Abstract
Background
Neuromodulation of the cerebello-thalamo-cortical (CTC) circuit via thalamic stimulation is an effective therapy for essential tremor (ET). In order to develop non-invasive neuromodulation approaches, clinically relevant thalamo-cortical connections must be elucidated.
Methods
Twenty-eight subjects (18 ET patients and 10 controls) underwent MRI diffusional kurtosis imaging (DKI). A deterministic fiber-tracking algorithm based on DKI was used, with a seeding region placed at the ventral intermediate nucleus (Vim—located based on intraoperative physiology) to the ending regions at the supplementary motor area (SMA), pre-SMA, or primary motor cortex. One-tailed t-tests were performed to compare groups, and associations with tremor severity were determined by Pearson correlations. All p-values were adjusted for multiple comparisons using Bonferroni correction.
Results
There was a decrease in the mean diffusivity (MD) in patients compared to controls in all three tracts: Vim-M1 (ET 0.87, control 0.96, p < 0.01), Vim-SMA (ET 0.86, control 0.96, p < 0.05), and Vim-pre-SMA (ET 0.87, control 0.95, p < 0.05). There was a significant positive correlation between Tremor Rating Scale score and MK (r = 0.471, p = 0.033) and mean FA (r = 0.438, p = 0.045) for the Vim-SMA tract, and no significant correlation for the Vim-pre-SMA or Vim-M1 tracts was found.
Discussion
Patients with ET demonstrated a reinforcement of Vim-cortical connectivity, with higher Vim-SMA connectivity being associated with greater tremor severity. This finding suggests that the Vim-SMA connection is relevant to the underlying pathophysiology of ET, and inhibition of the SMA may be an effective therapeutic approach.
Keywords: Essential tremor, structural connectivity, thalamus, thalamo-cortical, kurtosis, tractography
Introduction
Essential tremor (ET) is a common and potentially debilitating disorder for which there are limited treatment options.1 ET is described as a familial tremor present with action and posture; however, this phenotype may include multiple conditions, which are not currently unified by one underlying neurophysiologic mechanism.2 There are several lines of evidence to support the notion that tremor originates in the cerebellum3 and then propagates through the cerebello-thalamo-cortical (CTC) loop.4–6 Specifically, thalamic connection to the primary motor cortex (M1) has been well studied in ET7; however, the role of other cortical areas implicated in this network has not been elucidated. Neuromodulation of the CTC via thalamic stimulation is effective for the treatment of ET8; however, cortical stimulation targeting the CTC would provide a practical, cost-effective, and safer non-invasive option. Nonetheless, studies assessing cortical stimulation have led to inconsistent results.9–11 Chuang et al. reported a significant reduction in tremor amplitude after continuous theta burst stimulation (cTBS) of both motor and pre-motor cortices, but the improvement was short lived.12 Moro et al. reported sustained effect with subdural motor cortex stimulation up to 1 year,13 and Picillo reported a case of subdural cTBS with benefit up to 3 months.11 Another study reported subclinical improvement with M1 cTBS after a single session.14 Papa et al. reported improvement in tremor following 1 Hz rTMS to the cerebellum sustained up to 3 weeks.9
Among the cortical components of the CTC, the SMA appears to exert a key role in the pathophysiology of the disease. Gallea et al. reported functional and structural connectivity changes in the SMA in patients compared to controls, which correlated with tremor scores.15 Indeed, our group reported significant improvement following 15 sessions 1 Hz rTMS of the pre-SMA which was sustained at 3 months.10
In this study, we aim to evaluate the critical neuroanatomy of CTC abnormalities related to ET that may be amenable to non-invasive neuromodulation. We compared thalamo-cortical structural connectivity using diffusional kurtosis imaging (DKI) with the objective of determining the most clinically relevant components within the CTC in ET. DKI has been shown to be superior to DTI for detecting crossing fibers.16–18 We feel this approach is ideal for performing tractography in this region of the brain. These findings could further inform future interventional studies of cortical targets for the treatment of tremor in ET.
