Abstract
Purpose of review
Although type 2 diabetes (T2D) is one of the most important risk factors that leads to the development of de novo heart failure, there are limited data, particularly from a practical/qualitative standpoint, about predictors of heart failure in this population.
Recent findings
Sodium-glucose cotransporter-2 (SGLT-2) inhibitors have been shown to prevent the development of heart failure and the composite of heart failure and cardiovascular death in patients with T2D without known heart failure who have either established atherosclerotic vascular disease or multiple risk factors. The concept of primary prevention of heart failure has led many clinicians to inquire if there are specific risk/enrichment factors that may predict an increased risk of heart failure.
Summary
In this review, we identify some general and diabetes-specific risk factors that are associated with an increased risk of developing heart failure in people with T2D.
Keywords: heart failure, risk prediction, type 2 diabetes
INTRODUCTION
Atherosclerotic events were previously believed to be the primary driver of morbidity and mortality in patients with type 2 diabetes (T2D); however, recent evidence suggests that heart failure is now a predominant cardiovascular outcome that is at least as common as ischemic events [1▪]. The burden of an increased risk of hospitalization for heart failure appears to be present across the spectrum of clinical states in patients with T2D [2,3]. Furthermore, death from heart failure forms a significant burden of cause-specific cardiovascular death among patients with T2D and established atherosclerotic cardiovascular disease (ASCVD) [4]. Despite the burden of heart failure morbidity and mortality among patients with T2D, until recently, heart failure as an outcome of interest has been ignored [5]. Heart failure has traditionally been overlooked as an outcome of interest among patients in antihyperglycemic therapy trials [5,6]. The 2008 US Food and Drug Administration (FDA) guidance, which mandated that sponsors must demonstrate cardiovascular safety of antihyperglycemic therapies, did not provide any comment on assessment of heart failure safety [7,8]. The emergence of heart failure as an outcome of interest arose when the dipeptidyl peptidase-4 (DPP-4) inhibitors saxagliptin and alogliptin [9,10] demonstrated an increased risk of hospitalizations for heart failure. The FDA has issued a warning across the entire DPP-4 inhibitor class on the risk of heart failure on the basis of data from the SAVOR-TIMI 53 (saxagliptin) and EXAMINE (alogliptin) trials. Furthermore, recent trials involving the sodium-glucose cotransporter-2 (SGLT-2) inhibitors empagliflozin [11], canagliflozin [12], and dapagliflozin [13▪] have demonstrated a reduction in the risk of heart failure outcomes among patients with T2D who have established cardiovascular disease (CVD) or are at high cardiovascular risk.
While our understanding of the relationship between diabetes and heart failure expands, there is emerging interest in using antihyperglycemic therapies to prevent heart failure. A meta-analysis of the EMPA-REG OUTCOME (empagliflozin), CANVAS (canagliflozin), and DECLARE–TIMI 58 (dapagliflozin) trials demonstrated that SGLT-2 inhibitors reduced the risk of hospitalization for heart failure in patients with ASCVD [hazard ratio, 0.71 (95% confidence interval (CI), 0.62–0.82)] and without ASCVD but with multiple cardiovascular risk factors [hazard ratio, 0.64 (95% CI, 0.48–0.85)] [14▪▪]. Empagliflozin is associated with a reduction in the risk of postacute rehospitalization for heart failure and cardiovascular events [15]. The benefit of SGLT-2 inhibitors with regard to reductions in the risk of cardiovascular death or hospitalization for heart failure is seen across the spectrum of renal function, with a similar magnitude of risk reduction among those with an estimated glomerular filtration rate (eGFR) of 30 to less than 60, 60–90, or more than 90 ml/min/1.73 m2[16,17]. Data from the CREDENCE trial, which enrolled patients with T2D with albuminuric kidney disease [eGFR 30–90 ml/min/1.73 m2 and albumin-to-creatinine (ACR) ratio >300–5000 mg/g], demonstrated that canagliflozin, versus placebo, reduced the risk of hospitalization for heart failure [hazard ratio, 0.61 (95% CI, 0.47–0.80); P < 0.001] [18]. There are multiple potential mechanistic explanations for the benefit of SGLT-2 inhibitors including improvement in ventricular loading conditions due to reduction in preload (arising from natriuresis and osmotic diuresis), afterload reduction (improvement in blood pressure), improvement in cardiac metabolism and bioenergetics, reduction of necrosis and cardiac fibrosis, and alteration in adipokines, cytokine production, and epicardial adipose tissue mass [19].
