Supplemental Digital Content is Available in the Text.
Quantitative magnetic resonance spectroscopy of anterior insula reveals decreased glutamatergic neurotransmitter concentrations in patients with irritable bowel syndrome and hemisphere-specific associations with pain severity and pain-related coping.
Keywords: Irritable bowel syndrome, Functional magnetic resonance imaging, Quantitative magnetic resonance spectroscopy, Insula, Visceral pain, Coping
Abstract
Irritable bowel syndrome (IBS) is a visceral pain condition with psychological comorbidity. Brain imaging studies in IBS demonstrate altered function in anterior insula (aINS), a key hub for integration of interoceptive, affective, and cognitive processes. However, alterations in aINS excitatory and inhibitory neurotransmission as putative biochemical underpinnings of these functional changes remain elusive. Using quantitative magnetic resonance spectroscopy, we compared women with IBS and healthy women (healthy controls [HC]) with respect to aINS glutamate + glutamine (Glx) and γ-aminobutyric acid (GABA+) concentrations and addressed possible associations with symptoms. Thirty-nine women with IBS and 21 HC underwent quantitative magnetic resonance spectroscopy of bilateral aINS to assess Glx and GABA+ concentrations. Questionnaire data from all participants and prospective symptom-diary data from patients were obtained for regression analyses of neurotransmitter concentrations with IBS-related and psychological parameters. Concentrations of Glx were lower in IBS compared with HC (left aINS P < 0.05, right aINS P < 0.001), whereas no group differences were detected for GABA+ concentrations. Lower right-lateralized Glx concentrations in patients were substantially predicted by longer pain duration, while less frequent use of adaptive pain‐coping predicted lower Glx in left aINS. Our findings provide first evidence for reduced excitatory but unaltered inhibitory neurotransmitter levels in aINS in IBS. The results also indicate a functional lateralization of aINS with a stronger involvement of the right hemisphere in perception of abdominal pain and of the left aINS in cognitive pain regulation. Our findings suggest that glutaminergic deficiency may play a role in pain processing in IBS.
1. Introduction
Irritable bowel syndrome (IBS) is a chronic visceral pain disorder15,16 of an incompletely understood pathophysiology, yet growing evidence supports the notion that altered brain-gut interactions may underlie symptoms and their persistence.44 Comorbid psychopathologies and maladaptive pain-coping strategies are common15,59 and likely contribute to increased pain symptoms and emotional distress.59
In IBS, functional brain imaging studies consistently document alterations involving brain regions associated with emotion processing and modulation, particularly the anterior insula (aINS), a key node of the salience network.6,12,45 According to present models of insular function, interoceptive signals, including visceral pain, are received in the posterior insula and then processed in the aINS as a hub of interoceptive awareness, pain perception, and its cognitive and emotional modulation.6,12,49 Increasing evidence further supports a triple network model, in which the aINS plays a crucial role in switching between the task-positive executive control network and the task-negative default mode network.46,61 These findings suggest that the aINS does not only represent a key part of the limbic system but also is a hub of cognitive control and a facilitator of visceral pain.
Despite these well-recognized functional aINS alterations in the pathophysiology of visceral pain, in IBS the underlying changes in brain chemistry are poorly understood. Quantitative magnetic resonance spectroscopy (qMRS) allows for the detection and quantification of major excitatory and inhibitory neurochemicals in the human brain, namely glutamate (Glu) and γ‐aminobutyric acid (GABA), respectively. Glutamate is routinely reported with its precursor glutamine (Gln) as a combined measure (Glx)55 and GABA is typically reported with coedited macromolecular signals as GABA+.52 There is growing evidence of aberrant insular brain neurochemistry in several chronic pain conditions. Research findings have shown increased Glx concentration in the aINS in chronic pelvic pain2 and both reduced GABA and elevated Glu concentrations in fibromyalgia.20,27 In a study of patients with IBS, one qMRS study reported reduced hippocampal glutamatergic neurotransmission.51 Interestingly in this study, Glu concentrations in the left, but not right hippocampus of patients with IBS were negatively correlated with anxiety, pain catastrophizing, and pain duration, suggesting a lateralization on a biochemical level. Functional lateralization has also been observed for the aINS with the right insula being associated with sympathetic arousal, avoidance behavior, and energy expenditure, while the left insula seems to be related to parasympathetic activity, calm behavior, and energy nourishment.11,12 However, little is known about a possible lateralization of aINS involvement in pain processing and pain modulation and its possible biochemical basis in patients with IBS.
The aim of this study was to investigate aINS glutamatergic and GABAergic neurotransmitter concentrations in patients with IBS compared with age-matched healthy controls (HC). We further explored the predictive value of abdominal pain as a cardinal IBS symptom, as well as psychological factors relevant to IBS, such as anxiety, depression, and maladaptive pain-coping strategies, for concentrations of aINS neurotransmitters. Thus, this study focused on the question of whether or not neurotransmitter alterations and associations with disease-related or psychological measures would be left or right lateralized or show shared characteristics across hemispheres.
2. Methods
2.1. Study participants
Thirty-nine women with IBS, mean age 32.1 years (range 18-57 years) who met the Rome III criteria were recruited from the Gastroenterology Department, University Hospital in Linköping, Sweden. Twenty-one age-matched healthy women, mean age 32.1 years (range 20-55 years), without a medical history of gastrointestinal symptoms or complaints were recruited by local advertisement. The exclusion criteria for both groups were organic gastrointestinal disease, metabolic, neurological, or severe psychiatric disorders, nicotine intake within 2 months before the study, ferromagnetic implants, claustrophobia, and large tattoos. All participants were right-handed. Previous diagnoses of anxiety and/or depression in patients were extracted from their medical history documentation.
