There is moderately high-quality evidence to support the improved clinical utility of human papillomavirus (HPV) genotyping compared with qualitative HPV positivity to follow-up after treatment of high-grade cervical intraepithelial neoplasia.
OBJECTIVE:
To systematically examine human papillomavirus (HPV) genotyping compared with qualitative high-risk HPV result during follow-up after treatment of high-grade cervical intraepithelial neoplasia (CIN), for risk estimation of posttreatment high-grade CIN.
DATA SOURCES:
MEDLINE, Cochrane, and ClinicalTrials.gov were searched from January 2000 to April 2019 for prospective studies of women and retrospective studies of residual specimens from women, tested using HPV assays with genotype reporting.
METHODS OF STUDY SELECTION:
The primary outcome was posttreatment high-grade CIN after treatment of high-grade CIN. Risk of bias (individual study quality) was evaluated with a modified Newcastle-Ottawa Scale. Overall quality of evidence for the risk estimate outcomes was evaluated using modified GRADE methodology for observational diagnostic studies.
TABULATION, INTEGRATION, AND RESULTS:
Of the 233 identified abstracts, 33 full-text articles were retrieved, and seven studies were included in the synthesis. The risk of bias was deemed to be low. Either a positive qualitative HPV test result or a positive test result for the same genotype that was present pretreatment have a sensitivity for predicting posttreatment high-grade CIN that approaches 100%. However, the positive predictive value (PPV) for the same genotype result pretreatment and posttreatment (median 44.4%) is about double the PPV (median 22.2%) for qualitative HPV results. The PPV of a new HPV infection posttreatment approximates zero. Human papillomavirus genotyping discriminated risk of posttreatment high-grade CIN to a clinically significant degree for women after treatment procedures for high-grade CIN lesions, when same-genotype persistence was compared with new genotype infection.
CONCLUSION:
There is moderately high-quality evidence to support the improved clinical utility of HPV genotyping compared with qualitative HPV positivity to follow-up after treatment of high-grade CIN.
SYSTEMATIC REVIEW REGISTRATION:
PROSPERO: CRD42018091095.
FUNDING SOURCE:
Becton, Dickinson and Company, BD Life Sciences—Diagnostic Systems.
After treatment of high-grade cervical intraepithelial neoplasia (CIN 2–3), by excision, ablation, or laser, 5–16% of women are diagnosed with posttreatment high-grade disease or cancer.1,2 A study of 3,273 women, aged 25 years or older, treated for CIN 2, CIN 3, or adenocarcinoma in situ reported follow-up results with CIN 2 or worse as the primary outcome.2 Risk values varied by the antecedent screening results and the histopathology of the treated lesion. The 5-year risk for CIN 2 or worse was lower if the follow-up test results was negative: 4.2% after negative cytology, 3.7% after a negative qualitative high-risk human papillomavirus (HPV) test result, and 2.4% after negative cotesting results (cytology and HPV tests). Only after two negative cotest results was the 5-year risk for CIN 2 or worse (1.5%) approaching the 0.68% risk after a negative Pap test result that permits return to screening after 3 years.2
Cytology, HPV assay, or both (cotesting) have been used to determine whether a cancer precursor cleared or persisted after a treatment procedure such as large loop excision of the transformation zone, loop electrosurgical excision procedure (LEEP), laser, cone, or cryotherapy.3 Guidelines for follow-up after treatment of CIN 2–3 recommend cotesting at 4–6, 12, and 24 months after treatment of CIN 2–3 by excisional procedure. Guidelines for follow-up after treatment of adenocarcinoma in situ by excisional procedure recommend cotesting, colposcopy, and endocervical curettage after 4–6 months. The objectives of posttreatment follow-up testing are to confirm that the treatment was effective, to prevent invasive cancer, and to reassure women. It is a persistent high-risk HPV-induced lesion that places women at the highest risk for invasive cancers. Acquisition of a new infection of a different genotype confers a relatively low future risk, given the probability of clearance and the lengthy interval after HPV infection before the development of an invasive cancer.
A qualitative (pooled) HPV assay result is more sensitive than cytology for detection of CIN 2–3 after treatment, with equivalent specificity.3–8 A negative high-risk HPV assay result has a superior negative predictive value (NPV) compared with negative cytology or negative resection margins.7,9 Posttreatment negative cotesting would avert an additional 8.4 cases of CIN 3 or worse per 1,000 women-treated and reduce health care costs, compared with negative cytology alone.10
Partial genotype reporting of HPV16 and HPV18 and the group of other 12 high-risk genotypes demonstrated effective risk discrimination compared with pooled qualitative reporting (unadjusted odds ratio 8.0; 95% CI 2.1–30.4) for posttreatment recurrence.11 In addition, three retrospective cohort studies reported significantly different posttreatment CIN 2–3 or worse rates associated with an HPV 16 or 18 positive result at follow-up compared with a positive result for the other 12 HPV genotypes.12–14
To individualize the posttreatment surveillance, which may reduce patient anxiety, cost, and occurrence of cervical cancer, better identification of women with relevant HPV genotype presence may be a benefit. Reporting a genotype that was present before the treatment permits the distinction between same-genotype persistence and a new infection. The purpose of this systematic review is to compare genotyping, (beyond partial), with pooled HPV testing for determination of risk for women after treatment for high-grade CIN.
The secondary research question parameters of population, intervention, comparator, and outcomes (PICO) was: (P) women who underwent excisional or ablative treatment for CIN 2 or worse, (I) genotyping as a component of cotesting, (C) pooled HPV result as a component of cotesting, (O) CIN 2 or worse persistence or recurrence or “treatment failure” after excisional or ablative treatment of CIN 2 or worse. Secondary outcomes include the sensitivity, specificity, positive predictive value (PPV), and NPV for CIN 2 or worse.