While trials have highlighted the potential use of SGLT-2 inhibitors in preventing hospitalization for heart failure, there is growing need for clinicians to have a pragmatic approach in identifying patients at high risk of de novo heart failure among patients without baseline heart failure or established ASCVD. Identification of patients with T2D at risk of heart failure represents a strategy to potentially target therapies such as SGLT-2 inhibitors to those who may maximally benefit. To address this emerging clinical need, this review focuses on the framework for hospitalization for de novo heart failure. This framework focuses on describing readily available diabetes-specific, cardiovascular-specific, and noncardiovascular-specific risk factors that can identify patients at high risk for future heart failure, thereby warranting consideration of additional investigations and therapies such as SGLT-2 inhibitors.
Box 1.
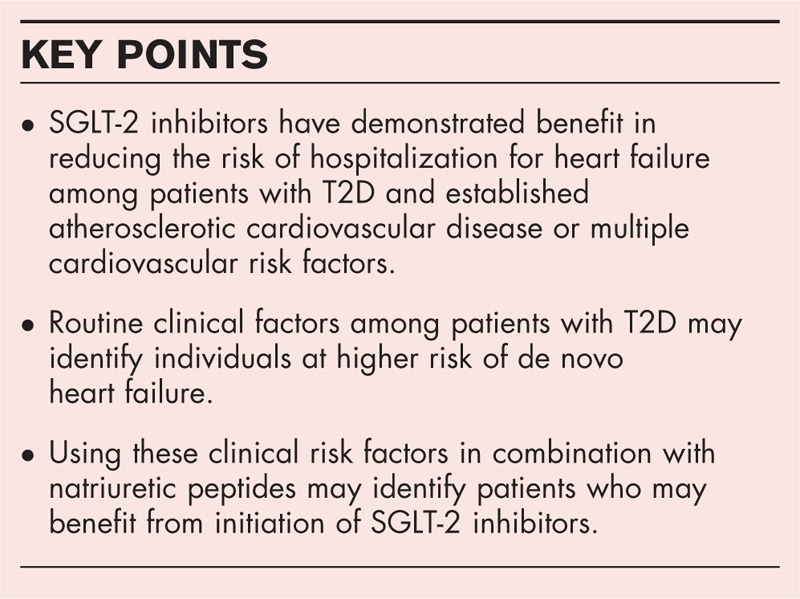
no caption available
DIABETES-SPECIFIC RISK FACTORS
Diabetes confers a two-fold increased risk of heart failure development, which substantially increases among patients with comorbid coronary artery disease [20]. Advances in our understanding of the pathophysiology of T2D have resulted in an emerging model of why patients with T2D are at increased risk of heart failure outcomes [21]. Diabetes-specific risk factors such as chronic hyperglycemia, insulin resistance, mitochondrial dysfunction, abnormal calcium handling, autonomic dysfunction, abnormal extracellular matrix remodeling, and enhanced renin–angiotensin–aldosterone system dysfunction are mechanisms inherent to patients with diabetes that potentially increase the risk of heart failure development [21–23].
There are several diabetes-specific variables readily identified in the outpatient setting that can aid healthcare providers in stratifying patients with T2D by risk of future heart failure. The presence of albuminuria, another easily obtainable clinical measure, is significantly associated with an increased risk of heart failure. In the Heart Outcomes Prevention Evaluation Study, after adjustment, a urine ACR more than 17.7 mg/g was associated with a significant increase in the relative risk of hospitalization for heart failure [3.23 (95% CI, 2.54–4.10)] [24]. Furthermore, each 3.5-mg/g increase in ACR increased the risk of hospitalization for heart failure by 10.6% (95% CI, 8.4–13.0%) [24].