Methods
Subjects
Twenty-eight subjects (18 ET patients and 10 controls) underwent clinical MRI with DKI. The ET patients were selected from the Medical University of South Carolina deep brain stimulation (DBS) clinic, prior DBS surgery. Healthy controls were age- and gender-matched; they were selected from the New York University Alzheimer’s Disease Center, as a control group, and had no evidence of dementia or mild cognitive impairment after neurologic, psychiatric evaluations, and neuropsychological batteries were administered to confirm this. Fahn–Tolosa–Marin Tremor Rating Scale (TRS) was performed as part of the DBS evaluation. This study was approved by the Institutional Review Board of the Medical University of South Carolina.
Magnetic resonance imaging
DKI datasets were acquired from 18 ET subjects and 10 controls. ET subjects were imaged on the Verio 3T (12), Avanto 1.5T (1), and Skyra 3T (5) MRI systems (running VB19) (Siemens Medical, Erlangen, Germany). The diffusion-encoding gradients were along 30 different directions with b-values of 0, 1,000, and 2,000 s/mm2. Voxel size was 3.0 × 3.0 × 3.0 mm3, FoV read was 222, and FoV phase was 100%. Echo time (TE) was 98 ms and the repetition time (TR) was 8,500 ms on 3T system. Echo time (TE) was 99 ms and the repetition time (TR) was 5,500 ms on 1.5T system.
Healthy controls were imaged on TimTrio 3T MRI (Siemens Medical, Erlangen, Germany). The diffusion-encoding gradients were along 30 different directions with b-values of 0, 1,000, and 2,000 s/mm2. Echo time (TE) was 96 ms, repetition time (TR) was 5,900 ms, voxel size was 2.7 × 2.7 × 2.7 mm3, FoV read was 222, and FoV phase was 100%.
Regions of Interest
The Vim region of interest (ROI) was determined based on intraoperative microelectrode recording (MER) findings. Intraoperatively, the Vim borders were defined by MER based on standard techniques.19 Based on these findings, the ROI center was identified, and coordinates were determined. A 6.0 × 6.0 × 6.0 mm3 (2 times the isotropic voxel size) ROI was created ensuring adjacent structures were not included.
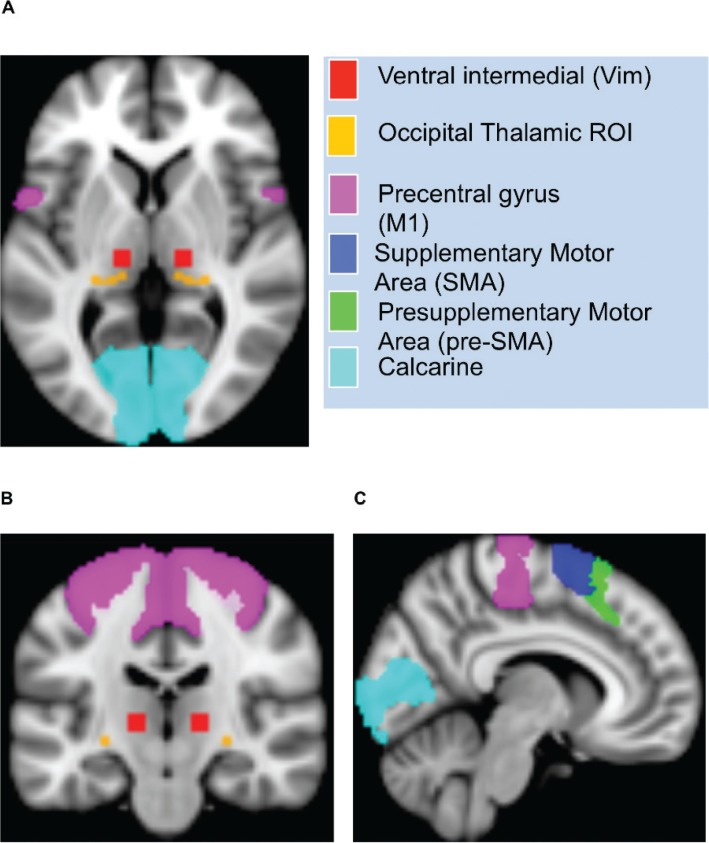
The SMA ROI was created from the AICHA Atlas.20 The pre-SMA ROI was created by combining the AICHA Atlas regions G_Supp_Motor_Area-2 and G_Supp_Motor_Area-1. The pulvinar ROI was derived from the Thalamic Connectivity Atlas, and the occipital cortex ROI was derived from the Harvard-Oxford Cortical Atlas. A threshold of 25% was applied to both ROIs. Regarding the ROIs derived from the AICHA Atlas and the Thalamic Connectivity Atlas, the left hemisphere was reflected on right for symmetry (see Figure 1).
