N3− to complexes, the intensity of the 585 nm band decreases, suggesting release of L1 and L2 from the aluminum complexes. Stoichiometry and binding mechanisms for both sensors are well characterized and established by the respective spectroscopic techniques. These results clearly demonstrate that our proposed sensors could be useful for the analysis of Al3+ and N3− in environmental samples and even for biological studies.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledged Morgan State University and the Institute of General Medical Science of the National Institute of Health (Grant No. UL1GM118973) for financial support.
Funding information
Institute of General Medical Science of the NIH, Grant/Award Number: UL1GM118973
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- [1].Xu Y, Mao S, Peng H, Wang F, Zhang H, Opeyemi A, Wu H, JOL 2017, 192, 56. [Google Scholar]
- [2].Perl D, Gajdusek D, Garruto R, Yanagihara R, Gibbs C, Science 1982, 217, 1053. [DOI] [PubMed] [Google Scholar]
- [3].Tang J, Li C, Li Y, Lu X, Qj H, Anal. Chim. Acta 2015, 888, 155. [DOI] [PubMed] [Google Scholar]
- [4].Zhan T, Feng X, Tong B, Shi J, Chen L, Zhi J, Dong Y, Chem. Commun 2012, 48, 416. [DOI] [PubMed] [Google Scholar]
- [5].Alvarez E, Fernandez M, Monterrooso C, For. Ecol. Manage 2005, 211, 227. [Google Scholar]
- [6].Upadhyay K, Kumar A, Org. Biomol. Chem 2010, 8, 4892. [DOI] [PubMed] [Google Scholar]
- [7].Zhao Y, Lin Z, Liao H, Duan C, Meng Q, Inorg. Chem. Commun 2006, 9, 966. [Google Scholar]
- [8].Sahana A, Banerjee A, Guha S, Lohar S, Chattopadhyay A, Mukhoppadhyay S, Das D, Analyst 2012, 137, 1544. [DOI] [PubMed] [Google Scholar]
- [9].Lohar S, Banerjee A, Sahana A, Banik A, Mukhopadhyay S, Das D, Anal. Methods 2013, 5, 442. [Google Scholar]
- [10].Morita T, Perrella MA, Lee ME, Kourembanas S, Proc. Natl. Acad. Sci 1995, 92, 1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jeong J, Rao B, Son Y, Sens. Actuators B 2015, 208, 75. [Google Scholar]
- [12].Gunnlaugsson T, Glynn M, Tocii GM, Kruger PE, Coord. Chem. Rev 2006, 250, 3094. [Google Scholar]
- [13].Weerasinghe AJ, Abebe F, Venter A, Sinn E, J. Fluoresc 2016, 26, 891. [DOI] [PubMed] [Google Scholar]
- [14].Weerasinghe AJ, Abebe F, Sinn E, Tetrahedron Lett. 2011, 52, 5648. [Google Scholar]
- [15].Wang HH, Xue L, Yu LL, Qian YY, Jiang H, Dyes Pigm. 2009, 91, 350. [Google Scholar]
- [16].Hue F, Su J, Sun Y, Yin C, Tong H, Nie Z, Dyes Pigm. 2010, 86, 50. [Google Scholar]
- [17].Li S, Zhang D, Wang M, Ma S, Liu J, Zhao Y, Ye Y, J. Fluoresc 2016, 26, 769. [DOI] [PubMed] [Google Scholar]
- [18].Wei Y, Aydin Y, Zhang Y, Liu Z, Guo M, Chem. Bio. Chem 2012, 13, 1569. [DOI] [PubMed] [Google Scholar]
- [19].Sikdar A, Panja S, Biswas P, J. Fluoresc 2012, 22, 443. [DOI] [PubMed] [Google Scholar]
- [20].Huang J, Xu Y, Qian X, J. Org. Chem 2009, 74, 2167. [DOI] [PubMed] [Google Scholar]
- [21].Huang W, Zhou P, Yan W, He C, Xiong L, Li F, Duan C, J. Environ. Monit 2009, 11, 330. [DOI] [PubMed] [Google Scholar]
- [22].Sarkar M, Banthia S, Samanta A, Tetrahedron Lett. 2009, 47, 7575. [Google Scholar]
- [23].Weerasinghe AJ, Schmiesing C, Sinn E, Tetrahedron Lett. 2009, 50, 6407. [Google Scholar]
- [24].Xiang Y, Tong A, Jin P, Ju Y, Org. Lett 2006, 8, 2863. [DOI] [PubMed] [Google Scholar]
- [25].Abebe F, Sinn E, Tetrahedron Lett. 2011, 52, 5234. [Google Scholar]
- [26].Lee E, Lee S, Heo N, Stucky G, Jun Y, Hong W, Chem. Commun 2012, 48, 3942. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.