The regional ethical review board in Linköping approved the study (Dnrs. 2013/506-32; 2014/264-32). All subjects gave their written informed consent in accordance with the Helsinki Declaration.
2.2. Magnetic resonance imaging data acquisition and analysis
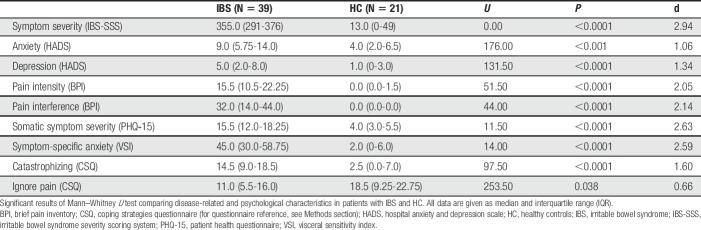
Participants were requested to fast for at least 4 hours (water was acceptable) and refrain from consuming alcohol and taking any IBS-related pain or sleep medications for at least 24 hours before the MR examination. Magnetic resonance data were collected on a 3 T Philips Ingenia (Philips Healthcare, Best, the Netherlands) equipped with a 32-channel head coil at the Center for Medical Image Science and Visualization (CMIV) at Linköping University, Sweden. Initially, T1-weighted 3D FFE images were acquired in all participants to exclude brain abnormalities and to ensure accurate voxel placement for subsequent spectroscopy measurements using the following parameters: inversion preparation and delay 900 ms, SAG-plane, FOV 256 × 240 × 170 mm3, resolution 1 × 1 × 1 mm3, flip angle 9°, TR 7 ms, TE 3.2 ms, TA 5:34. Subsequently, qMRS measures of the left and right aINS were accomplished using a MEGA-PRESS sequence47,48 (kindly provided by R.Edden, Johns Hopkins University) with the following parameters: TR/TE = 2000/68 ms, edited pulses ON at 1.90 ppm, edited pulses OFF at 7.46 ppm, water suppression MOIST, 40 dynamics in a voxel of 4.5 × 2.0 × 3.0 cm3 placed in the right and left aINS, respectively (Figs. 1A and B). In addition, a 2-dynamic unsuppressed water reference measurement was collected to obtain a reference of water in the tissue within the voxel. The MRS voxel was defined based on the parcellation in the Harvard-Oxford cortical structural atlas14,22,24,43 (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases) and was positioned based on individual high-resolution structural scans by an experienced radiographer specialized in magnetic resonance imaging (MRI)/MRS acquisition following a standardized protocol. Data were phase-corrected34 and frequency-aligned based on the water residual in the suppressed data. A difference spectrum was computed by subtracting the average OFF spectrum from the average ON spectrum and used as input to LCModel53 (Version 6.3-1L) to compute GABA+ concentrations (Fig. 1C). OFF dynamics were analyzed to assess concentrations of Glx (Fig. 1D). Concentrations were water-scaled using the water reference, resulting in concentrations with absolute units of mM. Data are provided with no correction for the contribution of cerebrospinal fluid.
Figure 1.
Typical qMRS volume of interest (voxel size 4.5 × 2.0 × 3.0 cm3) placement in the (A) right and (B) left aINS. The yellow box illustrates the voxel targeting the GABA signal at 3.0 ppm, and the white box illustrates the voxel the water residual at 4.7 ppm originates from, which shows the chemical shift displacement. Representative spectra with LCModel fitting depict (C) an averaged difference spectrum for the extraction of GABA+ and (D) an averaged MEGA-PRESS OFF spectrum for Glx extraction (labeled as 2 and 7, extracted solely from OFF-spectra) from a healthy volunteer (black line: postprocessed spectra before fitting; red line: LCModel fit). Residuals are shown at the top of each panel. (Assignments: 1, creatine (-2CH2-); 2, Glx (-2CH-); 3, choline (-N(CH3)3); 4, creatine (-N(CH3)); 5, GABA+ (-4CH2-); 6, tNA (-3CH2-); 7, Glx (-4CH2-); 8, GABA+ (-2CH2-); 9, tNA (-2CH3); 10, GABA+ (-3CH2-); 11 to 13, macromolecules and lipids, -CH2-).25 aINS, anterior insula; Glx, glutamate+glutamine; GABA+, γ-Aminobutyric acid + coedited macromolecular signals; qMRS, quantitative magnetic resonance spectroscopy; tNA, total N-Acetyl compounds (NAA + NAAG).
2.3. Questionnaires and symptom diary
Questionnaire data from all participants were collected for sample characterization and for the assessment of IBS-related and psychological variables. In addition, data extracted from symptom diaries were obtained from the patients with IBS, as described below.
2.3.1. Irritable bowel syndrome severity scoring system (IBS-SSS)
Irritable bowel syndrome severity scoring system is a 5-item questionnaire evaluating overall IBS symptom severity by assessing the frequency and the intensity of abdominal pain and distension, the satisfaction with bowel habits, and interference with daily life.21 Each item generates a score between 0 and 100 with a maximal sum score of 500. Sum scores indicate mild (75-175), moderate (175-300), or severe (>300) disease.
2.3.2. Visceral sensitivity index (VSI)
Visceral sensitivity index was implemented to assess gastrointestinal symptom-specific anxiety.36 The 15-item self-report questionnaire evaluates cognitive, emotional, and behavioral responses to fear of gastrointestinal sensations, symptoms, and the context in which the symptoms are experienced. Items are scored on a reversed 6-point scale ranging from 0 to 5, with sum scores between 0 and 75. Higher scores indicate more severe symptom-specific anxiety.