Figure 1.
Regions of Interest. Representation of sample ROI for Thalamic and Cortical Structures Represented by Color Scheme Shown on Legend, in the Axial (A), Sagittal (B), and Coronal (C) Views.
Structural connectivity
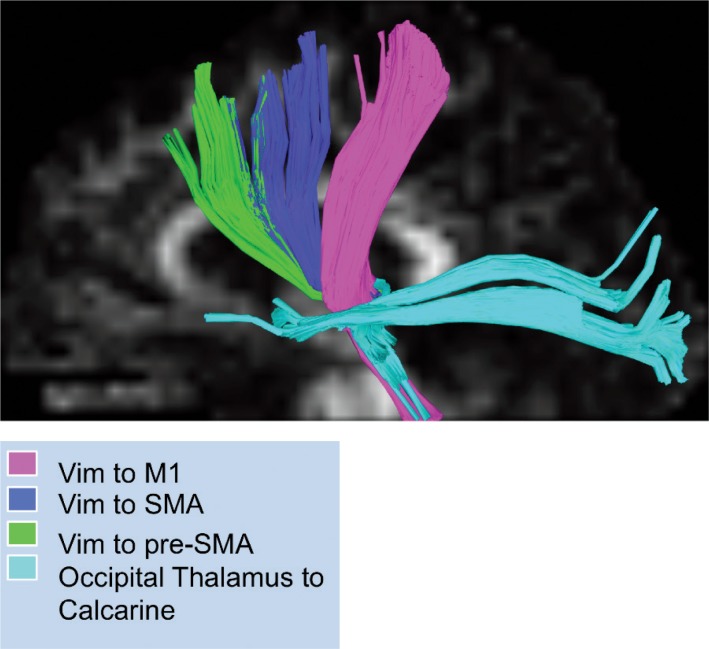
A deterministic fiber-tracking algorithm based on DKI was used, with a seeding region in the Vim ending in the SMA, pre-SMA, and M1 (see Figure 2). The anisotropy threshold for all subjects was 0.10, with the angular threshold at 35 degrees and step size at 1.4 mm. Tracts with length more than 300 mm were discarded, and a total of 100,000 seeds were placed.
Figure 2.
Tractography. Representation of DKI Tractography from Selected ROIs. Each tract is represented by the color scheme shown on legend.
Importantly, in order to avoid global non-specific brain differences in connectivity between ET and controls, the pulvinar to occipital cortex tract was used as a control to normalize the remaining tracts, since the pulvinar to occipital cortex tract is not directly related to the pathophysiology of ET. As such, all Vim-based tracts were normalized (per each individual) based on the pulvinar–occipital tracts according to the following formula (Vim tract/pulvinar tract), and all measures described below represent this ratio.
Statistics
Tests for normality were conducted for all tracts and all parameters, which confirmed a normal distribution. As such, one-tailed t-test (a priori hypothesis) was used when comparing FA Mean, MK Mean, and MD Mean between age-matched controls and ET subjects. In each tract, comparisons between groups for each metric (FA mean, MK mean, and MD mean) were conducted using Bonferroni-adjusted p-values (p-value*3).
Pearson’s correlation coefficients were calculated between TRS and FA Mean, MK Mean, and MD Mean for all three motor tracts. ANOVA was used when comparing FA Mean, MK Mean, and MD Mean between motor tracts in ET subjects. In each metric, comparisons between tracts in ET subjects were conducted using Bonferroni-adjusted p-values (p-value*3).
Results
Subjects
ET patients were 71.1 ± 8.8 years of age and 55.6% male, while controls were 69.4 ± 9.0 years of age and 30% male. Mean tremor severity was 55.6 ± 17.38 on the TRS. All patients in this cohort had severe, functionally debilitating tremor, which was treatment refractory and were cleared as such for DBS surgery. None of the subjects carried a diagnosis of ET-plus or had dystonic component to their tremor. All metrics of interest including age were normally distributed.