Depending on local practice patterns, the use of various antihyperglycemic therapies is associated with an increased risk of heart failure. Insulin use is associated with an increased risk of future heart failure events and may be used as a marker to identify a patient who requires further heart failure risk stratification [23,25]. Among 8063 patients with T2D without heart failure in the Kaiser Permanente Northwest diabetes registry, insulin added to an initial antihyperglycemic agent, when compared with the addition of a sulfonylurea, was associated with a 2.33-fold increased risk of de novo heart failure [26]; the risk of heart failure associated with insulin initiation was 2.66-fold greater than that with metformin. The thiazolidinediones rosiglitazone and pioglitazone have both been associated with an increased risk of heart failure [27]. The Rosiglitazone Evaluated for Cardiovascular Outcomes in Oral Agent Combination Therapy for Type 2 Diabetes trial demonstrated that rosiglitazone was associated with an increased risk of hospitalization for heart failure [hazard ratio, 2.6 (95% CI, 1.1–4.1); P = 0.001]. Similarly, the Prospective Pioglitazone Clinical trial in Macrovascular Events trial demonstrated an increase in the risk of hospitalization for heart failure with pioglitazone [hazard ratio, 1.41 (95% CI, 1.10–1.80); P = 0.007]. Sulfonylurea use may also be a marker of an increased risk of future hospitalizations for heart failure; in a retrospective observational study of 1243 general practices in England, sulfonylurea use, compared with metformin use, was associated with a 43% increased risk of heart failure [adjusted hazard ratio, 1.43 (95% CI, 1.30–1.57)] [28]. The DPP-4 inhibitor saxagliptin, versus placebo, demonstrated an increased risk of hospitalization for heart failure among patients with T2D at high risk of cardiovascular outcomes [hazard ratio, 1.27 (95% CI, 1.07–1.52); P = 0.007] [29]. The DPP-4 inhibitor alogliptin, versus placebo, demonstrated a nonsignificant increase in the risk of hospitalization for heart failure [hazard ratio, 1.19 (95% CI, 0.90–1.58)] [30,31]. Neither linagliptin nor sitagliptin demonstrated an increased risk of hospitalization for heart failure [32,33]. However, the FDA has now indicated a labeling change on all DPP-4 inhibitors to indicate a potential increase in the risk of heart failure events [34–36].
Other diabetes-specific clinical variables may provide prognostic information regarding risk of future heart failure events. Duration of diabetes is associated with an increased risk of a composite of cardiovascular outcomes, including heart failure. In a cohort of patients from the Framingham Heart Study, for each 10-year increase in the duration of diabetes, patients had a 25% numerical increase in the risk of cardiovascular events, including hospitalization for heart failure [multivariable adjusted hazard ratio, 1.25 (95% CI, 0.99–1.57)] [37]. Glycemic control also provides prognostic information regarding risk of heart failure. The UK Prospective Diabetes Study demonstrated a 16% reduction in the risk of heart failure associated with a 1% reduction in glycated hemoglobin, thereby highlighting that patients with nonoptimal glycemic control have an increased risk of heart failure events [38]. In the EMPA-REG OUTCOME trial, the presence of any microvascular disease (including neuropathy, retinopathy, and nephropathy) was associated with an increased risk of hospitalization for heart failure [hazard ratio, 1.63 (95% CI, 1.06–2.49); P = 0.025] [39].
CARDIOVASCULAR RISK FACTORS
In addition to diabetes-specific risk factors, traditional cardiovascular risk factors also increase the risk of heart failure among patients with T2D [21,40]. The role of hypertension in the development of heart failure with preserved ejection fraction and heart failure with reduced ejection fraction has been well established among patients with and without diabetes [41]. In addition, obesity also appears to be associated with an increasing risk of de novo heart failure. Among individuals without heart failure in the Framingham Heart Study, in adjusted models, there was an 8% increased risk of heart failure development per 1% increase in BMI among women [hazard ratio, 1.08 (95% CI, 1.06–1.11)] and a 7% increase among men [hazard ratio, 1.07 (95% CI, 1.04–1.11)] [42]. A history of myocardial infarction was independently associated with all-cause death and cardiovascular death in patients with established ASCVD [4,40]. Current consensus guidelines already recommend the use of SGLT-2 inhibitors or glucagon-like peptide-1 receptor agonists among these patients to reduce the risk of future heart failure outcomes [43]. While the evidence among patients with T2D is not as well established, the presence of atrial fibrillation is also associated with an increased risk of heart failure [44].