ROLE OF THE FUNDING SOURCE
Authors Fabio Bottari, Anna D. Iacobone, Rita Passerini, Maria T. Sandri, Eleonora P. Preti, and Clementina E. Cocuzza did not receive any support from Becton, Dickinson and Company and were responsible for data interpretation, risk of bias assessment, overall quality of evidence assessment, and preparation of the manuscript. Devin S. Gary and Jeffrey C. Andrews were full-time employees of Becton, Dickinson and Company and contributed to drafting and revision, the interpretation of the data, and critically assessed the manuscript for important intellectual content. Jeffrey C. Andrews facilitated conception and design of the study, contributed to data acquisition, and facilitated data analyses for the manuscript. All authors have attended meetings with manufacturers of HPV assays discussed in this work. Clementina E. Cocuzza was not compensated for work on this project, does not hold stock, and received no bonuses from any of the manufacturers. Maria T. Sandri has in the past served as paid advisor to Roche and received honoraria from Roche and BD. The authors had access to relevant aggregated study data and original articles required to understand and report research findings. The authors take responsibility for the presentation and publication of the research findings, have been fully involved at all stages of publication and presentation development, and are willing to take public responsibility for all aspects of the work. All individuals included as authors and contributors who made substantial intellectual contributions to the research, data analysis, and publication or presentation development are listed appropriately. The role of the any funding source in the design, execution, analysis, reporting is fully disclosed. The authors' personal interests, financial or nonfinancial, relating to this research and its publication have been disclosed.
SOURCES
MEDLINE, Cochrane Database of Systematic Reviews, Health Technology Assessment, and ClinicalTrials.gov electronic databases were searched from January 2000 and April 2019. The study protocol was developed and the review performed in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews),15 and the Institutes of Medicine Standards for Systematic Reviews.16 No similar published systematic review was found and no similar study protocol was found. This study protocol was registered with PROSPERO in 2018 (PROSPERO: CRD42018091095).17 The search string was: (HPV OR “human papillomavirus”) AND (genotyp*) AND (cervical or cervix) AND (cancer OR *cancer OR carcinoma OR lesion* OR CIN OR “Cervical intraepithelial neoplasia” OR screening OR persistence OR “test of cure” OR test-of-cure)) in Title, Abstract, Keywords; Online Publication Date between January 2000 and April 2019.
STUDY SELECTION
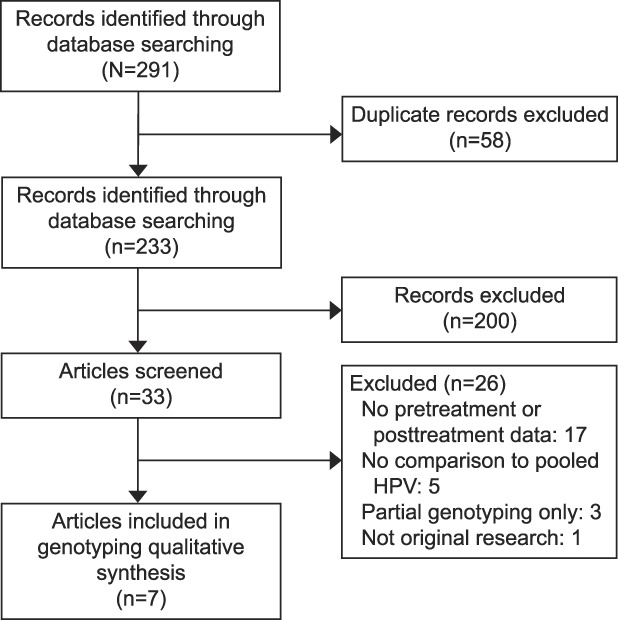
All retrieved titles and abstracts were assessed for possible relevance by applying inclusion criteria (prospective controlled trials and observational studies of women and retrospective studies of residual specimens from women; patients were women who underwent excisional or ablative treatment for CIN 2 or worse, genotyping testing was done before and after the treatment, testing after treatment was at an interval of 6 months or greater, CIN 2 or worse persistence or recurrence or “treatment failure” after excisional or ablative treatment of CIN 2 or worse was reported) and exclusion criteria (full-text not available in English, only partial genotype reporting). Full-text review of the articles that passed abstract review was performed and data was extracted. Figure 1 reports the PRISMA flow diagram.
Fig. 1. The PRISMA (Preferred Reporting of Items for Systematic Reviews) flow diagram detailing the search and selection process. HPV, human papillomavirus.
Bottari. Human Papillomavirus Genotyping After High-Grade CIN Treatment. Obstet Gynecol 2019.
Data extraction tables were developed in Excel, piloted, and used for study characteristics and for risk estimates with 95% CIs. Additional analysis was performed using Minitab to generate receiver operator characteristic curves using binary logistic regression and to calculate area under the curve (AUC).
Risk of bias (individual study quality) was evaluated with a modified Newcastle-Ottawa Scale18 that included six domains: selection (eligibility criteria, forming the cohort, selection of participants), detection (measurement of test result), outcome (assessment, length of follow-up), attrition (loss to follow-up), reporting (failure to adequately control confounding, failure to measure all known prognostic factors), and other bias. Summary assessment of risk of bias for individual studies, combining all authors' evaluations, was assessed as high, low, or unclear. Each author assessed the overall quality of evidence for the risk estimate outcomes (all included studies) using a modified GRADE (Grading of Recommendations, Assessment, Development and Evaluation)19 methodology for observational diagnostic studies and included the summary assessment of risk of bias for the individual studies, indirectness, imprecision, inconsistency, publication bias, magnitude of effect, and whether all plausible confounders or other biases diminished confidence in the estimated effect. Summary levels of certainty, combining all authors' evaluations, were assessed as high, moderate, or low.