Tractography
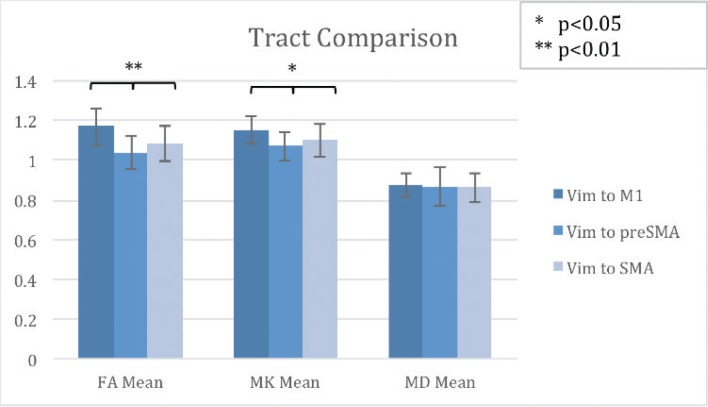
We compared the normalized ratios of mean fractional anisotropy (FA), mean kurtosis (MK), and mean diffusivity (MD) in all three tracts of interest (Vim-M1, Vim-SMA, and Vim-pre-SMA) in ET patients. We found the FA mean to be higher in Vim-M1 (1.17) compared to Vim-pre-SMA (1.04) and higher in Vim-SMA (1.08) compared to Vim-pre-SMA (1.04), p < 0.01, F statistic 10.412. We also found the MK mean to be higher in Vim-M1 (1.15) compared to Vim-pre-SMA (1.07) and higher in Vim-SMA (1.1) compared to Vim-pre-SMA (1.07), p < 0.05, F statistic 5.04. There were no statistically significant differences in MD (see Figure 3).
Figure 3.
Tract Comparison by metric in ET patients. Comparison of Each Track by FA Mean, MK Mean, and MD Mean.
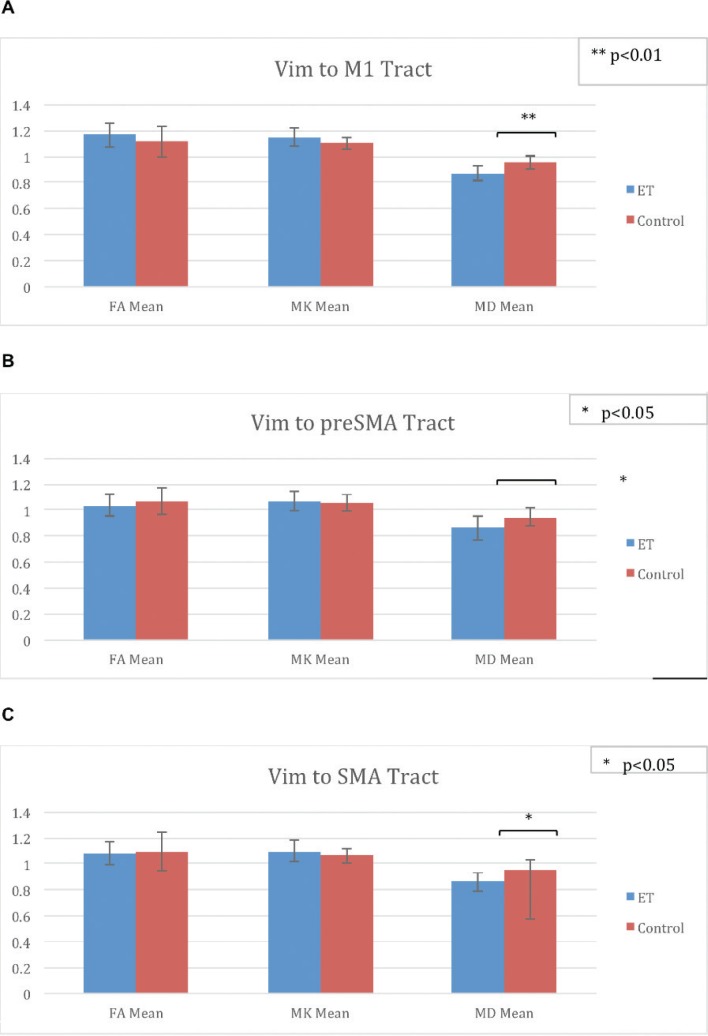
When comparing ET patients to controls, there was no statistically significant difference in FA or MK in any of the tracts. There was a statistically significant decrease in MD in patients (0.87) compared to controls (0.96) in p < 0.01 in the Vim-M1 tract. There was a trend towards increased FA and MK along this tract as well. There was a statistically significant decrease in MD in patients (0.87) compared to controls (0.95) in p < 0.05 in the Vim-pre-SMA tract. There was a statistically significant decrease in MD in patients (0.86) compared to controls (0.96) in p < 0.05 in the Vim-SMA tract (see Figure 4).