NONCARDIOVASCULAR RISK FACTORS
Noncardiovascular risk factors are often overlooked as a means to stratify patients for future heart failure risk. However, there are several readily available clinical variables that may identify patients at risk for heart failure. Age is a well established variable for heart failure development [45]. While not specific to patients with T2D obstructive sleep apnea has also been demonstrated to increase the risk of de novo heart failure. In the prospective Sleep Heart Health Study, obstructive sleep apnea predicted de novo heart failure in men [per 10-U increase in apnea–hypopnea index; hazard ratio, 1.13 (95% CI, 1.02–1.26)]; furthermore, men with apnea–hypopnea index at least 30 were 58% more likely to develop heart failure than those with apnea–hypopnea index less than 5 [46]. While treatment of the underlying sleep apnea should occur per practice guidelines, the presence of obstructive sleep apnea among patients with T2D may identify a patient at higher risk of developing heart failure.
RISK STRATIFICATION FOR INCIDENT HEART FAILURE AND PRIMARY PREVENTION OF HEART FAILURE
Trials evaluating the cardiovascular safety of SGLT-2 inhibitors have identified among patients with established CVD or cardiovascular risk factors that SGLT-2 inhibitors are associated with a reduced risk of heart failure events [11,12,13▪]. Additional trials evaluating the efficacy of these therapies in patients with established heart failure are underway [ClinicalTrials.gov identifiers: NCT03057977 (EMPEROR-Reduced), NCT03057951 (EMPEROR-Preserved), NCT03036124 (DAPA-HF), and NCT03619213 (DELIVER)]. Heart failure prevention among patients with T2D represents an important public health strategy. While underlying medical conditions should be managed per practice guidelines, these conditions can be used to identify patients who may be at higher risk of heart failure (Fig. 1). If patients are receiving DPP-4 inhibitors (saxagliptin and alogliptin) or thiazolidinediones, healthcare providers may consider discontinuing these therapies if there are concerns of increased risk of heart failure. Once these risk factors are identified among patients with T2D without baseline heart failure or ASCVD, we recommend measurement of natriuretic peptides [ideally N-terminal probrain natriuretic peptide (NTproBNP)] to further stratify for future risk of incident heart failure. Patients with T2D without CVD and with increased NTproBNP (>125 pg/ml) are at higher risk of incident heart failure and will benefit from intensified multidisciplinary team management and treatment with renin–angiotensin system antagonists and beta-blockers [47]. Using an even lower natriuretic peptide cutoff to identify patients at increased risk of incident heart failure may stratify by risk patients who may benefit from therapies to prevent heart failure such as SGLT-2 inhibitors. In the Natriuretic Peptides Studies Collaboration among 95 617 patients without baseline CVD, an NTproBNP more than 50 pg/ml was associated with an increased risk of incident heart failure [48]. We would recommend considering initiating an SGLT-2 inhibitor in patients with an NTproBNP at least 50 pg/ml to prevent de novo heart failure. Using NTproBNP to identify patients who may benefit from initiation of SGLT-2 inhibitors, while not included in current clinical practice guidelines, represents one potential approach for the primary prevention of heart failure. If the NTproBNP is at least 125 pg/ml, we recommend further screening with an echocardiogram to determine any underlying structural heart disease as recommended by consensus guidelines (Fig. 1) [40,49–51]. If patients have an NTproBNP less than 50 pg/ml, clinicians can continue to monitor but may consider SGLT-2 inhibitors if concerns for de novo heart failure emerge. Patients with a low NTproBNP may indeed receive benefit from SGLT-2 inhibitors regarding reduced risk of de novo heart failure, but more data will be needed.
FIGURE 1.
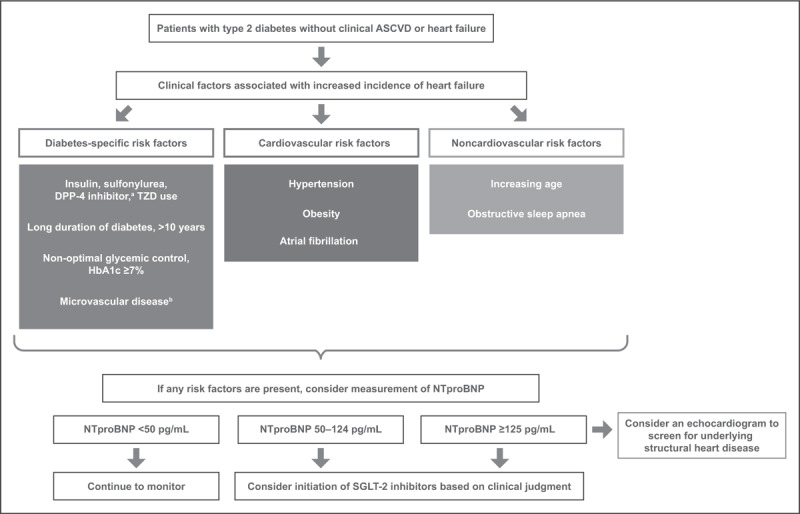
Potential framework for identifying patients without baseline heart failure or established atherosclerotic cardiovascular disease who may benefit from sodium-glucose cotransporter-2 inhibitors. aSaxagliptin and alogliptin. bIncluding neuropathy, retinopathy, or nephropathy. ASCVD, atherosclerotic cardiovascular disease; HbA1c, glycated hemoglobin; NTproBNP, N-terminal probrain natriuretic peptide; SGLT-2, sodium-glucose cotransporter-2; TZD, thiazolidinedione.