RESULTS
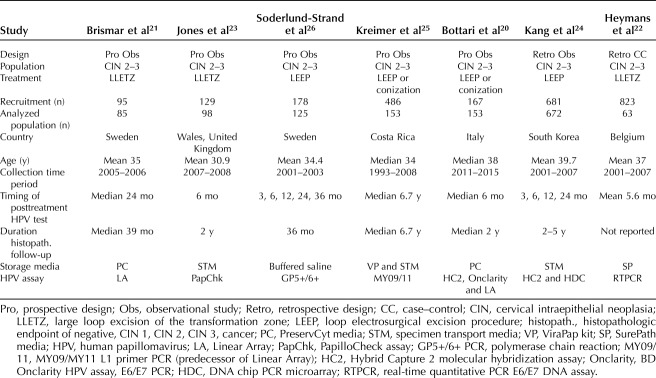
The search identified 233 unique abstracts: 200 did not meet inclusion criteria or met exclusion criteria, and 33 underwent full-text review. From this review, 26 studies were excluded for reasons listed in the PRISMA flow diagram in Figure 1. Seven studies that reported genotyping results, compared with pooled HPV results, and the risk of CIN 2 or worse after treatment, were included in the data extraction and synthesis.20–26 The seven studies had a combined population sample size of 1,543 patients (Table 1). Five of the studies were prospective observational,20,21,23,25,26 specifically diagnostic accuracy cross-sectional studies20,21,23,25–27; one of the five was a post hoc analysis of patients enrolled in a clinical study.25 Two of the studies were retrospective observational,22,24 specifically diagnostic accuracy cross-sectional studies27; one was a case–control study.22
Table 1.
Characteristics of the Included Studies
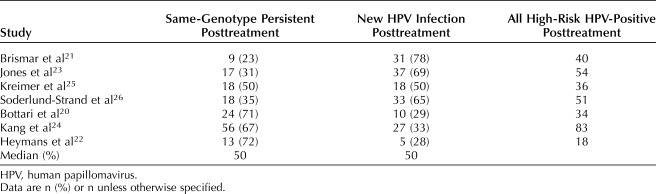
Of patients testing positive for HPV after treatment of CIN 2 or worse, the proportion of new infections ranged from 28–78%, with a mean and median of 50%; those with same-genotype persistence ranged from 23–72%, with a mean and median of 50% (Table 2).20–26
Table 2.
Proportions of Same-Genotype Persistence Compared With New Human Papillomavirus Infection (With Clearance of Pretreatment Genotypes)
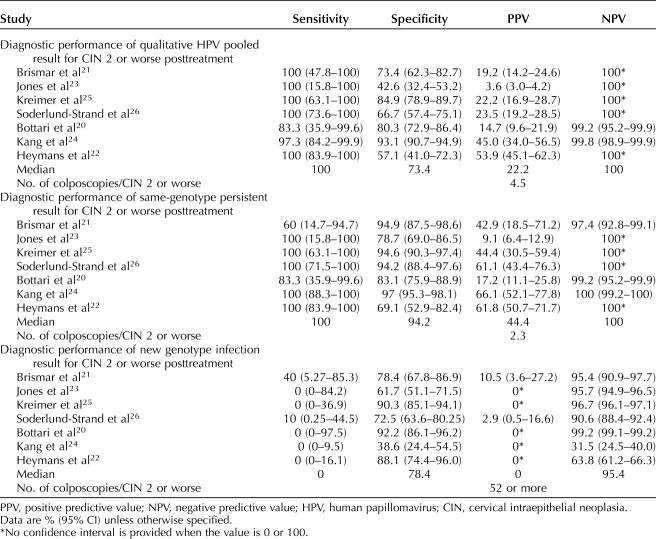
A prospective observational study analyzed the results for 90 women in Sweden who underwent large loop excision of the transformation zone treatment of CIN 2 or worse. Follow-up testing included cytology, qualitative HPV result, and genotype results (Table 1).14 The majority of HPV-positive results at follow-up after conization were new infections (77.5%), compared with same-genotype persistence (22.5% of HPV-positive, 10% of all patients). Whereas margin status and presence of CIN 3 or worse were poor predictors of outcome (sensitivity less than 50%), the presence of pooled high-risk HPV predicted 100% of CIN 2 or worse lesions detected posttreatment (sensitivity 100% [95% CI 47.8–100], specificity 73.4% [95% CI 62.3–82.7], PPV 19.2% [95% CI 14.2–25.6], NPV 100%). Persistent, same-genotype high-risk HPV infection predicted high-grade residual disease with a sensitivity of 60% (95% CI 14.7–94.7), specificity 94.9% (95% CI 87.5–98.6), PPV 42.9% (95% CI 18.5–71.2), NPV 97.4% (95% CI 92.8–99.1). In contrast, new genotype infection predicted high-grade residual disease with a sensitivity of 40% (95% CI 5.3–85.3), specificity 78.4% (95% CI 67.8–86.9), PPV 10.5% (95% CI 3.6–27.2), NPV 95.4% (95% CI 90.9–97.7) (Table 3 and Fig. 2). The PPV of same-genotype persistence was statistically significantly greater than the PPV of new genotype infection.21
Table 3.