2.3.3. Hospital anxiety and depression scale (HADS)
The hospital anxiety and depression scale was used to measure symptoms of depression and anxiety.71 The tool consists of 7 items for a depression (HADS-D) and an anxiety subscale (HADS-A), respectively, with scores on each subscale ranging from 0 to 21. Cutoff values are indicated as ≥8 for subclinical (suspicious) anxiety or depression and ≥11 as definite cases on both the HADS-D and HADS-A, respectively.5
2.3.4. Coping strategies questionnaire (CSQ)
The coping strategies questionnaire was used to evaluate cognitive and behavioral strategies to cope with pain,56 involving 6 subscales for cognitive strategies (ignoring pain, reinterpreting pain, diverting attention, calming self-statements, catastrophizing, and praying/hoping) and 2 subscales for behavioral strategies (increasing activity and increasing pain behavior). Sum scores between 0 and 36 for each subscale indicate how frequently a coping strategy is used.
2.3.5. Patient health questionnaire (PHQ-15)
The PHQ-15 was used to assess the somatic symptom severity across 15 different somatic symptoms.35 Sum scores ranging from 0 to 30 indicate severity of somatic symptoms.
2.3.6. Brief pain inventory (BPI)
The brief pain inventory is a validated pain assessment tool measuring both the intensity of pain (4 items, sensory dimension) and interference of pain with the patient's life (7 items, reactive dimension).8 Each item is scored on a 0 to 10 scale with sum scores ranging from 0 to 40 for pain severity and from 0 to 70 for interference and higher scores indicating higher levels of pain severity and interferences, respectively.
2.3.7. MacArthur scale of subjective social status (SES ladder)
All participants completed the MacArthur Scale of Subjective Social Status to assess the common sense of the individual's social status, which has been shown to be tightly linked to psychological functioning and health status.1,60 Using a symbolic stepladder image, the tool provides a score between 0 and 10 as a summative measure of the subjective social status.
2.3.8. Gastrointestinal symptom diary (GSD)
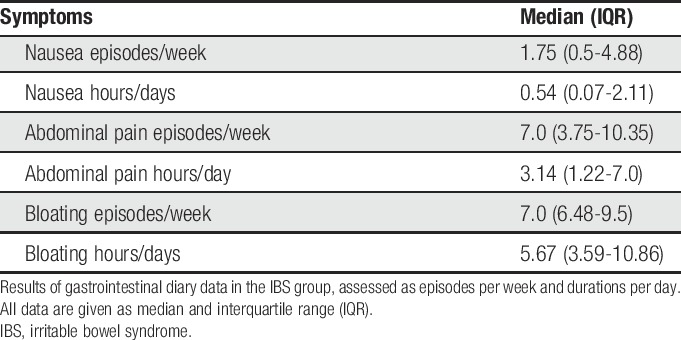
Patients with IBS recorded their gastrointestinal symptoms on 14 consecutive days using validated diary cards54 (Supplementary Fig. 1, available at http://links.lww.com/PAIN/A788). The symptoms (abdominal pain, nausea, and bloating) as well as every single bowel movement and stool consistency (defined by the Bristol Stool Chart39) were reported along a 24-hour time axis. The values were manually scored, and the mean frequency of symptom episodes per week and symptom duration per day was extracted from the diary data.
2.4. Statistical analyses
Statistical analyses were performed using SPSS version 25 (IBM Corporation, Armonk, NY) and GraphPad Prism 7 (https://www.graphpad.com/scientific-software/prism). As not all measures passed the Shapiro–Wilk normality test, the Mann–Whitney U test was used to address group comparisons in Glx and GABA+ concentrations and questionnaire data. χ2 test was used for group comparisons of medical journal data regarding previously diagnosed anxiety and/or depression and medication use. In a second step, multiple linear regression analyses using a stepwise approach (criteria: probability to enter ≤0.05, probability to remove ≥0.10) were conducted in patients with IBS to explore whether IBS-related measures (particularly pain reports) and psychological factors significantly predicted concentrations of aINS Glx and GABA+, respectively. Statistical significance was set at P < 0.05. Results are reported as median and interquartile range, unless indicated otherwise, and effect sizes are given as Cohen's d for results from U test and as Cramer's V for χ2 test.
3. Results
3.1. Clinical characteristics
Results of sociodemographic and clinical characteristics are given in Table 1. There was no significant difference in age between the groups. Women with IBS displayed significantly lower socioeconomic status in comparison with healthy women. Based on medical history documentation, 13 patients with IBS were previously diagnosed with anxiety and/or depression as a frequent comorbidity in IBS. Sixteen patients were on stable doses of IBS-related pain treatment with neuromodulators, in line with current recommendations.62 Results of the Mann–Whitney U test comparing IBS and HC groups with respect to IBS-related and psychological variables are given in Table 2. As expected, patients with IBS showed significantly higher IBS and somatic symptom severity, pain intensity, and interference, and reported increased severity of anxiety, depression, and gastrointestinal symptom-specific anxiety. Patients also reported a significantly higher tendency to use maladaptive pain-coping strategies, ie, they more frequently showed a catastrophizing coping style and less frequently ignored pain. No other differences in pain-coping strategies as assessed with the coping strategies questionnaire were detected.
Table 1.
Sociodemographic and clinical characteristics of patients with IBS and healthy controls.
Table 2.
Characterization of patients with IBS and healthy controls with respect to disease-related and psychological measures.
Thirty-three patients filled in the Gastrointestinal Symptom Diary cards. Data from 6 patients were not available due to poor compliance. Descriptive statistics of the extracted values are presented in Table 3. On average, patients with IBS reported 7 episodes of abdominal pain per week with a mean of more than 3 hours of daily pain duration. Abdominal distension was reported as frequently and persisting longer, whereas nausea was less frequent.