Figure 4.
Differences between ET patients and Controls. FA Mean, MK Mean, and MD Mean for patients and controls in (A) Vim-M1, (B) Vim-preSMA, and (C) Vim-SMA Tracts.
Clinical correlation
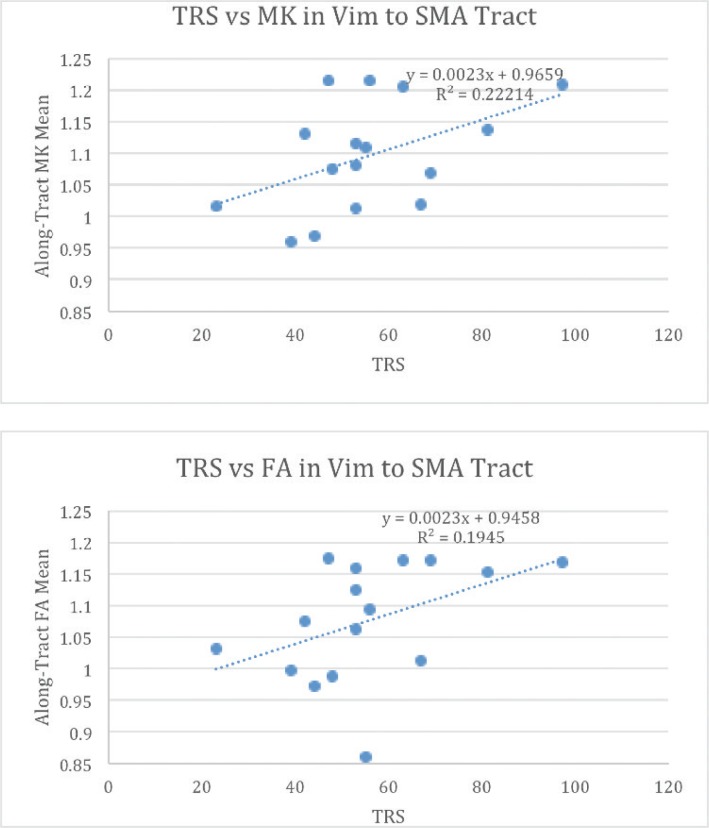
There was a significant positive correlation between Tremor Rating Scale (TRS) score and MK (r = 0.471, p = 0.033) for the Vim-SMA tract. There was also a significant positive correlation between TRS score and mean FA (r = 0.438, p = 0.045) for the Vim-SMA tract. There was no significant correlation for FA, MK, or MD, for the Vim → pre-SMA or Vim → M1 tracts (see Figure 5).
Figure 5.
Relationship between Tractography metrics and Tremor Severity. MK and FA Plotted against Tremor Severity represented by total tremor rating scale (TRS) score.
Discussion
We report the findings of the first study utilizing DKI deterministic tractography to investigate thalamo-cortical connectivity in ET. We also report the feasibility of our approach using intraoperative mapping to guide ROI selection within the thalamus, determining the clinically relevant sensorimotor region of the thalamus using detailed intraoperative physiology. Comparisons of multiple metrics along the three tracts of interest in ET patients revealed greater FA and MK values in the Vim to M1, or Vim to SMA compared to the Vim to pre-SMA tract, but no difference in MD. This may be explained by the fact that each parameter represents different physical properties along the tract. Specifically, FA is an index of directional dependence of diffusion, MD refers to the amplitude of diffusion or displacement of the water molecules, and MK is a measure of heterogeneity of the tissue microenvironment. Therefore, there are differences in the directionality of diffusion as well as the heterogeneity of the microenvironment of the tract, but no significant difference in the amplitude of diffusion. Given that this is an area of high white matter fiber crossing, differences in heterogeneity of the tissue structure are captured, while differences in diffusion are not as evident.