CONCLUSION
In this review, we described some of the routinely available clinical parameters that can aid in identifying patients who may have an increased risk of future heart failure (Fig. 1). While we have not derived these variables as a part of a validated model, the consistency of these variables suggesting a higher risk of heart failure across the literature encourages their use to stratify patients by risk in routine practice. Referring such patients to clinicians with expertise in managing the cardiovascular complications of diabetes may also aid in optimizing therapies to reduce future cardiovascular risk. Given the burden of heart failure among patients with T2D, strategies to easily identify those at risk for heart failure remain a critical first step to reduce the high burden of heart failure morbidity and mortality.
Acknowledgements
Editorial support was provided by Susan M. Kaup, PhD, of inScience Communications (Philadelphia, Pennsylvania, USA) in accordance with Good Publication Practice (GPP-3) and funded by AstraZeneca.
Financial support and sponsorship
The development of this article was supported by AstraZeneca.
Conflicts of interest
S.V. holds a Tier 1 Canada Research Chair in Cardiovascular Surgery and reports receiving research grants and/or speaking honoraria from Amgen, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novartis, Novo Nordisk, Sanofi, Servier, and Valeant. He is a member of the scientific excellence committee of the EMPEROR-Preserved and EMPEROR-Reduced trials, a member of the scientific committee of the DETERMINE-A and DETERMINE-B trials, a member of the global expert panel of the SELECT study, and a national lead investigator of the DAPA-HF, DELIVER, DETERMINE-A, DETERMINE-B, EMPEROR-Preserved, EMPEROR-Reduced, SELECT, and SOLOIST studies. A.S. has received research support from Akcea, PharmaSolutions, Alberta Innovates Health Solutions, Bayer-Canadian Cardiovascular Society, Boehringer-Ingelheim, Roche Diagnostics, and Takeda. N.K. serves as the Clinical Champion for Diabetes UK, the Clinical Network Lead for Diabetes-Greater Manchester, and the Diabetes Research Lead for Greater Manchester Clinical Research Network. He is a member of the Primary Care Diabetes Society Committee and a Community Diabetes Consultant for the Manchester University Foundation Trust. He has received educational speaking honoraria from AstraZeneca, Novo Nordisk, Sanofi, Napp, and Takeda, has received educational and travel grants from AstraZeneca and Novo Nordisk, and has served on advisory boards for Ascensia, AstraZeneca, Novo Nordisk, Roche, and Sanofi Pasteur. J.B. has received research support from the National Institutes of Health, Patient Centered Outcomes Research, and the European Union. He serves on the speaker bureau for Novartis, Janssen, and Novo Nordisk. He serves as a consultant and/or serves on steering committees, clinical events committees, or data safety monitoring boards for Abbott, Adrenomed, Amgen, Array, Astra Zeneca, Bayer, BerlinCures, Boehringer Ingelheim, Bristol-Myers Squib, Cardiocell, Corvidia, CVRx, G3 Pharmaceutical, Innolife, Janssen, Lantheus, LinaNova, Luitpold, Medscape, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Roche, Sanofi, StealthPeptide, SC Pharma, V-Wave Limited, Vifor, and ZS Pharma.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪.Verma S, Jüni P, Mazer CD. Pump, pipes, and filter: do SGLT2 inhibitors cover it all? Lancet 2019; 393:3–5. [DOI] [PubMed] [Google Scholar]; The article describes a novel paradigm for heart failure risk reduction among patients with type 2 diabetes (T2D).