Table 3.
Gastrointestinal symptom diary data collected in the IBS sample.
3.2. Group differences in anterior insula neurotransmitter levels
Analyses of qMRS data revealed significant group differences in aINS neurotransmitter concentrations when comparing patients with IBS and HC. Specifically, patients showed significantly reduced concentrations of Glx in aINS bilaterally with more pronounced effects for the right hemisphere (left aINS U = 228.5; P = 0.017, right aINS U = 170; P < 0.001; Fig. 2 A and B). GABA+ concentrations in aINS did not significantly differ between groups (Fig. 3 A and B).
Figure 2.
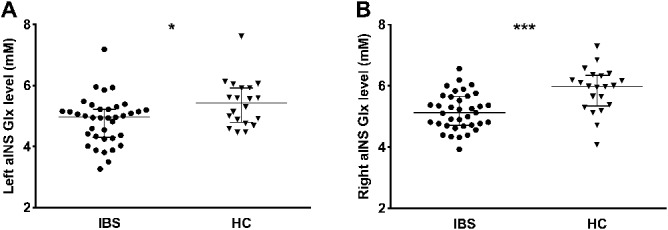
Results from group comparisons of Glx concentrations in (A) left and (B) right aINS in HC and patients with IBS. All data are given as median and interquartile range (IQR). Significantly lower bilateral aINS Glx concentrations were observed in patients with IBS compared to HC. *P < 0.05; ***P < 0.001. aINS, anterior insula; HC, healthy controls; IBS, irritable bowel syndrome.
Figure 3.
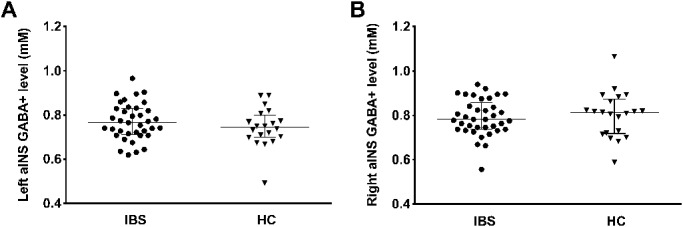
Results from group comparisons of GABA+ concentrations in (A) left and (B) right aINS between HC and patients with IBS. All data are given as median and interquartile range (IQR). No significant group differences were detected for GABA+ in either hemisphere. aINS, anterior insula; HC, healthy controls; IBS, irritable bowel syndrome.
3.3. Regression analyses
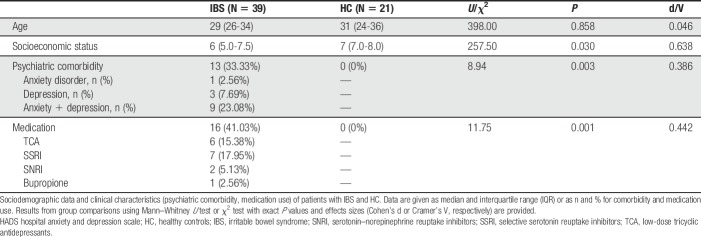
Stepwise regression analyses conducted in patients with IBS revealed hemisphere-specific predictors of Glx concentrations. Specifically, abdominal pain duration as assessed by gastrointestinal symptom diaries significantly predicted right aINS Glx concentrations (R2 = 0.354; F = 15.32; P = 0.001). In addition, a model including pain behaviors as a coping strategy was identified (R2 = 0.117; F = 5.99; P = 0.021). A longer pain duration per day (ß = −0.624; t = −4.44; P < 0.001; Fig. 4A) and less frequently used pain behaviors to cope with pain (ß = 0.344; t = 2.45; P = 0.021; Fig. 4B) independently predicted lower Glx concentrations.
Figure 4.
Scatterplots with regression curves and 95% confidence intervals depicting significant results from stepwise regression: (A) abdominal pain duration and (B) pain behaviors as predictors of Glx concentration in the right aINS, (C) diverting attention as a predictor of left aINS Glx and (D) calming self-statements as a significant predictor of GABA+ concentrations in the left aINS. aINS, anterior insula.
For the left hemisphere, diverting attention (an adaptive pain-coping strategy) significantly explained variance in aINS Glx concentrations (R2 = 0.228; F = 8.28; P = 0.008). Here, lower Glx in left aINS was significantly predicted by less frequent use of the adaptive coping style (ß = 0.478; t = 2.88; P = 0.008; Fig. 4C). Regarding GABA+ concentrations, regression analyses for the left hemisphere identified calming self-statements as an adaptive pain-coping strategy to be a significant predictor (R2 = 0.187; F = 6.46; P = 0.017), with a lower tendency to use the adaptive coping style predicting higher concentrations of the inhibitory neurotransmitter (ß = −0.433; t = −2.54; P = 0.017; Fig. 4D). No predictor for concentrations of right aINS GABA+ was found.
4. Discussion
Our study examined aINS neurotransmitter levels in women with IBS, a mechanism that might contribute to aberrant insular function consistently observed in chronic visceral pain disorders.3,7,9,19,32,38,50 We observed decreased concentrations of the excitatory neurotransmitter Glx in bilateral aINS in women with IBS compared with healthy women, whereas no significant group differences were found in aINS GABA+ concentrations. Furthermore, we demonstrated that longer duration of pain predicted lower Glx concentrations in the right aINS while lower Glx concentrations in the left aINS were predicted by less use of adaptive pain-coping strategies.