The network involved in ET has been studied extensively in recent years with a variety of coherence analyses of tremor frequency as measured by surface electromyography (EMG), cortical oscillations captured with multiple electroencephalography (EEG) techniques including magnetoencephalography or MEG and high density EEG, and dynamic source imaging. In this analysis, the cortical area found to have high coherence with cerebellar oscillations in ET was primarily M1; however, premotor cortex and SMA were also implicated.21 Earlier studies have also shown coherence between M1 and tremor on EMG.7 Due to the anatomical location of SMA, it is difficult to capture in scalp EEG studies, which makes tractography a more useful measure for this particular connection.
Although tractography has been proposed to aid in surgical targeting, very few studies have been reported of structural connectivity changes in ET compared to controls. A recent study showed a reduction in structural connectivity from the thalamus to the pre-motor cortex, which is in line with our findings of decreased thalamo-cortical connectivity but they did not look at other motor cortices.22 A more recent study used probabilistic tractography to segment the thalamus and then modeled of volume tissue activated (VTA) with DBS to see which thalamic segments were within the model of VTA. They found the thalamic segments corresponding to SMA and prefrontal cortex to be associated with tremor reduction.23
In our study, we focused on seeding the motor thalamus as identified by physiology and used DKI tractography to investigate associations between MK, MD and FA along each tract with tremor severity. DKI has been shown to have less systematic error than DTI enabling the detection of crossing fiber bundles.18 This is critical in answering questions of thalamo-cortical connectivity given the anatomic location of these connections. With this approach, we were able to identify a positive association with MK and FA with tremor severity in the Vim-SMA tract, specifically, which was not present along the other tracts. We interpret these findings as an indication that the Vim-SMA tract is more closely linked to tremor propagation than other thalamo-cortical tracts. Furthermore, using DKI tractography we are able to quantify metrics within the tract and how these are associated with tremor severity, which may have significant therapeutic implications. Specifically, we found that as the connectivity between Vim and SMA strengthens, tremor worsens, which would suggest that inhibition of the SMA may be an effective therapeutic approach.
Thalamo-cortical connections are complex and include afferent and efferent connections and interneurons that are thought to have a modulatory role of the motor thalamus.24 The role of the SMA in ET has been thought to be mostly compensatory to a primary cerebellar problem, based on evidence from opposing changes of the SMA and cerebellum. Decreased cerebellar volume is positively correlated with tremor frequency, while increased SMA volume is positively correlated with tremor intensity.15 One hypothesis is that the compensatory role of the SMA is effective early on and eventually overwhelmed, leading to the emergence of tremor clinically. If so, enhancing the SMA compensatory activity should yield tremor reduction. An alternative hypothesis is that increased SMA connectivity is a maladaptive response, which explains why increases in volume and connectivity correlate with worsening tremor severity. In this case, SMA inhibition is more likely to reduce tremor severity.
However, previous attempts at inhibiting cortical areas in ET revealed mixed results. Possible explanations for these findings include: (1) suboptimal targeting, (2) suboptimal dosing, and (3) targeting a less relevant cortical region. In our study inhibiting the pre-SMA, we saw sustained improvement in tremor severity from pre- to post-treatment without a difference between active and sham. If in fact, the Vim-SMA connection is the relevant clinical connection, the observed tremor reduction may have been related more to the stimulation of the adjacent SMA proper than pre-SMA itself. Our findings therefore inform future studies targeting cortical structure for the treatment of ET suggesting the SMA as the more relevant cortical-thalamic connection and inhibition as the more likely effective approach.
Limitations of our study include the use of different MRI scanners within the ET subject group and with the control group. We addressed this limitation by normalizing our results to the pulvinar-occipital cortex tract. There are no reported or expected differences along this tract in ET. However, the possibility that some of the observed changes are due to different scanners cannot be excluded. It is also important to note that although corrections for multiple comparisons were made when comparing across tracts and imaging metrics, we did not correct for multiple comparisons in the clinical correlation analysis. Therefore, we interpret these findings as exploratory and should be replicated in a larger cohort.