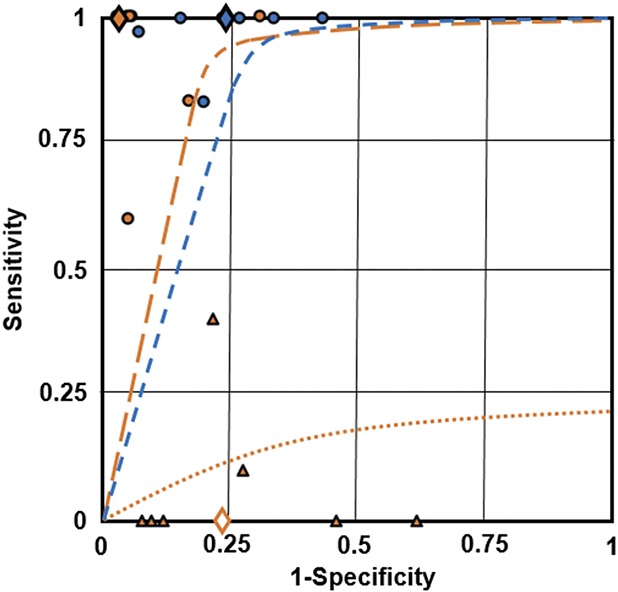
Fig. 2. Receiver operating characteristics curves (sensitivity against 1-specificity) for three posttreatment human papillomavirus (HPV) results. Median values from the three screening strategies for sensitivity and specificity values obtained from the seven included studies: blue circle point values and hashed line represent qualitative HPV-positive, orange circle point values and hashed line represent same-genotype persistence, and orange triangle point values and orange dotted lines represent new HPV genotype infection posttreatment. The overall screening group (from the seven studies) median values for sensitive and 1-specificity are plotted as single points and represent qualitative HPV-positive (blue diamond), same-genotype persistence (orange diamond) and new infection (white diamond). Receiver operating characteristics analysis revealed area under the curve values close to 1 for qualitative HPV and same-genotype persistence; the new infection area under the curve value was 0.24.
Bottari. Human Papillomavirus Genotyping After High-Grade CIN Treatment. Obstet Gynecol 2019.
A study of 98 women in Wales, treated for CIN 2 or worse by large loop excision of the transformation zone were evaluated before treatment and 6 months after treatment with cytology, qualitative HPV testing, and genotyping (Table 1).23 Genotyping before and after treatment revealed that 83% (95% CI 75.7–88.8) of HPV infections detected before treatment were cleared; 17% (95% CI 11.2–24.3) of patients had posttreatment positive qualitative HPV test results. Of the patients with a positive HPV result posttreatment, and 69% had a new HPV genotype infection, and 31% had same-genotype persistence. Posttreatment, qualitative HPV test results predicted CIN 2 or worse with sensitivity of 100% (95% CI 34.2–100), specificity 39.6 (95% CI 30.4–49.6), PPV 3.3% (95% CI 0.9–11.4), and NPV of 100% (95% CI 90.8–100). There were two cases out of 98 with posttreatment CIN 2 or worse and therefore sparse data for comparing pooled HPV results to genotyping. Same-genotype persistence results predicted CIN 2 or worse with sensitivity of 100% (95% CI 34.2–100), specificity 95% (95% CI 88.4–97.8), PPV 28.6% (95% CI 8.3–64.1), and NPV 100% (95% CI 94.9–100) (Table 3 and Fig. 2). The specificity for same-genotype persistence was statistically significantly higher than the specificity for qualitative pooled HPV positivity. New HPV genotype infection results predicted CIN 2 or worse with sensitivity of 0% (95% CI 0–84.2), specificity 54.2% (95% CI 42.9–65.2), PPV 0%, and NPV 95.7% (95% CI 94.9–96.5).23
A post hoc analysis of 347 women enrolled in the Guanacaste, Costa Rica Natural History Study cohort, who were treated for high-grade CIN reported pretreatment and posttreatment cytology, HPV, and genotyping results (Table 1).25 Of the 36 women with positive pooled HPV results, 50% had persistent, same-genotype infection, and 50% had a new infection. Posttreatment, qualitative HPV test results predicted CIN 2 or worse with sensitivity of 100% (95% CI 63.1–100), specificity 85.0 (95% CI 78.9–89.7), PPV 22.2% (95% CI 16.9–28.7), and NPV of 100%. Same-genotype persistence results predicted CIN 2 or worse with sensitivity of 100% (95% CI 63.1–100), specificity 94.6% (95% CI 90.3–97.4), PPV 44.4% (95% CI 30.5–59.4), and NPV 100% (Table 3 and Fig. 2). The specificity and PPV for same-genotype persistence were statistically significantly higher than the specificity and PPV for qualitative pooled HPV positivity. No posttreatment disease was observed among women with new HPV infections during the follow-up period. New HPV genotype infection results predicted CIN 2 or worse with sensitivity of 0% (95% CI 0–36.9), specificity 90.3% (95% CI 85.1–94.1), PPV 0%, and NPV 95.4% (95% CI 95.2–95.6).25
A study of 178 women in Sweden who were treated for CIN 2 or worse with conization and had cytology, qualitative HPV, and genotyping tests before and treatment were followed at 3, 6, 12, 24, and 36 months posttreatment (Table 1).26 Of the 51 patients with a qualitative pooled HPV-positive result at follow-up, 25 were same-genotype persistent (49%), and 26 were new genotype infections (51%). Posttreatment, qualitative HPV test results predicted CIN 2 or worse with sensitivity of 100% (95% CI 73.6–100), specificity 66.7 (95% CI 57.4–75.1), PPV 23.5% (95% CI 19.2–28.5), and NPV of 100%. Same-genotype persistence results predicted CIN 2 or worse with sensitivity of 100% (95% CI 71.5–100), specificity 94.2% (95% CI 88.4–97.6), PPV 61.1% (95% CI 43.4–76.3), and NPV 100% (Table 3 and Fig. 2). The specificity and PPV for same-genotype persistence were statistically significantly higher than the specificity and PPV for qualitative pooled HPV positivity. New HPV genotype infection results predicted CIN 2 or worse with sensitivity of 10% (95% CI 0.3–44.5), specificity 72.5% (95% CI 63.6–80.3), PPV 2.9% (95% CI 0.5–16.6), and NPV 95.4% (90.6% CI 88.4–92.4).26