The aINS is considered a key node of interoceptive awareness with crucial relevance to pain processing and modulation.10,12,13,58 It is structurally and functionally connected with the limbic system41 and other regions involved in emotion processing and regulation,66 supporting a central role of aINS particularly in emotional aspects of pain. As a core region of the brain's salience network, the aINS further seems to be involved in switching between the default mode network and the central executive network in the face of salient events46,61 and serves an integrative function in the context of pain.4 The region is therefore likely of particular relevance in adaptive attentional, cognitive, and emotional responses to pain. Extensive reorganization of insular connectivity within the salience, default mode, and central executive networks29,31,65 has previously been described in patients with IBS, further supporting the assumption of substantial alterations within pain- and emotion-regulatory networks in IBS. Reduced Glx concentrations in bilateral aINS, as observed herein, could therefore reflect dysfunctional excitatory neurotransmission that might contribute to compromised regulatory attentional, cognitive, and emotional pain-related processing in patients suffering from IBS. At the same time, given extensive projections of aINS also to brainstem regions associated with descending pain modulation,63 reduced excitation of aINS could further detrimentally affect communication pathways with descending pain inhibitory circuits, as supported by findings also from other chronic pain conditions,58,64,68 and thereby impact the perception of pain.
In support of our spectroscopic findings, reduced hippocampal Glu concentrations in patients have previously been reported in the only other existing MRS study conducted in IBS.51 Studies in other chronic pain conditions highly comorbid with IBS, however, revealed partly inconsistent results, such as unaltered aINS neurochemistry in fibromyalgia,17,18,27,28 or increased Glu concentrations in posterior insula in this disease group.27 Finally, As-Sanie et al.2 demonstrated increased aINS Glx concentrations in women with endometriosis-associated chronic pelvic pain in comparison with healthy participants. While these inconsistent findings might be due to methodological differences, they could also indicate disease-specific neurochemical alterations, possibly adding to the chemical heterogeneity of the living human brain, as previously described.26,29,30,52
Interestingly, excitatory neurotransmitter concentrations in patients seemed to show hemisphere-specific relations to abdominal pain and pain‐coping, providing novel evidence on a biochemical level to support a functional asymmetry of aINS.10,12,69 Specifically, abdominal pain duration experienced by women with IBS was the most substantial predictor of lower Glx concentrations in the right but was not associated with Glx levels in the left aINS. The right aINS is considered to contain representation of sympathetic afferent stimuli such as pain and temperature.11 Accordingly, Zhou et al. demonstrated a positive correlation between spontaneous neural activity in the right INS and disease severity in functional dyspepsia,70 which is a visceral pain disorder known to overlap with IBS in up to 50% of patients.67 In IBS, successful treatment (hypnotherapy and educational intervention) was not only associated with a significant improvement in symptom severity, but also led to a significant attenuation in right aINS activation during rectal distensions.40 Our findings extend these observations of a distinct association between pain perception and right insular function in visceral pain conditions to a biochemical level.
We further found reduced left, but not right, aINS excitatory neurochemistry to be related to less use of adaptive cognitive coping strategies, specifically diverting attention when confronted with pain. Adaptive pain-coping strategies are considered as behavioral contributors to an effective top-down modulation of pain. Also, a stronger involvement of left rather than right aINS in top-down behavioral modulation has recently been proposed.33 Moreover, Zautra et al.69 showed that activating the left aINS by increased parasympathetic input (slow breathing) was correlated with decreases in affective responses to acute pain stimulation in healthy volunteers, whereas this regulatory mechanism appeared to be blunted in patients with fibromyalgia. Our findings complement and extend this evidence of a specific role of left aINS in adaptive top-down pain modulation, suggesting that reduced excitatory neurotransmitter levels in IBS might contribute to alterations involving a compromised capability to adaptively cope with pain.
Taken together, the observed pattern of increased pain associated with lower Glx concentrations in right aINS and less use of adaptive cognitive coping related to lower left insular Glx concentrations provide novel biochemical evidence suggesting an asymmetry of insular function of putative relevance in pain processing and its cognitive regulation in IBS.
In a heterogeneous group of conditions such as IBS, our study has several strengths. First, a recall bias is known to be an inherent problem of retrospective reports.37 The prospective pain data collection from gastrointestinal diaries applied in this study therefore likely provides a more reliable measure of symptoms in patients. Second, our study included well-phenotyped and age-matched samples of women with IBS and healthy women. Given that brain chemistry is reportedly sex- and age-dependent,26,29,30,52 together with the rationale that IBS is a disease with female predominance,15,16 including only women strengthens the study results. However, this approach might at the same time limit generalizability of this study; therefore, follow-up studies on insular neurotransmission are needed to address biochemical changes also in men suffering from IBS and to allow for the investigation of possible, yet unknown sex differences in insular biochemistry.
Furthermore, the current study has some limitations with respect to the spectroscopy measurements applied, which need to be addressed. The region of investigation in spectroscopic measures involves different tissue types, such as gray matter, white matter, and cerebrospinal fluid. The current data were not corrected for possibly different contribution of these tissue types. While we therefore cannot exclude that group differences observed in Glx concentrations may have been affected by distinct features in tissue contribution in women with IBS and healthy women, a recent report using high-resolution MRI demonstrated differences in concentrations of GABA to be substantially larger when comparing gray matter and white matter than was the case for Glu.23 Given that, even using a sequence optimized for GABA, no group differences in concentrations of the inhibitory neurotransmitter were observed, our findings are unlikely to be mainly attributable to differences in tissue contribution.
Finally, the MEGA-PRESS technique used in this study is considered “state of the art” for a noninvasive evaluation of GABA concentrations.52,57 While assessing Glx from MEGA-PRESS “OFF” spectra represents a good proxy for conventional measures of Glx concentrations in brain tissue,42,53 the sequence is primarily optimized for the detection of GABA. Specifically, since the echo time used in the editing sequence in this study was chosen for optimal GABA+ quantification, this may have led to a loss in signal-to-noise ratio of Glx due to the faster relaxation of Glu compared with GABA. Further research focusing on the excitatory neurotransmitter and applying an acquisition protocol optimized for the detection of Glu are needed to substantiate the reported observations.