Footnotes
Citation: Revuelta G, McGill C, Jensen JH, Bonilha L. Characterizing thalamo-cortical structural connectivity in essential tremor with diffusional kurtosis imaging tractography. Tremor Other Hyperkinet Mov. 2019; 9. doi: 10.7916/tohm.v0.690
Editor: Elan D. Louis, Yale University, USA
Funding: This study was supported by NIH Grants 1K23NS091391-01A1, R01DC014021, RF1AG057602, R01NS097775, R01AG054159, and R01AG055132.
Financial Disclosures: Dr. Revuelta has received research funding from the South Carolina Clinical & Translational Research (SCTR) Institute, supported by NIH/NCATS Grant Number UL1TR000062 and by NIH NINDS Grant Number 1K23NS091391-01A1, Boston Scientific and the National Center of Neuromodulation for Rehabilitation. Dr. Revuelta has received educational grants from Medtronic and Abbott, and honoraria from Lundbeck. Ms. McGill has no independent funding to disclose. Dr. Jensen has received financial support from NIH grants RF1AG057602, R01NS097775, R01AG054159, and R01AG055132, and from a grant awarded by the Litwin Foundation. Dr. Bonilha’s research is supported by the NIH NIDCD grant number R01DC014021, by the American Heart Association grant number SFDRN26030003 and by Medtronic Inc.
Conflict of Interest: Jens Jensen is a co-inventor on US patents 8,811,706, 9,478,026, and 9,965,962, which are related to the MRI techniques utilized in this work; the other authors do not report any conflict of interest related to the research reported in this article.
Ethics Statement: This study was reviewed by the authors’ institutional ethics committee and was considered exempted from further review.
References
- 1.Zesiewicz TA, Elble RJ, Louis ED, et al. . Evidence-based guideline update: treatment of essential tremor: report of the quality standards subcommittee of the American Academy of Neurology. Neurology 2011;77(19):1752–1755. doi: 10.1212/WNL.0b013e318236f0fd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Espay AJ, Lang AE, Erro R, et al. . Essential pitfalls in “essential” tremor. Mov Disord 2017;32(3):325–331. doi: 10.1002/mds.26919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis ED, Kerridge CA, Chatterjee D, et al. . Contextualizing the pathology in the essential tremor cerebellar cortex: a patholog-omics approach. Acta Neuropathol 2019. doi: 10.1007/s00401-019-02043-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallett M. Tremor: pathophysiology. Parkinsonism Relat Disord 2014;20(Suppl 1):S118–S122. doi: 10.1016/S1353-8020(13)70029-4 [DOI] [PubMed] [Google Scholar]
- 5.Raethjen J, Deuschl G. The oscillating central network of essential tremor. Clin Neurophysiol 2012;123(1):61–64. doi: 10.1016/j.clinph.2011.09.024 [DOI] [PubMed] [Google Scholar]
- 6.Lenka A, Bhalsing KS, Panda R, et al. . Role of altered cerebello-thalamo-cortical network in the neurobiology of essential tremor. Neuroradiology 2017;59(2):157–168. doi: 10.1007/s00234-016-1771-1 [DOI] [PubMed] [Google Scholar]
- 7.Schnitzler A, Munks C, Butz M, Timmermann L, Gross J. Synchronized brain network associated with essential tremor as revealed by magnetoencephalography. Mov Disord. 2009;24(11):1629–1635. doi: 10.1002/mds.22633 [DOI] [PubMed] [Google Scholar]
- 8.Deuschl G, Raethjen J, Hellriegel H, Elble R. Treatment of patients with essential tremor. Lancet Neurol 2011;10(2):148–161. doi: 10.1016/S1474-4422(10)70322-7 [DOI] [PubMed] [Google Scholar]
- 9.Popa T, Russo M, Vidailhet M, et al. . Cerebellar rTMS stimulation may induce prolonged clinical benefits in essential tremor, and subjacent changes in functional connectivity: an open label trial. Brain Stimul 2013;6(2):175–179. doi: 10.1016/j.brs.2012.04.009 [DOI] [PubMed] [Google Scholar]