A study of 167 women in Milan who were treated for high-grade CIN and were assessed by cytology and qualitative pooled HPV testing and genotyping pretreatment and at 6 months after LEEP or laser conization reported results in 2019 (Table 1).20 Of the 34 patients with a qualitative pooled HPV-positive result at follow-up, 24 were same-genotype persistent (71%), and 10 were new genotype infections (29%). Posttreatment, qualitative HPV test results predicted CIN 2 or worse with sensitivity of 83.3% (95% CI 35.9–99.6), specificity 80.3 (95% CI 72.9–86.4), PPV 14.7% (95% CI 9.6–22), and NPV of 99.2% (95% CI 95.2–99.9). Same-genotype persistence results predicted CIN 2 or worse with sensitivity of 83.3% (95% CI 35.6–99.6), specificity 83.1% (95% CI 75.9–88.9), PPV 17.2% (95% CI 11.1–25.8), and NPV 99.2% (95% CI 95.2–99.9) (Table 3 and Fig. 2). New HPV genotype infection results predicted CIN 2 or worse with sensitivity of 0% (95% CI 0–97.5), specificity 92.2% (95% CI 86.1–96.2), PPV 0%, and NPV 99.2% (90.6% CI 99.1–99.2).20
A retrospective study of 672 women in South Korea who had been treated with LEEP for CIN 2 or worse, had pretreatment and posttreatment cytology, qualitative pooled HPV and genotype results (Table 1).24 A total of 37 women (5.5%) had posttreatment CIN 2 or worse, and 100% of them tested positive for the same HPV genotype before and after treatment. Posttreatment, qualitative HPV test results predicted CIN 2 or worse with sensitivity of 97.3% (95% CI 98.9–99.9), specificity 93.1 (95% CI 90.7–94.9), PPV 45% (95% CI 34–56.5), and NPV of 99.8% (95% CI 95.2–99.9). Same-genotype persistence results predicted CIN 2 or worse with sensitivity \of 100% (95% CI 88.3–100), specificity 97% (95% CI 95.3–98.1), PPV 66.1% (95% CI 52.1–77.8), and NPV 1,002% (95% CI 99.2–100) (Table 3 and Fig. 2). The specificity for same-genotype persistence was statistically significantly higher than the specificity for qualitative pooled HPV positivity. New HPV genotype infection results predicted CIN 2 or worse with sensitivity of 0% (95% CI 0–9.5), specificity 38.6% (95% CI 24.4–54.5), PPV 0%, and NPV 31.5% (90.6% CI 24.5–40).24
A case–control registry-based study in Belgium enrolled 21 women with biopsy-proven recurrence of CIN 2 or worse (cases), compared with 42 women without recurrence (controls), with a follow-up period of at least 24 months after treatment of CIN 2 or worse (Table 1).22 HPV testing at 6 months posttreatment was significantly more sensitive compared with follow-up cytology (risk ratio: 1.31; 95% CI 1.10–1.54), but less specific (risk ratio: 0.85; 95% CI 0.81–0.90) to predict treatment failure or recurrence. All women who developed a recurrence tested positive for HPV. Of the 18 patients with a qualitative pooled HPV-positive result at follow-up, 13 were same-genotype persistent (72%), and five were new genotype infections (28%). Posttreatment, qualitative HPV test results predicted CIN 2 or worse with sensitivity of 100% (95% CI 83.9–100), specificity 57.1 (95% CI 41–72.3), PPV 53.9% (95% CI 45.1–62.3), and NPV of 100%. Same-genotype persistence results predicted CIN 2 or worse with sensitivity of 100% (95% CI 83.9–100), specificity 69.1% (95% CI 52.9–82.4), PPV 61.8% (95% CI 50.7–71.7), and NPV 100% (Table 3 and Fig. 2). New HPV genotype infection results predicted CIN 2 or worse with sensitivity of 0% (95% CI 0–16.1), specificity 88.1% (95% CI 74.4–96.0), PPV 0%, and NPV 63.8% (90.6% CI 61.2–66.3). The PPV and NPV of same genotype detection were statistically significantly different from the PPV and NPV of new genotype infection.22
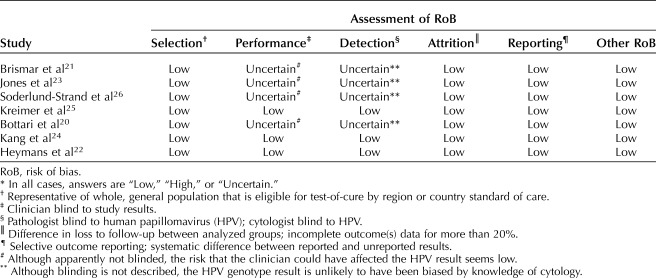
Risk of bias for each of the seven included studies was assessed by the modified Newcastle-Ottawa scale, with six domains, by four authors individually (F.B., A.D.I., D.S.G., J.C.A.). Majority decision determined the answers; in the event of a tie, a fifth author (M.T.S.) would have voted to resolve, but this was not required. The results are reported in Table 4.
Table 4.