Together, our findings of decreased glutamatergic yet unaltered GABAergic neurotransmitter concentrations in patients with IBS and left and right lateralized associations with abdominal pain and coping provide novel insights into brain molecular mechanisms potentially contributing to IBS pathophysiology by affecting circuits of salience and pain modulation. Our observations support the assumption that glutamatergic deficiency might be involved in a failure to adaptively engage aINS in pain processing and modulation, which could contribute to more pain symptoms and increased symptom burden in patients with IBS.
Conflict of interest statement
The authors have no conflict of interest to declare.
Partial study sample was published as conference abstract at the Neurogastroenterology and Motility Conference in Cork, Ireland in 2017.
Supplementary Material
Acknowledgments
The authors are very grateful to the following for providing the segmentations used to create these atlases: David Kennedy and Christian Haselgrove, Centre for Morphometric Analysis, Harvard; Bruce Fischl, the Martinos Center for Biomedical Imaging, MGH (NIH grants P41-RR14075, R01 RR16594-01A1, R01 NS052585-01); Janis Breeze and Jean Frazier from the Child and Adolescent Neuropsychiatric Research Program, Cambridge Health Alliance (NIH grants K08 MH01573, K01 MH01798); Larry Seidman and Jill Goldstein from the Department of Psychiatry of Harvard Medical School.
This work was supported by the County Council of Östergötland, the AFA research foundation DNR. 140407, the Bengt-Ihre fund, and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) ‐ DFG IC 81/1-1 and project number 316803389 ‐ SFB 1280. This study was also supported by Kurt and Helena Widéns Research Fund, and the “Seeing Organ Function” project funded by the Knut and Alice Wallenberg Foundation. The funding agencies had no role in the conception, analysis, or interpretation of the data.
Author contributions: O. Bednarska: planning and conducting the study, collecting and interpreting data, drafting the manuscript, and has approved the final draft submitted. A. Icenhour: planning and conducting the study, collecting and interpreting data, drafting the manuscript, and has approved the final draft submitted. S. Tapper: planning and conducting the study, collecting and interpreting data, drafting the manuscript, and has approved the final draft submitted. S.T. Witt: planning and conducting the study, collecting and interpreting data, drafting the manuscript, and has approved the final draft submitted. A. Tisell: planning and conducting the study, and has approved the final draft submitted. P. Lundberg: planning the study, drafting the manuscript, and has approved the final draft submitted. S. Elsenbruch: planning the study, drafting the manuscript, and has approved the final draft submitted. M. Engström: planning the study, drafting the manuscript, and has approved the final draft submitted. S. Walter: planning and conducting the study, collecting and interpreting data, drafting the manuscript, and has approved the final draft submitted.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/A788.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
O. Bednarska and A. Icenhour contributed equally.
References
- [1].Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychol 2000;19:586–92. [DOI] [PubMed] [Google Scholar]
- [2].As-Sanie S, Kim J, Schmidt-Wilcke T, Sundgren PC, Clauw DJ, Napadow V, Harris RE. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain 2016;17:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Baliki MN, Baria AT, Apkarian AV. The cortical rhythms of chronic back pain. J Neurosci 2011;31:13981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bastuji H, Frot M, Perchet C, Hagiwara K, Garcia-Larrea L. Convergence of sensory and limbic noxious input into the anterior insula and the emergence of pain from nociception. Sci Rep 2018;8:13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52:69–77. [DOI] [PubMed] [Google Scholar]
- [6].Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex 2013;23:739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ching YY, Wang C, Tay T, Loke YM, Tang PH, Sng BL, Zhou J. Altered sensory insular connectivity in chronic postsurgical pain patients. Front Hum Neurosci 2018;12:483-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cleeland CS, Ryan KM. Pain assessment: global use of the Brief pain inventory. Ann Acad Med Singapore 1994;23:129–38. [PubMed] [Google Scholar]
- [9].Cottam WJ, Iwabuchi SJ, Drabek MM, Reckziegel D, Auer DP. Altered connectivity of the right anterior insula drives the pain connectome changes in chronic knee osteoarthritis. PAIN 2018;159:929–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 2002;3:655–66. [DOI] [PubMed] [Google Scholar]
- [11].Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn Sci 2005;9:566–71. [DOI] [PubMed] [Google Scholar]
- [12].Craig AD. How do you feel-now? The anterior insula and human awareness. Nat Rev Neurosci 2009;10:59–70. [DOI] [PubMed] [Google Scholar]
- [13].Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci 2011;1225:72–82. [DOI] [PubMed] [Google Scholar]
- [14].Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31:968–80. [DOI] [PubMed] [Google Scholar]