- 10.Badran BW, Glusman CE, Austelle CW, et al. . A double-blind, sham-controlled pilot trial of pre-supplementary motor area (Pre-SMA) 1 Hz rTMS to treat essential tremor. Brain Stimul 2016;9(6):945–947. doi: 10.1016/j.brs.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picillo M, Moro E, Edwards M, Di Lazzaro V, Lozano AM, Fasano A. Subdural continuous theta burst stimulation of the motor cortex in essential tremor. Brain Stimul 2015;8(4):840–842. doi: 10.1016/j.brs.2015.05.003 [DOI] [PubMed] [Google Scholar]
- 12.Chuang WL, Huang YZ, Lu CS, Chen RS. Reduced cortical plasticity and GABAergic modulation in essential tremor. Mov Disord 2014;29(4):501–507. doi: 10.1002/mds.25809 [DOI] [PubMed] [Google Scholar]
- 13.Moro E, Schwalb JM, Piboolnurak P, et al. . Unilateral subdural motor cortex stimulation improves essential tremor but not Parkinson’s disease. Brain 2011;134(Pt 7):2096–2105. doi: 10.1093/brain/awr072 [DOI] [PubMed] [Google Scholar]
- 14.Hellriegel H, Schulz EM, Siebner HR, Deuschl G, Raethjen JH. Continuous theta-burst stimulation of the primary motor cortex in essential tremor. Clin Neurophysiol 2012;123(5):1010–1015. doi: 10.1016/j.clinph.2011.08.033 [DOI] [PubMed] [Google Scholar]
- 15.Gallea C, Popa T, Garcia-Lorenzo D, et al. . Intrinsic signature of essential tremor in the cerebello-frontal network. Brain 2015;138(Pt 10):2920–2933. doi: 10.1093/brain/awv171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen JH, Helpern JA, Tabesh A. Leading non-Gaussian corrections for diffusion orientation distribution function. NMR Biomed 2014;27(2):202–211. doi: 10.1002/nbm.3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glenn GR, Helpern JA, Tabesh A, Jensen JH. Optimization of white matter fiber tractography with diffusional kurtosis imaging. NMR Biomed 2015;28(10):1245–1256. doi: 10.1002/nbm.3374 [DOI] [PubMed] [Google Scholar]
- 18.Glenn GR, Kuo LW, Chao YP, Lee CY, Helpern JA, Jensen JH. Mapping the orientation of white matter fiber bundles: a comparative study of diffusion tensor imaging, diffusional kurtosis imaging, and diffusion spectrum imaging. AJNR Am J Neuroradiol 2016;37(7):1216–1222. doi: 10.3174/ajnr.A4714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gross RE, Krack P, Rodriguez-Oroz MC, Rezai AR, Benabid AL. Electrophysiological mapping for the implantation of deep brain stimulators for Parkinson’s disease and tremor. Mov Disord 2006;21(Suppl 14):S259–S283. doi: 10.1002/mds.20960 [DOI] [PubMed] [Google Scholar]
- 20.Joliot M, Jobard G, Naveau M, et al. . AICHA: an atlas of intrinsic connectivity of homotopic areas. J Neurosci Methods 2015;254:46–59. doi: 10.1016/j.jneumeth.2015.07.013 [DOI] [PubMed] [Google Scholar]
- 21.Muthuraman M, Raethjen J, Koirala N, et al. . Cerebello-cortical network fingerprints differ between essential, Parkinson’s and mimicked tremors. Brain 2018;141(6):1770–1781. doi: 10.1093/brain/awy098 [DOI] [PubMed] [Google Scholar]
- 22.Caligiuri ME, Arabia G, Barbagallo G, et al. . Structural connectivity differences in essential tremor with and without resting tremor. J Neurol 2017;264(9):1865–1874. doi: 10.1007/s00415-017-8553-5 [DOI] [PubMed] [Google Scholar]
- 23.Middlebrooks EH, Tuna IS, Almeida L, et al. . Structural connectivity-based segmentation of the thalamus and prediction of tremor improvement following thalamic deep brain stimulation of the ventral intermediate nucleus. NeuroImage Clin 2018;20:1266–1273. doi: 10.1016/j.nicl.2018.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galvan A, Hu X, Smith Y, Wichmann T. Effects of optogenetic activation of corticothalamic terminals in the motor thalamus of awake monkeys. J Neurosci 2016;36(12):3519–3530. doi: 10.1523/JNEUROSCI.4363-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]