Modified Newcastle-Ottawa Scale (Risk of Bias Tool for Quality Assessment of Observational Studies)*
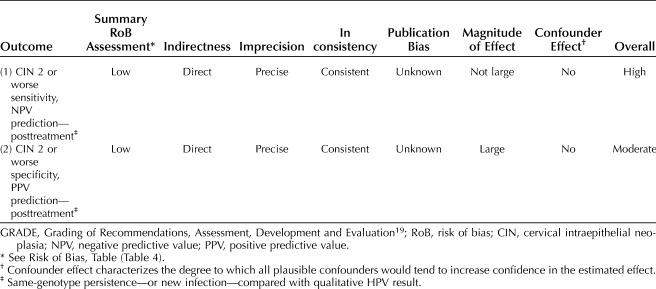
The overall quality of evidence for the risk estimate outcomes (all included studies) using a modified GRADE methodology for observational diagnostic studies was assessed, and the summary assessment of risk of bias for the individual studies, indirectness, imprecision, inconsistency, magnitude of effect, and whether all plausible confounders or other biases increased confidence in the estimated effect was considered by four authors individually (F.B., A.D.I., D.S.G., J.C.A.). Majority decision determined the answers; in the event of a tie, a fifth author (M.T.S.) would have voted to resolve, but this was not required. The results are reported in Table 5. For the outcome CIN 2 or worse prediction posttreatment by genotyping (same-genotype persistence or new infection) compared with qualitative HPV, sensitivity and NPV, the overall quality of evidence was adjudged moderate. For the outcome CIN 2 or worse prediction posttreatment by genotyping (same-genotype persistence or new infection) compared with qualitative HPV, specificity and PPV, the overall quality of evidence was adjudged moderate. Further research is likely to have an effect on our confidence in the estimate of risk. Confidence in the risk estimates is constrained by the size individual studies. However, the risk discrimination afforded by genotyping is significantly greater than by qualitative HPV pooled results, and this observed effect is unlikely to change, although the magnitude could be altered by more information from future studies.
Table 5.
Overall Quality of Evidence for Outcomes (Modified GRADE)
The sensitivity of qualitative HPV-positive results and same genotype positive results, posttreatment with known HPV genotype status pretreatment approaches 100% for both tests; however, differences were identified between the two diagnostic approaches for specificity and PPV values (Table 2). The receiver operator characteristic curve in Figure 2 represents the optimal balance (sensitivity vs specificity) for diagnostic capacity (visualized at the curve inflection to identify the discrete balance threshold) and presents diagnostic comparisons between pooled HPV positivity, genotype-specific persistence, and new genotype infection. Relative to the comparator (pooled HPV persistence) genotype-specific persistence results in an inflection point providing better sensitivity and specificity values compared with new genotype infection. When compared directly to pooled HPV persistence, genotype-specific persistence shows a similar AUC. However, the inflection point of the curve for genotype-specific persistence is located to the left of that for pooled HPV persistence (0.85 vs 0.7, respectively), indicating greater PPV with genotype-specific persistence compared with pooled HPV persistence. The PPV for same genotype positive results posttreatment (median 44.4%) is approximately double the PPV (22.2%) for qualitative HPV-positive results (Table 2). The PPV of a new HPV infection posttreatment approximates zero. Pooled HPV persistence, on the other hand, results in 6–8% higher sensitivity at the inflection point compared with genotype-specific persistence. However, as mentioned above and as shown (plotted medians) in Figure 2, the overall median sensitivity was determined to be 100% for same-genotype persistence and pooled HPV persistence. Clearance of HPV posttreatment, with negative HPV assay results provides a NPV approximating 100% (Table 3 and Fig. 2).
DISCUSSION
The seven studies included in this review demonstrated that qualitative HPV pooled results have good clinical utility when following women after treatment for CIN 2 or worse. However, these studies reported that new HPV infection (with clearance of the pretreatment genotypes) accounted for a median of 60% of positive HPV results posttreatment. The risk of CIN 2 or worse after treatment was very low in the case of new HPV infection; zero for five studies, 2.9% for one study, and 10.5% for one study. The risk of CIN 2 or worse after treatment was statistically and clinically significantly in the case of same-genotype persistence; a median PPV of 44.4% (range 17.2–66.1%). A PPV of 44.4% is in the risk range for recommending repeat colposcopy and biopsy, and consideration of repeat treatment.2 The median PPV for new HPV infection (with clearance of the genotypes detected pretreatment) is in risk range for recommending retesting (surveillance without immediate colposcopy).2
If all HPV positivity after treatment of CIN 2 or worse was the same genotype that was identified pretreatment, there would be no need for genotyping. Because these studies showed that a mean of 50% of HPV detected posttreatment were new HPV infections (range 28–78%) (Table 2), there is a role for genotype testing pretreatment and posttreatment in women with CIN 2 or worse.
Within the United States, in 2019, the FDA approved HPV assays are limited to reporting of genotypes 16, 18, and 45. Outside the United States, there are several HPV genotyping assays. The VALGENT framework group classified these as qualitative (pooled result for 13–14 oncogenic genotypes); partial or limited genotyping assay (individual reporting of HPV16 and 18 and the remaining high-risk HPV in a group of 12 others); extended genotyping requires the assay to report at least six individual genotypes and the remaining in one or more groups; and full genotyping requires the assay to report all high-risk genotypes individually.28
HPV genotype reporting pretreatment and posttreatment for women with CIN 2 or worse provides better specificity and significantly improved PPV compared with qualitative HPV pooled assay results, without diminishing sensitivity or NPV (Fig. 2). Although the areas under the curve (Fig. 2) for qualitative HPV persistence and genotype-specific resistance are similar in this synthesis, certain points should be made before a proper interpretation can be made regarding their comparative ability to discriminate between true and false positive or negative results. First, these results were derived from a small sample size and included analysis with median performance values—therefore a stringent statistical analysis is not possible. Second, the genotype make-up of the qualitative HPV persistence approach was not known; it could have contained a disproportionately high prevalence of high-risk genotypes (eg, HPV 16, 31, or 33) that could lead to CIN 2 or worse lesions relatively early (12–18 months) and therefore do not apply to the persistence aspect of HPV screening that is considered in this systematic review. As discussed above, up to 70% of the qualitative HPV persistence cases in question could have involved same-genotype persistence, which would greatly reduce the hypothesized difference in performance relative to same-genotype persistence.