- [15].Drossman DA, Morris CB, Schneck S, Hu YJ, Norton NJ, Norton WF, Weinland SR, Dalton C, Leserman J, Bangdiwala SI. International survey of patients with IBS: symptom features and their severity, health status, treatments, and risk taking to achieve clinical benefit. J Clin Gastroenterol 2009;43:541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, Niesler B, Quigley EM, Rajilic-Stojanovic M, Schemann M, Schwille-Kiuntke J, Simren M, Zipfel S, Spiller RC. Irritable bowel syndrome. Nat Rev Dis Primers 2016;2:16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fayed N, Andres E, Viguera L, Modrego PJ, Garcia-Campayo J. Higher glutamate+glutamine and reduction of N-acetylaspartate in posterior cingulate according to age range in patients with cognitive impairment and/or pain. Acad Radiol 2014;21:1211–7. [DOI] [PubMed] [Google Scholar]
- [18].Fayed N, Andres E, Rojas G, Moreno S, Serrano-Blanco A, Roca M, Garcia-Campayo J. Brain dysfunction in fibromyalgia and somatization disorder using proton magnetic resonance spectroscopy: a controlled study. Acta Psychiatr Scand 2012;126:115–25. [DOI] [PubMed] [Google Scholar]
- [19].Flodin P, Martinsen S, Altawil R, Waldheim E, Lampa J, Kosek E, Fransson P. Intrinsic brain connectivity in chronic pain: a resting-state fMRI study in patients with rheumatoid arthritis. Front Hum Neurosci 2016;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Foerster BR, Petrou M, Edden RA, Sundgren PC, Schmidt-Wilcke T, Lowe SE, Harte SE, Clauw DJ, Harris RE. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum 2012;64:579–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997;11:395–402. [DOI] [PubMed] [Google Scholar]
- [22].Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN, Herbert MR, Bent EK, Koneru VK, Dieterich ME, Hodge SM, Rauch SL, Grant PE, Cohen BM, Seidman LJ, Caviness VS, Biederman J. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry 2005;162:1256–65. [DOI] [PubMed] [Google Scholar]
- [23].Ganji SK, An Z, Banerjee A, Madan A, Hulsey KM, Choi C. Measurement of regional variation of GABA in the human brain by optimized point-resolved spectroscopy at 7 T in vivo. NMR Biomed 2014;27:1167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Goldstein JM, Seidman LJ, Makris N, Ahern T, O'Brien LM, Caviness VS, Jr, Kennedy DN, Faraone SV, Tsuang MT. Hypothalamic abnormalities in schizophrenia: sex effects and genetic vulnerability. Biol Psychiatry 2007;61:935–45. [DOI] [PubMed] [Google Scholar]
- [25].Govind V, Young K, Maudsley AA. Corrigendum: proton NMR chemical shifts and coupling constants for brain metabolites. Govindaraju V, Young K, Maudsley AA, NMR Biomed. 2000; 13: 129–153. NMR Biomed 2015;28:923–4. [DOI] [PubMed] [Google Scholar]
- [26].Grachev ID, Apkarian AV. Chemical heterogeneity of the living human brain: a proton MR spectroscopy study on the effects of sex, age, and brain region. Neuroimage 2000;11:554–63. [DOI] [PubMed] [Google Scholar]
- [27].Harris RE, Sundgren PC, Craig AD, Kirshenbaum E, Sen A, Napadow V, Clauw DJ. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum 2009;60:3146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Harris RE, Napadow V, Huggins JP, Pauer L, Kim J, Hampson J, Sundgren PC, Foerster B, Petrou M, Schmidt-Wilcke T, Clauw DJ. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology 2013;119:1453–64. [DOI] [PubMed] [Google Scholar]
- [29].Hong JY, Kilpatrick LA, Labus JS, Gupta A, Katibian D, Ashe-McNalley C, Stains J, Heendeniya N, Smith SR, Tillisch K, Naliboff B, Mayer EA. Sex and disease-related alterations of anterior insula functional connectivity in chronic abdominal pain. J Neurosci 2014;34:14252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hong JY, Kilpatrick LA, Labus J, Gupta A, Jiang Z, Ashe-McNalley C, Stains J, Heendeniya N, Ebrat B, Smith S, Tillisch K, Naliboff B, Mayer EA. Patients with chronic visceral pain show sex-related alterations in intrinsic oscillations of the resting brain. J Neurosci 2013;33:11994–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Icenhour A, Witt ST, Elsenbruch S, Lowen M, Engstrom M, Tillisch K, Mayer EA, Walter S. Brain functional connectivity is associated with visceral sensitivity in women with Irritable Bowel Syndrome. Neuroimage Clin 2017;15:449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ichesco E, Puiu T, Hampson JP, Kairys AE, Clauw DJ, Harte SE, Peltier SJ, Harris RE, Schmidt-Wilcke T. Altered fMRI resting-state connectivity in individuals with fibromyalgia on acute pain stimulation. Eur J Pain 2016;20:1079–89. [DOI] [PubMed] [Google Scholar]
- [33].Kann S, Zhang S, Manza P, Leung HC, Li CR. Hemispheric lateralization of resting-state functional connectivity of the anterior insula: association with age, gender, and a novelty-seeking trait. Brain Connect 2016;6:724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Klose U. In vivo proton spectroscopy in presence of eddy currents. Magn Reson Med 1990;14:26–30. [DOI] [PubMed] [Google Scholar]
- [35].Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med 2002;64:258–66. [DOI] [PubMed] [Google Scholar]
- [36].Labus JS, Bolus R, Chang L, Wiklund I, Naesdal J, Mayer EA, Naliboff BD. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther 2004;20:89–97. [DOI] [PubMed] [Google Scholar]
- [37].Lackner JM, Jaccard J, Keefer L, Firth R, Carosella AM, Sitrin M, Brenner D. The accuracy of patient-reported measures for GI symptoms: a comparison of real time and retrospective reports. Neurogastroenterol Motil 2014;26:1802–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Larsson MB, Tillisch K, Craig AD, Engstrom M, Labus J, Naliboff B, Lundberg P, Strom M, Mayer EA, Walter SA. Brain responses to visceral stimuli reflect visceral sensitivity thresholds in patients with irritable bowel syndrome. Gastroenterology 2012;142:463–72.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997;32:920–4. [DOI] [PubMed] [Google Scholar]