- 2.Rawshani A, Rawshani A, Franzén S, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2018; 379:633–644. [DOI] [PubMed] [Google Scholar]
- 3.Sharma A, Cannon CP, White WB, et al. Early and chronic dipeptidyl-peptidase-IV inhibition and cardiovascular events in patients with type 2 diabetes mellitus after an acute coronary syndrome: a landmark analysis of the EXAMINE trial. J Am Heart Assoc 2018; 7:e007649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma A, Green JB, Dunning A, et al. Causes of death in a contemporary cohort of patients with type 2 diabetes and atherosclerotic cardiovascular disease: insights from the TECOS trial. Diabetes Care 2017; 40:1763–1770. [DOI] [PubMed] [Google Scholar]
- 5.McMurray JJV, Gerstein HC, Holman RR, Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol 2014; 2:843–851. [DOI] [PubMed] [Google Scholar]
- 6.Sharma A, Bhatt DL, Calvo G, et al. Heart failure event definitions in drug trials in patients with type 2 diabetes. Lancet Diabetes Endocrinol 2016; 4:294–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Medicines Agency Guideline on clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. 2012; Available at: https://www.fda.gov/media/71297/download. [Accessed 5 May 2019]. [Google Scholar]
- 8.US Food and Drug Administration Guidance for industry diabetes mellitus – evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. 2008; Available at: https://www.fda.gov/media/71297/download. [Accessed 5 May 2019]. [Google Scholar]
- 9.Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326. [DOI] [PubMed] [Google Scholar]
- 10.Packer M. Worsening heart failure during the use of DPP-4 inhibitors: pathophysiological mechanisms, clinical risks, and potential influence of concomitant antidiabetic medications. JACC Heart Fail 2018; 6:445–451. [DOI] [PubMed] [Google Scholar]
- 11.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 12.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377:644–657. [DOI] [PubMed] [Google Scholar]
- 13▪.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380:347–357. [DOI] [PubMed] [Google Scholar]; The DECLARE–TIMI 58 trial demonstrated that among a trial population of patients with a high proportion of patients without established atherosclerotic cardiovascular disease (CVD), dapagliflozin reduced the risk of cardiovascular death or hospitalization for heart failure.
- 14▪▪.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019; 393:31–39. [DOI] [PubMed] [Google Scholar]; The meta-analysis demonstrated the benefit of sodium-glucose cotransporter-2 inhibitors to reduce the risk of cardiovascular death and heart failure hospitalization among patients with T2D with established atherosclerotic CVD or multiple cardiovascular risk factors.
- 15.Savarese G, Sattar N, Januzzi J, et al. Empagliflozin is associated with a lower risk of postacute heart failure rehospitalization and mortality. Circulation 2019; 139:1458–1460. [DOI] [PubMed] [Google Scholar]
- 16.Neuen BL, Ohkuma T, Neal B, et al. Cardiovascular and renal outcomes with canagliflozin according to baseline kidney function. Circulation 2018; 138:1537–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wanner C, Lachin JM, Inzucchi SE, et al. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation 2018; 137:119–129. [DOI] [PubMed] [Google Scholar]
- 18.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380:2295–2306. [DOI] [PubMed] [Google Scholar]
- 19.Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia 2018; 61:2108–2117. [DOI] [PubMed] [Google Scholar]
- 20.Cavender MA, Steg PG, Smith SC, et al. Impact of diabetes mellitus on hospitalization for heart failure, cardiovascular events, and death. Circulation 2015; 132:923–931. [DOI] [PubMed] [Google Scholar]
- 21.Sharma A, Ezekowitz JA. Diabetes, impaired fasting glucose, and heart failure: it's not all about the sugar. Eur J Heart Fail 2014; 16:1153–1156. [DOI] [PubMed] [Google Scholar]
- 22.Dei Cas A, Khan SS, Butler J, et al. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail 2015; 3:136–145. [DOI] [PubMed] [Google Scholar]
- 23.Sharma A, Cooper LB, Fiuzat M, et al. Antihyperglycemic therapies to treat patients with heart failure and diabetes mellitus. JACC Heart Fail 2018; 6:813–822. [DOI] [PubMed] [Google Scholar]
- 24.Gerstein HC, Mann JFE, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286:421–426. [DOI] [PubMed] [Google Scholar]