Limitations of this review include that there were only seven studies, with a total of 1,543 patients, of which 316 had positive HPV results posttreatment for CIN 2 or worse. The results of the seven studies were synthesized, to the degree possible, while retaining and presenting the individual results. The population factors were mildly heterogeneous; the years of study varied; the techniques for treatment likely varied between the six countries. The genotyping test under investigation was different in each of the studies, and only one of the tests had validated clinical cutoffs. The comparator qualitative HPV test differed across the studies. The timing of the follow-up HPV test, and the duration of histopathologic follow-up varied significantly across the studies. Most recent guidelines from Gruppo Italiano Screening Citologico (GISCi) and ASCCP indicate 6 and 12 months, respectively, as the most suitable period for carrying out the first follow-up visit after treatment of CIN 2 or worse.29,30 Taken in total, the elements and degrees of heterogeneity resulted in our judgment that formal meta-analysis would not be appropriate. If performed, meta-analysis would require a Bayesian methodology and several assumptions and inference judgments would be needed.
Additional limitations to be considered include that HPV genotyping is an established research test, but a relatively recent clinical assay; availability and access to clinical genotype testing may be limited. Although it is possible that, in future, higher reimbursement could be assigned to HPV assays with genotyping capabilities, in 2019 the reimbursement for genotyping tests is the same as that for qualitative pooled results. The genotyping results are commonly reported in the same run as the HPV qualitative result. Therefore, the cost and resources are the same for qualitative compared with genotyping, but genotyping provides more information to the clinician and the woman. Finally, although not a focus of this systematic review, there are rare cases of premalignant and invasive cervical lesions are related to non–high-risk HPV genotypes, which would not be detected by either qualitative HPV assays with clinical indications, nor genotyping assays with clinical indications.31
New genotype infection has very low ability to discriminate between true and false screening results; a clear difference in AUC values between new genotype infection and genotype-specific persistence is demonstrated in Figure 2. In addition, those women with same-genotype persistence posttreatment are at significant risk of CIN 2 or worse and would undergo the same management as for qualitative HPV-positive results. Those women with clearance of the pretreatment genotype and detection of a new HPV genotype infection posttreatment are at a significantly lower risk of CIN 2 or worse, and by the principle of similar management for similar risk, could be managed by repeat testing (either HPV assay or HPV and cytology), thus avoiding an immediate repeat colposcopy and reducing the risk of over-treatment.
Footnotes
Financial Disclosure Devin S. Gary and Jeffrey C. Andrews were full-time employees of Becton, Dickinson and Company and contributed to drafting and revision, the interpretation of the data, and critically assessed the manuscript for important intellectual content. All authors have attended meetings with manufacturers of HPV assays discussed in this work. Clementina E. Cocuzza was not compensated for work on this project, does not hold stock, and received no bonuses from any of the manufacturers. Maria T. Sandri has in the past served as paid advisor to Roche and received honoraria from Roche and BD. The other authors did not report any potential conflicts of interest.
Presented in part at the International Papillomavirus Conference, February 28–March 4, 2017, Cape Town, South Africa; EUROGIN International Multidisciplinary HPV Congress, October 8–11, 2017, Amsterdam, Netherlands; International Workshop on Lower Genital Tract Pathology, April 12–13, 2018, Rome, Italy; ASCCP annual meeting, April 18–21, 2018, Las Vegas, Nevada; EUROGIN International Multidisciplinary HPV Congress, December 2–5, 2018, Lisbon, Portugal; and SCCPS annual conference, March 2–3, 2019, Singapore.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/B496.
REFERENCES
- 1.Del Mistro A, Matteucci M, Insacco EA, Onnis G, Da Re F, Baboci L, et al. Long-Term clinical outcome after treatment for high-grade cervical lesions: a retrospective monoinstitutional cohort study. Biomed Res Int 2015;2015:984528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katki HA, Schiffman M, Castle PE, Fetterman B, Poitras NE, Lorey T, et al. Five-year risk of recurrence after treatment of CIN 2, CIN 3, or AIS: performance of HPV and Pap cotesting in posttreatment management. J Low Genit Tract Dis 2013;17(5 suppl 1):S78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuschieri K, Bhatia R, Cruickshank M, Hillemanns P, Arbyn M. HPV testing in the context of post-treatment follow up (test of cure). J Clin Virol 2016;76(suppl 1):S56–61. [DOI] [PubMed] [Google Scholar]
- 4.Arbyn M, Paraskevaidis E, Martin-Hirsch P, Prendiville W, Dillner J. Clinical utility of HPV-DNA detection: triage of minor cervical lesions, follow-up of women treated for high-grade CIN: an update of pooled evidence. Gynecol Oncol 2005;99(3 suppl 1):S7–11. [DOI] [PubMed] [Google Scholar]
- 5.Arbyn M, Ronco G, Anttila A, Meijer CJ, Poljak M, Ogilvie G, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine 2012;30(suppl 5):F88–99. [DOI] [PubMed] [Google Scholar]