- [40].Lowen MB, Mayer EA, Sjoberg M, Tillisch K, Naliboff B, Labus J, Lundberg P, Strom M, Engstrom M, Walter SA. Effect of hypnotherapy and educational intervention on brain response to visceral stimulus in the irritable bowel syndrome. Aliment Pharmacol Ther 2013;37:1184–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lu C, Yang T, Zhao H, Zhang M, Meng F, Fu H, Xie Y, Xu H. Insular cortex is critical for the perception, modulation, and chronification of pain. Neurosci Bull 2016;32:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Maddock RJ, Caton MD, Ragland JD. Estimating glutamate and Glx from GABA-optimized MEGA-PRESS: off-resonance but not difference spectra values correspond to PRESS values. Psychiatry Res Neuroimaging 2018;279:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Makris N, Goldstein JM, Kennedy D, Hodge SM, Caviness VS, Faraone SV, Tsuang MT, Seidman LJ. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophrenia Res 2006;83:155–71. [DOI] [PubMed] [Google Scholar]
- [44].Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med 2011;62:381–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mayer EA, Labus JS, Tillisch K, Cole SW, Baldi P. Towards a systems view of IBS. Nat Rev Gastroenterol Hepatol 2015;12:592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 2010;214:655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed 1998;11:266–72. [DOI] [PubMed] [Google Scholar]
- [48].Mullins PG, McGonigle DJ, O'Gorman RL, Puts NA, Vidyasagar R, Evans CJ, Edden RA. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage 2014;86:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Namkung H, Kim SH, Sawa A. The insula: an underestimated brain area in clinical neuroscience, psychiatry, and neurology. Trends Neurosci 2017;40:200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum 2010;62:2545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Niddam DM, Tsai SY, Lu CL, Ko CW, Hsieh JC. Reduced hippocampal glutamate-glutamine levels in irritable bowel syndrome: preliminary findings using magnetic resonance spectroscopy. Am J Gastroenterol 2011;106:1503–11. [DOI] [PubMed] [Google Scholar]
- [52].O'Gorman RL, Michels L, Edden RA, Murdoch JB, Martin E. In vivo detection of GABA and glutamate with MEGA-PRESS: reproducibility and gender effects. J Magn Reson Imaging 2011;33:1262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993;30:672–9. [DOI] [PubMed] [Google Scholar]
- [54].Ragnarsson G, Bodemar G. Division of the irritable bowel syndrome into subgroups on the basis of daily recorded symptoms in two outpatients samples. Scand J Gastroenterol 1999;34:993–1000. [DOI] [PubMed] [Google Scholar]
- [55].Ramadan S, Lin A, Stanwell P. Glutamate and glutamine: a review of in vivo MRS in the human brain. NMR Biomed 2013;26:1630–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. PAIN 1983;17:33–44. [DOI] [PubMed] [Google Scholar]
- [57].Schubert F, Gallinat J, Seifert F, Rinneberg H. Glutamate concentrations in human brain using single voxel proton magnetic resonance spectroscopy at 3 Tesla. Neuroimage 2004;21:1762–71. [DOI] [PubMed] [Google Scholar]
- [58].Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007;27:2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Sherwin LB, Leary E, Henderson WA. The association of catastrophizing with quality-of-life outcomes in patients with irritable bowel syndrome. Qual Life Res 2017;26:2161–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Singh-Manoux A, Marmot MG, Adler NE. Does subjective social status predict health and change in health status better than objective status? Psychosom Med 2005;67:855–61. [DOI] [PubMed] [Google Scholar]
- [61].Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A 2008;105:12569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Tornblom H, Drossman DA. Psychotropics, antidepressants, and visceral analgesics in functional gastrointestinal disorders. Curr Gastroenterol Rep 2018;20:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron 2007;55:377–91. [DOI] [PubMed] [Google Scholar]
- [64].Truini A, Tinelli E, Gerardi MC, Calistri V, Iannuccelli C, La Cesa S, Tarsitani L, Mainero C, Sarzi-Puttini P, Cruccu G, Caramia F, Di Franco M. Abnormal resting state functional connectivity of the periaqueductal grey in patients with fibromyalgia. Clin Exp Rheumatol 2016;34(2 suppl 96):S129–33. [PubMed] [Google Scholar]
- [65].Weng Y, Qi R, Liu C, Ke J, Xu Q, Wang F, Zhang LJ, Lu GM. Disrupted functional connectivity density in irritable bowel syndrome patients. Brain Imaging Behav 2017;11:1812–22. [DOI] [PubMed] [Google Scholar]
- [66].Wiech K, Jbabdi S, Lin CS, Andersson J, Tracey I. Differential structural and resting state connectivity between insular subdivisions and other pain-related brain regions. PAIN 2014;155:2047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Yarandi SS, Christie J. Functional dyspepsia in review: pathophysiology and challenges in the diagnosis and management due to coexisting gastroesophageal reflux disease and irritable bowel syndrome. Gastroenterol Res Pract 2013;2013:351086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yu R, Gollub RL, Spaeth R, Napadow V, Wasan A, Kong J. Disrupted functional connectivity of the periaqueductal gray in chronic low back pain. Neuroimage Clin 2014;6:100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zautra AJ, Fasman R, Davis MC, Craig AD. The effects of slow breathing on affective responses to pain stimuli: an experimental study. PAIN 2010;149:12–8. [DOI] [PubMed] [Google Scholar]
- [70].Zhou G, Liu P, Wang J, Wen H, Zhu M, Zhao R, von Deneen KM, Zeng F, Liang F, Gong Q, Qin W, Tian J. Fractional amplitude of low-frequency fluctuation changes in functional dyspepsia: a resting-state fMRI study. Magn Reson Imaging 2013;31:996–1000. [DOI] [PubMed] [Google Scholar]
- [71].Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.