- 25.Packer M. Potentiation of insulin signaling contributes to heart failure in type 2 diabetes. JACC Basic Transl Sci 2018; 3:415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nichols GA, Koro CE, Gullion CM, et al. The incidence of congestive heart failure associated with antidiabetic therapies. Diabetes Metab Res Rev 2005; 21:51–57. [DOI] [PubMed] [Google Scholar]
- 27.Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet 2007; 370:1129–1136. [DOI] [PubMed] [Google Scholar]
- 28.Hippisley-Cox J, Coupland C. Diabetes treatments and risk of heart failure, cardiovascular disease, and all cause mortality: cohort study in primary care. BMJ 2016; 354:i3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scirica BM, Braunwald E, Raz I, et al. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation 2014; 130:1579–1588. [DOI] [PubMed] [Google Scholar]
- 30.White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369:1327–1335. [DOI] [PubMed] [Google Scholar]
- 31.Zannad F, Cannon CP, Cushman WC, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 2015; 385:2067–2076. [DOI] [PubMed] [Google Scholar]
- 32.McGuire DK, Alexander JH, Johansen OE, et al. Linagliptin effects on heart failure and related outcomes in individuals with type 2 diabetes mellitus at high cardiovascular and renal risk in CARMELINA. Circulation 2019; 139:351–361. [DOI] [PubMed] [Google Scholar]
- 33.McGuire DK, Van de Werf F, Armstrong PW, et al. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol 2016; 1:126–135. [DOI] [PubMed] [Google Scholar]
- 34.Tradjenta [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2017. [Google Scholar]
- 35.Januvia [package insert]. Whitehouse, NJ: Merck & Co., Inc.; 2018. [Google Scholar]
- 36.US Food and Drug Administration. FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. 2016; Available at: https://www.fda.gov/media/96895/download [Accessed 5 May 2019]. [Google Scholar]
- 37.Fox CS, Sullivan L, D’Agostino RB, Wilson PWF. The significant effect of diabetes duration on coronary heart disease mortality: the Framingham Heart Study. Diabetes Care 2004; 27:704–708. [DOI] [PubMed] [Google Scholar]
- 38.Stratton IM, Adler AI, Neil AW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000; 321:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verma S, Wanner C, Zwiener I, et al. Influence of microvascular disease on cardiovascular events in type 2 diabetes. J Am Coll Cardiol 2019; 73:2780–2782. [DOI] [PubMed] [Google Scholar]
- 40.Seferovic PM, Petrie MC, Filippatos GS, et al. Type 2 diabetes mellitus and heart failure: a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018; 20:853–872. [DOI] [PubMed] [Google Scholar]
- 41.Messerli FH, Rimoldi SF, Bangalore S. The transition from hypertension to heart failure. JACC Heart Fail 2017; 5:543–551. [DOI] [PubMed] [Google Scholar]
- 42.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med 2002; 347:305–313. [DOI] [PubMed] [Google Scholar]
- 43.Das SR, Everett BM, Birtcher KK, et al. 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease. J Am Coll Cardiol 2018; 72:3200–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chatterjee NA, Chae CU, Kim E, et al. Modifiable risk factors for incident heart failure in atrial fibrillation. JACC Heart Fail 2017; 5:552–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhingra R, Vasan RS. Age as a risk factor. Med Clin North Am 2012; 96:87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gottlieb DJ, Yenokyan G, Newman AB, et al. A prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the Sleep Heart Health Study. Circulation 2010; 122:352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP Selected PreventiOn of cardiac eveNts in a populaTion of dIabetic patients without A history of Cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol 2013; 62:1365–1372. [DOI] [PubMed] [Google Scholar]
- 48.Willeit P, Kaptoge S, Welsh P, et al. Natriuretic Peptides Studies Collaboration Natriuretic peptides and integrated risk assessment for cardiovascular disease: an individual-participant-data meta-analysis. Lancet Diabetes Endocrinol 2016; 4:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ezekowitz JA, O’Meara E, McDonald MA, et al. 2017 Comprehensive update of the Canadian cardiovascular society guidelines for the management of heart failure. Can J Cardiol 2017; 33:1342–1433. [DOI] [PubMed] [Google Scholar]
- 50.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 51.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 37:2129–2200. [DOI] [PubMed] [Google Scholar]