- 6.Kocken M, Uijterwaal MH, de Vries AL, Berkhof J, Ket JC, Helmerhorst TJ, et al. High-risk human papillomavirus testing versus cytology in predicting post-treatment disease in women treated for high-grade cervical disease: a systematic review and meta-analysis. Gynecol Oncol 2012;125:500–7. [DOI] [PubMed] [Google Scholar]
- 7.Mariani L, Sandri MT, Preti M, Origoni M, Costa S, Cristoforoni P, et al. HPV-testing in follow-up of patients treated for CIN2+ lesions. J Cancer 2016;7:107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alonso I, Torne A, Puig-Tintore LM, Esteve R, Quinto L, Campo E, et al. Pre- and post-conization high-risk HPV testing predicts residual/recurrent disease in patients treated for CIN 2-3. Gynecol Oncol 2006;103:631–6. [DOI] [PubMed] [Google Scholar]
- 9.Zielinski GD, Bais AG, Helmerhorst TJ, Verheijen RH, de Schipper FA, Snijders PJ, et al. HPV testing and monitoring of women after treatment of CIN 3: review of the literature and meta-analysis. Obstet Gynecol Surv 2004;59:543–53. [DOI] [PubMed] [Google Scholar]
- 10.Legood R, Smith M, Lew JB, Walker R, Moss S, Kitchener H, et al. Cost effectiveness of human papillomavirus test of cure after treatment for cervical intraepithelial neoplasia in England: economic analysis from NHS Sentinel Sites Study. BMJ 2012;345:e7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruickshank ME, Sharp L, Chambers G, Smart L, Murray G. Persistent infection with human papillomavirus following the successful treatment of high grade cervical intraepithelial neoplasia. BJOG 2002;109:579–81. [DOI] [PubMed] [Google Scholar]
- 12.Andrade CE, Scapulatempo-Neto C, Longatto-Filho A, Vieira MA, Tsunoda AT, Da Silva ID, et al. Prognostic scores after surgical treatment for cervical intraepithelial neoplasia: a proposed model and possible implications for post-operative follow-up. Acta Obstet Gynecol Scand 2014;93:941–8. [DOI] [PubMed] [Google Scholar]
- 13.Gok M, Coupe VM, Berkhof J, Verheijen RH, Helmerhorst TJ, Hogewoning CJ, et al. HPV16 and increased risk of recurrence after treatment for CIN. Gynecol Oncol 2007;104:273–5. [DOI] [PubMed] [Google Scholar]
- 14.Venturoli S, Ambretti S, Cricca M, Leo E, Costa S, Musiani M, et al. Correlation of high-risk human papillomavirus genotypes persistence and risk of residual or recurrent cervical disease after surgical treatment. J Med Virol 2008;80:1434–40. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Institute of Medicine (IOM). Finding what works in Health care: standards for systematic reviews. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 17.Booth A, Clarke M, Ghersi D, Moher D, Petticrew M, Stewart L. An international registry of systematic-review protocols. Lancet 2011;377:108–9. [DOI] [PubMed] [Google Scholar]
- 18.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Retrieved March 27, 2019. [Google Scholar]
- 19.Schunemann H, Brozek J, Guyatt G, Oxman A. GRADE handbook for grading quality of evidence and strength of recommendations. 2013. Available at: https://gdt.gradepro.org/app/handbook/handbook.html. Retrieved March 27, 2019. [Google Scholar]
- 20.Bottari F, Iacobone AD, Boveri S, Preti EP, Franchi D, Mariani L, et al. Onclarity HPV Extended genotyping in the management of CIN2+ lesions. J Low Genit Tract Dis 2019;23:39–42. [DOI] [PubMed] [Google Scholar]
- 21.Brismar S, Johansson B, Borjesson M, Arbyn M, Andersson S. Follow-up after treatment of cervical intraepithelial neoplasia by human papillomavirus genotyping. Am J Obstet Gynecol 2009;201:17.e1–8. [DOI] [PubMed] [Google Scholar]
- 22.Heymans J, Benoy IH, Poppe W, Depuydt CE. Type-specific HPV geno-typing improves detection of recurrent high-grade cervical neoplasia after conisation. Int J Cancer 2011;129:903–9. [DOI] [PubMed] [Google Scholar]
- 23.Jones J, Saleem A, Rai N, Shylasree TS, Ashman S, Gregory K, et al. Human Papillomavirus genotype testing combined with cytology as a 'test of cure' post treatment: the importance of a persistent viral infection. J Clin Virol 2011;52:88–92. [DOI] [PubMed] [Google Scholar]
- 24.Kang WD, Oh MJ, Kim SM, Nam JH, Park CS, Choi HS. Significance of human papillomavirus genotyping with high-grade cervical intraepithelial neoplasia treated by a loop electrosurgical excision procedure. Am J Obstet Gynecol 2010;203:72.e1–6. [DOI] [PubMed] [Google Scholar]
- 25.Kreimer AR, Schiffman M, Herrero R, Hildesheim A, Gonzalez P, Burk RD, et al. Long-term risk of recurrent cervical human papillomavirus infection and precancer and cancer following excisional treatment. Int J Cancer 2012;131:211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soderlund-Strand A, Kjellberg L, Dillner J. Human papillomavirus type-specific persistence and recurrence after treatment for cervical dysplasia. J Med Virol 2014;86:634–41. [DOI] [PubMed] [Google Scholar]
- 27.Andrews J, Likis FE. Study design algorithm. J Low Genit Tract Dis 2015;19:364–8. [DOI] [PubMed] [Google Scholar]
- 28.Arbyn M, Depuydt C, Benoy I, Bogers J, Cuschieri K, Schmitt M, et al. VALGENT: a protocol for clinical validation of human papillomavirus assays. J Clin Virol 2016;76(suppl 1):S14–21. [DOI] [PubMed] [Google Scholar]
- 29.Carozzi FM, Iossa A, Scalisi A, Sideri M, Andersson KL, Confortini M, et al. hr-HPV testing in the management of women with ASC-US+ and in the follow-up of women with cytological abnormalities and negative colposcopy. Recommendations of the Italian group for cervical cancer screening (GISCi). Epidemiol Prev 2015;39(3 suppl 1):84–90. [PubMed] [Google Scholar]
- 30.Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis 2013;17(5 suppl 1):S1–27. [DOI] [PubMed] [Google Scholar]
- 31.Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst 1995;87:796–802. [DOI] [PubMed] [Google Scholar]